Abstract
Background
The impact of atrial fibrillation (AF) in intermediate surgical risk patients with severe aortic stenosis who undergo either transcatheter or surgical aortic valve replacement (AVR) is not well established.
Methods and Results
Data were assessed in 2663 patients from the PARTNER (Placement of Aortic Transcatheter Valve) 2A or S3i trials. Analyses grouped patients into 3 categories according to their baseline and discharge rhythms (ie, sinus rhythm [SR]/SR, SR/AF, or AF/AF). Among patients with transcatheter AVR (n=1867), 79.2% had SR/SR, 17.6% had AF/AF, and 3.2% had SR/AF. Among patients with surgical AVR (n=796), 71.7% had SR/SR, 14.1% had AF/AF, and 14.2% had SR/AF. Patients with transcatheter AVR in AF at discharge had increased 2‐year mortality (SR/AF versus SR/SR; hazard ratio [HR], 2.73; 95% CI, 1.68–4.44; P<0.0001; AF/AF versus SR/SR; HR, 1.56; 95% CI, 1.16–2.09; P=0.003); patients with SR/AF also experienced increased 2‐year mortality relative to patients with AF/AF (HR, 1.77; 95% CI, 1.04–3.00; P=0.03). For patients with surgicalAVR, the presence of AF at discharge was also associated with increased 2‐year mortality (SR/AF versus SR/SR; HR, 1.93; 95% CI, 1.25–2.96; P=0.002; and AF/AF versus SR/SR; HR, 1.67; 95% CI, 1.06–2.63; P=0.027). Rehospitalization and persistent advanced heart failure symptoms were also more common among patients with transcatheter AVR and surgical AVR discharged in AF, and major bleeding was more common in the transcatheter AVR cohort.
Conclusions
The presence of AF at discharge in patients with intermediate surgical risk aortic stenosis was associated with worse outcomes—especially in patients with baseline SR—including increased all‐cause mortality at 2‐year follow‐up.
Registration
URL: https://www.clinicaltrials.gov; Unique identifiers: NCT01314313 and NCT03222128.
Keywords: aortic stenosis, atrial fibrillation, transcatheter aortic valve replacement
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Atrial Fibrillation, Mortality/Survival, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- DAPT
dual antiplatelet therapy
- NYHA
New York Heart Association
- SAVR
surgical aortic valve replacement
- SR
sinus rhythm
- TAVR
transcatheter aortic valve replacement
Clinical Perspective
What Is New?
We examined relationships between atrial fibrillation (AF) and clinical outcomes in 3 categories of patients with severe aortic stenosis and intermediate surgical risk—those with baseline and discharge sinus rhythm, baseline and discharge AF, and baseline sinus rhythm and AF at discharge.
AF at discharge was associated with adverse outcomes, most notably all‐cause mortality, and patients with transcatheter aortic valve replacement and baseline sinus rhythm who developed AF had an even greater hazard for mortality relative to patients with baseline AF.
What Are the Clinical Implications?
AF at discharge, particularly if not present at baseline, is associated with worse outcomes in patients with intermediate risk patients and severe aortic stenosis undergoing either transcatheter aortic valve replacement or surgical aortic valve replacement.
Thus, clinicians may want to consider such patients as a higher risk group for adverse outcomes, warranting continued follow‐up and consideration for AF‐related therapies (ie, rate/rhythm control and thromboembolism prevention).
Atrial fibrillation (AF) is the most common clinical significant arrhythmia and a frequent complication of many cardiac procedures, 1 , 2 particularly valve surgery and coronary artery bypass grafting, where its incidence ranges from 5% to 40%. 3 , 4 , 5 AF is a known risk factor for mortality following surgical aortic valve replacement (SAVR). 6 AF also commonly occurs following transcatheter aortic valve replacement (TAVR), which has emerged as an effective and less invasive alternative to SAVR for most patients with severe AS and trileaflet anatomy, irrespective of surgical risk profile. 7 , 8 , 9 , 10 The incidence of AF after TAVR has been reported in the range of 6.0% to 35.0%, with higher proportions occurring with more invasive access site. 11 , 12 The PARTNER (Placement of Aortic Trancatheter Valve) trial was one of the first studies to show the clinical impact of AF after TAVR. 13 The study was performed in patients with high surgical risk and severe aortic stenosis (AS) and showed an association between the presence of AF at discharge and increased 30‐day and 1‐year mortality.
However, there are limited data reporting the impact of AF on patients with intermediate risk and AS undergoing TAVR as well as SAVR. In this paper, we examine the association between AF on outcomes, including mortality, bleeding, and stroke at 2 years in patients with intermediate surgical risk patients and AS undergoing either TAVR or SAVR in the PARTNER 2A and S3i trials.
Methods
Study Population
This study analyzed data from patients who underwent TAVR or SAVR in either the PARTNER 2A or S3i trials. 9 , 14 In the PARTNER 2A trial, participants with symptomatic, severe AS and intermediate surgical risk were randomized in a 1:1 fashion to either SAVR or TAVR with a SAPIEN XT valve using a femoral approach or a transapical or direct aortic approach if the transfemoral approach was not feasible. The PARTNER S3i registry included a cohort of patients with intermediate surgical risk patients and severe AS who underwent TAVR with a SAPIEN 3 valve via either a transfemoral or transthoracic route. The study was approved by the institutional review board at each participating site, and all patients provided informed consent to participate in the trials. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Outcomes
Patients in both trials had an ECG, echocardiogram, and clinical evaluation performed at baseline, discharge, 30 days, 6 months, 1 year, and 2 years post‐procedure. All ECGs were interpreted in core laboratory work. AF was defined as AF or atrial flutter present on the baseline and/or follow‐up ECGs. Time to first clinical outcomes were compared among patients from the following 3 groups: baseline sinus rhythm (SR) and discharge SR (SR/SR), baseline SR and discharge AF (SR/AF), and baseline AF and discharge AF (AF/AF). Those patients with baseline AF and discharge SR were not analyzed because of their relatively low representation (n=42; 21 in the TAVR cohort and 21 in the SAVR cohort). The primary end point was all‐cause mortality at 2 years. Secondary end points included cardiovascular mortality, bleeding complications and stroke/transient ischemic attack (TIA), which were all defined and adjudicated according to the previously described PARTNER protocol. 8 Outcomes were assessed at 3 separate time points post‐procedure: 30 days, 1 year, and 2 years. Adverse clinical events through 2 years were adjudicated by an independent clinical events committee.
Statistical Analysis
Continuous variables are presented as means with SDs and were compared using ANOVA or the Kruskal–Wallis test for nonnormally distributed data. Categorical variables are presented as counts and percentages and were compared with the χ2 or Fisher's exact test. Time to first event rates are reported using Kaplan‐Meier estimates and compared among groups with a log‐rank test. Hazard ratios (HRs) with 95% CIs for SR/AF versus AF/AF versus SR/SR were generated with Cox regression models. Postdischarge mortality at 30 days, 1 year and 2 years was evaluated with multivariable Cox regression models separately for patients who underwent TAVR or SAVR. Multivariable models incorporated clinically relevant variables including New York Heart Association (NYHA) III/IV, previous myocardial infarction, previous stroke/TIA, cardiomyopathy, pulmonary hypertension, and anticoagulation use. Stepwise model selections were applied in the models. A 2‐sided P value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
The study included 2663 patients from the PARTNER 2A or S3i trials, with 1867 in the TAVR arm and 796 in the SAVR arm (Figure 1). In the TAVR cohort, 1478 patients (79.2%) had SR/SR, 60 patients (3.2%) had SR/AF, and 329 patients (17.6%) had AF/AF. Among patients with TAVR in the SR/AF group, AF was detected at 30‐day follow‐up in 36.5% of patients (n=19), 29.5% of patients at 1‐year follow‐up (n=13), and 29.4% of patients at 2‐year follow‐up (n=5). For patients with SAVR, 571 patients (71.7%) had SR/SR, 113 patients (14.2%) had SR/AF, and 112 patients (14.1%) had AF/AF. Among patients with SAVR in the SR/AF group, AF was detected at 30‐day follow‐up in 43.3% of patients (n=42), 26.6% of patients at 1‐year follow‐up (n=20), and 25.5% of patients at 2‐year follow‐up (n=12).
Figure 1. Flow diagram illustrating the study cohort.
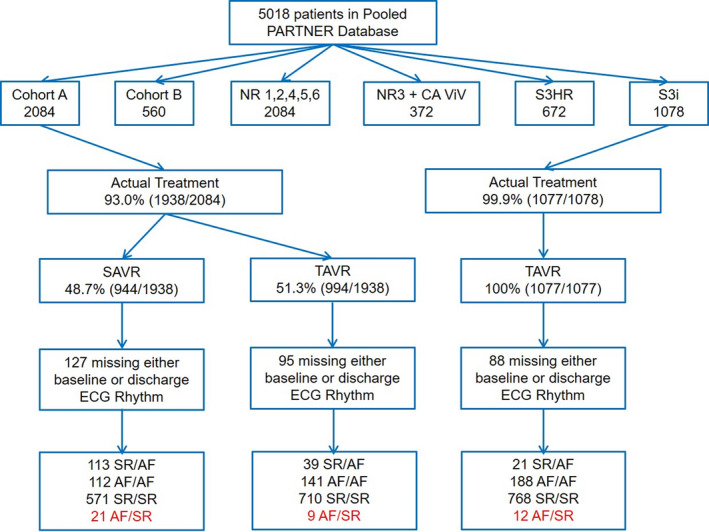
The final study cohort included 2663 individuals, 1867 TAVR recipients and 796 SAVR recipients. AF indicates atrial fibrillation; PARTNER, Placement of Aortic Transcatheter Valve; SAVR, surgical aortic valve replacement; SR, sinus rhythm; and TAVR, transcatheter aortic valve replacement.
Baseline characteristics of patients are noted in Tables 1 (patients with TAVR) and 2 (patients with SAVR). Echocardiographic parameters for TAVR and SAVR recipients are provided in Tables S1 and S2, respectively. For patients with TAVR, the mean age was 81.7±6.6 years, with men composing 58% of the cohort. Mean Society of Thoracic Surgeons (STS) score was 5.5±1.7% and was highest in the SR/AF group (6.2±2.2%). There were no significant differences among patients with TAVR with respect to body mass index, diabetes mellitus, hypertension, or prior congestive heart failure. The SR/AF group had the highest rate of prior myocardial infarctions and the AF/AF group had the highest rate of preoperative stroke or TIA.
Table 1.
Baseline Characteristics of Patients With TAVR
| Overall | SR/SR | SR/AF | AF/AF | P Value, All Groups | P Value, SR/AF vs AF/AF | P Value, SR/AF vs SR/SR | P Value, SR/SR vs AF/AF | |
|---|---|---|---|---|---|---|---|---|
| No. | 1867 | 1478 | 60 | 329 | … | … | … | … |
| Age, y (SD) | 81.7 (6.6) | 81.5 (6.7) | 83.0 (6.6) | 82.4 (6.3) | 0.03 | 0.47 | 0.09 | 0.03 |
| % Male | 58.0% | 55.3% | 61.7% | 69.6% | <0.0001 | 0.22 | 0.33 | <0.0001 |
| % White | 94.4% | 93.8% | 89.7% | 97.5% | 0.009 | 0.003 | 0.20 | 0.008 |
| Body mass index, kg/m2 (SD) | 28.7 (6.2) | 28.8 (6.3) | 27.3 (5.0) | 28.6 (5.9) | 0.17 | 0.09 | 0.06 | 0.69 |
| Cardiovascular comorbidity | ||||||||
| Diabetes mellitus | 36.0% | 36.7% | 33.3% | 33.7% | 0.55 | 0.95 | 0.60 | 0.32 |
| Hypertension | 93.0% | 93.0% | 96.7% | 92.7% | 0.53 | 0.26 | 0.27 | 0.87 |
| Hyperlipidemia | 81.6% | 81.9% | 88.3% | 78.7% | 0.15 | 0.09 | 0.20 | 0.18 |
| Smoking (previous or current tobacco use) | 49.9% | 48.4% | 53.3% | 55.9% | 0.04 | 0.71 | 0.45 | 0.01 |
| Prior myocardial infarction | 16.7% | 16.5% | 28.3% | 15.2% | 0.04 | 0.01 | 0.02 | 0.56 |
| Prior coronary artery bypass graft | 25.9% | 26.4% | 23.3% | 24.3% | 0.66 | 0.87 | 0.60 | 0.44 |
| Prior heart failure | 84.6% | 84.1% | 90.0% | 85.7% | 0.38 | 0.37 | 0.22 | 0.47 |
| Prior stroke/transient ischemic attack | 17.0% | 16.2% | 10.0% | 21.9% | 0.02 | 0.03 | 0.20 | 0.01 |
| NYHA symptoms | ||||||||
| NYHA I | 0.1% | 0.1% | 0.0% | 0.0% | 0.88 | N/A | 0.84 | 0.64 |
| NYHA II | 25.2% | 26.2% | 18.3% | 22.2% | 0.15 | 0.50 | 0.17 | 0.13 |
| NYHA III | 60.4% | 59.7% | 66.7% | 62.3% | 0.40 | 0.52 | 0.28 | 0.38 |
| NYHA IV | 14.4% | 14.1% | 15.0% | 15.5% | 0.79 | 0.92 | 0.84 | 0.50 |
| Society of Thoracic Surgeons, renal disease defined by serum creatinine ≥2 mg/dL Score | 5.5 (1.7) | 5.5 (1.7) | 6.2 (2.2) | 5.8 (1.8) | <0.0001 | 0.11 | 0.0006 | 0.0007 |
| Congestive heart failure, hypertension, age, diabetes, previous stroke/transient ischemic attack, vascular disease, and sex category | 5.5 (1.2) | 5.5 (1.2) | 5.5 (1.0) | 5.5 (1.2) | 0.91 | 0.73 | 0.67 | 0.91 |
| Renal disease | 6.1% | 5.7% | 8.3% | 7.3% | 0.41 | 0.78 | 0.39 | 0.26 |
| Liver disease | 1.4% | 1.2% | 1.7% | 2.1% | 0.44 | 0.82 | 0.76 | 0.20 |
| Chronic obstructive pulmonary disease | 30.6% | 29.9% | 30.0% | 33.5% | 0.44 | 0.59 | 0.99 | 0.20 |
All values presented as means with SD in parentheses, or percentages. AF/AF indicates baseline AF/discharge AF; NYHA, New York Heart Association; SR/AF, baseline SR/discharge AF; SR/SR, baseline sinus rhythm/discharge sinus rhythm; and TAVR, thoracic aortic valve replacement..
Table 2.
Baseline Characteristics of Patients With SAVR
| Overall | SR/SR | SR/AF | AF/AF | P Value, All Groups | P Value, SR/AF vs AF/AF | P Value, SR/AF vs SR/SR | P Value, SR/SR vs AF/AF | |
|---|---|---|---|---|---|---|---|---|
| No. | 796 | 571 | 113 | 112 | … | … | … | … |
| Age, y (SD) | 81.6 (6.8) | 81.3 (6.9) | 81.9 (7.1) | 82.7 (5.6) | 0.11 | 0.36 | 0.39 | 0.04 |
| % Male | 55.0% | 50.3% | 64.6% | 69.6% | <0.0001 | 0.42 | 0.005 | 0.0002 |
| % White | 93.7% | 92.8% | 93.8% | 98.1% | 0.12 | 0.10 | 0.73 | 0.04 |
| Body mass index, kg/m2 (SD) | 28.2 (6.1) | 28.1 (6.2) | 28.8 (6.3) | 28.4 (5.6) | 0.58 | 0.62 | 0.32 | 0.70 |
| Cardiovascular comorbidity | ||||||||
| Diabetes mellitus | 35.2% | 36.6% | 33.6% | 29.5% | 0.33 | 0.50 | 0.55 | 0.15 |
| Hypertension | 94.3% | 94.7% | 94.7% | 92.0% | 0.50 | 0.41 | 0.98 | 0.25 |
| Hyperlipidemia | 81.4% | 81.8% | 84.1% | 76.8% | 0.34 | 0.17 | 0.56 | 0.22 |
| Smoking (previous or current tobacco use) | 50.3% | 49.9% | 48.7% | 53.6% | 0.73 | 0.46 | 0.81 | 0.48 |
| Prior myocardial infarction | 17.6% | 17.5% | 19.5% | 16.1% | 0.80 | 0.51 | 0.62 | 0.71 |
| Prior coronary artery bypass graft | 25.3% | 24.5% | 27.4% | 26.8% | 0.75 | 0.91 | 0.51 | 0.61 |
| Prior heart failure | 84.3% | 83.4% | 87.6% | 85.7% | 0.48 | 0.68 | 0.26 | 0.54 |
| Prior stroke/transient ischemic attack | 17.3% | 17.2% | 16.8% | 18.8% | 0.91 | 0.70 | 0.93 | 0.69 |
| NYHA symptoms | ||||||||
| NYHA I | 0.0% | 0.0% | 0.0% | 0.0% | N/A | N/A | N/A | N/A |
| NYHA II | 24.4% | 24.7% | 31.0% | 16.1% | 0.03 | 0.008 | 0.17 | 0.05 |
| NYHA III | 56.7% | 57.4% | 51.3% | 58.9% | 0.44 | 0.25 | 0.24 | 0.76 |
| NYHA IV | 18.9% | 17.9% | 17.7% | 25.0% | 0.20 | 0.18 | 0.96 | 0.08 |
| Society of Thoracic Surgeons, renal disease defined by serum creatinine ≥2 mg/dL Score | 5.8 (1.9) | 5.7 (1.8) | 5.9 (2.1) | 5.9 (1.7) | 0.26 | 0.93 | 0.23 | 0.18 |
| Congestive heart failure, hypertension, age, diabetes, previous stroke/transient ischemic attack, vascular disease, and sex category | 5.6 (1.2) | 5.6 (1.2) | 5.5 (1.2) | 5.5 (1.1) | 0.61 | 0.93 | 0.42 | 0.48 |
| Renal disease | 5.4% | 4.7% | 7.1% | 7.1% | 0.41 | 0.99 | 0.30 | 0.29 |
| Liver disease | 2.4% | 2.1% | 4.4% | 1.8% | 0.30 | 0.25 | 0.15 | 0.83 |
| Chronic obstructive pulmonary disease | 29.6% | 29.6% | 27.4% | 31.5% | 0.80 | 0.50 | 0.64 | 0.69 |
All values presented as means with SD in parentheses, or percentages. AF/AF indicates baseline AF/discharge AF; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; SR/AF, baseline SR/discharge AF; and SR/SR, baseline sinus rhythm/discharge sinus rhythm.
For patients with SAVR, the mean age was 81.6±6.8 years. Men comprised 55% of the cohort and were overrepresented in the AF groups (69.6% of patients with AF/AF, 64.6% of patients with SR/AF). Mean STS score of patients with SAVR was 5.8±1.9%. There were no significant differences among patients with SAVR with respect to STS score, body mass index, diabetes mellitus, hypertension, or congestive heart failure.
Clinical Outcomes
Clinical outcomes including all‐cause and cardiovascular mortality, bleeding, stroke/TIA, rehospitalizations, and NYHA class III/IV symptoms at 1 and 2 years are noted in Tables 3 (patients with TAVR) and 4 (patients with SAVR). At discharge, anticoagulation (ie, warfarin, heparin, enoxaparin, or dabigatran) was prescribed for patients with SAVR and TAVR as follows: for patients with SR/AF—57.6% TAVR versus 53.6% SAVR; odds ratio [OR], 1.18, 95% CI, 0.62 to 2.23; for patients with AF/AF—76.3% TAVR versus 77.5% SAVR; OR, 0.94; 95% CI, 0.56 to 1.56; for patients with SR/SR—17.0% TAVR versus 23.6% SAVR; OR, 0.66; 95% CI, 0.84 to 5.52; P‐interaction nonsignificant between different groups. However, patients with TAVR discharged in AF were substantially more likely to be discharged with dual antiplatelet therapy (DAPT) compared with patients with SAVR: for patients with SR/AF—52.5% TAVR versus 22.3% SAVR; OR, 3.85; 95% CI, 1.96 to 7.59; for patients with AF/AF—28.0% TAVR versus 12.6% SAVR; OR, 2.69, 95% CI, 1.46 to 4.95; patients with for SR/SR—63.7% TAVR versus 22.2% SAVR; OR, 6.16; 95% CI, 4.92 to 7.71; P‐interaction=0.0125 between TAVR and SAVR for patients with AF/AF versus those with SR/SR.
Table 3.
TAVR Outcomes
| Overall | SR/SR | SR/AF | AF/AF | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Overall | SR/AF vs AF/AF | SR/AF vs SR/SR | AF/AF vs SR/SR | |||||
| 1‐y | ||||||||
| Number of patients | 1687 | 1347 | 50 | 291 | … | … | … | … |
| Mortality (all cause) | 8.1% | 7.3% | 16.7% | 10.1% | 0.01 | 0.13 | 0.006 | 0.09 |
| Cardiovascular mortality | 4.5% | 4.1% | 6.8% | 5.9% | 0.23 | 0.76 | 0.28 | 0.15 |
| Any bleeding | 44.2% | 42.2% | 64.9% | 50.0% | 0.0001 | 0.005 | <0.0001 | 0.08 |
| Life‐threatening bleeding | 11.4% | 10.4% | 32.3% | 12.4% | <0.0001 | <0.0001 | <0.0001 | 0.98 |
| Stroke or TIA | 8.0% | 8.2% | 6.8% | 7.5% | 0.85 | 0.89 | 0.73 | 0.64 |
| Rehospitalization | 12.6% | 11.2% | 19.8% | 17.8% | 0.001 | 0.77 | 0.06 | 0.001 |
| NYHA III/IV | 7.2% | 6.1% | 12.2% | 11.1% | 0.005 | 0.81 | 0.08 | 0.003 |
| 2‐y | ||||||||
| Number of patients | 1539 | 1239 | 42 | 258 | … | … | … | … |
| Mortality (all cause) | 13.7% | 12.0% | 30.0% | 18.3% | <0.0001 | 0.03 | <0.0001 | 0.003 |
| Cardiovascular mortality | 8.0% | 7.0% | 12.7% | 11.9% | 0.008 | 0.82 | 0.10 | 0.004 |
| Any bleeding | 48.2% | 46.0% | 64.9% | 55.9% | 0.0002 | 0.01 | 0.0001 | 0.05 |
| Life‐threatening bleeding | 13.4% | 12.2% | 32.3% | 15.8% | <0.0001 | <0.0001 | <0.0001 | 0.71 |
| Stroke or TIA | 11.8% | 11.2% | 13.4% | 14.7% | 0.90 | 0.76 | 0.99 | 0.66 |
| Rehospitalization | 16.8% | 15.2% | 21.9% | 23.3% | 0.001 | 0.87 | 0.15 | 0.0004 |
| NYHA III/IV | 8.6% | 7.6% | 10.5% | 13.0% | 0.03 | 0.67 | 0.51 | 0.007 |
All values presented as percentages. AF/AF indicates baseline AF/discharge AF; NYHA, New York Heart Association; SR/AF, baseline SR/discharge AF; SR/SR, baseline sinus rhythm/discharge sinus rhythm; TAVR, thoracic aortic valve replacement; and TIA, transient ischemic attack.
Table 4.
SAVR Outcomes
| Overall | SR/SR | SR/AF | AF/AF | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Overall | SR/AF vs AF/AF | SR/AF vs SR/SR | AF/AF vs SR/SR | |||||
| 1‐y | ||||||||
| Number of patients | 688 | 506 | 94 | 88 | … | … | … | … |
| Mortality (all cause) | 11.4% | 9.0% | 16.0% | 18.9% | 0.002 | 0.59 | 0.02 | 0.002 |
| Cardiovascular mortality | 7.0% | 5.6% | 11.0% | 10.3% | 0.04 | 0.87 | 0.03 | 0.06 |
| Any bleeding | 80.6% | 81.1% | 79.9% | 78.6% | 0.71 | 0.80 | 0.65 | 0.45 |
| Life‐threatening bleeding | 47.8% | 49.6% | 40.9% | 45.8% | 0.19 | 0.46 | 0.08 | 0.42 |
| Stroke or TIA | 8.9% | 8.8% | 8.6% | 10.4% | 0.86 | 0.61 | 0.86 | 0.64 |
| Rehospitalization | 14.6% | 11.7% | 15.9% | 28.4% | <0.0001 | 0.03 | 0.22 | <0.0001 |
| NYHA III/IV | 6.6% | 6.5% | 9.3% | 4.9% | 0.50 | 0.28 | 0.34 | 0.60 |
| 2‐y | ||||||||
| Number of patients | 616 | 458 | 77 | 81 | … | … | … | … |
| Mortality (all cause) | 16.8% | 14.2% | 25.2% | 21.7% | 0.003 | 0.62 | 0.002 | 0.03 |
| Cardiovascular mortality | 10.3% | 8.3% | 18.8% | 12.4% | 0.003 | 0.25 | 0.0008 | 0.14 |
| Any bleeding | 81.5% | 81.9% | 81.1% | 79.6% | 0.73 | 0.78 | 0.68 | 0.46 |
| Life‐threatening bleeding | 49.1% | 50.8% | 44.0% | 45.8% | 0.26 | 0.68 | 0.14 | 0.33 |
| Stroke or TIA | 10.4% | 10.6% | 8.6% | 11.5% | 0.77 | 0.47 | 0.54 | 0.78 |
| Rehospitalization | 17.5% | 14.5% | 19.2% | 31.6% | <0.0001 | 0.04 | 0.21 | <0.0001 |
| NYHA III/IV | 6.8% | 6.6% | 5.6% | 9.5% | 0.59 | 0.37 | 0.75 | 0.37 |
All values presented as percentages. AF/AF indicates baseline AF/discharge AF; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; SR/AF, baseline SR/discharge AF; SR/SR, baseline sinus rhythm/discharge sinus rhythm; and TIA, transient ischemic attack.
Patients with TAVR in the SR/AF group experienced the highest all‐cause mortality when compared with the AF/AF and SR/SR groups at 1 year (16.7% SR/AF versus 10.1% AF/AF versus 7.3% SR/SR, P=0.01 overall) and 2 years (30.0% SR/AF versus 18.3% AF/AF versus 12.0% SR/SR, P<0.0001 overall). At 2 years, patients with AF experienced an increased risk of mortality compared to patients in SR/SR: for SR/AF versus SR/SR (HR, 2.73; 95% CI, 1.68–4.44; P<0.0001; Table 5) and for AF/AF versus SR/SR (HR, 1.56; 95% CI 1.16–2.09, P=0.003). Patients who were admitted in SR and discharged in AF (SR/AF) had an incremental mortality relative to patients with baseline AF as well (HR, 1.77; 95% CI, 1.04–3.00; P=0.03) (Figure 2A). This association with mortality remained significant even when adjusting for TAVR access site (for SR/AF versus SR/SR, HR, 2.72; 95% CI, 1.61–4.60; P=0.0002; for AF/AF versus SR/SR, HR, 2.25; 95% CI, 1.49–3.40; P=0.0001). Multiple interaction effects were explored, and notably there was no significant interaction between baseline left ventricular function or mitral regurgitation severity on the relationship between AF and all‐cause mortality. With regard to cardiovascular mortality, similar differences emerged only at 2 year follow up (12.7% SR/AF versus 11.9% AF/AF versus 7.0% SR/SR, P=0.008 overall). Patients with AF, especially those in the SR/AF group, experienced more bleeding events, including life‐threatening or disabling events. The AF groups also had higher rates of rehospitalizations at 1 year (19.8% SR/AF and 17.8% AF/AF versus 11.2% SR/SR, P=0.001 overall) and at 2 years (21.9% SR/AF and 23.3% AF/AF versus 15.2% SR/SR, P=0.001 overall). No significant differences in rates of stoke or TIA emerged at 1‐ or 2‐year follow‐up.
Table 5.
Cox‐Proportional Hazard Models for the Primary and Secondary End Points at 2‐Year Follow‐Up
| Outcome | TAVR | SAVR | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All‐cause mortality | ||||
| SR/AF vs AF/AF | 1.77 (1.04–3.00) | 0.0318 | 1.15 (0.66–1.98) | 0.6208 |
| SR/AF vs SR/SR | 2.73 (1.68–4.44) | <0.0001 | 1.93 (1.25–2.96) | 0.0024 |
| AF/AF vs SR/SR | 1.56 (1.16–2.09) | 0.0031 | 1.67 (1.06–2.63) | 0.0268 |
| Any bleeding | ||||
| SR/AF vs AF/AF | 1.59 (1.11–2.26) | 0.0115 | 1.05 (0.79–1.41) | 0.7762 |
| SR/AF vs SR/SR | 1.89 (1.36–2.62) | <0.001 | 0.95 (0.76–1.19) | 0.6834 |
| AF/AF vs SR/SR | 1.20 (1.00–1.43) | 0.0473 | 0.90 (0.72–1.13) | 0.4582 |
| Stroke or transient ischemic attack | ||||
| SR/AF vs AF/AF | 0.87 (0.37–2.08) | 0.7591 | 0.73 (0.31–1.73) | 0.4710 |
| SR/AF vs SR/SR | 1.00 (0.44–2.27) | 0.9935 | 0.80 (0.40–1.62) | 0.5362 |
| AF/AF vs SR/SR | 1.09 (0.75–1.57) | 0.6589 | 1.09 (0.59–2.03) | 0.7811 |
AF/AF indicates baseline AF/discharge AF; HR, hazard ratio; MI, myocardial infarction; SAVR, surgical aortic valve replacement; SR/AF, baseline SR/discharge AF; SR/SR, baseline sinus rhythm/discharge sinus rhythm; and TAVR, transcatheter aortic valve replacement.
Figure 2. Major clinical outcomes for TAVR and SAVR among rhythm categories.
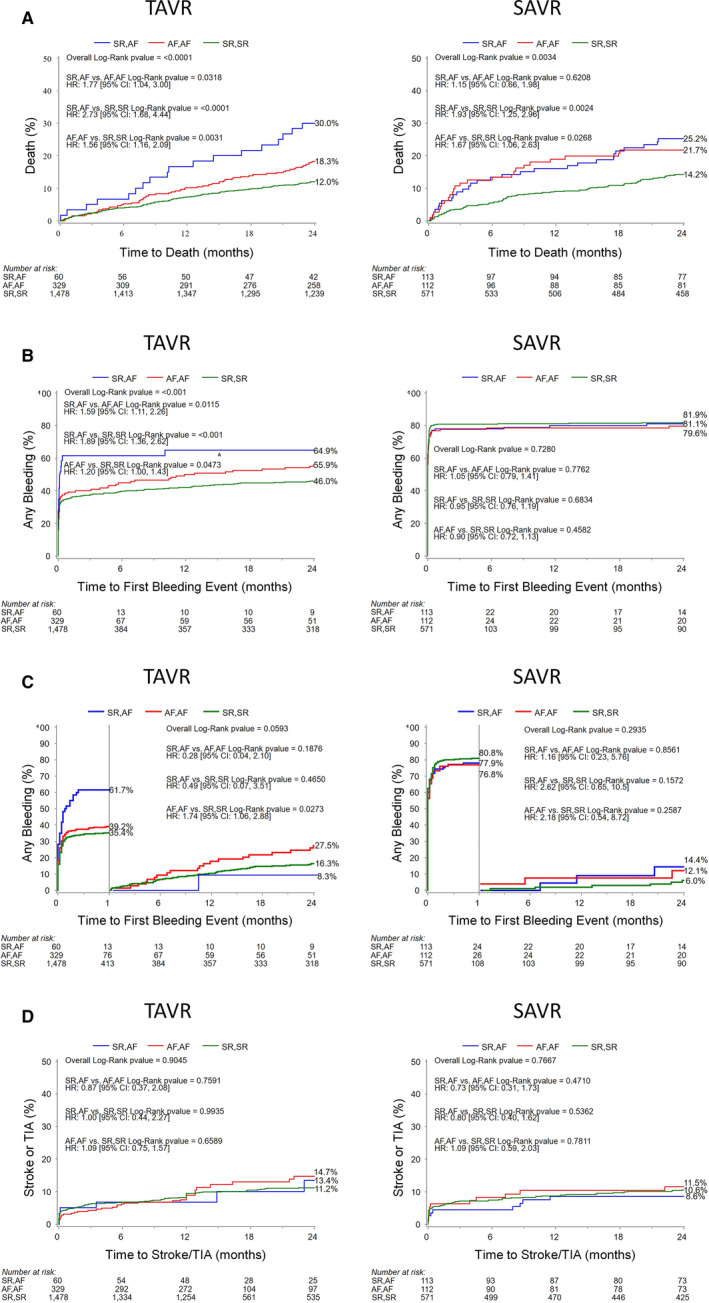
Kaplan‐Meier curves for TAVR and SAVR for time to (A) all‐cause mortality, (B) any bleeding events, (C) bleeding events landmarked after 30 days, and (D) stroke/transient ischemic attack at 2‐year follow‐up. Panel A illustrates the increased mortality associated with any form of AF post‐procedure. Note the increased bleeding events in SR/AF TAVR patients highlighted in (B). AF indicates atrial fibrillation; HR, hazard ratio; SAVR, surgical aortic valve replacement; SR, sinus rhythm; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack.
Patients with SAVR in the AF/AF and SR/AF groups also experienced significantly higher mortality rates compared with patients with SR/SR (1 year: 18.9% AF/AF versus 16.0% SR/AF versus 9.0% SR/SR, P=0.002 overall; 2 years: 21.7% AF/AF versus 25.2% SR/AF versus 14.2% SR/SR, P=0.003 overall). The presence of AF at baseline or discharge resulted in an increased hazard of death at 2 years relative to sinus rhythm (for SR/AF versus SR/SR, HR, 1.93; 95% CI, 1.25–2.96; P=0.002; for AF/AF versus SR/SR, HR, 1.67; 95% CI, 1.06–2.63; P=0.027, Figure 2A and Table 5). However, for patients admitted in SR and discharged in AF, there was not an association with increased mortality relative to patients with baseline AF among patients with SAVR (HR, 1.15; 95% CI, 0.66–1.98; P=0.62). Cardiovascular mortality, specifically, was also increased in patients with AF at the 1‐ and 2‐year landmarks (1 year: 10.3% AF/AF and 11.0% SR/AF versus 9.0% SR/SR, P=0.04 overall; 2 year: 12.4% AF/AF and 18.8% SR/AF versus 8.3% SR/SR, P=0.003 overall). The AF groups also manifested higher rates of rehospitalization at 1 year (28.4% AF/AF and 15.9% SR/AF versus 11.7% SR/SR, P<0.0001 overall) and 2 years (31.6% AF/AF and 19.2% SR/AF versus 14.5% SR/SR, P<0.0001 overall).
For bleeding events, patients with TAVR, but not SAVR, admitted in SR and discharged in AF experienced an increased risk of bleeding events relative to their SR/SR counterparts (HR, 1.89; 95% CI, 1.36–2.62; P<0.001) and patients with baseline AF (HR, 1.59; 95% CI, 1.11–2.26; P=0.01) (Figure 2B and Table 5). Landmark analysis after 30 days showed that patients with TAVR admitted in SR and discharged in AF experienced a higher hazard for bleeding in the first 30 days (SR/AF versus AF/AF, HR, 1.81; 95% CI, 1.25–2.60; P=0.0012; SR/AF versus SR/SR, HR, 2.05; 95% CI, 1.47–2.86; P<0.0001; AF/AF versus SR/SR, HR, 1.14; 95% CI, 0.94–1.38; P=0.1864), but a nonsignificant difference in bleeding relative to patients with SR/SR and AF/AF during the subsequent 23 months (Figure 2C). The risk of bleeding events in the SAVR cohort was not significantly different across the 3 groups of patients.
Functional status as reflected by NYHA class was also worse in the AF groups (Tables 3 and 4). The percentage of patients with TAVR with NYHA class III/IV symptoms was not different at baseline among the different groups (81.7% SR/AF versus 77.8% AF/AF versus 73.7% SR/SR, P=0.14), but patients in the SR/AF group displayed a trend toward increased risk of residual NYHA III/IV symptoms relative to patients in the SR/SR group. Patients in the AF/AF group had the highest risk of NYHA III/IV heart failure symptoms relative to patients in the SR/SR group: discharge (relative risk [RR], 1.09; 95% CI, 1.01–1.19; P=0.02), 30 days (RR, 1.04; 95% CI, 1.00–1.09; P=0.02), 1 year (RR, 1.06; 95% CI, 1.01–1.10; P=0.003), and 2 years (RR, 1.06; 95% CI, 1.01–1.12; P=0.007). Although patients in the SAVR cohort were more likely to have NYHA III/IV symptoms at discharge if they were in the AF/AF group when compared with the SR/SR group (RR, 1.26; 95% CI, 1.01–1.56; P=0.02), this association was not maintained throughout the remainder of the follow‐up period. Patients in the SR/AF group were more likely to have advanced heart failure symptoms relative to patients in the SR/SR group only at 30‐day follow‐up (RR, 1.13; 95% CI, 1.01–1.25; P=0.006). Event rates for the stroke/TIA outcome at 2 years were also not significantly different among any groups in either the TAVR or SAVR cohorts, but moderate‐severe mitral regurgitation exerted an interaction effect (P‐interaction=0.04) on the relationship between AF and stroke.
Table 6 details results of multivariable analysis for clinical outcomes at 30 days, 1 year, and 2 years. At the 30‐day time point, no variables of interest predicted mortality in the TAVR cohort, while the presence of cardiomyopathy (HR, 4.11; 95% CI, 1.20–14.06; P=0.02) predicted mortality in the population with SAVR. In contrast, predictors of mortality were similar at the 1‐ and 2‐year marks among patients with TAVR and SAVR (including for patients admitted in SR and discharged in AF and those with baseline AF and prior myocardial infarction). Prior stroke or TIA emerged as independent predictors of mortality at 2 years in the TAVR group. Procedure type (ie, TAVR versus SAVR) did not affect the association between these predictors and 2 year mortality (P‐interaction nonsignificant).
Table 6.
All‐Cause Mortality: Multivariable Analysis
| Timepoint | Variable | TAVR | SAVR | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| 30 d | SR/AF vs SR/SR | 3.86 (0.44–34.28) | 0.22 | 3.34 (0.60–18.50) | 0.17 |
| AF/AF vs SR/SR | 1.60 (0.28–9.08) | 0.60 | 2.23 (0.29–16.99) | 0.44 | |
| STS risk score >8 | 1.02 (0.13–7.93) | 0.98 | 2.01 (0.42–9.70) | 0.38 | |
| Cardiomyopathy | 2.58 (0.72–9.27) | 0.15 | 4.11 (1.20–14.06) | 0.02 | |
| NYHA III/IV symptoms | 0.68 (0.23–1.98) | 0.48 | 0.86 (0.22–3.27) | 0.82 | |
| Previous MI | 1.55 (0.49–4.86) | 0.45 | 0.80 (0.17–3.77) | 0.78 | |
| Previous stroke/TIA | 0.72 (0.16–3.20) | 0.66 | 2.30 (0.67–7.90) | 0.19 | |
| 1 y | SR/AF vs SR/SR | 2.79 (1.37–5.68) | 0.005 | 2.19 (1.21–3.97) | 0.010 |
| AF/AF vs SR/SR | 2.13 (1.22–3.72) | 0.008 | 3.17 (1.62–6.21) | 0.0007 | |
| STS risk score >8 | 1.88 (1.14–3.10) | 0.01 | 1.76 (1.02–3.04) | 0.04 | |
| Cardiomyopathy | 1.06 (0.61–1.84) | 0.84 | 1.23 (0.67–2.27) | 0.50 | |
| NYHA III/IV symptoms | 0.87 (0.60–1.25) | 0.45 | 0.92 (0.57–1.50) | 0.74 | |
| Previous MI | 1.64 (1.13–2.37) | 0.009 | 1.47 (0.91–2.36) | 0.11 | |
| Previous stroke/TIA | 1.29 (0.87–1.91) | 0.21 | 1.06 (0.61–1.82) | 0.84 | |
| 2 y | SR/AF vs SR/SR | 3.23 (1.91–5.45) | <0.0001 | 2.28 (1.42–3.66) | 0.0007 |
| AF/AF vs SR/SR | 2.30 (1.53–3.47) | <0.0001 | 2.39 (1.33–4.31) | 0.004 | |
| STS risk score >8 | 1.64 (1.10–2.43) | 0.02 | 1.48 (0.93–2.37) | 0.10 | |
| Cardiomyopathy | 1.16 (0.78–1.73) | 0.47 | 1.37 (0.85–2.21) | 0.20 | |
| NYHA III/IV symptoms | 1.02 (0.76–1.36) | 0.90 | 0.96 (0.65–1.42) | 0.83 | |
| Previous MI | 1.48 (1.11–1.96) | 0.008 | 1.48 (1.00–2.18) | 0.050 | |
| Previous stroke/TIA | 1.37 (1.02–1.84) | 0.03 | 1.22 (0.80–1.86) | 0.37 | |
AF/AF indicates baseline AF/discharge AF; HR, hazard ratio; MI, myocardial infarction; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; SR/AF, baseline SR/discharge AF; SR/SR, baseline sinus rhythm/discharge sinus rhythm; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; and TIA, transient ischemic attack.
Discussion
Four major conclusions from this analysis add to our understanding of the impact of AF on clinical outcomes following aortic valve intervention in patients with severe AS at intermediate surgical risk. First, those patients in AF at the discharge time point, including patients with SR/AF, experienced worse outcomes after undergoing either TAVR or SAVR. In particular, 1‐ and 2‐year all‐cause mortality, as well as rehospitalization rates, were increased in patients with TAVR and SAVR with AF. Second, the development of AF at discharge occurred more frequently in patients with SAVR than in patients with TAVR, though at a lower than expected rate based on prior literature. Nevertheless, the impact on 2‐year mortality of AF at discharge for patients with baseline SR is higher for patients with TAVR versus SAVR (HR, 2.73 post‐TAVR versus 1.93 post‐SAVR), possibly owing to the more transient course of AF after SAVR. Third, advanced heart failure symptoms were more common in patients with AF at discharge, though only the specific subgroup of patients with TAVR with AF/AF were more likely than patients with SR/SR to have advanced heart failure symptoms at long‐term follow‐up. Fourth, bleeding complications were increased in patients with TAVR but not SAVR discharged in AF, and in particular in patients with SR/AF, a high percentage of whom were treated with DAPT therapy as well as anticoagulation.
These data add to prior findings from the original PARTNER study, which noted worse outcomes in a cohort of patients at high risk with severe AS undergoing TAVR or SAVR who had or developed AF. 13 Our study also complements observational findings from other nonrandomized trials. For example, the SOURCE XT study (Edwards SAPIEN XT Aortic Bioprosthesis Multi‐Region Outcome Registry) was a multicenter, prospective registry of consecutive patients treated with the SAPIEN XT valve that assessed the baseline frequency of AF and whether preexisting AF or AF at discharge affected clinical outcomes. Both preexisting AF and AF at discharge were associated with increased all‐cause and cardiovascular mortality and bleeding events relative to SR. 15 FRANCE‐2 (French Aortic National Corevalve and Edwards registry) reported findings from the multicenter French national transcatheter aortic valve implantation registry and results mirrored findings from SOURCE XT and the PARTNER experience, with increased 1‐year mortality in patients with AF and worse outcomes in patients with AF who had nonfemoral TAVR access. The incidence of stroke (4.1%) was also similar relative to earlier studies. 16
More recently, analyses from Medicare claims data (72 660 patients) and the STS/American College of Cardiology Transcatheter Valve Therapy registry (13 556 patients) reported postoperative AF in 6.8% and 8.4% of patients treated with TAVR, respectively. 17 , 18 In the later study, older age, female sex, higher STS score, and nonfemoral access were associated with AF. Importantly, postoperative AF was associated with 1‐year all‐cause mortality (adjusted HR, 1.39; 95% CI, 1.19–1.59), but the authors did not specifically segregate the mortality analysis by STS risk. Additional post hoc data informing the risk of AF in patients with intermediate risk from randomized trials like ours are limited. The SURTAVI trial (Surgical Replacement and Transcatheter Aortic Valve Implantation), for example, evaluated a self‐expanding transcatheter valve in patients with intermediate surgical risk and reported baseline rates of AF different from those noted in our study (28.1% in the TAVR group, 26.5% in the SAVR group). At 30 days, AF occurred in 12.9% of the TAVR group versus 43.4% in the SAVR group, but the association between AF and outcomes was not reported.
Given these data, it is increasingly clear that AF is associated with negative outcomes, not only for patients at high risk with surgical AS but also for those at intermediate risk. This finding should be considered when tailoring treatment strategies for both patients with high and intermediate risk TAVR and SAVR. With the recognition that post‐procedural AF portends worse outcomes, clinicians should consider (1) how best to monitor patients with AF post‐TAVR or ‐SAVR, (2) and how to treat AF when it is detected, that is, whether to use a rhythm control strategy with antiarrhythmics, cardioversion, or invasive procedures such as catheter ablation and concomitant Cox‐Maze with SAVR versus a more conservative rate control strategy, while also (3) balancing stroke and bleeding risks when considering anticoagulation prescription. 19
In the burgeoning market of noninvasive monitors including wearable patches, watches, and smartphones, as well as minimally invasive subcutaneous monitors, the development and validation of approaches for diagnosing and monitoring AF following TAVR will be crucial. Clinicians routinely caring for patients with TAVR need to use these monitors to (1) detect AF when it arises, (2) quantify its burden, and (3) evaluate the effectiveness of treatment targeted to controlling either the rate or rhythm. With more granular and robust data regarding post‐TAVR and post‐SAVR AF, further studies will be able to tailor treatment strategies using a combination of rate control and/or antiarrhythmic medications, cardioversion, and catheter ablation, thereby minimizing overall risk for patients with post‐procedural AF.
Thromboembolism prevention is important in patients with AS and AF, as a large percentage of these patients possess relatively high CHADS2‐VASC (congestive heart failure, hypertension, age, diabetes, previous stroke/TIA, vascular disease, and sex category) scores. 20 Interestingly, stroke/TIA rates were low at 2‐year follow‐up and not significantly different between patients in different rhythm categories, emphasizing the value of medical therapy in mitigating this risk. Nevertheless, the occurrence of bleeding on DAPT and anticoagulants (including anticoagulant type) must be weighed against antiembolic benefits derived from these agents. For patients with TAVR discharged in AF, particularly SR/AF, bleeding events were significantly increased, with landmark analysis noting a significantly steep rise by 1 month. The increased risk in patients with AF requiring anticoagulants may be associated with more than double the use of DAPT therapy in patients with TAVR versus SAVR. This finding is in keeping with a number of small studies conducted early in the TAVR experience that suggested DAPT and triple therapy (DAPT plus anticoagulation) were associated with worse bleeding outcomes. 21 , 22 The recently published Popular‐TAVI (Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation) study has also provided insight regarding the interaction between antiplatelet therapy and anticoagulation, reporting that among those patients with TAVR with an indication for anticoagulation, oral anticoagulation alone was associated with reduced bleeding compared with oral anticoagulation plus clopidogrel and was noninferior with respect to major adverse ischemic events. 23 Furthermore, the option of left atrial appendage closure is being analyzed in the WATCH‐TAVR trial (WATCHMAN for Patients With Atrial Fibrillation Undergoing Transcatheter Aortic Valve Replacement) and it may provide a safe alternative for thromboembolism prevention in patients with AF who require antiplatelet therapy. 24 In summary, strong conclusions with respect to optimal anticoagulation strategies cannot yet be made, and further research is required to transcend the current equipoise regarding the optimal antithrombotic regimen for patients who develop AF following TAVR or SAVR.
Study Limitations
This study was a post hoc analysis of prospective trials with adjudicated ECG and clinical outcome data analyzed at discrete time points: baseline, discharge, 1 year, and 2 years. As such, continuous cardiac rhythm monitoring was not available. Thus, patients with a change in rhythm status between admission and discharge, including those who developed postoperative AF during hospitalization and subsequently converted to SR before discharge, were not identified in the group of patients analyzed as having developed AF. As baseline and discharge rhythms were analyzed, caution must be taken not to extrapolate results of this study to patients with postoperative AF, or paroxysmal or persistent AF, as such categorizations cannot be made solely based on a baseline and discharge ECG. Furthermore, ECGs cannot reflect the burden of AF and account for any associations between the duration of arrhythmia and clinical outcomes. Nevertheless, the discharge rhythm of AF remains a challenging real‐world clinical situation with consequent outcomes that requires careful follow‐up. Similarly, data regarding patients with paroxysmal episodes of AF after discharge were not available. In addition, this study did not gather information regarding the nature of AF or atrial flutter in any of the patients nor did it elicit details of antiarrhythmic treatment or surgical therapies for atrial fibrillation (ie, the maze procedure), which may have affected survival in patients with AF/AF relative to patients with SR/AF. Lastly, although adjustments for several covariates were made, the potential for unmeasured confounders remains. This is particularly relevant for the influence of access site on rhythm category among recipients of TAVR.
Conclusions
The presence of AF at discharge, particularly if not present at baseline, is a strong determinant of poor outcomes in patients with intermediate risk and severe AS undergoing either TAVR or SAVR, including mortality at 1 and 2 years. In addition to increased mortality, rehospitalizations and advanced heart failure symptoms were also more likely in those patients with baseline AF or those who developed AF by discharge relative to patients in SR. Patients who developed AF in the TAVR cohort were also more likely than their SR counterparts to suffer from bleeding; this finding was not demonstrated in patients with SAVR and may be related to higher use of DAPT along with anticoagulant therapy in patients with TAVR. Considering the relatively high prevalence of AF in the population with SAVR and TAVR, further investigation is required to show the impact of AF treatments, from rate and rhythm control strategies to thromboembolism prevention, on clinical outcomes in order to personalize appropriate treatment for each patient with AS.
Sources of Funding
None.
Disclosures
George is a consultant for MitreMedical, CardioMech, WL Gore, Atricure, Neptune Medical. Nazif is a consultant for Edwards Lifesciences, Medtronic, Boston Scientific. Malaisrie is a consultant for Edwards Lifesciences, Medtronic, Abbott. Makkar reports research grants from Edwards Life Sciences, Abbott, Medtronic and Boston Scientific; personal proctoring fee from Edwards Life Sciences; and travel support from Edwards, Abbott and Boston Scientific. Mack reports institutional research support (no direct financial compensation) from Edwards Lifesciences. Thourani reports research funding and consulting for Abbott, Allergen, Boston Scientific, Cryolife, Edwards Lifescience, Gore, Jenavalve. Leon reports institutional research support (no direct financial compensation) from Edwards Lifesciences, Medtronic, Boston Scientific, Abbott. Consultant/Advisory Board for Medtronic, Boston Scientific, Gore, Meril Lifesciences, and Abbott. Kodali reports institutional research support (no direct financial compensation) from Edwards Lifesciences, Medtronic, Abbott. Consultant for Abbott, Admedus, Meril Lifesciences. Equity options from Biotrace Medical and Thubrikar Aortic Valve, Inc. Biviano is on the Medical Advisory Board for Biosense Webster and Boston Scientific. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
(J Am Heart Assoc. 2021;10:e019584. DOI: 10.1161/JAHA.120.019584.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019584
For Sources of Funding and Disclosures, see page 11.
References
- 1. Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81:119–125. DOI: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lip GY. Can we predict stroke in atrial fibrillation? Clin Cardiol. 2012;35(suppl 1):21–27. DOI: 10.1002/clc.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, Tarazi R, Shroyer AL, Sethi GK, Grover FL, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–513; discussion 511–503. DOI: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, Arnar DO, Gudbjartsson T. Atrial fibrillation following cardiac surgery: risk analysis and long‐term survival. J Cardiothorac Surg. 2012;7:87. DOI: 10.1186/1749-8090-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mostafa A, El‐Haddad MA, Shenoy M, Tuliani T. Atrial fibrillation post cardiac bypass surgery. Avicenna J Med. 2012;2:65–70. DOI: 10.4103/2231-0770.102280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filardo G, Hamilton C, Hamman B, Hebeler RF Jr, Adams J, Grayburn P. New‐onset postoperative atrial fibrillation and long‐term survival after aortic valve replacement surgery. Ann Thorac Surg. 2010;90:474–479. DOI: 10.1016/j.athoracsur.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 7. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. DOI: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 8. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. DOI: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 9. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. DOI: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 10. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. DOI: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 11. Motloch LJ, Reda S, Rottlaender D, Khatib R, Müller‐Ehmsen J, Seck C, Strauch J, Madershahian N, Erdmann E, Wahlers T, et al. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg. 2012;93:124–131. DOI: 10.1016/j.athoracsur.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 12. Tanawuttiwat T, O'Neill BP, Cohen MG, Chinthakanan O, Heldman AW, Martinez CA, Alfonso CE, Mitrani RD, Macon CJ, Carrillo RG, et al. New‐onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol. 2014;63:1510–1519. DOI: 10.1016/j.jacc.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 13. Biviano AB, Nazif T, Dizon J, Garan H, Fleitman J, Hassan D, Kapadia S, Babaliaros V, Xu KE, Parvataneni R, et al. Atrial fibrillation is associated with increased mortality in patients undergoing transcatheter aortic valve replacement: insights from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Interv. 2016;9:e002766. DOI: 10.1161/CIRCINTERVENTIONS.115.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. DOI: 10.1016/S0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- 15. Tarantini G, Mojoli M, Windecker S, Wendler O, Lefevre T, Saia F, Walther T, Rubino P, Bartorelli AL, Napodano M, et al. Prevalence and impact of atrial fibrillation in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement: an analysis from the SOURCE XT prospective multicenter registry. JACC Cardiovasc Interv. 2016;9:937–946. DOI: 10.1016/j.jcin.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 16. Gilard M, Eltchaninoff H, Iung B, Donzeau‐Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, et al. Registry of transcatheter aortic‐valve implantation in high‐risk patients. N Engl J Med. 2012;366:1705–1715. DOI: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 17. Vora AN, Dai D, Matsuoka R, Harrison JK, Hughes GC IV, Sherwood MW, Piccini JP, Bhardwaj B, Lopes RD, Cohen D, et al. Incidence, management, and associated clinical outcomes of new‐onset atrial fibrillation following transcatheter aortic valve replacement: an analysis from the STS/ACC TVT registry. JACC Cardiovasc Interv. 2018;11:1746–1756. DOI: 10.1016/j.jcin.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 18. Mentias A, Saad M, Girotra S, Desai M, Elbadawi A, Briasoulis A, Alvarez P, Alqasrawi M, Giudici M, Panaich S, et al. Impact of pre‐existing and new‐onset atrial fibrillation on outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:2119–2129. DOI: 10.1016/j.jcin.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandrasekhar J, Hibbert B, Ruel M, Lam BK, Labinaz M, Glover C. Transfemoral vs non‐transfemoral access for transcatheter aortic valve implantation: a systematic review and meta‐analysis. Can J Cardiol. 2015;31:1427–1438. DOI: 10.1016/j.cjca.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 20. Nombela‐Franco L, Webb JG, de Jaegere PP, Toggweiler S, Nuis R‐J, Dager AE, Amat‐Santos IJ, Cheung A, Ye J, Binder RK, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation. 2012;126:3041–3053. DOI: 10.1161/CIRCULATIONAHA.112.110981. [DOI] [PubMed] [Google Scholar]
- 21. Poliacikova P, Cockburn J, de Belder A, Trivedi U, Hildick‐Smith D. Antiplatelet and antithrombotic treatment after transcatheter aortic valve implantation—comparison of regimes. J Invasive Cardiol. 2013;25:544–548. [PubMed] [Google Scholar]
- 22. Czerwińska‐Jelonkiewicz K, Witkowski A, Dąbrowski M, Banaszewski M, Księżycka‐Majczyńska E, Chmielak Z, Kuśmierski K, Hryniewiecki T, Demkow M, Orłowska‐Baranowska E, et al. Antithrombotic therapy—predictor of early and long‐term bleeding complications after transcatheter aortic valve implantation. Arch Med Sci. 2013;9:1062–1070. DOI: 10.5114/aoms.2013.39794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nijenhuis VJ, Brouwer J, Delewi R, Hermanides RS, Holvoet W, Dubois CLF, Frambach P, De Bruyne B, van Houwelingen GK, Van Der Heyden JAS, et al. Anticoagulation with or without clopidogrel after transcatheter aortic‐valve implantation. N Engl J Med. 2020;382:1696–1707. DOI: 10.1056/NEJMoa1915152. [DOI] [PubMed] [Google Scholar]
- 24. ClinicalTrials.gov . Watch‐TAVR, watchman for patients with atrial fibrillation undergoing transcatheter aortic valve replacement. 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


