Abstract
Background
Esophageal thermal injury (ETI) is a byproduct of atrial fibrillation (AF) ablation using thermal sources. The most severe form of ETI is represented by atrioesophageal fistula, which has a high mortality rate. Late gadolinium enhancement (LGE) magnetic resonance imaging (MRI) allows identification of ETI. Hence, we sought to evaluate the utility of LGE‐MRI as a method to identify ETI across the entire spectrum of severity.
Methods and Results
All AF radiofrequency ablations performed at the University of Utah between January 2009 and December 2017 were reviewed. Patients with LGE‐MRI within 24 hours following AF ablation as well as patients who had esophagogastroduodenoscopy in addition to LGE‐MRI were identified. An additional patient with atrioesophageal fistula who had AF ablation at a different institution and had MRI and esophagogastroduodenoscopy at the University of Utah was identified. A total of 1269 AF radiofrequency ablations were identified. ETI severity was classified on the basis of esophageal LGE pattern (none, 60.9%; mild, 27.5%; moderate, 9.9%; severe, 1.7%). ETI resolved in most patients who underwent repeat LGE‐MRI at 3 months. All patients with esophagogastroduodenoscopy‐confirmed ETI had moderate‐to‐severe LGE 24 hours after ablation MRI. Moderate‐to‐severe LGE had 100% sensitivity and 58.1% specificity in detecting ETI, and a negative predictive value of 100%. Atrioesophageal fistula was visualized by both computed tomography and LGE‐MRI in one patient.
Conclusions
LGE‐MRI is useful in detecting and characterizing ETI across the entire severity spectrum. LGE‐MRI exhibits an extremely high sensitivity and negative predictive value in screening for ETI after AF ablation.
Keywords: ablation, atrial fibrillation, atrioesophageal fistula, EGD, esophageal thermal injury, LGE‐MRI
Subject Categories: Atrial Fibrillation, Magnetic Resonance Imaging (MRI), Catheter Ablation and Implantable Cardioverter-Defibrillator, Complications
Nonstandard Abbreviations and Acronyms
- AEF
atrioesophageal fistula
- ETI
esophageal thermal injury
- LGE
late gadolinium enhancement
- PV
pulmonary vein
- PVI
pulmonary vein
isolation
- RFA
radiofrequency ablation
Clinical Perspective
What Is New?
Late gadolinium enhancement magnetic resonance imaging (LGE‐MRI) is highly sensitive for detecting postablation esophageal thermal injury (ETI).
LGE‐MRI has a high negative predictive value to exclude the presence of ETI in the absence of moderate or severe esophageal enhancement.
Postablation ETI is frequently detected by LGE‐MRI, which resolves in the majority of patients.
What Are the Clinical Implications?
LGE‐MRI is a valuable noninvasive diagnostic tool for detection and monitoring of postablation ETI.
LGE‐MRI may be used to assess ETI across the entire spectrum of severity, including atrioesophageal fistula.
Pulmonary vein isolation (PVI) using radiofrequency is an established treatment for atrial fibrillation (AF). 1 PVI involves ablation on the posterior wall of the left atrium (LA). For many patients with persistent AF and significant substrate outside of the pulmonary veins (PVs), additional strategies such as posterior wall isolation, scar homogenization, and complex fractionated atrial electrogram ablation are often used. 2 , 3 , 4 , 5 The esophagus runs adjacent to the posterior wall of the LA within a distance of as small as 2.5 mm. 6 Thus, a major limitation of radiofrequency ablation (RFA) at the posterior wall is the risk of esophageal thermal injury (ETI). 7 , 8 Late gadolinium enhancement (LGE) magnetic resonance imaging (MRI) is an effective tool for detection of acute tissue injury such as inflammation and edema, and therefore has the potential to serve as a noninvasive test to detect and follow the progression of ETI. 9 , 10 , 11 Existing data on the role of LGE‐MRI on detection of ETI and correlation of postablation LGE‐MRI lesions and esophagogastroduodenoscopy (EGD) findings are scarce. The purpose of this study was to evaluate the value of LGE‐MRI in the characterization of ETI across the entire spectrum of severity after AF RFA.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design and Patient Population
Patient data were obtained from the Atrial Fibrillation Research Registry at the Comprehensive Arrhythmia Research and Management Center (CARMA), University of Utah, Salt Lake City, Utah. 12 This registry is an observational, retrospective database of patients who undergo LGE‐MRI as part of routine arrhythmia care at the University of Utah. The database study protocol was reviewed and approved by the University of Utah Institutional Review Board and was Health Insurance Portability and Accountability Act compliant. Patient requirement for consent has been waived.
All AF RFAs performed at the University of Utah between January 2009 and December 2017 were reviewed. The inclusion criteria were (1) patients aged 18 years or older and (2) patients referred for AF RFA. A cohort of patients who underwent a same‐day (<24 hours) post‐RFA LGE‐MRI was identified and examined. Furthermore, a subset of patients who underwent both a same‐day post‐RFA LGE‐MRI and an early post‐RFA esophagogastroduodenoscopy (EGD) was identified. The algorithm we used at the University of Utah hospital is shown in Figure 1.
Figure 1. Postablation University of Utah esophageal thermal injury evaluation algorithm.
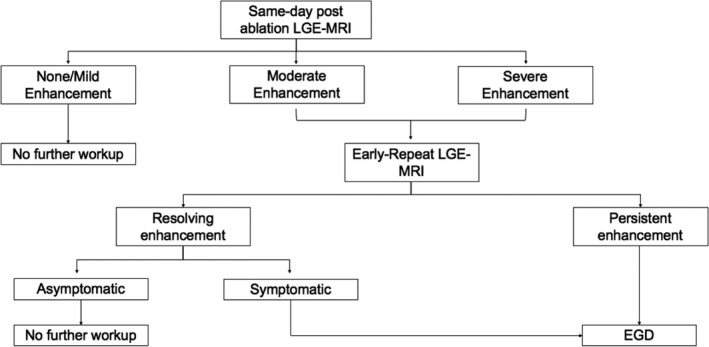
EGD indicates esophagogastroduodenoscopy; and LGE‐MRI, late gadolinium enhancement magnetic resonance imaging.
In addition, a patient with atrioesophageal fistula (AEF) secondary to an AF RFA performed at another institution who had LGE‐MRI and EGD at our institution was identified.
RFA Procedure
The left atrial RFA procedure was previously described in detail. 13 , 14 Briefly, a multipole catheter was used to record right atrial and coronary sinus electrograms and as the reference catheter for 3‐dimensional electroanatomical mapping with CARTO (Biosense Webster, Diamond Bar, CA) or ESI/NavX (Abbott, North Chicago, IL). Two transseptal punctures were performed under intracardiac echocardiography guidance using a phased‐array catheter (AccuNav, Siemens Medical Solutions USA, Mountain View, CA; CartoSound, Biosense Webster; ViewFlex Xtra, Abbott). A mapping catheter (Lasso or Pentaray, Biosense Webster; Reflexion Spiral, St. Jude Medical, Saint Paul, MN) and an RFA catheter (ThermoCool NaviStar, Biosense Webster; Tacticath, Abbott) were advanced into the LA for mapping and ablation. Ablation lesions were placed in a circular fashion in the PV antral region until electrical isolation of the PVs was achieved. PVI was successful in all patients acutely. Radiofrequency delivery was interrupted if the impedance rose suddenly or if a burst in microbubble density was seen by intracardiac echocardiography. Additional LA posterior wall ablation was performed as previously described. 13 Some operators reduced the power to 25 to 30 W on the posterior wall, whereas other operators used a high‐power, short‐duration (40 or 50 W) strategy with limited force and duration for posterior wall radiofrequency applications. The decision on the ablation strategy was at the operator’s discretion. A temperature probe was used in every procedure.
MRI Image Acquisition
Protocol for postablation LGE‐MRI was previously described. 10 , 15 Briefly, MRI studies were performed using either a 1.5‐T Avanto or a 3‐T Verio clinical MRI scanner (Siemens Healthcare, Erlangen, Germany). High‐resolution LGE images were acquired using 3‐dimensional electrocardiogram‐gated, respiratory‐navigated, inversion‐recovery prepared, spoiled gradient‐recalled echo pulse sequence. An LGE‐MRI scan was initiated about 15 minutes following injection of 0.1 mmol/kg gadobenate dimeglumine (Multihance; Bracco Diagnostics, Princeton, NJ). The scan parameters were as follows: axial imaging volume with a voxel size of 1.25×1.25×2.5 mm, inversion time=270–350 ms, and generalized autocalibrating partially parallel acquisition with reduction factor R=2 in phase‐encoding (right–left) direction. Typical scan parameters for LGE‐MRI at the 1.5‐T scanner were: echo time=2.4 ms, repetition time=5.2 ms, flip angle=20°. Scan parameters for LGE‐MRI at the 3‐T scanner were: echo time/repetition time=1.4/3.1 ms, flip angle=14°. The navigator was positioned on the right hemidiaphragm, and the navigator acceptance window was ±3 mm. Inversion recovery preparation was applied every heartbeat, and fat saturation was performed immediately before data acquisition during the stationary phase of the LA cardiac cycle. The inversion time value for the LGE‐MRI scan was identified using an inversion time scout scan.
Esophageal LGE was classified as none, no observable LGE of the esophagus; mild, minimal or focal LGE; moderate, transmural or near transmural LGE of the anterior wall of the esophagus; and severe, transmural LGE involving more than 5 mm of the esophagus or in more than one location (Figure 2). 15 The interpretation of the severity was performed by a reading by a radiologist or imaging cardiologist at the time of image acquisition.
Figure 2. Representative examples of different degrees of esophageal late gadolinium enhancement on magnetic resonance imaging.
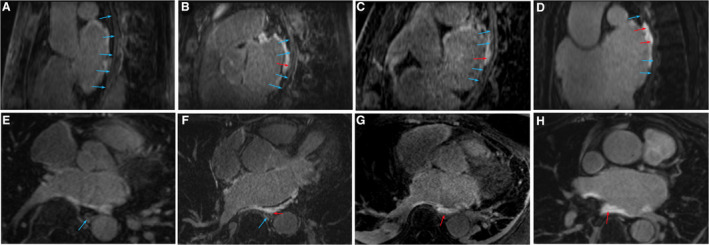
Longitudinal (A) and transversal (E) view of the esophagus without enhancement. Longitudinal (B) and transversal (F) view of the esophagus with mild enhancement. Longitudinal (C) and transversal (G) view of the esophagus with moderate enhancement. Longitudinal (D) and transversal (H) view of the esophagus with severe enhancement. Blue arrows indicate esophageal wall without enhancement. Red arrows indicate areas with anterior esophageal wall enhancement.
Esophagogastroduodenoscopy
EGD was performed under moderate sedation/analgesia (conscious sedation). The endoscope was introduced through the mouth, and advanced to the second part of duodenum. Throughout the procedure, the patient's blood pressure, pulse, and oxygen saturations were monitored continuously. The findings were collected from the EGD reports.
Statistical Analysis
We used the mean and standard deviation for continuous variables with normal distribution, median and interquartile range for continuous variables with nonnormal distribution, and percentages for categorical variables to describe the distribution of baseline characteristics. Pearson χ2 and Fisher exact tests were used to compare the categorical variables. A McNemar test was used to compare paired variables. A 2‐sided P<0.05 was considered statistically significant. To access interrater agreement, 56 random scans were independently scored by 2 readers, and weighted κ was calculated. LGE‐MRI sensitivity was determined by dividing the total number of RFA procedures resulting in moderate/severe enhancement on LGE‐MRI and ETI evidence on EGD (true positives) over the total number of EGD procedures showing evidence of ETI (true positive+false negatives). Specificity was calculated by dividing the number of RFA procedures resulting in no moderate/severe enhancement on LGE‐MRI and no evidence of ETI on EGD (true negatives) over the total number of EGD procedures showing no evidence of ETI (true negatives+false positives). Negative predictive value was calculated by dividing the number of RFA procedures resulting in no moderate/severe enhancement on LGE‐MRI and no evidence of ETI on EGD (true negatives) over the total number of LGE‐MRI tests without evidence of moderate/severe enhancement (true negatives+false negatives). Statistical analyses were performed using R version 3.4.0 open‐source software (R Foundation, Vienna, Austria).
Results
Post‐AF Ablation ETI Detection by LGE‐MRI
A total of 2102 AF RFA procedures performed at the University of Utah Hospital between January 2007 and December 2017 were identified. LGE‐MRI was performed within 24 hours of 1269 AF RFA procedures (60% of the total AF procedures) in 1074 patients (Figure 3). The mean age was 65±11 years, and 66% of the patients were men. The Table shows the baseline clinical characteristics of all patients. The severity of post‐RFA ETI was as follows: none after 773 AF RFA procedures (60.9%), mild enhancement after 349 AF RFA procedures (27.5%), moderate enhancement after 126 AF RFA procedures (9.9%), and severe enhancement after 21 AF RFA procedures (1.7%). The AF RFA technique was available in 1034 cases (81.5% of all cases). PVI alone was performed in 345 cases (33.4%), whereas PVI in addition to posterior wall isolation and/or debulking was performed in 689 cases (66.6%). Nevertheless, the distribution of LGE severity was similar between patients who underwent PVI alone and those who underwent PVI and posterior wall isolation/posterior wall debulking (none/mild: 89.3% versus 89.4%, moderate: 8.4% versus 9.0%, and severe 2.3% versus 1.6%, P=0.65) (Figure 4).
Figure 3. AF ablation procedures flowchart based on LGE‐MRI findings.
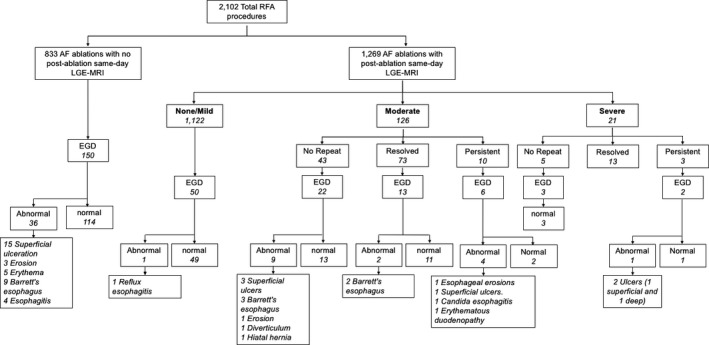
AF indicates atrial fibrillation; EGD, esophagogastroduodenoscopy; LGE‐MRI, late gadolinium enhancement magnetic resonance imaging; and RFA, radiofrequency ablation.
Table 1.
Baseline Characteristics of Patients With Late Gadolinium Enhancement Magnetic Resonance Imaging Within 24 Hours After Atrial Fibrillation Ablation (N=1074)
| Baseline Clinical Characteristics | Value |
|---|---|
| Age, y | 65.2±11.3 |
| Men, % (n) | 66.0% (709) |
| Coronary artery disease, % (n) | 19.9% (214) |
| Coronary artery bypass graft surgery, % (n) | 8.6% (92) |
| Cardiomyopathy, % (n) | 9.1% (98) |
| Chronic obstructive pulmonary disease, % (n) | 16.6% (178) |
| Congestive heart failure, % (n) | 12.7% (136) |
| Diabetes mellitus, % (n) | 17.1% (184) |
| Hyperlipidemia, % (n) | 16.6% (178) |
| Hypertension, % (n) | 65.0% (698) |
| Mitral valve regurgitation, % (n) | 6.1% (66) |
| Previous myocardial infarction, % (n) | 5.1% (55) |
| Obstructive sleep apnea, % (n) | 27.2% (292) |
| History of stroke/TIA, % (n) | 10.4% (112) |
| Tobacco use, % (n) | 26.8% (288) |
| Atrial fibrillation type, % (n) | |
| Paroxysmal | 39.3% (422) |
| Persistent | 60.7% (652) |
| Radiofrequency ablation technique, % (n), n=1034 | |
| Pulmonary vein isolation | 33.4% (345) |
| Pulmonary vein isolation with posterior wall isolation or debulking | 66.6% (689) |
TIA indicates transient ischemic attack.
Figure 4. Severity of late gadolinium enhancement magnetic resonance imaging between cases treated with and without posterior wall ablation.
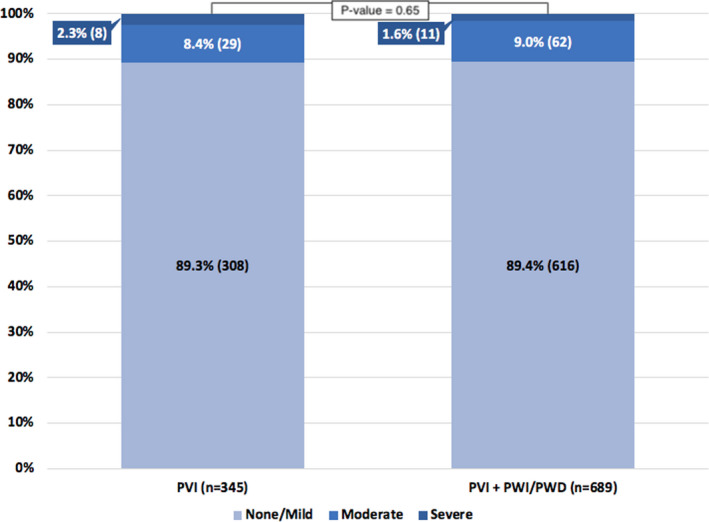
PVI indicates pulmonary vein isolation; PWD, posterior wall debulking; and PWI, posterior wall isolation.
Progression of ETI on LGE‐MRI
One hundred seventy‐five AF RFA procedures with same‐day LGE‐MRI were followed with early‐repeat LGE‐MRI (within 14 days of AF RFA), 805 AF RFA procedures with same‐day LGE‐MRI were followed with late‐repeat LGE‐MRI (after 14 days of AF RFA), and 289 AF RFA procedures with same‐day LGE‐MRI were not followed with repeat LGE‐MRI. With the exception of an increased prevalence of cardiomyopathy and heart failure among patients with no follow‐up MRI, all other baseline characteristic differences are statistically insignificant compared with patients with follow‐up MRI (Table S1).
In the subset of patients with early‐repeat LGE‐MRI (n=175), the severity of ETI was none (17.7%), mild (26.9%), moderate (46.3%), and severe (9.1%) on the same‐day LGE‐MRI and improved in most patients on the early‐repeat LGE‐MRI as follows: none (54.9%), mild (37.7%), moderate (6.3%), and severe (1.1%) (Figure 5). In patients with moderate‐to‐severe enhancement on LGE‐MRI (n=97), 78 patients (80.4%) had improvement in LGE, 17 patients (17.5%) had stable LGE, and 2 patients (2.1%) with moderate enhancement progressed to severe enhancement on early‐repeat imaging. There was an extremely significant improvement in moderate/severe LGE versus none/mild LGE on repeat imaging compared with same‐day imaging (P<0.0001).
Figure 5. Progression of LGE‐MRI–detected esophageal thermal injury.
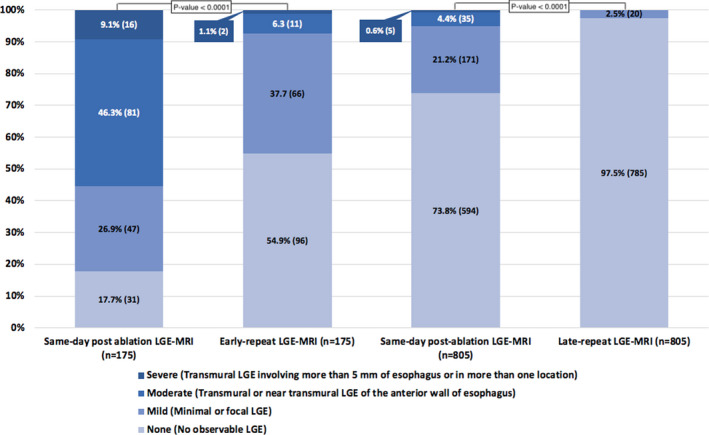
LGE indicates late gadolinium enhancement; and MRI, magnetic resonance imaging.
Data on MRI scanner type were available for 982 cases; 301 cases (30.7%) were performed with a 1.5‐T MRI scanner and 681 cases (69.3%) were performed with a 3‐T MRI scanner. A statistically significant difference in distribution of LGE‐MRI severity was found between patients scanned with a 1.5‐T and 3‐T scanner (none/mild: 91.4% versus 87.8%, moderate: 8.3% versus 9.5%, and severe 0.3% versus 2.6%, respectively; Fisher exact test P=0.028) (Figure 6).
Figure 6. Severity of late gadolinium enhancement magnetic resonance imaging (MRI) between cases scanned with 1.5‐T vs 3‐T MRI.
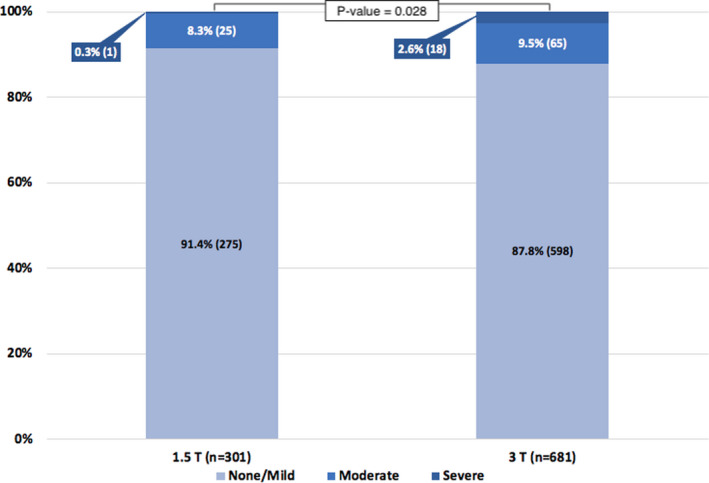
T indicates tesla.
Severity of ETI detected by same‐day post–AF ablation LGE‐MRI resolved in most patients on late‐repeat LGE‐MRI (n=805). On the same‐day after AF ablation, LGE‐MRI severity was none (73.8%), mild (21.2%), moderate (4.4%), severe (0.6%), and on late‐repeat LGE‐MRI was none (97.5%) and mild (2.5%) (Figure 5). There was a significant improvement in moderate/severe LGE versus none/mild LGE on repeat imaging compared with same‐day imaging (P<0.0001). None of the patients had evidence of AEF on LGE‐MRI. All patients with moderate‐to‐severe LGE who had late‐repeat MRI (n=40) had improvement in LGE on MRI, and only 9.5% of patients with evidence of ETI on same‐day MRI had evidence of mild ETI at 3 months.
The interrater agreement of MRI imaging interpretation was considered substantial (weight κ=0.741). 16
EGD in Patients With LGE‐MRI Post‐AF Ablation–Detected ETI
Ninety‐seven patients had early post–AF ablation LGE‐MRI and EGD. The average patient was aged 64.3±12 years, and 59.7% were men. Fifty‐seven percent had hypertension, 9% had heart failure, 11% had diabetes mellitus, and 11% had a prior transient ischemic attack or stroke.
The median duration between same‐day MRI and EGD was 1 day (interquartile range, 1–3] after AF ablation. Nine patients had evidence of ETI on EGD. Eight out of 9 patients with EGD‐confirmed ETI had moderate‐to‐severe ETI on LGE‐MRI (Figure 7). Moderate‐to‐severe enhancement on LGE‐MRI had 94.1% sensitivity and 64.5% specificity for detecting an abnormality on EGD. The negative predictive value of moderate‐to‐severe enhancement on LGE‐MRI was 98% to exclude an abnormality on EGD. LGE‐MRI had 100% sensitivity and 58.1% specificity for detecting EGD‐confirmed ETI and 100% negative predictive value to exclude ETI in the absence of moderate or severe enhancement on LGE‐MRI. The 3‐T MRI scanners’ sensitivity and specificity were 100% and 67.6%, respectively, and the 1.5‐T MRI scanners’ sensitivity and specificity were 100% and 33.3%, respectively. Among cases with moderate or severe enhancement on LGE‐MRI (n=147), 99 cases underwent early‐repeat LGE‐MRI. The majority of early‐repeat MRI (86 cases, 86.9%) showed resolving enhancement, whereas 13 cases (13.1%) showed persistent enhancement on MRI.
Figure 7. Endoscopic evidence of esophageal thermal injury (ETI) and corresponding late gadolinium enhancement magnetic resonance imaging findings.
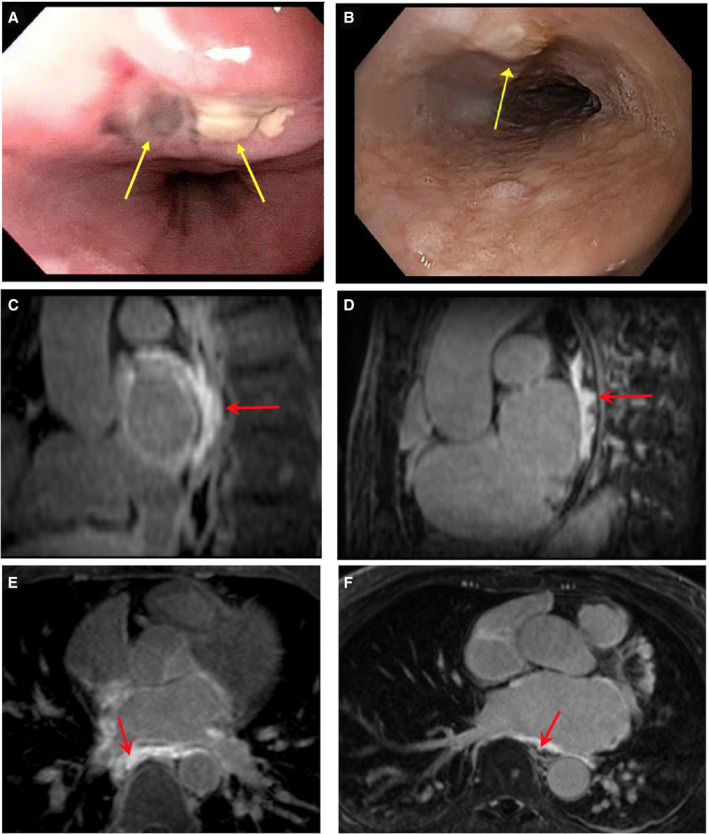
A, A 5‐mm nonbleeding crated ulcer and a superficial ulcer (yellow arrow). Transverse (C) and longitudinal (E) view of the esophagus showing transmural esophageal enhancement (red arrow). B, Localized mild mucosal changes characterized by nodularity and altered texture (yellow arrow). Transverse (D) and longitudinal (F) view of the esophagus showing near transmural delayed gadolinium enhancement of the esophagus. Yellow arrows indicate area of ETI on esophagogastroduodenoscopy. Red arrows indicate areas of anterior esophageal wall enhancement.
Assessment of Atrioesophageal Fistula by LGE‐MRI
A 65‐year‐old man with a past medical history of symptomatic persistent AF presented 28 days after AF RFA at an outside hospital with odynophagia, melena, and hematochezia. The patient had no postablation LGE‐MRI at the outside hospital. The patient was afebrile and had no neurological signs and symptoms. The patient underwent an EGD at an outside hospital, which showed a bleeding ulcer that was injected with epinephrine. The patient was stabilized and was transferred to the University of Utah Hospital. Upon admission, a repeat EGD was performed and showed a healing esophageal ulcer with a fibrinous exudate at the ulcer base. Chest computed tomography was performed using a small volume of water‐soluble oral contrast material, and images were acquired with the patient in supine and prone positions. Presence of extraluminal contrast was not detected in either position. Thickening of the esophageal wall was observed just posterior to the left atria. Two locules of air were adjacent to this thickening (Figure 8A and 8D). Computed tomography findings suggested a focal, contained esophageal perforation. A cardiac MRI study was performed on the following day. Study protocol included double inversion recovery–prepared T2‐weighted turbo spin echo, contrast‐enhanced angiography, and 3‐dimensional LGE‐MRI. Extensive edema of the posterior left atrial wall, pericardium, and esophagus was observed on double inversion recovery T2‐weighted turbo spin echo images (Figure 8E). No contrast extravasation from the LA was detected on contrast‐enhanced magnetic resonance angiography (Figure 8B and 8F). Perforation of the anterior wall of the esophagus contained by a blood clot was observed on magnetic resonance angiography (Figure 8B and 8F) and LGE images (Figure 8C and 8G), suggesting the presence of a self‐contained AEF with a blood clot. Enhancement of the anterior wall of the esophagus adjacent to the perforation was seen on LGE images (Figure 8C and 8G). Edema on double inversion recovery T2‐weighted fast spin‐echo and enhancement on LGE‐MRI revealed inflammation of the esophageal wall. Later on, the patient had a transient ischemic attack manifesting as a brief episode of left hemiparesis. The patient was taken emergently to the operating room to evaluate and treat the AEF. The presence of the AEF was confirmed, and it was treated surgically with AEF resection and esophageal and atrial repair (Figure 9). The patient had no persistent neurological deficits after the operation. A month after the operation, he was discharged to an inpatient rehabilitation facility where he stayed for 3 weeks before going home. A few months later, he developed gradually worsening shortness of breath and fatigue. A transthoracic echocardiogram showed evidence of aortic valve endocarditis. The patient’s blood cultures grew Enterococcus faecalis and he was treated with long‐term IV antibiotics. The patient underwent robotic‐assisted aortic valve replacement 5 years later for aortic valve insufficiency and stenosis. The patient had no symptomatic AF recurrences or heart failure.
Figure 8. Computed tomography (CT) and magnetic resonance imaging of an atrioesophageal fistula.
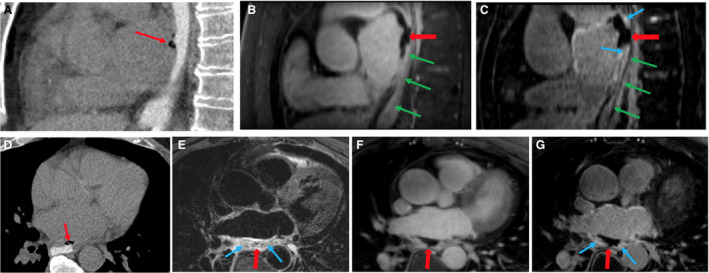
Top row: sagittal view. A, CT shows esophagus lumen filled with oral contrast and presence of air locules between the esophagus and left atrium (LA) (narrow red arrow). B, Magnetic resonance angiography (MRA) shows perforation of the anterior wall of the esophagus and blood clot (wide red arrow). Green arrows mark the esophagus. C, Late gadolinium enhancement (LGE) shows disruption of the anterior wall of the esophagus and a blood clot (wide red arrow). The anterior wall of the esophagus adjacent to the posterior LA wall is enhanced, indicating an inflammation process (narrow blue arrows). Bottom row: axial view. D, CT shows esophagus lumen filled with oral contrast and presence of air locule between esophagus and LA (narrow red arrow). E, Double inversion recovery T2‐weighted turbo spin echo shows edema caused by inflammation of the posterior LA wall, adjacent pericardium, and esophagus (narrow blue arrows) and the presence of a blood clot (wide red arrow). F, MRA shows perforation of the anterior wall of the esophagus and a blood clot (wide red arrow). G, LGE shows perforation of the anterior wall of the esophagus and a blood clot (wide red arrow). The posterior LA wall and pericardium are enhanced (narrow blue arrows).
Figure 9. Time course of events after atrial fibrillation (AF) radiofrequency (RF) ablation.
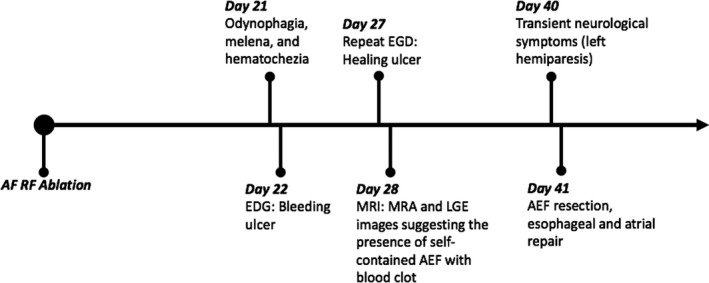
AEF indicates atrioesophageal fistula; EGD, esophagogastroduodenoscopy; LGE, late gadolinium enhancement; MRA, magnetic resonance angiography; and MRI, magnetic resonance imaging.
Discussion
Despite the development of techniques to prevent ETI and AEF formation, ETI is still expected in the postablation period. ETI has been reported to occur in up to 40% of cases. 7 , 8 , 11 , 17 , 18 In severe forms, ETI can be complicated by the formation of AEF. AEF has been reported to occur in <0.1% of cases. 11 , 19 , 20 However, it is associated with a high mortality rate, ranging between 55% and 85%. 11 , 21 , 22 Early detection of ETI is critical in the postablation period, and if treated early may potentially prevent formation of fatal AEF. In this study, we demonstrated the high sensitivity and negative predictive value of LGE‐MRI in detecting EGD‐confirmed ETI across the entire spectrum of injury severity.
LGE‐MRI has been used to assess the extent and progression of ETI in the postablation period. 10 Badger et al. have shown that postablation esophageal wall enhancement on MRI is transient and completely reversible. 10 Similarly, Schmidt et al. have shown complete resolution of endoscopic ETI changes in all patients with postablation ETI. 23
In this study, we describe the feasibility of LGE‐MRI in screening for postablation ETI. Our study demonstrates that ETI is common after AF RFA. However, the majority of post‐RFA AF ETIs are mild in nature. Moderate‐to‐severe ETI is identified only in a minority of cases. In our study, LGE‐MRI had high sensitivity in detecting moderate‐to‐severe ETI. In addition, LGE‐MRI exhibits a high negative predictive value to exclude the presence of EGD‐detected ETI in the absence of moderate or severe esophageal enhancement. All cases of EGD‐confirmed ETI had evidence of moderate‐to‐severe LGE on MRI. Halbfass et al. have demonstrated that only patients with esophageal ulceration developed AEF. 24 Thus, moderate‐to‐severe LGE on MRI can be interpreted as an early sign for esophageal ulceration and increased risk for AEF formation.
Badger et al. showed that contrast enhancement on LGE‐MRI was present in 5 out of 41 patients at 24 hours after ablation in MRI studies. 10 Three patients underwent EGD, which revealed evidence of ETI. 10 They also showed that contrast enhancement on LGE‐MRI resolved over time. 10 On the other hand, Gorman et al. have shown no correlation between immediate MRI imaging and EGD findings. 25 Immediate postablation MRI was more sensitive in detecting ETI compared with EGD. 25 The high sensitivity of LGE‐MRI in detecting evidence of ETI compared with EGD can be attributed to the fact that LGE‐MRI evaluates the full thickness of the esophageal wall, whereas EGD only visualizes the luminal esophageal surface.
Our findings also show that postablation ETI is by far a transient reaction to RFA. Severity of ETI on LGE‐MRI decreased significantly on repeat LGE‐MRI scans, reflecting resolution of transient esophageal injury. This has been consistently demonstrated in prior smaller studies. 10 , 15 , 25 The transient nature of ETI may also explain why 24 hours after RFA, LGE‐MRI detected more cases of moderate/severe ETI than findings on EGD studies performed a few days later.
AEF usually presents 2 weeks after ablation with constitutional symptoms such as fever, chest discomfort, fatigue, gastrointestinal symptoms, and neurological symptoms. 11 Intravenous contrast‐enhanced chest computed tomography has been considered to be a valuable imaging tool in diagnosing AEF. Typical computed tomography findings suggestive of AEF includes extravasation of IV contrast from the LA into the esophagus or posterior mediastinum, pneumomediastinum, irregular or ulcerated appearance of PV, left atrial wall thickening, gas within the LA or PVs, and PV fat stranding. 11 , 26 , 27 In our AEF case, MRI provided better characterization of the esophageal wall by showing evidence of edema on double inversion recovery T2‐weighted turbo spin echo images and enhancement on LGE images, suggestive of esophageal wall inflammation, which is likely an early sign of ETI and impending AEF. Additionally, LGE‐MRI and MRA images revealed the presence of self‐contained esophageal wall perforation with a blood clot, which was not seen on computed tomography imaging.
Only cases with persistent enhancement on early‐repeat MRI scans had evidence of ETI on EGD, whereas all cases with resolved enhancement on MRI showed no evidence of ETI on EGD. Thus, early‐repeat LGE‐MRI can be used to monitor the progression of ETI before performing EGD.
Limitations
The findings of this study have to be considered in light of some limitations. First, this is a single‐center retrospective study and is subject to limitations related to the retrospective nature of the study design. External validation in large randomized controlled trials comparing the use of LGE‐MRI and EGD would be appropriate. Second, only 175 cases out of 1269 cases with same‐day postablation LGE‐MRI were followed with early‐repeat LGE‐MRI after AF RFA, and thus early‐repeat MRI results might not be accurately representative of all cases. However, it would be unreasonable to justify obtaining an early‐repeat LGE‐MRI after AF RFA cases when same‐day LGE‐MRI shows mild or no esophageal LGE in the absence of other indications such as symptoms suggestive of ETI or AEF. Third, 24‐hour postablation LGE‐MRI was not performed in 39.6% of all AF RFA cases (833 cases out of 2102 total cases) because of major factors precluding patients from having LGE‐MRI, such as the presence of an implanted cardiac device, reduced creatinine clearance, scheduling issues, insurance denial, and patient refusal, which would limit generalizability of our study results in special AF patient populations (eg, patients with implanted cardiac devices and severely reduced kidney function). Lastly, because of the rarity of AEF complication after AF RFA, it would be challenging to identify LGE‐MRI precursor findings; however, the case we reported proposes LGE‐MRI findings that could suggest the presence of AEF. Extensive multicenter studies assessing patients with AEF are needed to further characterize AEF precursor findings on imaging.
Conclusions
Our results indicate that LGE‐MRI exhibits an extremely high sensitivity and negative predictive value for detecting EGD‐confirmed ETI after AF RFA. Our results also show that all patients with EGD‐confirmed ETI had evidence of moderate‐to‐severe enhancement on same‐day LGE‐MRI. Repeat LGE‐MRI has demonstrated that ETI is a transient phenomenon. Because of its high sensitivity and negative predictive value in detecting ETI after ablation, LGE‐MRI is an attractive noninvasive modality to evaluate for ETI before EGD. Furthermore, MRI can be used to diagnose AEF by providing better tissue characterization of the esophagus and surrounding structures.
Sources of Funding
None.
Disclosures
Mr Morris reports equity interest in Marrek, Inc. Dr Kholmovski reports consulting fees from and equity interest in Marrek Inc. Dr Marrouche reports consulting fees from Abbott, Biotronik, Wavelet Health, Cardiac Design, Medtronic, Preventice, Vytronus, Biosense Webster, Marrek Inc., and Boston Scientific; research funding from Abbott, Boston Scientific, GE Healthcare, Siemens, Biotronik, Vytronus, and Biosense Webster; ownership interest in Marrek Inc. and Cardiac Designs; contracted research with Biosense Webster, Medtronic, St. Jude Medical, and Boston Scientific; and reports consulting fees from Biotronik and Preventice. The remaining authors have no disclosures to report.
Supporting information
Table S1
(J Am Heart Assoc. 2021;10:e018924. DOI: 10.1161/JAHA.120.018924.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018924
For Sources of Funding and Disclosures, see page 11.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. DOI: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim J‐S, Shin SY, Na JO, Choi CU, Kim SH, Kim JW, Kim EJ, Rha S‐W, Park CG, Seo HS, et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation? A prospective randomized clinical trial. Int J Cardiol. 2015;181:277–283. DOI: 10.1016/j.ijcard.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 3. He X, Zhou Y, Chen Y, Wu L, Huang Y, He J. Left atrial posterior wall isolation reduces the recurrence of atrial fibrillation: a meta‐analysis. J Interv Card Electrophysiol. 2016;46:267–274. DOI: 10.1007/s10840-016-0124-7. [DOI] [PubMed] [Google Scholar]
- 4. Saini A, Huizar JF, Tan A, Koneru JN, Ellenbogen KA, Kaszala K. Scar homogenization in atrial fibrillation ablation: evolution and practice. J Atr Fibrillation. 2017;10:1645. DOI: 10.4022/jafib.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin CA, Curtain JP, Gajendragadkar PR, Begley DA, Fynn SP, Grace AA, Heck PM, Virdee MS, Agarwal S. Ablation of complex fractionated electrograms improves outcome in persistent atrial fibrillation of over 2 year's duration. J Atr Fibrillation. 2018;10:1607. DOI: 10.4022/jafib.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsao HM, Wu MH, Higa S, Lee KT, Tai CT, Hsu NW, Chang CY, Chen SA. Anatomic relationship of the esophagus and left atrium: implication for catheter ablation of atrial fibrillation. Chest. 2005;128:2581–2587. DOI: 10.1378/chest.128.4.2581. [DOI] [PubMed] [Google Scholar]
- 7. Garg L, Garg J, Gupta N, Shah N, Krishnamoorthy P, Palaniswamy C, Bozorgnia B, Natale A. Gastrointestinal complications associated with catheter ablation for atrial fibrillation. Int J Cardiol. 2016;224:424–430. DOI: 10.1016/j.ijcard.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 8. Kiuchi K, Okajima K, Shimane A, Kanda G, Yokoi K, Teranishi J, Aoki K, Chimura M, Tsubata H, Miyata T, et al. Incidence of esophageal injury after pulmonary vein isolation in patients with a low body mass index and esophageal temperature monitoring at a 39°C setting. J Arrhythm. 2015;31:12–17. DOI: 10.1016/j.joa.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three‐dimensional navigator‐gated delayed enhancement mr imaging: initial experience. Radiology. 2007;243:690–695. DOI: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 10. Badger TJ, Adjei‐Poku YA, Burgon NS, Kalvaitis S, Shaaban A, Sommers DN, Blauer JJE, Fish; EN, Akoum N, Haslem TS, et al. Initial experience of assessing esophageal tissue injury and recovery using delayed‐enhancement mri after atrial fibrillation ablation. Circulation. Arrhythmia and electrophysiology. 2009;2:620–625. DOI: 10.1161/CIRCEP.109.871939. [DOI] [PubMed] [Google Scholar]
- 11. Marashly Q, Chelu MG. Ablation approaches and imaging modalities to lower risk of atrioesophageal injury during catheter ablation for atrial fibrillation. Curr Cardiovasc Risk Rep. 2020;14:1–10. DOI: 10.1007/s12170-019-0635-8. [DOI] [Google Scholar]
- 12. Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, et al. Association of left atrial fibrosis detected by delayed‐enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57:831–838. DOI: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Segerson NM, Daccarett M, Badger TJ, Shabaan A, Akoum N, Fish EN, Rao S, Burgon NS, Adjei‐poku Y, Kholmovski E, et al. Magnetic resonance imaging‐confirmed ablative debulking of the left atrial posterior wall and septum for treatment of persistent atrial fibrillation: rationale and initial experience. J Cardiovasc Electrophysiol. 2010;21:126–132. DOI: 10.1111/j.1540-8167.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 14. Badger TJ, Daccarett M, Akoum NW, Adjei‐Poku YA, Burgon NS, Haslam TS, Kalvaitis S, Kuppahally S, Vergara G, McMullen L, et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed‐enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010;3:249–259. DOI: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baher A, Kheirkhahan M, Rechenmacher SJ, Marashly Q, Kholmovski EG, Siebermair J, Acharya M, Aljuaid M, Morris AK, Kaur G, et al. High‐power radiofrequency catheter ablation of atrial fibrillation: using late gadolinium enhancement magnetic resonance imaging as a novel index of esophageal injury. JACC Clin Electrophysiol. 2018;4:1583–1594. DOI: 10.1016/j.jacep.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. DOI: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 17. Wazni O, Wilkoff B, Saliba W. Catheter ablation for atrial fibrillation. N Engl J Med. 2011;365:2296–2304. DOI: 10.1056/NEJMct1109977. [DOI] [PubMed] [Google Scholar]
- 18. Tilz RR, Chun KRJ, Metzner A, Burchard A, Wissner E, Koektuerk B, Konstantinidou M, Nuyens D, Potter TD, Neven K, et al. Unexpected high incidence of esophageal injury following pulmonary vein isolation using robotic navigation. J Cardiovasc Electrophysiol. 2010;21:853–858. DOI: 10.1111/j.1540-8167.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- 19. Cappato R, Calkins H, Chen S‐A, Davies W, Iesaka Y, Kalman J, Kim Y‐H, Klein G, Natale A, Packer D, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. DOI: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts‐Thomson KC, Brooks AG, Sanders P. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol. 2013;6:1082–1088. DOI: 10.1161/CIRCEP.113.000768. [DOI] [PubMed] [Google Scholar]
- 21. Han HC, Ha FJ, Sanders P, Spencer R, Teh AW, O'Donnell D, Farouque O, Lim HS. Atrioesophageal fistula: clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol. 2017;10:e005579. DOI: 10.1161/CIRCEP.117.005579. [DOI] [PubMed] [Google Scholar]
- 22. Ghia KK, Chugh A, Good E, Pelosi F, Jongnarangsin K, Bogun F, Morady F, Oral H. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol. 2009;24:33–36. DOI: 10.1007/s10840-008-9307-1. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt M, Nolker G, Marschang H, Gutleben K‐J, Schibgilla V, Rittger H, Sinha A‐M, Ritscher G, Mayer D, Brachmann J, et al. Incidence of oesophageal wall injury post‐pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace. 2008;10:205–209. DOI: 10.1093/europace/eun001. [DOI] [PubMed] [Google Scholar]
- 24. Halbfass P, Müller P, Nentwich K, Krug J, Roos M, Hamm K, Barth S, Szöllösi A, Mügge A, Schieffer B, et al. Incidence of asymptomatic oesophageal lesions after atrial fibrillation ablation using an oesophageal temperature probe with insulated thermocouples: a comparative controlled study. Europace. 2017;19:385–391. DOI: 10.1093/europace/euw070. [DOI] [PubMed] [Google Scholar]
- 25. Gorman DR, Peterson KA, Fang J, Olpin J, Sommers DO, McFadden M, Morshedzadeh JH, Akoum N, Daccarett M, Marrouche N, et al. Cross‐sectional imaging obtained immediately following radiofrequency atrial fibrillation ablation does not predict endoscopic evidence of esophageal injury. Dig Dis Sci. 2011;56:3453–3458. DOI: 10.1007/s10620-011-1829-1. [DOI] [PubMed] [Google Scholar]
- 26. Malamis AP, Kirshenbaum KJ, Nadimpalli S. CT radiographic findings: atrio‐esophageal fistula after transcatheter percutaneous ablation of atrial fibrillation. J Thorac Imaging. 2007;22:188–191. DOI: 10.1097/01.rti.0000213569.63538.30. [DOI] [PubMed] [Google Scholar]
- 27. Preis O, Digumarthy SR, Wright CD, Shepard JA. Atrioesophageal fistula after catheter pulmonary venous ablation for atrial fibrillation: imaging features. J Thorac Imaging. 2007;22:283–285. DOI: 10.1097/RTI.0b013e318054e26f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


