Abstract
Background
Regional patient characteristics, care quality, and outcomes may differ based on a variety of factors among patients hospitalized for heart failure (HF). Regional disparities in outcomes of cardiovascular disease have been suggested across various regions in the United States. This study examined whether there are significant differences by region in quality of care and short‐term outcomes of hospitalized patients with HF across the United States.
Methods and Results
We examined regional demographics, quality measures, and short‐term outcomes across 4 US Census Bureau regions in patients hospitalized with HF and enrolled in the GWTG‐HF (Get With The Guidelines–Heart Failure) registry from 2010 to 2016. Differences in length of stay and mortality by region were examined with multivariable logistic regression. The study included 423 333 patients hospitalized for HF in 488 hospitals. Patients in the Northeast were significantly older. Completion of achievement measures, with few exceptions, were met with similar frequency across regions. Multivariable analysis demonstrated significantly lower in‐hospital mortality in the Midwest compared with the Northeast (hazard ratio, 0.64; 95% CI, 0.51–0.8; P<0.00001). The length of stay varied significantly by region with a significantly higher risk‐adjusted length of stay in the Northeast compared with other regions.
Conclusions
Although we did not find any substantial differences by region in quality of care in patients hospitalized for HF, risk‐adjusted inpatient mortality was found to be lower in the Midwest compared with the Northeast, and may be secondary to unmeasured differences in patient characteristics, and to longer length of stay in the Northeast.
Keywords: heart failure, quality and outcomes, regional variations
Subject Categories: Heart Failure, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- GWTG‐HF
Get With The Guidelines–Heart Failure
- HFrEF
heart failure with reduced ejection fraction
Clinical Perspective
What Is New?
Regional variations in heart failure outcomes in the United States have previously been demonstrated suggesting quality of care as a target for improvement.
Our contemporary analysis of the GWTG‐HF (Get With The Guidelines–Heart Failure) database suggests there are no substantial US regional variations in the quality of delivered heart failure care.
What Are the Clinical Implications?
Heart failure outcomes, including inpatient mortality, length of stay, and 30‐day readmission rates among Centers for Medicare and Medicaid Services patients, did vary regionally despite a lack of differences in quality of care, suggesting the regional variations in outcomes may be driven more by patient characteristics.
Heart failure (HF) therapies have been successful in reduction of symptoms and hospitalizations and improvement in survival. 1 Despite previous decreases in national trends for HF hospitalization and in‐hospital mortality, a plateau has been seen in more recent years. The frequency of HF hospitalizations has remained almost constant over the past decade, with nearly 1 million hospitalizations per year. 2 , 3 Inpatient mortality is associated with presenting patient characteristics such as age, low blood pressure, low serum sodium, and elevated blood urea nitrogen/creatinine. 4 , 5 However, short‐term mortality has been shown to vary in intercontinental studies of patients with HF and may be linked to regional differences, not only in disease etiology and severity but also in quality of care and treatment. 6 , 7 Differences in cardiovascular outcomes have also been suggested across various regions in the United States, with higher rates of morbidity and mortality in the southeastern states. 8 , 9 , 10 A prior study examining HF hospitalization rates in the US between 1995 and 2004 demonstrated increasing hospitalization rates for adults aged 35 to 64 years with greater increases in the South and West regions. 11 In an analysis of the National Inpatient Sample of HF hospitalizations in 2013 to 2014, in‐hospital mortality was highest in the Northeast and lowest in the Midwest. 12 However, there is a lack of contemporary data on regional variations in outcomes for patients hospitalized for HF in the context of quality of care provided.
The objectives of our study were to compare regional differences in patient demographics, quality measures, and short‐term outcomes in patients hospitalized for HF within the United States. This may identify targets for improvement in care outside of inherent patient characteristics.
METHODS
Our study used data from GWTG‐HF (Get With The Guidelines–Heart Failure) registry, a national quality improvement initiative of the American Heart Association. Because data were collected for clinical care and quality improvement rather than primarily for research, the American Heart Association (the steward of the data according to contracts between the American Heart Association and participating hospitals) cannot provide the data, statistical analysis code, or other study materials to other researchers. The methods and design of the registry have been previously described. 13 , 14 , 15 The national GWTG‐HF registry identifies adults hospitalized with HF as a primary diagnosis or those who developed significant HF symptoms during hospitalization. It includes hospitals from all regions and of various types across the United States. Trained personnel abstract information on consecutive admissions for HF and enter data into an online system known as IQVIA. Registry data elements include patient characteristics (demographics, medical history, medications, examination/laboratory results, in‐hospital treatment, discharge status, and length of stay [LOS]) as well as hospital‐level characteristics. This study included patients enrolled from January 1, 2010, to December 31, 2016. Study sites with limited participation (>25% of missing medical history panel or sex) were excluded. Patients were excluded if there was no defined discharge status, discharged to hospice, left against medical advice, or if race or left ventricular ejection fraction (LVEF) were missing. Participating institutions were required to comply with local regulatory and privacy guidelines and, where required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. IQVIA (Durham, NC) is the data collection and coordination center for the GWTG programs. The Duke Clinical Research Institute (Durham, NC) serves as the data analysis center and analyzes aggregate deidentified data for research purposes.
In this study, geographical region was the primary independent variable. Participating hospitals were divided into 4 US Census Bureau regions (www.census.gov) including (1) Northeast: Pennsylvania, New Jersey, New York, Connecticut, Massachusetts, Vermont, New Hampshire, Maine; (2) South: Texas, Oklahoma, Arkansas, Louisiana, Mississippi, Alabama, Tennessee, Kentucky, West Virginia Virginia, Maryland, Delaware, North Carolina, South Carolina, Georgia, Florida; (3) Midwest: Kansas, Nebraska, South Dakota, North Dakota, Missouri, Iowa, Minnesota, Illinois, Wisconsin, Indiana, Ohio, Michigan; and (4) West: Montana, Idaho, Wyoming, Nevada, Utah, Colorado, Arizona, New Mexico, California, Oregon, Washington, Alaska, Hawaii. Main outcomes examined included risk‐adjusted in‐hospital mortality, LOS less than or equal to the median, and discharge home. Secondary outcomes included achievement measures and quality measures as defined by the GWTG‐HF. 16 Achievement measures include the appropriate use of angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin receptor blocker (ARB)/ARB–neprilysin inhibitors at discharge, evidence‐based beta‐blocker use, measurement of LVEF, and a postdischarge appointment for patients with HF. Quality measures include appropriate use of aldosterone antagonists, anticoagulation for atrial fibrillation, and hydralazine/nitrates at discharge, venous thromboembolism prophylaxis in hospital, cardiac resynchronization–defibrillator therapy/pacing therapy placement or prescription at discharge, implantable cardioverter‐defibrillator counseling or placement at discharge, influenza vaccination during flu season and pneumococcal vaccination before discharge, and follow‐up appointment provided within ≤7 days after discharge. A follow‐up appointment was determined as provided if the appointment was scheduled and documented in the medical record with a location, date, time, or home health visit. Achievement measure composite scores and overall composite scores were examined on the basis of a composite of all achievement measures or all achievement and quality measures.
Outcomes of all‐cause readmission and mortality within 30 days of discharge were examined in the subset of patients with fee‐for‐service Medicare. Patients were included if their deidentified records could be linked to data from the Centers for Medicare and Medicaid Services, and for whom at least 30‐day follow‐up was available, with data availability from January 1, 2010, to November 30, 2015. Prior studies have described this methodology, and the applicability has been demonstrated within GWTG‐HF. 17 , 18
Statistical Analysis
Patient demographics, medical history, medications, laboratory data, hospital treatment, HF‐specific quality measures, and outcomes were compared between regions using standardized mean differences. A standardized difference of >10 indicated a significant imbalance between groups. The association of region with in‐hospital mortality, LOS less than or equal to median (4 days), and discharge home was examined using adjusted logistic regression models with generalized estimation equations used to account for in‐hospital clustering. Multiple imputation with 25 imputations were used to impute for missing covariates. The full‐conditional specification imputation method was used. If a patient had missing medical history, it was assumed that the medical condition did not occur. Hospital characteristics were not imputed. Variables used for adjustment included patient demographics (age, sex, race), medical history (anemia, ischemic heart disease, cerebrovascular accident/transient ischemic attack, diabetes mellitus, hyperlipidemia, hypertension, chronic obstructive pulmonary disease/asthma, peripheral vascular disease, renal insufficiency, cigarette smoking in the past year), vital signs at admission (systolic blood pressure, heart rate, serum sodium, blood urea nitrogen, LVEF), and hospital characteristics (region, hospital type, number of beds, rural versus urban, heart transplant center). The Northeast region was the reference group. For the subgroup analysis examining the association of region with 30‐day outcomes, Cox regression models were used. Robust variance estimation was used to account for clustering. The Fine and Gray method was used to account for competing risk from mortality for the 30‐day readmission outcome. Factors used for adjustment in the models were the same as those used for analysis of in‐hospital outcomes. SAS version 9.4 (SAS Institute Inc., Cary, NC) software was used for all statistical analyses. All P values were 2‐sided, and statistical significance was defined as P<0.05.
RESULTS
Study Cohort
During the study period (2010–2016), a total of 501 238 patients hospitalized with HF were enrolled across 509 sites. Twenty‐one sites and 77 905 patients were excluded on the basis of criteria specified. The final cohort included 423 333 patients from 488 sites. The subgroup of patients with fee‐for‐service Medicare and 30‐day outcomes included 98 808 patients from 374 sites. Baseline characteristics of the study population are detailed in Table 1. The mean age of the cohort was 72.2 years with 48.2% female, 69.1% White, 19.4% Black, and 7.6% Hispanic patients. A history of atrial fibrillation was noted in 39%, diabetes mellitus in 45%, hypertension in 81%, coronary artery disease in almost 50%, and renal insufficiency in 25% of patients.
Table 1.
Baseline Patient and Hospital Characteristics
| Overall (N=423 333) | Northeast (N=127 154) | Midwest (N=106 129) | South (N=133 438) | West (N=56 612) | Abs. Stand. Diff. (*=Significant, >10%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Midwest vs Northeast | South vs Northeast | West vs Northeast | |||||||
| Age, y±SD | 72.2±14.6 | 74.9±13.7 | 72.0±14.6* | 70.4±14.8* | 70.3±15.6* | 20.9* | 31.7* | 31.4* | |
| Female, % | 48.2 | 48.8 | 49.5 | 48.1 | 44.5 | 1.3 | 1.6 | 8.6 | |
| Race/Ethnicity, % | |||||||||
| Black | 19.3 | 14.2 | 19.8* | 27.9* | 9.9* | 15.0* | 34.3* | 13.2* | |
| White | 69.1 | 76.5 | 74.9 | 60.0* | 63.4* | 3.8 | 36.1* | 28.9* | |
| Hispanic | 7.6 | 6.4 | 2.5* | 9.4* | 15.3* | 18.7* | 11.5* | 29.1* | |
| Other† | 3.9 | 2.9 | 2.8 | 2.6 | 11.4* | 1.33 | 0.71 | 21.9* | |
| Insurance, % | |||||||||
| Medicare | 55.7 | 60.3 | 52.5* | 55.8 | 51.1* | 15.7* | 9.1 | 18.5* | |
| Medicaid | 13.5 | 11.8 | 14.4 | 12.8 | 16.9* | 7.7 | 3.0 | 14.5* | |
| Other | 27.0 | 26.2 | 30.0 | 25.0 | 28.3 | 8.3 | 2.9 | 4.7 | |
| No insurance/ not documented | 3.9 | 1.7 | 3.1 | 6.5* | 3.7* | 9.5 | 24.4* | 12.4* | |
| Missing | 14.3 | 16.7 | 18.1 | 10.3 | 11.5 | ||||
| Medical history, % | |||||||||
| Atrial fibrillation | 39.2 | 43.7 | 40.3 | 34.7* | 37.5* | 6.8 | 18.4* | 12.5* | |
| COPD | 33.6 | 33.5 | 37.2 | 32.6 | 29.7 | 7.7 | 1.8 | 8.1 | |
| Diabetes mellitus | 45.0 | 43.6 | 46.3 | 46.7 | 42.1 | 5.4 | 6.3 | 3.0 | |
| Hyperlipidemia | 54.1 | 54.9 | 59.1 | 52.6 | 46.3* | 8.5 | 4.6 | 17.1* | |
| Hypertension | 81.1 | 79.5 | 83.6* | 83.0 | 76.0 | 10.7* | 9.0 | 8.3 | |
| PVD | 12.4 | 12.3 | 15.3 | 11.7 | 9.0* | 8.5 | 2.0 | 10.8* | |
| CAD | 49.6 | 53.7 | 49.8 | 49.3 | 40.2* | 7.8 | 9.0 | 27.3* | |
| Prior CABG | 20.0 | 21.2 | 21.4 | 20.0 | 15.0* | 0.5 | 2.9 | 16.1* | |
| Prior MI | 20.4 | 20.5 | 22.1 | 19.6 | 18.9 | 4.0 | 2.3 | 4.0 | |
| CVA/TIA | 15.9 | 15.7 | 17.6 | 15.6 | 13.5 | 4.9 | 0.3 | 6.3 | |
| Dialysis | 3.8 | 3.6 | 3.9 | 4.0 | 3.7 | 1.3 | 1.9 | 0.3 | |
| Renal insufficiency | 24.9 | 26.0 | 25.7 | 24.7 | 21.4* | 0.9 | 3.0 | 11.0* | |
| Smoking | 16.5 | 13.2 | 18.2* | 18.0* | 17.2* | 13.8* | 12.8* | 10.6* | |
| ICD/CRT‐D | 15.7 | 15.5 | 16.2 | 16.5 | 13.1 | 1.8 | 2.6 | 7.0 | |
| Medications, % | |||||||||
| ACEI | 33.7 | 31.4 | 34.5 | 35.4 | 33.7 | 6.7 | 8.5 | 4.9 | |
| ARB | 14.5 | 13.9 | 14.5 | 14.9 | 15.0 | 1.8 | 2.8 | 3.1 | |
| MRA | 12.7 | 11.0 | 12.7 | 13.3 | 15.3* | 5.1 | 6.8 | 12.6* | |
| Beta‐blocker | 68.5 | 70.0 | 70.0 | 68.1 | 64.2* | 0.1 | 4.1 | 12.3* | |
| Digoxin | 10.8 | 11.9 | 10.0 | 10.5 | 10.2 | 6.1 | 4.5 | 5.6 | |
| Loop diuretic | 60.0 | 61.1 | 61.6 | 58.4 | 56.3 | 1.0 | 5.4 | 9.8 | |
| Hydralazine/nitrate | 22.4 | 23.1 | 23.5 | 22.8 | 18.1* | 0.9 | 0.8 | 12.4* | |
| Statin | 51.3 | 54.2 | 52.8 | 48.5* | 49.2* | 2.9 | 11.4* | 10.1* | |
| Vital signs/lab | |||||||||
| BMI, kg/m2 | 29.2±6.2 | 29.0±5.7 | 29.4±6.2 | 29.2±6.4 | 29.1+6.6 | 6.6 | 4.4 | 2.6 | |
| SBP, mm Hg | 141.7±30.0 | 140.6±29.0 | 143.0±30.1 | 143.8±31.0* | 138.7±29.6 | 8.3 | 10.6* | 6.4 | |
| DBP, mm Hg | 78.0±18.9 | 76.7±18.1 | 77.7±19.2 | 79.9±19.6* | 77.8±18.8 | 5.2 | 17.0* | 6.1 | |
| BNP, pg/mL | 838 (408–1669) | 750 (342–1500) | 822 (411–1631) | 897 (439–1792)* | 916 (460–1789)* | 8.0 | 17.0* | 15.9* | |
| Heart rate | 85.6±19.8 | 84.4±19.4 | 85.5±19.8 | 86.7±19.9* | 86.7±20.5* | 5.4 | 11.5* | 11.5* | |
| Sodium, meq/L | 137.5±7.4 | 137.6±7.4 | 137.7±7.1 | 137.3±7.9 | 137.1±6.6 | 1.0 | 3.5 | 6.7 | |
| Creatinine, mg/dL | 1.3 (1.0–1.8) | 1.3 (1.0–1.8) | 1.3 (1.0–1.8) | 1.3 (1.0–1.9) | 1.3 (0.9–1.8) | 1.3 | 4.0 | 0.7 | |
| LVEF | |||||||||
| Preserved | 43.0 | 45.5 | 44.3 | 40.5* | 40.7 | 2.5 | 10.2* | 9.7 | |
| Borderline | 13.6 | 13.8 | 13.3 | 13.6 | 13.7 | 1.5 | 0.7 | 0.4 | |
| Reduced | 43.4 | 40.7 | 42.4 | 45.9* | 45.6 | 3.5 | 10.6* | 10.0 | |
| Hospital characteristics | |||||||||
| Teaching | 77.1 | 81.2 | 73.8* | 77.8 | 72.9* | 17.7* | 8.3 | 19.7* | |
| Number of beds | 383 (248–540) | 363 (245–539) | 411 (249–607)* | 405 (243–585)* | 374 (261–501)* | 19.4* | 27.8* | 10.9* | |
| Rural location | 3.3 | 4.4 | 1.0* | 4.4 | 2.4* | 21.0* | 0.1 | 11.0* | |
| Heart transplant hospital | 11.1 | 9.1 | 17.5* | 4.9* | 16.8* | 24.9* | 16.9* | 22.9* | |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy–defibrillator; CVA/TIA, cerebrovascular accident/transient ischemic attack; DBP, diastolic blood pressure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; PVD, peripheral vascular disease; and SBP, systolic blood pressure.
Abs. stand. difference=absolute standardized difference; SD >10 was considered significant; regions with significant SD for a variable compared with the Northeast region.
Other includes Asian, American Indian or Alaskan Native, Native Hawaiian or Pacific Islander, and unable to be determined.
Regional Variations in Patient Characteristics
Comparison of patient demographics showed significant differences in age, race, and insurance status among the 4 specified regions (Table 1). All regions had a significantly younger population when compared with the Northeast, which had a mean age of ≈75 years. This also translated into a larger proportion of patients aged >65 years in the Northeast region when compared with the Midwest, South, and West regions (78% versus 70%, 67%, and 66%, respectively). There were significant differences in race/ethnicity, with a larger proportion of White patients with HF in the Northeast and Midwest regions when compared with the South and West (77% and 75% versus 60% and 63%, respectively), a larger proportion of Black patients in the South (27.9%) and Midwest (19.8%) regions and a smaller proportion in the West (9.9%), compared with the Northeast (14.2%). The proportion of Hispanic patients was highest in the West and South and lower in the Midwest. Patients in the South (6.5%) and West (3.7%) also more frequently had lack of insurance coverage, with the lowest proportion without insurance noted in the Northeast (1.7%). Among Medicare and Medicaid enrollees, those in the Northeast were most likely to be enrolled in Medicare and those in the West most likely to be enrolled in Medicaid. There was no significant variation by sex among regions.
As shown in Table 1, when compared with the West, patients with HF in the Northeast more frequently had several comorbidities, including hyperlipidemia, peripheral vascular disease, coronary artery disease, prior coronary artery bypass grafting, and renal insufficiency (serum creatinine >2 g/dL). They also more frequently had atrial fibrillation when compared with patients in the South and West (44% versus 35% and 38%, respectively). Otherwise, patients in the South and the Midwest had similar frequency of most other comorbidities compared with the Northeast. Although the prevalence of comorbidities such as hypertension, renal insufficiency, and end‐stage renal disease were similar in the South compared with the Northeast and West, it should be noted that the patients were younger in the South, suggesting that patients in the South may be more affected by these comorbidities at a younger age. Similarly, the prevalence of coronary artery disease in the younger patients in the South was similar to older patients in the Midwest, but higher than a similarly aged younger patient group in the West. As shown in Table 1, some differences were noted by region in admission vital signs and laboratory evaluation. In the South, patients had significantly higher systolic and diastolic blood pressure when compared with the Northeast. Average admission heart rates as well as admission B‐type natriuretic peptide levels were significantly higher in the South and West regions when compared with the Northeast. Patients in the South and the West more frequently had HF with reduced ejection fraction (HFrEF), whereas patients in the Northeast and Midwest tended to have a higher frequency of HF with preserved ejection fraction.
Patients admitted in the Northeast were more likely to be hospitalized at a teaching hospital when compared with the Midwest and South (81.1% versus 73.8% and 72.9%, respectively). However, patients in the Northeast were less likely to be admitted to a heart transplant hospital and more likely to be in a rural location compared with the Midwest and South (Table 1).
Regional Variations in Quality of Care
As shown in Table 2, a review of the achievement measures at discharge for the prescription of ACEIs, ARBs, or ARB–neprilysin inhibitors (overall 94.5%) and evidence‐based beta‐blockers (89.9%) in appropriate patients without contraindications did not demonstrate any significant differences by region. The appropriate prescription of other medications collected as quality measures demonstrated no differences, including nitrate‐hydralazine combination, although aldosterone receptor antagonists were prescribed less frequently in the South, while the prescription of anticoagulation for atrial fibrillation was highest in the Northeast. In addition, rates of influenza (≈84%) and pneumococcal vaccinations (≈79%) across regions was similar.
Table 2.
Achievement and Quality Measures at Discharge
| Overall | Northeast | Midwest | South | West | Abs. Stand. Diff. (*=Significant, >10) | |||
|---|---|---|---|---|---|---|---|---|
| Midwest vs Northeast | South vs Northeast | West vs Northeast | ||||||
| Achievement measures (%) | ||||||||
| ACEI/ARB or ARB–neprilysin inhibitor | 94.5 | 95.1 | 95.1 | 94.3 | 93.3 | 0.01 | 3.83 | 7.79 |
| Evidence‐based beta‐blocker | 89.9 | 89.7 | 89.7 | 89.7 | 91.5 | 0.07 | 0.02 | 6.33 |
| Postdischarge appointment for HF | 61.3 | 60.1 | 69.1* | 58.6 | 56.2 | 18.90* | 3.02 | 7.90 |
| Measurement of LV function | 100 | 100 | 100 | 100 | 100 | 0.00 | 0.00 | 0.00 |
| Composite achievement measure | 85.8+21.6 | 86.0+21.5 | 88.4*+20.4 | 84.5+22.0 | 83.7*+22.6 | 11.30* | 7.17 | 10.51* |
| Quality measures, % | ||||||||
| Aldosterone antagonist | 41.9 | 44.4 | 41.1 | 37.8* | 48.7 | 6.84 | 13.6* | 8.56 |
| Hydralazine/nitrate | 26.4 | 26.7 | 27.3 | 25.8 | 25.1 | 1.34 | 1.93 | 3.75 |
| CRT‐D/CRT‐P placed or prescribed | 51.2 | 55.8 | 56.4 | 47.0* | 42.4* | 1.35 | 17.5* | 26.9* |
| ICD placed or prescribed | 63.7 | 71.2 | 67.0 | 60.3* | 53.2* | 9.3 | 23.1* | 37.9* |
| Follow‐up visit within ≤7 d | 75.9 | 80.1 | 77.2 | 71.0* | 76.4 | 7.1 | 21.5* | 9.0 |
| Anticoagulation for atrial fibrillation, n | 109 427 (81.5) | 37 131 (86.7) | 28 362 (82.1)* | 29 856 (77.2)* | 14 078 (77.6)* | 12.6* | 24.7* | 23.9* |
| DVT prophylaxis | 83.7 | 86.6 | 82.4* | 81.7* | 84.1 | 11.5* | 13.4* | 6.8 |
| Influenza vaccine during flu season | 84.1 | 82.6 | 84.6 | 84.4 | 85.8 | 5.5 | 5.0 | 8.8 |
| Pneumococcal vaccination | 79.1 | 79.9 | 79.3 | 78.2 | 78.7 | 1.6 | 4.3 | 3.0 |
| Overall composite performance measure | 80.1+19.9 | 81.9±19.6 | 81.4±19.7 | 77.8*±20.0 | 78.6*±19.8 | 2.7 | 20.9* | 16.7* |
The denominator for each measure is appropriate patients without contraindication for each measure. Composite achievement measure: composite of the 4 achievement measures=number of measure performed/number of eligible measure×100. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRT‐D/CRT‐P, cardiac resynchronization therapy with defibrillator/pacemaker only; DVT, deep venous thrombosis; HF, heart failure; ICD, implantable cardioverter‐defibrillator; and LV, left ventricle.
Abs. Stand. diff.=absolute standardized difference, >10% was considered significant; regions with abs. stand. diff. >10% for a variable compared with the Northeast region.
Among the nonmedication achievement measures at discharge (Table 2), there was no difference in the frequency of measurement of LVEF between regions, which was noted to be 100%. However, the highest frequency of an established postdischarge follow‐up appointment for HF (69%) was demonstrated in the Midwest compared with the other regions, which ranged from 56% to 60%. This measure documents the percentage of eligible patients with HF for whom a follow‐up appointment was scheduled and documented, including location, date, and time for follow‐up visits or location and date for home health visit. This difference also translated into the highest composite HF achievement score in the Midwest (88.4%) along with a lower score for the West (83.7%) when compared with the Northeast (86%; Figure 1). Table 2 also demonstrates some other regional variance in non–medication‐related quality measures including significantly lower placement or prescription of cardiac resynchronization‐defibrillator therapy/pacing therapy or implantable cardioverter‐defibrillator (if not already implanted) among appropriate patients during hospitalization or at discharge in the South and the West. Of note, there were up to 79% of patients with missing data regarding cardiac resynchronization‐defibrillator therapy/pacing therapy placement or prescription and up to 53% missing data regarding implantable cardioverter‐defibrillators in patients with HFrEF. The above variations were reflected in the overall composite scores of all achievement and quality measures, which demonstrated slightly lower rates of completion in the South and West (77.8% and 78.6%, respectively) compared with the Northeast and Midwest (81.9% and 81.4%, respectively).
Figure 1. Summary of quality measures across regions including completion of composite achievement measures and inpatient mortality.
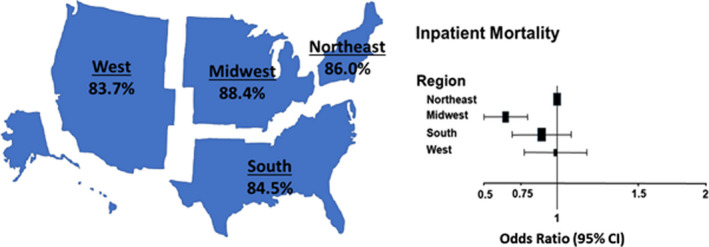
Left panel: percentage of regional completion of the composite achievement measure. Composite achievement measure included a composite of prescription of evidenced‐based ACEI/ARB or ARB–neprilysin inhibitor and beta‐blocker, documentation of a postdischarge appointment and measurement of left ventricular function. Right panel: adjusted odds ratios and 95% CI for inpatient mortality by region (reference=Northeast region). ACEI indicates angiotension‐converting enzyme inhibitor; and ARB, angiotensin receptor blocker.
Short‐Term Outcomes
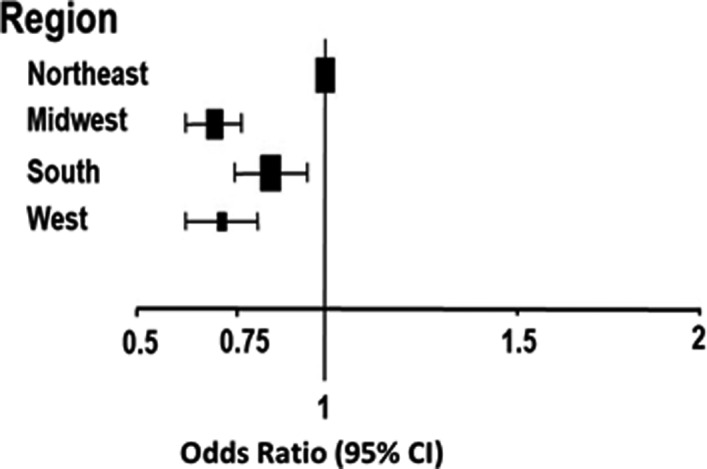
The in‐hospital crude mortality rate was numerically higher for the Northeast compared with other regions (Table 3), and the unadjusted model demonstrated higher odds of in‐hospital mortality in the Northeast compared with all other regions. However, after risk adjustment, no significant differences were seen for in‐patient mortality in the South and West regions compared with the Northeast, although a lower risk was still noted for the Midwest compared with the Northeast (odds ratio [OR], 0.64; 95% CI, 0.51–0.80) (Table 3 and Figure 1). Additionally, LOS varied significantly by region, with the Northeast having significantly higher risk‐adjusted LOS >4 days when compared with the Midwest, South, and West (Table 3 and Figure 2). The regional findings for risk‐adjusted in‐hospital mortality and LOS >4 days demonstrated the same trend, even if patients with HFrEF, HF with preserved ejection fraction, and HF with borderline LVEF were analyzed separately (Tables S1 and S2).
Table 3.
In‐Hospital Outcomes by Region
| Overall (N=423 333) | Northeast (N=127 154) | Midwest (N=106 129) | South (N=133 438) | West (N=56 612) | |
|---|---|---|---|---|---|
| In‐hospital mortality, % | 2.8 | 3.2 | 2.6 | 2.6 | 2.9 |
| Unadjusted OR (95% CI) | 1 [reference] |
0.76 (0.67–0.87) P<0.0001 |
0.78 (0.69–0.88) P<0.0001 |
0.86 (0.74–1.00) P=0.048 |
|
| Adjusted OR (95% CI)* | 1 [reference] |
0.64 (0.51–0.80) P=0.0001 |
0.87 (0.70–1.09) P=0.23 |
0.96 (0.78–1.20) P=0.74 |
|
| Discharge home, % | 77.5 | 73.4 [reference] | 76.4 | 81.0 | 80.3 |
| Unadjusted OR (95% CI) | 1 [reference] |
1.08 (0.97–1.20) P=0.18 |
1.57 (1.40–1.76) P<0.0001 |
1.46 (1.27–1.68) P<0.0001 |
|
| Adjusted OR (95% CI)* | 1 [reference] |
0.99 (0.87–1.12) P=0.87 |
1.21 (1.04–1.42) P=0.02 |
1.31 (1.12–1.52) P=0.0006 |
|
| Discharge to other healthcare facility (%) | 19.7 | 23.4 | 21.0 | 16.5 | 16.8 |
| Abs. stand. diff. vs Northeast | 5.8 | 17.4 | 16.5 | ||
| Median LOS, d (25th, 75th) | 4.0 (3, 6) | 4.0 (3, 6) | 4.0 (3, 6) | 4.0 (3, 6) | 4.0 (2, 6) |
| Abs. stand. diff. vs Northeast | 7.94 | 0.07 | 5.96 | ||
| LOS >4 d (%) | 41.5 | 43.9 | 39.9 | 42.4 | 37.6 |
| Unadjusted OR (95% CI) | 1 [reference] |
0.69 (0.62–0.77) P<0.0001 |
0.87 (0.79–0.96) P=0.05 |
0.71 (0.62–0.81) P<0.0001 |
|
| Adjusted OR (95% CI)* | 1 [reference] |
0.70 (0.63–0.78) P<0.0001 |
0.85 (0.76–0.95) P=0.005 |
0.72 (0.63–0.82) P<0.0001 |
Abs. stand. diff. indicates absolute standardized difference, >10% was considered significant; OR, odds ratio; and LOS, length of stay.
Factors for adjustment included age, sex, race, anemia, ischemic history, cerebrovascular accident/transient ischemic attack, diabetes mellitus, hyperlipidemia, hypertension, chronic obstructive pulmonary disease/asthma, peripheral vascular disease, renal insufficiency, cigarette smoking in the past year, vital signs and laboratory studies at admission (systolic blood pressure, heart rate, sodium, blood urea nitrogen, left ventricular ejection fraction), and hospital characteristics.
Figure 2. Adjusted odds ratios for length of stay by region (reference=Northeast region).
Fewer patients in the Northeast were discharged to home as opposed to being discharged to other facilities when compared with the South and West regions, even after adjustment for baseline characteristics, although no difference was noted between the Northeast and Midwest regions (Table 3).
Examination of mortality in the subgroup of patients with HF enrolled in fee‐for‐service Medicare and whose data were linked to Medicare files, demonstrated no significant differences in risk‐adjusted 30‐day mortality by region (Table 4). However, in this subgroup, a modestly lower risk of all‐cause readmission within 30 days was seen in the Midwest (OR, 0.92; 95% CI, 0.85–0.99) compared with the Northeast. Although a similar trend toward lower risk of readmission was also noted for the South and West, the differences were not statistically significant. Although data for socioeconomic status (SES) are not available for the entire cohort, there is county‐level data available for SES variables that could be linked to zip code data available only for the Medicare fee‐for‐service subgroup. A previous study using such data for the GWTG Medicare patients had demonstrated the modest impact of SES variables at the county level of median household income and percentage of patients with at least a high school diploma on 30‐day outcomes. 19 We performed an analysis examining baseline differences of these variables by region and repeating the adjusted 30‐day mortality and rehospitalization analysis with the addition of these 2 SES variables as covariates. The percentage of high school graduates was slightly higher in the Midwest and was lower in the South and West compared with the Northeast. Also, the median household income was lower in the Midwest and the South compared with the Northeast (Table S3). The adjusted risk of mortality at 30 days remained similar by region, and the modestly lower 30 day risk of rehospitalization in all regions compared with the Northeast was nominally similar to the original analysis but was now statistically significant (Table S4).
Table 4.
Fee‐for‐Service Medicare Subgroup: 30‐Day Outcomes
| 30 d Mortality | 30‐d Mortality Unadjusted HR (95% CI) | 30‐d Mortality Adjusted* HR (95% CI) | 30‐d All‐Cause Readmission | 30‐d All‐Cause Readmission Unadjusted HR (95% CI) | 30‐d All‐Cause Readmission Adjusted* HR (95% CI) | |
|---|---|---|---|---|---|---|
| Northeast | 5% | 1 [reference] | 1 [reference] | 17.06% | 1 [reference] | 1 [reference] |
| Midwest | 4.36% |
0.91 (0.85–0.99) P=0.02 |
1.08 (0.95–1.22) P=0.23 |
14.55% |
0.90 (0.86–0.93) P<0.0001 |
0.92 (0.85–0.99) P=0.03 |
| South | 4.57% |
0.94 (0.88–1.01) P=0.10 |
1.11 (0.99–1.24) P=0.07 |
15.67% |
0.94 (0.90–0.98) P=0.001 |
0.93 (0.86–1.01) P=0.08 |
| West | 4.09% |
0.87 (0.79–0.97) P=0.01 |
1.02 (0.87–1.19) P=0.82 |
13.67% |
0.86 (0.81–0.91) P<0.0001 |
0.90 (0.80–1.00) P=0.05 |
HR indicates hazard ratio.
Factors for adjustment included age, sex, race, anemia, ischemic history, cerebrovascular accident/transient ischemic attack, diabetes mellitus, hyperlipidemia, hypertension, chronic obstructive pulmonary disease/asthma, peripheral vascular disease, renal insufficiency, cigarette smoking in the past year, vital signs and laboratory studies at admission (systolic blood pressure, heart rate, sodium, blood urea nitrogen, left ventricular ejection fraction), and hospital characteristics.
DISCUSSION
This analysis of the GWTG‐HF registry examined regional variations in patient demographics, quality of HF care, and short‐term outcomes in patients hospitalized with HF. There were some regional differences noted in patient characteristics. However, the differences in quality of in‐hospital care were small and did not vary substantially or systematically by geographic region. There were some regional variations of outcomes in‐hospital, which were attenuated with adjustment for patient characteristics and with examination of 30‐day outcomes in a subset. These findings suggest that in the context of hospitals participating in a national HF quality improvement program, overall similar in‐hospital quality of care can be achieved irrespective of region. To our knowledge these findings are novel in that regional variations across the United States in both a comprehensive set of quality measures along with short‐term outcomes in HF have not been recently compared in a large contemporary cohort using abstracted clinical data, such as used in our study.
It is reassuring that within the context of hospitals participating in a national HF quality improvement program, the 2 most established groups of medications for HFrEF, that is, ACEIs/ARBs and evidence‐based beta‐blockers were prescribed at high rates in appropriate patients, and LVEF was measured or documented well in all regions, even though there were significant differences by region in patient demographics, especially age and race, and in comorbidities. The use of ARB–neprilysin inhibitors was low because of the time period of the study; no conclusions could be drawn on their regional use. 20 Furthermore, the rates of use of other lifesaving medications such as aldosterone receptor blockers (42% overall) and isosorbide‐hydralazine (26%) in appropriate patients without contraindications was lower across all regions and identifies an area for improvement. However, the use of isosorbide‐hydralazine has increased compared with that reported from the same database in 2013 when it was 12.6% of appropriate patients. 21 We could not examine differences in doses of guideline‐directed medications given that these data were not available in the GWTG‐HF database. However, the issue of dose is important given recent data from another contemporary US registry that demonstrated that most eligible patients with HFrEF do not receive target doses of medical therapy. 22 Also, some observed differences in therapies by region deserve mention. First, although the reasons for lower rates of implantable cardioverter‐defibrillator and cardiac resynchronization‐defibrillator therapy/pacing therapy implanted or prescribed in the West and South are not clear from these data, it is possible that lower rates of insured patients in the West and South may have contributed to the observed disparity. Second, the lower rates of anticoagulants prescribed for atrial fibrillation in the West and South regions could also be contributed to by lack of definite follow‐up appointment, if needed, for lab monitoring and dose adjustment. On the other hand, the higher rate of discharge of patients to other healthcare facilities rather than home could make monitoring of lab work and medications easier, possibly contributing to higher rates of anticoagulation prescription in the Northeast. Furthermore, in the Northeast, it is likely that older patients with a greater number of comorbidities may have higher thromboembolic risk scores, contributing to higher use of anticoagulants.
In unadjusted analyses, there was higher inpatient mortality, longer LOS, and higher rates of 30‐day mortality and 30‐day readmission after discharge in the Northeast region compared with other regions. These findings were likely driven by differences in regional patient characteristics and perhaps hospital characteristics based on significant attenuation of the differences in inpatient mortality, and 30‐day mortality and readmission with risk‐adjusted analyses. Adjustment for age and comorbidities tried to account for the older age and higher comorbidity burden seen in patients in the Northeast. There was a persistent modest difference in risk‐adjusted inpatient mortality and 30‐day readmission, which was higher in the Northeast compared with the Midwest. Systematic differences in quality of care do not appear to be a significant contributor to the differences in mortality. Part of the differences could reflect residual confounding from higher severity of illness or other unmeasured patient or hospital characteristics, for example, more frequent rural location. Although the longer LOS for patients in the Northeast may suggest greater severity of illness and greater time taken for discharge to places other than home, the longer LOS also provides a longer time window for events culminating in inpatient mortality. Our results are concordant with findings from an analysis of the National Inpatient Sample of HF hospitalizations in 2013 to 2014, in which risk‐adjusted rate of in‐hospital mortality was highest in the Northeast and lowest in the Midwest. 12 The Northeast region had the longest LOS and the lowest risk‐adjusted rate of home discharge, as was seen in our study population. Interestingly, in another recent regional comparison among a national sample of patients implanted with left ventricular assist devices, the pattern of higher LOS with higher rates of discharge to extended care facilities as compared with home was similarly noted for patients in the Northeast, even though in‐hospital mortality did not differ by regions. 23 Our study extends results of these other studies by demonstrating that the overall quality of care was similar or even better (eg, rates for anticoagulation) in the Northeast, suggesting that differences in quality of care may not be the major contributor to differences of in‐hospital outcomes observed. Furthermore, the similar 30‐day mortality after discharge by regions in the Medicare fee‐for‐service subset is reassuring.
Study Strengths and Limitations
Our study was possible given the large nationally representative GWTG‐HF data set with standardized data collection using clinical chart abstraction. However, given the observational nature of the data, there may be residual confounding variables that may affect the results. Second, the hospitals included in the GWTG‐HF registry are voluntary participants and may be more motivated toward meeting quality measures and tracking outcomes, which may limit the generalizability of our findings to all US hospitals and regions. Even with that caveat, there was a good representation of different hospital sizes, teaching versus nonteaching hospitals, and hospitals with and without specialty programs such as heart transplantation. Some of these differences were captured in the data and used for adjustment in the multivariable models. Finally, the subgroup analysis for 30‐day outcomes included only patients enrolled in fee‐for‐service Medicare plans and therefore may not be completely representative of the overall cohort, especially those who are younger and without insurance, given that the average age differs by region. Furthermore, we do not have data for SES status in the overall cohort. Given that SES differences by region may exist in the overall cohort, we are unable to evaluate if SES status accounts for some of the observed differences in in‐hospital outcomes.
CONCLUSIONS
There were no substantial or systematic regional differences in HF quality of care. Modest differences in in‐hospital outcomes did not appear to correlate with the quality‐of‐care measures. However, our findings are noted in the context of a network of hospitals participating in a quality improvement initiative, with caution in generalizing to all centers across the United States. Continued regional surveillance of HF care through systems such as GWTG‐HF may provide ongoing improvement in HF care throughout the United States.
Sources of Funding
The GWTG‐HF program of the American Heart Association is supported in part by Medtronic, Ortho‐McNeil, and the American Heart Association Pharmaceutical Roundtable, and has been funded in the past through support from GlaxoSmithKline.
Disclosures
Dr Fonarow reports consulting for Abbott, Amgen, Bayer, CHF Solutions, Janssen, Medtronic, and Novartis. Dr DeVore reports research support from the American Heart Association; Amgen; AstraZeneca; Bayer; Intra‐Cellular Therapies; Luitpold Pharmaceuticals; Medtronic; the National, Heart, Lung, and Blood Institute; Novartis; and PCORI; and consulting with AstraZeneca, Bayer, LivaNova, Mardil Medical, Novartis, and Procyrion. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
(J Am Heart Assoc. 2021;10:e018696. DOI: 10.1161/JAHA.120.018696.)
This manuscript was sent to John Jefferies, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018696
For Sources of Funding and Disclosures, see page 10.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. DOI: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 2. Akintoye E, Briasoulis A, Egbe A, Dunlay SM, Kushwaha S, Levine D, Afonso L, Mozaffarian D, Weinberger J. National trends in admission and in‐hospital mortality of patients with heart failure in the United States (2001–2014). J Am Heart Assoc. 2017;6:e006955. DOI: 10.1161/JAHA.117.006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 4. Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Predictors of in‐hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). J Am Coll Cardiol. 2008;52:347–356. DOI: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 5. Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. DOI: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 6. Blair JEA, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, et al. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol. 2008;52:1640–1648. DOI: 10.1016/j.jacc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 7. Howlett JG, Ezekowitz JA, Podder M, Hernandez AF, Diaz R, Dickstein K, Dunlap ME, Corbalán R, Armstrong PW, Starling RC, et al. Global variation in quality of care among patients hospitalized with acute heart failure in an international trial: findings from the acute study clinical effectiveness of nesiritide in decompensated heart failure trial (ASCEND‐HF). Circ Cardiovasc Qual Outcomes. 2013;6:534–542. DOI: 10.1161/CIRCOUTCOMES.113.000119. [DOI] [PubMed] [Google Scholar]
- 8. Allen NB, Holford TR, Bracken MB, Goldstein LB, Howard G, Wang Y, Lichtman JH. Geographic variation in one‐year recurrent ischemic stroke rates for elderly Medicare beneficiaries in the USA. Neuroepidemiology. 2010;34:123–129. DOI: 10.1159/000274804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall WD, Ferrario CM, Moore MA, Hall JE, Flack JM, Cooper W, Simmons JD, Egan BM, Lackland DT, Perry M Jr, et al. Hypertension‐related morbidity and mortality in the southeastern United States. Am J Med Sci. 1997;313:195–209. DOI: 10.1097/00000441-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 10. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. DOI: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 11. Zhang W, Watanabe‐Galloway S. Ten‐year secular trends for congestive heart failure hospitalizations: an analysis of regional differences in the United States. Congest Heart Fail. 2008;14:266–271. DOI: 10.1111/j.1751-7133.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 12. Akintoye E, Briasoulis A, Egbe A, Adegbala O, Sheikh M, Singh M, Alliu S, Ahmed A, Asleh R, Kushwaha S, et al. Regional variation in mortality, length of stay, cost, and discharge disposition among patients admitted for heart failure in the United States. Am J Cardiol. 2017;120:817–824. DOI: 10.1016/j.amjcard.2017.05.058. [DOI] [PubMed] [Google Scholar]
- 13. Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–186. DOI: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 14. Luo N, Ballew NG, O'Brien EC, Greiner MA, Peterson PN, Hammill BG, Hardy NC, Laskey WK, Heidenreich PA, Chang C‐L, et al. Early impact of guideline publication on angiotensin‐receptor neprilysin inhibitor use among patients hospitalized for heart failure. Am Heart J. 2018;200:134–140. DOI: 10.1016/j.ahj.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 15. Smaha LA. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148:S46–S48. DOI: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16. Heart failure: fact sheet. Get with the Guidelines Heart Failure. American Heart Association. [Google Scholar]
- 17. Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3:44–53. DOI: 10.1001/jamacardio.2017.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. DOI: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eapen ZJ, McCoy LA, Fonarow GC, Yancy CW, Miranda ML, Peterson ED, Califf RM, Hernandez AF. Utility of socioeconomic status in predicting 30‐day outcomes after heart failure hospitalization. Circ Heart Fail. 2015;8:473–480. DOI: 10.1161/CIRCHEARTFAILURE.114.001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo N, Fonarow GC, Lippmann SJ, Mi X, Heidenreich PA, Yancy CW, Greiner MA, Hammill BG, Hardy NC, Turner SJ, et al. Early adoption of sacubitril/valsartan for patients with heart failure with reduced ejection fraction: insights from Get With the Guidelines‐Heart Failure (GWTG‐HF). JACC Heart Fail. 2017;5:305–309. DOI: 10.1016/j.jchf.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 21. Golwala HB, Thadani U, Liang L, Stavrakis S, Butler J, Yancy CW, Bhatt DL, Hernandez AF, Fonarow GC. Use of hydralazine‐isosorbide dinitrate combination in African American and other race/ethnic group patients with heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2:e000214. DOI: 10.1161/JAHA.113.000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365–2383. DOI: 10.1016/j.jacc.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Briasoulis A, Inampudi C, Akintoye E, Adegbala O, Asleh R, Alvarez P, Bhama J. Regional variation in mortality, major complications, and cost after left ventricular assist device implantation in the United States (2009 to 2014). Am J Cardiol. 2018;121:1575–1580. DOI: 10.1016/j.amjcard.2018.02.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


