Abstract
Background
Supine hypertension affects a majority of patients with autonomic failure; it is associated with end‐organ damage and can worsen daytime orthostatic hypotension by inducing pressure diuresis and volume loss during the night. Because sympathetic activation prevents blood pressure (BP) from falling in healthy subjects exposed to heat, we hypothesized that passive heat had a BP‐lowering effect in patients with autonomic failure and could be used to treat their supine hypertension.
Methods and Results
In Protocol 1 (n=22), the acute effects of local heat (40–42°C applied with a heating pad placed over the abdomen for 2 hours) versus sham control were assessed in a randomized crossover fashion. Heat acutely decreased systolic BP by −19±4 mm Hg (versus 3±4 with sham, P<0.001) owing to decreases in stroke volume (−18±5% versus −4±4%, P=0.013 ) and cardiac output (−15±5% versus −2±4%, P=0.013). In Protocol 2 (proof‐of‐concept overnight study; n=12), we compared the effects of local heat (38°C applied with a water‐perfused heating pad placed under the torso from 10 pm to 6 am) versus placebo pill. Heat decreased nighttime systolic BP (maximal change −28±6 versus −2±6 mm Hg, P<0.001). BP returned to baseline by 8 am. The nocturnal systolic BP decrease correlated with a decrease in urinary volume (r=0.57, P=0.072) and an improvement in the morning upright systolic BP (r=−0.76, P=0.007).
Conclusions
Local heat therapy effectively lowered overnight BP in patients with autonomic failure and supine hypertension and offers a novel approach to treat this condition. Future studies are needed to assess the long‐term safety and efficacy in improving nighttime fluid loss and daytime orthostatic hypotension.
Registration
URL: https://www.clinicaltrials.gov; Unique identifiers: NCT02417415 and NCT03042988.
Keywords: autonomic failure, heat, orthostatic hypotension, supine hypertension, thermoregulation
Subject Categories: Autonomic Nervous System, Clinical Studies, Hypertension, Treatment, Hemodynamics
Nonstandard Abbreviations and Acronyms
- AF
autonomic failure
- CO
cardiac output
- CVC
cutaneous vascular conductance
- DBP
diastolic blood pressure
- HR
heart rate
- OH
orthostatic hypotension
- PP
pulse pressure
- SBP
systolic blood pressure
- SV
stroke volume
- Tcore
core body temperature
Clinical Perspective
What Is New?
In patients with autonomic failure and supine hypertension, low levels of local passive heat had an acute and profound blood pressure‐lowering effect, with a rapid onset of action and recovery.
In a proof‐of‐concept study, overnight therapy with local passive heat (38°C) effectively lowered nocturnal blood pressure in these patients.
Nocturnal diuresis and morning orthostatic hypertension improved in patients with greater blood pressure responses.
What Are the Clinical Implications?
Supine hypertension complicates the management of orthostatic hypertension in patients with autonomic failure; treatment of one condition often worsens the other.
Local passive heat therapy has the advantage of rapid onset and offset of the blood pressure effects and may improve nocturnal diuresis and morning orthostatic hypertension in some patients.
Overnight heat therapy may offer a novel nonpharmacologic approach to treat the nocturnal supine hypertension of autonomic failure, but future studies are needed to assess the long‐term efficacy and safety of this approach.
Primary autonomic failures (AF) are neurodegenerative disorders characterized by disabling orthostatic hypotension (OH) due to loss of compensatory sympathetic activation and absence of baroreflex buffering. 1 , 2 A majority of these patients also have supine hypertension, which has been associated with cardiac and renal end‐organ damage. 3 , 4 Moreover, there is evidence that nocturnal supine hypertension begets daytime OH by inducing a significant volume loss during the night because of pressure natriuresis. 5 There is, however, concern about treating supine hypertension for fear that antihypertensive medications will worsen OH. 6
In most patients, supine hypertension can be controlled during the day simply by avoiding the supine position, but it remains a management problem during the night. The reluctance to treat this condition would be overcome if we had a treatment that would selectively lower nocturnal blood pressure (BP), reduce nighttime natriuresis, and thereby improve morning OH. Many medications successfully reduce nighttime BP. 5 , 7 , 8 , 9 , 10 , 11 Most of them, however, fail to reduce nighttime diuresis and none improves daytime OH, likely because of the lingering effects of these drugs. 5 , 7 , 8 , 9 , 10 , 11 Thus, there is an unmet need to develop an alternative approach that would effectively treat nocturnal hypertension while improving daytime OH.
The cutaneous vasculature, one of the largest capacitance beds in the human body, is controlled mainly by the thermoregulatory system. Heat exposure induces skin vasodilation, redistributing blood from central vascular beds to the skin. 12 , 13 BP is normally maintained owing to autonomically mediated compensatory hemodynamic responses. 14 , 15 In AF, these hemodynamic responses are impaired, and thus, heat‐induced hypotension likely contributes to the severe worsening of orthostatic symptoms these patients complain about when exposed to ambient heat. 16 This clinical observation prompted us to explore whether heat had an acute BP‐lowering effect in patients with AF, and whether this effect can be used to treat their nocturnal hypertension. We tested these hypotheses in 2 steps: Protocol 1 assessed the acute hemodynamic effects of local application of controlled passive heat, and Protocol 2 determined, in a proof‐of‐concept study, whether overnight application of local controlled heat improves nocturnal hypertension, nocturia, and morning orthostatic tolerance.
Methods
The data that support the findings of this study will be made available by the corresponding author to any researcher upon reasonable request.
Subjects
Thirty‐one patients with severe primary AF (20 with pure autonomic failure, 10 with Parkinson disease, and 1 with probable multiple system atrophy) were recruited from referrals to Vanderbilt University Autonomic Dysfunction Center. Clinical diagnoses were defined using current criteria. 1 , 2 , 17 , 18 All patients had neurogenic OH and supine hypertension defined as systolic BP (SBP) ≥150 mm Hg or diastolic BP (DBP) ≥90 mm Hg. 19 Nineteen patients participated in Protocol 1, 9 in Protocol 2, and 3 patients participated in both protocols. Patients were excluded if they had secondary causes of AF (eg, diabetes mellitus or amyloidosis), if they were bedridden, or if they had any significant cardiac or renal illness. The Vanderbilt University Institutional Review Board approved this study, and written informed consent was obtained from each patient before initiating the study (http://clinicaltrials.gov identifier: NCT02417415 and NCT03042988).
General Protocol
Patients were studied as inpatients in the Clinical Research Center at Vanderbilt University Medical Center and were fed a low‐monoamine, caffeine‐free diet containing 150 mEq sodium and 70 mEq potassium per day. Medications affecting BP, blood volume, and the autonomic nervous system, including fludrocortisone, pressor drugs, or antihypertensive medications, were withheld for ≥5 half‐lives before studies. All other medications were held constant during admission. The screening consisted of a medical history, physical examination, ECG, routine safety laboratory assessments, and standardized autonomic function tests, including orthostatic stress test, respiratory sinus arrhythmia, and Valsalva maneuver. 20 BP and heart rate (HR) were obtained using an automated oscillometric sphygmomanometer (Dinamap ProCare, GE Healthcare), finger photoplethysmography (Nexfin, BMEYE), and continuous ECG. During the orthostatic test, blood samples were obtained for norepinephrine while patients were supine and upright, as described previously. 7 Plasma norepinephrine was measured by high‐performance liquid chromatography with electrochemical detection. 21 All patients were screened for nocturnal supine hypertension by assessing BP in duplicate at 2‐hour intervals from 8:00 pm to 8:00 am with an automated sphygmomanometer (Dinamap ProCare, GE Healthcare).
Protocol 1: Acute Hemodynamic Effects of Local Controlled Passive Heating
Patients were studied on 2 separate days in a randomized crossover manner with either local controlled passive heat stress (40–42°C) or sham control. Studies were conducted in a temperature‐controlled room (24–26°C) in the afternoon when BP is higher, 22 in a post‐void state, and ≥2.5 hours after meals.
On each study day, patients remained supine throughout the study covered by a thin blanket for comfort. BP and HR were measured every 5 minutes with an automated oscillometric sphygmomanometer (Dinamap ProCare, GE Healthcare), and continuously with finger photoplethysmographic volume‐clamp BP device (Nexfin, BMEYE) and ECG. Skin temperature was monitored with a wireless temperature sensor (Dermal Patch, VitalSense, Philips Respironics) placed on the right lower abdominal quadrant (underneath the heating pad). Core body temperature (Tcore) was monitored with a telemetric thermometer pill (Jonah™ ingestible capsule, VitalSense, Mini Mitter) that was swallowed >2 hours before studies. 23
After 30 minutes of normothermic baseline measurements, passive heat was applied over the abdomen and pelvis with a commercial electric heating pad (61×30 cm, Sunbeam Products), placed over 2 layers of clothing, to provide local heating at 40 to 42°C (“high” temperature setting) continuously for up to 2 hours. Outcome measurements were recorded during the intervention and for ≤30 minutes of recovery. All procedures were identical in the sham control day, but the heating pad was turned off.
In a subset of participants, we included measurements of systemic and skin hemodynamics at baseline and at 2 hours after each intervention to assess the hemodynamic mechanisms underlying the BP effects of passive heat (secondary objective). Specifically, stroke volume (SV) was estimated using impedance cardiography, as described previously. 24 Cardiac output (CO) was then calculated by multiplying SV by the HR obtained from oscillometric BP measurements. Systemic vascular resistance (SVR) was estimated by dividing oscillometric mean arterial pressure (MAP) by CO. Skin blood flow was measured on the right lower abdominal quadrant (underneath the heating pad), and on the right lateral calf (a distal site unexposed to heat) using laser‐Doppler flow probes (DRT4, Moor Instruments Inc.), to assess local (abdomen) and reflex‐induced (calf) vascular changes due to local heating. Skin temperature on the right lateral calf was monitored with a wireless temperature sensor (Dermal Patch, VitalSense, Philips Respironics). Cutaneous vascular conductance (CVC) was estimated as the ratio of skin blood flow to arm cuff MAP, to assess changes in vasomotor tone. 25 Systemic and skin hemodynamic data were reduced to average values measured during a 5 to 15 minute period and expressed as percent changes from baseline.
Protocol 2: Proof‐of‐Concept Study to Assess the Efficacy of Overnight Heat Therapy for the Treatment of Nocturnal Supine Hypertension
In a randomized, 2‐night crossover study, we compared the effects of overnight therapy with controlled local passive heat (38°C) versus a placebo pill. Studies were conducted from 8 pm to 8 am, and ≥2.5 hours after the last meal. Patients were instructed to remain supine throughout the night. Fluid intake was restricted to avoid the pressor effect of water drinking. 26 BP and HR were measured twice in a row at 2‐hour intervals from 8 pm to 8 am by an automated sphygmomanometer (Dinamap ProCare, GE Healthcare). Internal body temperature was measured at the same time intervals with a thermistor thermometer (SureTemp Plus, Welch Allyn Inc.) placed in the sublingual sulcus. Urine was collected throughout the night for determination of volume.
Overnight heat therapy was applied using a conductive warming therapy system (Norm‐O‐Temp, Gentherm Medical, LLC), consisting of a water heater and circulating pump connected to a 79.4×60.3 cm water‐perfused pad (Gelli‐roll 194P, Gentherm Medical, LLC). This is a Food and Drug Administration approved device for whole‐body heat therapy before, during, and after surgical procedures and in patients in intensive care units and emergency rooms. 27 The heating pad was placed at 8 pm over the mattress, covered by a sheet, under the patient's torso and proximal thighs, to ensure that it remained in place throughout the night. After ≈2 hours of baseline (8 pm–10 pm), heat therapy was applied by perfusing water at 38°C through the pad for ≈8 hours (10 pm–6 am). This temperature induces local skin vasodilation while being safe and well tolerated for prolonged contact with the skin. 28 , 29 At 6 am, the heating pad was removed from the patient's bed, and at 8 am, patients were asked to stand for ≤10 minutes. BP and HR were measured at 1, 3, 5, and 10 minutes of standing, or as long as tolerated, to assess morning orthostatic tolerance.
Adverse event information was collected on each study day in both protocols. In Protocol 1, the skin under the heating pad was assessed for signs of irritation or burns every 20 to 30 minutes during passive heating and end of recovery.
Statistical Analysis
In Protocol 1, our primary objective was to test the hypothesis that local controlled passive heat stress would decrease supine BP compared with sham control (normothermia) in patients with AF and supine hypertension. The primary outcome was the change from the averaged baseline in supine SBP (∆SBP) during the 2‐hour intervention period. Secondary outcomes included changes from baseline in DBP, MAP, HR, pulse pressure (PP), and abdominal skin temperature and Tcore during the intervention and recovery periods. We used 2‐way repeated‐measures ANOVA to test effects of treatment, time, and their interaction on primary and secondary outcomes. If a significant overall treatment difference was found, paired comparisons of outcome variables across time were performed using paired t tests with Bonferroni correction as post hoc test. In the secondary objective, BP, HR, and percent changes from baseline in CO, SV, SVR, skin blood flow, and CVC at 2 hours post‐intervention were compared between treatments using Wilcoxon signed‐rank tests or paired t tests depending on the data distribution.
In Protocol 2, we hypothesized that overnight heat therapy would decrease nocturnal supine BP compared with placebo and that this BP‐lowering effect of heat would be associated with lower nocturnal urine volume and improved morning orthostatic tolerance. We chose a “antihypertensive” placebo pill, that would supposedly lower BP, as our control rather than sham heat, cognizant that patient would not be blinded by the later approach. The primary outcome was the change from baseline (8 pm) in the supine SBP (ΔSBP) during the intervention period (10 pm–6 am). Differences between treatments in the primary outcome and changes from baseline in HR, DBP, MAP, and PP were analyzed using the same approach described in Protocol 1. Secondary outcomes included nocturnal diuresis, body weight, and morning orthostatic tolerance, defined as the area under the curve of standing SBP during the orthostatic test at 8 am (calculated by the trapezoidal rule [mean upright SBP×standing time]). 24 This is a composite score that integrates both the standing time and the upright SBP. 30 Comparisons were made only for patients who could stand after all treatments, as previously reported. 30 Baseline measurements and secondary outcomes were compared between treatments using Wilcoxon signed‐rank tests or paired t tests depending on the data distribution. Data are presented as mean±SEM unless otherwise noted. All of the tests were 2‐tailed, and a P value of <0.05 was considered significant. Analyses were performed with SPSS 23.0 (IBM Corp).
Sample Size and Power Calculations
In Protocol 1, power calculation was based on preliminary data from 4 patients. The difference in the change from baseline in supine SBP between local controlled passive heating and sham control groups at 2 hours post‐intervention was of 29 mm Hg, with SD of the difference of 33 mm Hg, which was consistent with the clinically significant hypotensive effect of nitric oxide potentiation with sildenafil and other antihypertensive tested in the same patient population. 9 Assuming this effect size and variance, a sample size of 22 patients would have 97% power to detect a difference in mean values between treatments with an α level of 0.05 using paired t test analysis. In Protocol 2, power calculation was based on preliminary data from overnight studies with passive heat in 3 patients. The difference in the maximal changes from baseline in supine SBP between overnight heat therapy and placebo was of 28 mm Hg, with SD of the difference of 23 mm Hg. A sample size of 12 patients would have 97% power to detect a difference in mean values between treatments with an α level of 0.05 using paired t test analysis. Sample calculation was performed with G*Power, Version 3.1.9.4. 31
Results
Patient Characteristics and Autonomic Testing
We studied a total of 31 patients with severe AF and supine hypertension (73±1 years, body mass index 27±1 kg/m2, supine BP 170±4/90±2, 21 men): 22 patients in Protocol 1 and 12 patients in Protocol 2. Patient clinical and autonomic characteristics are shown in Tables 1 and 2. A history of essential hypertension preceded the diagnosis of AF in 42% of patients and supine hypertension started after the onset of AF in the remainder. The majority of patients (58%) had a history of worsening of orthostatic symptoms in hot environments (heat intolerance) before study participation. All patients exhibited a profound decrease in BP on standing (OH) without an adequate compensatory increase in HR and severe impairment of autonomic reflexes (Table 2). Respiratory sinus arrhythmia was blunted, suggesting parasympathetic dysfunction. Evidence of sympathetic dysfunction included an exaggerated decrease in SBP during phase II and the absence of BP overshoot during phase IV of the Valsalva maneuver.
Table 1.
Patient Characteristics
| Parameters, Unit | Protocol 1 (n=22) | Protocol 2 (n=12) |
|---|---|---|
| Sex, male/female | 16/7 | 8/4 |
| Age, y | 73±1 | 76±2 |
| Body mass index, kg/m2 | 27±1 | 27±1 |
| Disease duration, y | 7±1 | 7±1 |
| Diagnosis, % (n) | ||
| Pure autonomic failure | 64 (14) | 75 (9) |
| Parkinson disease+autonomic failure | 32 (7) | 25 (3) |
| Multiple system atrophy | 4 (1) | … |
| Medical history of essential hypertension, % (n) | 45 (10) | 42 (5) |
| Heat intolerance, % (n) | 68 (15) | 50 (6) |
| Supine | ||
| Systolic BP, mm Hg | 170±5 | 175±5 |
| Diastolic BP, mm Hg | 92±3 | 89±3 |
| Heart rate, bpm | 67±3 | 62±3 |
| Plasma norepinephrine, pg/mL | 132±16 | 164±31 |
| Upright | ||
| Systolic BP, mm Hg | 100±7 | 97±8 |
| Diastolic BP, mm Hg | 59±3 | 58±5 |
| Heart rate, bpm | 77±3 | 77±4 |
| Plasma norepinephrine, pg/mL | 199±30 | 242±42 |
Data are presented as mean±SEM. Protocol 1, acute hemodynamic effects of local heat stress vs sham. Protocol 2, overnight treatment with local passive heat vs placebo. BP indicates blood pressure.
Table 2.
Autonomic Function Tests and Orthostatic Stress Test
| Parameters, Unit | Protocol 1 (n=22) | Protocol 2 (n=12) | Normals* |
|---|---|---|---|
| Orthostatic change in systolic BP, mm Hg | −68±6 | −77±9 | ≤20 |
| Orthostatic change in heart rate, bpm | 9±2 | 13±3 | 5–10 |
| Sinus arrhythmia ratio | 1.05±0.01 | 1.06±0.02 | 1.2±0.1 |
| Depressor response to Valsalva in phase II, mm Hg | −69±5 | −74±8 | ≤20 |
| BP response to Valsalva phase IV, mm Hg † | −38±5 | −45±5 | >20 |
| Valsalva ratio | 1.16±0.04 | 1.21±0.1 | 1.5±0.2 |
Values are expressed as mean±SEM. Pressor responses are given as changes in systolic blood pressure (BP).
Normal values are from the Autonomic Dysfunction Database at Vanderbilt University Medical Center.
A negative value for phase IV of the Valsalva maneuver indicates that the BP overshoot was absent.
Protocol 1: Acute Hemodynamic Effects of Local Heating
Twenty‐two patients completed both treatment arms. Tcore was obtained in 12 patients. Of the remaining 10 patients, 7 refused to take the telemetry pill, and the pill was no longer commercially available in 3. Baseline Tcore was similar between trial days (36.7±0.1 versus 36.8±0.1°C for heat and sham respectively; P=0.330); baseline abdominal skin temperature was slightly lower on the heating day compared with that of sham (33.4±0.3 versus 33.9±0.3°C, respectively; P=0.001). The heating pad increased abdominal skin temperature by 6.3±0.5°C (to 39.7±0.4°C) after 1 hour of heating, and by 5.9±0.4°C (to 39.3±0.4°C) after 2 hours; whereas sham control produced no significant change (1.1±0.2 [to 35.0±0.2] and 1.1±0.2°C [to 35.1±0.2°C], respectively; Figure 1A). Tcore had a small, but statistically significant, increase during heating compared with sham (P=0.010 for treatment effect and P<0.001 for treatment×time interaction, 2‐way repeated‐measures ANOVA); Tcore increased by 0.2±0.1°C (to 36.9±0.1°C) at 1 hour and by 0.4±0.1°C (to 37.2±0.1°C) at 2 hours post‐intervention compared with 0.0±0.1 (36.8±0.1) and 0.1±0.0°C (36.9±0.1°C) respectively with sham (P<0.001 for both paired comparisons).
Figure 1. Acute effects of local passive heat (Protocol 1).
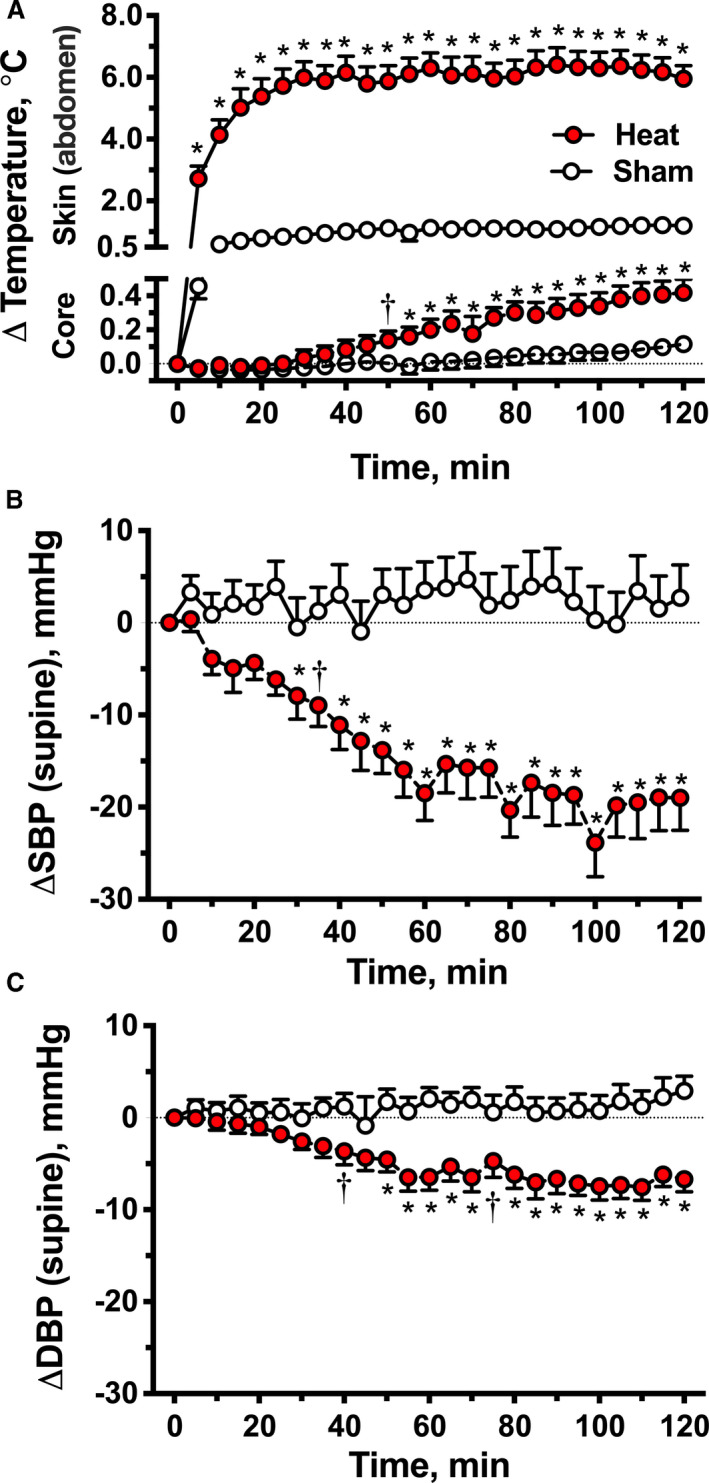
Changes from baseline in abdominal skin and core body temperatures (A), supine systolic blood pressure (ΔSBP, B) and diastolic blood pressure (ΔDBP, C) during 2 hours of local heat (40–42°C applied over the abdomen and pelvis with an electric heating pad) or sham control. Local passive heat decreased supine SBP and DBP and produced a small, but significant, increase in core body temperature compared with sham control. Values are expressed as mean±SEM. Overall differences were analyzed by 2‐way repeated‐measures ANOVA (P≤0.01 for treatment effect and P<0.001 for treatment×time interaction in all comparisons). *P<0.001 and † P<0.05 vs sham, adjusted for multiple comparisons using Bonferroni correction.
Baseline supine BP was similar between heat and sham trials; SBP was 167±4 and 159±4 mm Hg, respectively (P=0.079), DBP was 92±2 and 88±2 mm Hg (P=0.074), and MAP was 117±3 and 112±2 mm Hg (P=0.074). Local heating significantly decreased supine BP compared with sham control (P<0.001 for treatment effects and for treatment×time interactions for comparisons in SBP, DBP, and MAP, 2‐way repeated measures ANOVA; Figure 1B and 1C). SBP started to decrease significantly after 25 minutes of heating, with an average reduction of 19±3 mm Hg at 1 hour and 19±4 mm Hg at 2 hours post‐intervention compared with 4±3 and 3±4 mm Hg, respectively, with sham control (P<0.001 for both paired comparisons). The SBP reduction preceded the increase in Tcore. Similarly, DBP had a sustained decrease of 7±1 mm Hg at 1 and 2 hours of passive heat (versus 2±1 mm Hg at 1 hour and 3±2 mm Hg at 2 hours in the sham control group; P<0.001 for both paired comparisons), whereas MAP decreased by 11±2 mm Hg at 1 and 2 hours post‐intervention (versus 3±2 mm Hg at 1 and 2 hours with sham control; P<0.001 for both paired comparisons). PP also followed the same trend (Figure S1). Baseline PP was similar between interventions (heat: 76±3 versus sham: 72±4 mm Hg; P=0.131), and significantly decreased during the 2 hours of heating compared with sham control (−12±2 mm Hg at 1 and 2 hours of heating versus 2±2 and 0±2 mm Hg, respectively, with sham control; P<0.001 for both paired comparisons) suggesting that the BP reduction during heat was driven by a decrease in SV. Thirty minutes after removing the heating pad, SBP, DBP, MAP, and PP returned to almost baseline values (−3±8, −1±4, −1±2 and −3±2 mm Hg, respectively).
Systemic hemodynamics were measured in 13 patients and abdominal and calf skin blood flow and CVC in 9 (Table 3 and Figure 2). The heat‐induced decrease in BP was associated with a significant decrease in CO (−15±5%; Figure 2A) and SV (−18±5%) compared with sham control, with no significant changes in SVR (9±6%) or HR (3±1 bpm). Skin blood flow in the abdomen significantly increased with local heating compared with sham control (Figure 2B), whereas calf skin blood flow (a site unexposed to heat) was similar in both groups. Abdominal and calf CVC followed a similar trend (Figure 2B). Calf skin temperature did not significantly increase compared with sham control (Table 3).
Table 3.
Systemic and Skin Hemodynamic Changes During Acute Local Controlled Passive Heat Stress*
| Parameters, Unit | 2 h | P Value † | |
|---|---|---|---|
| Heat | Sham | ||
| Systemic hemodynamic parameters (n=13) | |||
| Systolic blood pressure, mm Hg | −17±4 | 0±4 | 0.013 |
| Diastolic blood pressure, mm Hg | −8±2 | 1±2 | 0.008 |
| Mean arterial pressure | −11±2 | 0±2 | 0.007 |
| Heart rate, bpm | 3±1 | 2±1 | 0.541 |
| Cardiac output, % | −15±5 | −2±4 | 0.013 |
| Stroke volume, % | −18±5 | −4±4 | 0.013 |
| Systemic vascular resistance, % | 9±6 | 4±5 | 0.435 |
| Skin blood flow (n=9) | |||
| Abdomen, % | 118±58 | 3±8 | 0.020 |
| Calf, % | 26±6 | 7±13 | 0.232 |
| Cutaneous vascular conductance (n=9) | |||
| Abdomen, % | 143±62 | 0±4 | 0.012 |
| Calf, % | 39±6 | 14±18 | 0.232 |
| Calf skin temperature, °C (n=9) | 0.7±0.3 | 1.0±0.6 | 0.922 |
Data are presented as mean±SEM. The acute effects on systemic and skin hemodynamic parameters and calf skin temperature at 2 hours of local controlled passive heating (40–42°C applied over the abdomen and pelvis with an electric heating pad) or sham control are expressed as absolute or percent changes from baseline.
The P values were generated by Wilcoxon signed‐rank tests or paired t tests depending on the data distribution.
Figure 2. Acute effects of local controlled passive heat on systemic and skin hemodynamics (Protocol 1).
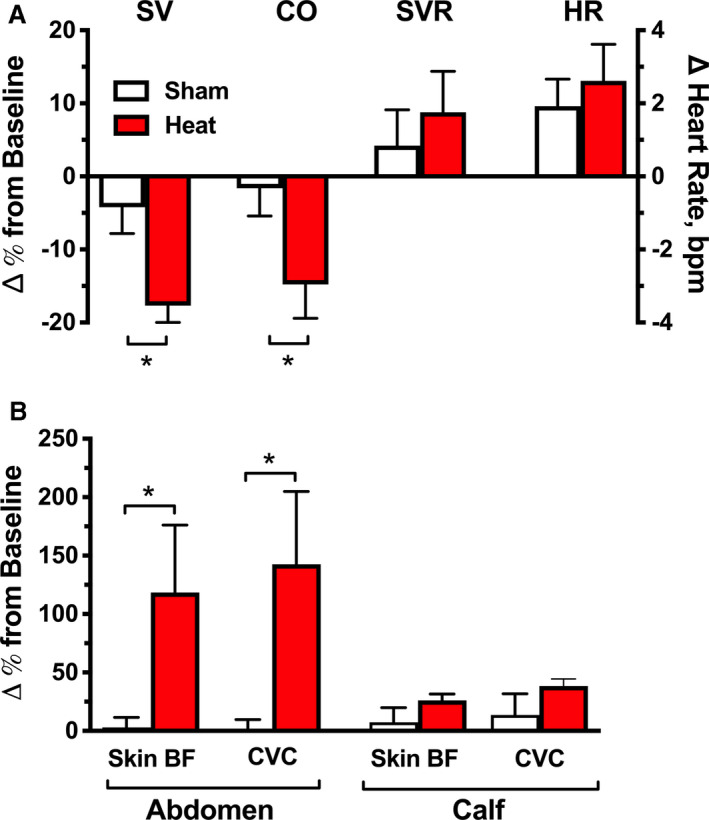
A, Percent changes from baseline in systemic hemodynamic parameters at 2 hours of controlled passive heating (40–42°C applied over the abdomen and pelvis with an electric heating pad) or sham control. Local passive heat significantly decreased cardiac output (CO) and stroke volume (SV) compared with sham control, whereas systemic vascular resistance (SVR) and heart rate (HR) were similar between groups. B, Percent changes from baseline in abdominal and calf skin blood flow (skin BF) and cutaneous vascular conductance (CVC) at 2 hours post‐intervention. Compared with sham control, passive heat significantly increased local skin BF and CVC in the abdomen (directly under the heating pad) but had no significant effect in the calf, a distal site not exposed to the heating source. Values are expressed as mean±SEM. *P<0.05 vs sham.
Protocol 2: Effects of Overnight Heat Therapy on Nocturnal Supine Hypertension
Baseline supine SBP (8 pm) was similar between treatment arms (heat: 166±4 versus placebo: 162±5 mm Hg; P=0.371). Overnight heat therapy significantly decreased supine SBP compared with placebo (P=0.008 for treatment effect and P<0.001 for treatment×time interaction, 2‐way repeated‐measures ANOVA; Figure 3A), resulting in a maximal reduction of 28±6 mm Hg at 4 hours (2 am) post‐intervention (versus −2±6 mm Hg for placebo; P<0.001). MAP and PP also decreased significantly during overnight heat, with a maximal reduction of 15±3 and 20±4 mm Hg, respectively, after 4 hours post‐intervention (versus −1±4 and 0±5 mm Hg for placebo; P<0.001 for both comparisons; Figure S2). DBP followed a similar trend but did not reach significance. HR had a similar small decrease during both trials (P<0.001 for time effect, P=0.744 for treatment effect and P=0.786 for treatment×time interaction), with a maximal reduction at 2 hours post‐intervention in both trials (heat: −4±1 and placebo: −5±2 bpm). Overnight heat therapy had no significant effect on sublingual temperature when compared with placebo (P=0.052 for time effect, P=0.736 for treatment effect and P=0.919 for treatment×time interaction). The change from baseline in sublingual temperature at 2 AM (time of maximal BP reduction) was −0.1±0.1°C (36.6±0.1°C) versus −0.2±0.1°C (36.5±0.1°C) with placebo.
Figure 3. Effect of overnight heat therapy on nighttime BP (Protocol 2).
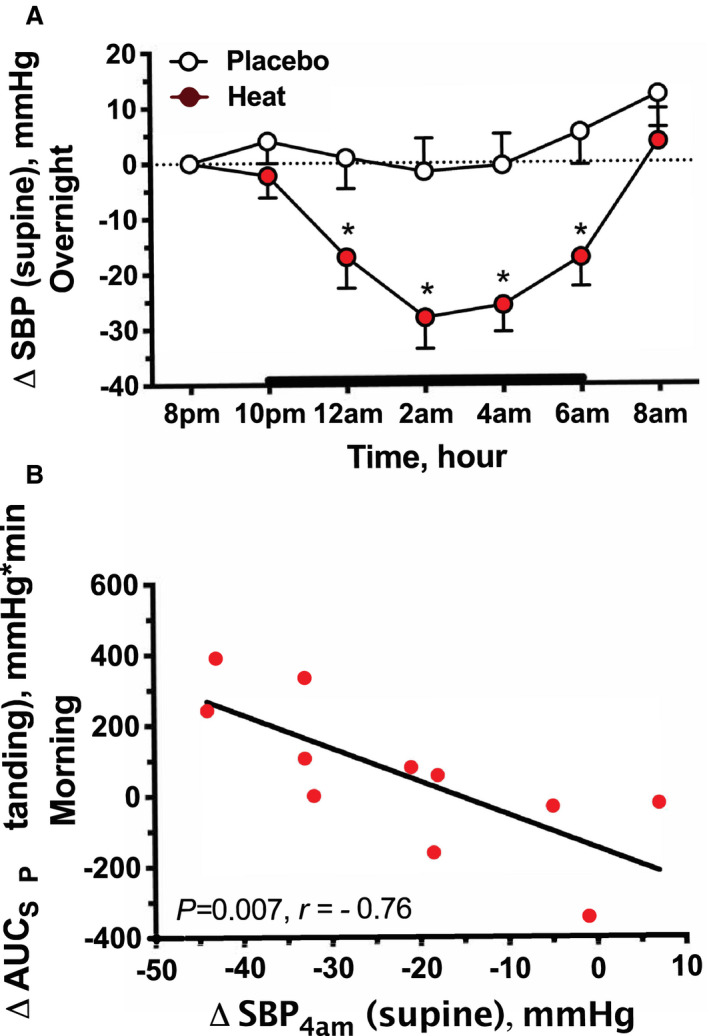
A, The time course of the change in systolic blood pressure (ΔSBP) in patients with autonomic failure and nocturnal supine hypertension. Passive heat (38°C) was applied with a water‐perfused heating pad placed under the patient's torso and proximal thighs from 10 pm to 6 am (bold line). Changes from baseline (8 pm) in supine SBP (∆SBP) are expressed as mean±SEM. Overnight heat therapy decreased SBP compared with placebo (P<0.01 for treatment effect and treatment×time interaction, 2‐way repeated measures ANOVA). *P<0.01 vs placebo, adjusted for multiple comparisons using Bonferroni correction. B, The relation between the depressor response to overnight heat therapy (calculated as the difference in the supine SBP at 4 am between heat and placebo nights, ∆SBP4AM) and the change in orthostatic tolerance the morning after heat treatment (estimated as the difference in the area under the curve of standing SBP between overnight heat therapy and placebo groups, ∆AUCSBP). Patients with greater reductions in supine SBP with overnight heat had greater improvements in morning orthostatic tolerance.
Nocturnal urine volume was collected in 11 patients; 1 patient did not collect urine because of incontinence. There was no difference in nocturnal urine volume between trials (heat: 964±168 versus placebo: 993±139 mL; P=0.999). Morning orthostatic tolerance, estimated as the area under the curve of standing SBP during a 10‐minute test in the 10 patients who were able to stand after both treatment arms, did not differ between treatments (heat: 497±106 versus placebo: 433±121 mm Hg×minute; P=0.350). In a post hoc analysis, we assessed if the magnitude of the depressor response to overnight heat (defined as the difference in the supine SBP at 4 am between heat and placebo nights) correlated with the nocturnal urine volume and with the change in orthostatic tolerance the morning after. We found a trend toward a positive correlation between the SBP response to overnight heat and nocturnal urine volume (P=0.072, r=0.57), and a significant negative correlation with the change in morning orthostatic tolerance (estimated as the difference in area under the curve of standing SBP between treatments, P=0.007, r=−0.76; Figure 3B), suggesting that patients with greater reductions in supine SBP with overnight heat had lower nocturnal urine volumes and greater improvement in morning orthostatic tolerance.
Adverse Events
All participants tolerated well the acute and overnight heat therapy. No adverse events were reported by participants. There were no signs of burn injuries or skin irritation during studies. No participant requested to lower the temperature or remove the heating pad before study completion, and none reported difficulty sleeping during studies with overnight heat therapy.
Discussion
The main findings of this study were that (1) acute application of low levels of local passive heat to AF patients with supine hypertension had a profound BP‐lowering effect with a rapid onset of action and recovery; (2) this BP effect was associated with local skin vasodilation and reductions in CO and SV; (3) when applied for 8 hours overnight, local heat lowered nocturnal BP, in a magnitude comparable to that of pharmacologic antihypertensive treatment we have previously studied; (4) nocturnal urinary loss or morning orthostatic tolerance did not improve with overnight heat therapy in the overall group, but these parameters improved in patients with greater BP responses to overnight heat; and (5) nocturnal body temperature was not significantly affected by overnight heat application. Taken together, overnight heat therapy may offer a novel nonpharmacologic approach to treat the nocturnal supine hypertension of AF, but future studies are needed to assess the long‐term efficacy and safety of this approach.
In healthy subjects, local passive heating increases skin blood flow by 2 independent mechanisms: an initial brisk vasodilation mediated by local sensory nerve activity followed by a slower prolonged vasodilation predominantly because of release of endothelial nitric oxide. 32 , 33 If heating is intense enough to increase core temperature, a compensatory sympathetically mediated reflex skin vasodilation occurs in nonheated areas to allow for heat dissipation. 12 , 13 , 15 , 28 This can result in large increases in skin vascular conductance and reductions in effective circulatory volume and cardiac preload. 34 BP, however, is normally maintained because of sympathetically mediated reductions in blood flow and volume in the splanchnic and renal vasculature and increases in HR and CO. 14 These compensatory autonomic mechanisms, critical to maintaining normotension during heat stress, are impaired in patients with AF. Furthermore, heat dissipation is also impaired in AF because sweating is abolished or significantly decreased. 35 On the other hand, nitric oxide‐dependent local skin vasodilation induced by heat is largely preserved. 33 , 36 Thus, heat‐induced skin vasodilation uncoupled from compensatory hemodynamic responses may cause acute reductions in BP. This is compounded by the severe baroreflex impairment characteristic of AF, evidenced by the marked pressor and depressor responses to stimuli that would normally produce little, if any, effect in healthy subjects. For example, upright posture lowered systolic BP by 68 to 77 mm Hg in our patients. Meals also have a similar dramatic hypotensive effect, whereas water drinking can produce substantial increases in BP. 26 , 37 , 38 This constellation of factors, therefore, may explain why these patients complain of severe worsening of their orthostatic symptoms when exposed to ambient heat. 16
The purpose of this study was to assess whether we can take advantage of this phenomenon to develop a novel treatment for supine hypertension in AF. We chose local passive heating instead of whole‐body heating because of our interest in finding a practical treatment option and because local passive heat has the potential safety advantage that body parts unexposed to heat would allow for heat loss, and thus, minimize the risk for hyperthermia. 15 We found that local passive heating acutely produced a profound reduction in supine BP (Protocol 1). This effect had a rapid onset and offset of action (≈30 minutes after application and removal of heat) and does not appear to require increases in Tcore because the BP decreased before any significant change in Tcore. Because the BP reduction was associated with a decrease in PP, SV, and CO, we speculate that these hemodynamic changes were primarily driven by an uncompensated reduction in cardiac preload. Indeed, HR did not change during passive heating whereas local skin blood flow and CVC increased, consistent with previous studies. 33 , 36 Furthermore, absence of contraction of resistance and capacitance vessels in the splanchnic circulation may have contributed to the decrease in SV during passive heating, as suggested by studies in animals undergoing celiac sympathectomy or ganglionic blockade that showed attenuated contraction of these vessels during whole‐body heating. 39 , 40 We did not observe a decrease in SVR and the reason for this finding is not apparent from our studies. Skin blood flow and CVC increased only in areas exposed to heat (abdomen) but not in areas distal from the heating source (calf), suggesting that the sympathetically mediated reflex skin vasodilation was either impaired or not engaged by the relatively small increase in Tcore (0.4°C) observed in our patients. 15 It is possible, therefore, that the area of local skin vasodilation induced by heat in our patients was not large enough to be reflected in SVR. Additional studies are required to define the contribution of resistance and capacitance changes in the skin vasculature to the large BP‐lowering effect of heat in patients with AF.
Based on the findings In Protocol 1, we designed a proof‐of‐concept study to assess the efficacy of overnight heat therapy for the treatment of supine hypertension in AF (Protocol 2). We used a lower level of passive heat (38°C) for the overnight studies because it has been shown to be safe, well tolerated, and able to induce local skin vasodilation. 28 , 29 while minimizing the risk of hyperthermia and low‐temperature burns due to the prolonged heat exposure. 41 We found that overnight heat therapy was effective in controlling nocturnal hypertension. The magnitude of this effect, with a trough of ≈28±6 mm Hg in supine SBP, is equivalent to many of the antihypertensive medications we have previously tested (Figure 4). 5 , 7 , 8 , 9 , 10 , 11 Importantly, BP was restored to baseline values by the next morning. The intervention was well tolerated, and no safety concerns were identified. Overnight heat did not prevent the normal decrease in nighttime body temperature, an important observation relevant to the safety of this approach. Disappointingly, nocturnal urinary loss or morning orthostatic tolerance were not improved when analyzing the study population as a whole. Nonetheless, we found that patients with greater BP reductions with overnight heat reduced nocturnal diuresis and improved orthostatic tolerance the next morning. This conclusion, however, has the limitation of being derived from a post hoc analysis.
Figure 4. Maximal BP‐lowering effects of pharmacologic treatment for nocturnal supine hypertension and overnight heat therapy.
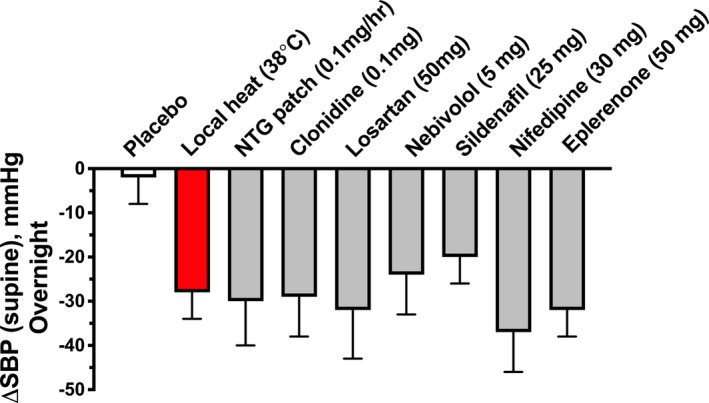
Maximal reductions from baseline in nocturnal supine systolic BP (ΔSBP) were measured after a single dose of placebo pill, 0.1 mg/hour nitroglycerin patch, 11 0.1 mg clonidine PO, 11 50 mg losartan PO, 7 5 mg nebivolol PO, 10 25 mg sildenafil PO, 10 30 mg nifedipine PO, 5 and 50 mg eplerenone PO 8 given at 8 pm. Local heat therapy (38°C) was applied from 10 pm to 6 am. The maximal reduction in nocturnal supine SBP during local heat was similar to that of pharmacological therapy. Values are expressed as mean±SEM.
Supine hypertension not only increases the risk for cardiac and renal end‐organ damage 3 , 4 but also induces nocturnal pressure natriuresis, resulting in volume depletion and worsening of morning OH. 5 Because supine hypertension coexists with OH in the majority of patients with AF, 19 effective treatment of one is often achieved at the expense of the other. Treatment of OH with pressor agents, with few exceptions, produces greater increases in supine than in standing BP, 37 whereas pharmacologic treatment of supine hypertension can worsen OH. Thus, it has been argued that treatment of OH should be prioritized over treatment of supine hypertension. 6 The reluctance to treat supine hypertension would be overcome if we found a therapy that can effectively lower BP during the night and the effects rapidly reversed the next morning, so that the reduction in pressure diuresis and volume loss would improve daytime OH. Sleeping in a head‐up tilt position is one such therapy 42 ; however, the degree of tilt required to effectively reduce BP limits the usefulness of this approach. Our results suggest that local heat provides a novel treatment option for this condition. It compares favorably with medications previously tested; several agents effectively lower nocturnal BP in these patients such as nitroglycerin, sildenafil, clonidine, nebivolol, losartan, nifedipine, and eplerenone (Figure 4). 5 , 7 , 8 , 9 , 10 , 11 Of these, only clonidine and losartan reduced nocturnal sodium excretion, and none improved morning OH. 7 , 11
Several studies in hypertensive and heart failure patients have shown that frequent, short‐term exposure to high levels of environmental heat (eg, sauna baths) is a relatively safe intervention that may be associated with reduced cardiovascular risk and improved hemodynamic function, including BP reduction. 43 , 44 These observations, however, cannot be extrapolated to our study population given the unique clinical characteristics of autonomic failure. In addition, our study has some limitations that should be considered before recommending local heat as a therapeutic option but at the same time open opportunities for further research. First, despite the large BP decrease, the effect on nocturia and morning OH was not consistent. The sample size was too small to identify predictors of response to overnight heat. Nonetheless, local heat compares favorably against all the pharmacological agents previously tested. Second, for safety reasons we used a medical‐grade device to apply overnight heat, which may be impractical for home use. Several types of heated mattress pads, which operate within the range of temperatures used in this study, are available for general use but translating our findings into clinical practice will require further studies to assess safety and efficacy under real‐life conditions, chronic use, and its effects when combined with other pharmacologic and nonpharmacologic approaches. Finally, the majority of our participants had peripheral forms of AF. Thus, we have not determined whether this approach was effective in patients with multiple system atrophy in whom supine hypertension is driven by residual sympathetic tone. 45
Conclusions
In conclusion, our results confirmed the importance of the autonomic nervous system in the maintenance of BP during passive heat stress. In patients with AF and supine hypertension, even low levels of local passive heating produced an acute and reversible BP reduction that was effective in controlling their nocturnal hypertension. Heat application was well tolerated, and under the controlled conditions of our study, no safety concerns were identified. In a post hoc analysis, patients with greater decreases in nocturnal BP also showed a reduction in nocturia and improvement in early morning orthostatic tolerance. Therefore, overnight heat therapy may offer a novel nonpharmacologic approach to treat the supine hypertension of AF, but further research is needed to translate these findings to clinical practice and to assess the long‐term efficacy and safety of this approach. Autonomic failure provides a unique human model to examine BP regulation in the absence of autonomic influences. Thus, our findings not only have important implications for the treatment of these patients but also may have an impact on our understanding of the role of the autonomic nervous system in the cardiovascular responses to heat stress.
Sources of Funding
This work was supported by the National Institutes of Health (NIH) grants R01 HL144568, PO1 HL56693, U54 NS065736, R01 HL122847 and UL1 TR000445 (National Center for Advancing Translational Sciences). Additional support was provided by the American Heart Association grant 14CRP20380211 (Okamoto). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Disclosures
Biaggioni received 2 donated units of the Norm‐O‐Temp system and Gelli‐roll pads from Cincinnati Sub‐Zero for the conduct of the overnight studies. The remaining authors have no disclosures to report.
Supporting information
Figures S1–S2
Acknowledgments
We acknowledge the patients who volunteered for these studies and the Clinical Research Center nurses who made this study possible.
(J Am Heart Assoc. 2021;10:e018979. DOI: 10.1161/JAHA.120.018979.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–126. DOI: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 2. Gibbons CH, Schmidt P, Biaggioni I, Frazier‐Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264:1567–1582. DOI: 10.1007/s00415-016-8375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL, Biaggioni I, Robertson D. Renal impairment of pure autonomic failure. Hypertension. 2009;54:1057–1061. DOI: 10.1161/HYPERTENSIONAHA.109.136853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;355:725–726. DOI: 10.1016/S0140-6736(99)05320-9. [DOI] [PubMed] [Google Scholar]
- 5. Jordan J, Shannon JR, Pohar B, Paranjape SY, Robertson D, Robertson RM, Biaggioni I. Contrasting effects of vasodilators on blood pressure and sodium balance in the hypertension of autonomic failure. J Am Soc Nephrol. 1999;10:35–42. [DOI] [PubMed] [Google Scholar]
- 6. Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15:954–966. DOI: 10.1016/S1474-4422(16)30079-5. [DOI] [PubMed] [Google Scholar]
- 7. Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension. 2013;61:701–706. DOI: 10.1161/HYPERTENSIONAHA.111.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnold AC, Okamoto LE, Gamboa A, Black BK, Raj SR, Elijovich F, Robertson D, Shibao CA, Biaggioni I. Mineralocorticoid receptor activation contributes to the supine hypertension of autonomic failure. Hypertension. 2016;67:424–429. DOI: 10.1161/HYPERTENSIONAHA.115.06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gamboa A, Shibao C, Diedrich A, Paranjape SY, Farley G, Christman B, Raj SR, Robertson D, Biaggioni I. Excessive nitric oxide function and blood pressure regulation in patients with autonomic failure. Hypertension. 2008;51:1531–1536. DOI: 10.1161/HYPERTENSIONAHA.107.105171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okamoto LE, Gamboa A, Shibao CA, Arnold AC, Choi L, Black BK, Raj SR, Robertson D, Biaggioni I. Nebivolol, but not metoprolol, lowers blood pressure in nitric oxide‐sensitive human hypertension. Hypertension. 2014;64:1241–1247. DOI: 10.1161/HYPERTENSIONAHA.114.04116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. Clonidine for the treatment of supine hypertension and pressure natriuresis in autonomic failure. Hypertension. 2006;47:522–526. DOI: 10.1161/01.HYP.0000199982.71858.11. [DOI] [PubMed] [Google Scholar]
- 12. Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol. 1977;69:154–166. DOI: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- 13. Rowell LB, Brengelmann GL, Detry JM, Wyss C. Venomotor responses to local and remote thermal stimuli to skin in exercising man. J Appl Physiol. 1971;30:72–77. DOI: 10.1152/jappl.1971.30.1.72. [DOI] [PubMed] [Google Scholar]
- 14. Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586:293–301. DOI: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Compr Physiol. 2015;5:17–43. DOI: 10.1002/cphy.c140015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pathak A, Lapeyre‐Mestre M, Montastruc J‐L, Senard J‐M. Heat‐related morbidity in patients with orthostatic hypotension and primary autonomic failure. Mov Disord. 2005;20:1213–1219. DOI: 10.1002/mds.20571. [DOI] [PubMed] [Google Scholar]
- 17. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. DOI: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. DOI: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 19. Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. The hypertension of autonomic failure and its treatment. Hypertension. 1997;30:1062–1067. DOI: 10.1161/01.HYP.30.5.1062. [DOI] [PubMed] [Google Scholar]
- 20. Mosqueda‐Garcia R. Evaluation of autonomic failure. In: Disorders of the Autonomic Nervous System. Luxembourg, Europe: Harwood Academic Publishers GmbH; 1995:25–59. [Google Scholar]
- 21. Goldstein DS, Eisenhofer G, Stull R, Folio CJ, Keiser HR, Kopin IJ. Plasma dihydroxyphenylglycol and the intraneuronal disposition of norepinephrine in humans. J Clin Invest. 1988;81:213–220. DOI: 10.1172/JCI113298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Omboni S, Smit AA, van Lieshout JJ, Settels JJ, Langewouters GJ, Wieling W. Mechanisms underlying the impairment in orthostatic tolerance after nocturnal recumbency in patients with autonomic failure. Clin Sci (Lond). 2001;101:609–618. DOI: 10.1042/cs1010609. [DOI] [PubMed] [Google Scholar]
- 23. Domitrovich JW, Cuddy JS, Ruby BC. Core‐temperature sensor ingestion timing and measurement variability. J Athl Train. 2010;45:594–600. DOI: 10.4085/1062-6050-45.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas SH. Impedance cardiography using the Sramek‐Bernstein method: accuracy and variability at rest and during exercise. Br J Clin Pharmacol. 1992;34:467–476. [PMC free article] [PubMed] [Google Scholar]
- 25. Chaseling GK, Crandall CG, Gagnon D. Skin blood flow measurements during heat stress: technical and analytical considerations. Am J Physiol Regul Integr Comp Physiol. 2020;318:R57–R69. DOI: 10.1152/ajpregu.00177.2019. [DOI] [PubMed] [Google Scholar]
- 26. Jordan J, Shannon JR, Grogan E, Biaggioni I, Robertson D. A potent pressor response elicited by drinking water. Lancet. 1999;353:723. DOI: 10.1016/S0140-6736(99)99015-3. [DOI] [PubMed] [Google Scholar]
- 27. Accessdata.fda.gov . 2020. 510(K) premarket notification. [online] Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K120081. Accessed May 11, 2020.
- 28. Kamijo Y, Lee K, Mack GW. Active cutaneous vasodilation in resting humans during mild heat stress. J Appl Physiol (1985). 2005;98:829–837. DOI: 10.1152/japplphysiol.00235.2004. [DOI] [PubMed] [Google Scholar]
- 29. Low DA, Shibasaki M, Davis SL, Keller DM, Crandall CG. Does local heating‐induced nitric oxide production attenuate vasoconstrictor responsiveness to lower body negative pressure in human skin. J Appl Physiol (1985). 2007;102:1839–1843. DOI: 10.1152/japplphysiol.01181.2006. [DOI] [PubMed] [Google Scholar]
- 30. Okamoto LE, Shibao C, Gamboa A, Choi L, Diedrich A, Raj SR, Black BK, Robertson D, Biaggioni I. Synergistic effect of norepinephrine transporter blockade and α‐2 antagonism on blood pressure in autonomic failure. Hypertension. 2012;59:650–656. DOI: 10.1161/HYPERTENSIONAHA.111.184812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. DOI: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 32. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985). 2001;91:1619–1626. DOI: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 33. Charkoudian N, Eisenach JH, Atkinson JL, Fealey RD, Joyner MJ. Effects of chronic sympathectomy on locally mediated cutaneous vasodilation in humans. J Appl Physiol (1985). 2002;92:685–690. DOI: 10.1152/japplphysiol.00758.2001. [DOI] [PubMed] [Google Scholar]
- 34. Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank‐Starling relations in humans. J Physiol. 2009;587:3383–3392. DOI: 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coon EA, Cheshire WP. Sweating disorders. Continuum (Minneap Minn). 2020;26:116–137. DOI: 10.1212/CON.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 36. Yamanaka Y, Asahina M, Mathias CJ, Akaogi Y, Koyama Y, Hattori T. Skin vasodilator response to local heating in multiple system atrophy. Mov Disord. 2007;22:2405–2408. DOI: 10.1002/mds.21742. [DOI] [PubMed] [Google Scholar]
- 37. Okamoto LE, Diedrich A, Baudenbacher FJ, Harder R, Whitfield JS, Iqbal F, Gamboa A, Shibao CA, Black BK, Raj SR, et al. Efficacy of servo‐controlled splanchnic venous compression in the treatment of orthostatic hypotension: a randomized comparison with midodrine. Hypertension. 2016;68:418–426. DOI: 10.1161/HYPERTENSIONAHA.116.07199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shibao C, Gamboa A, Diedrich A, Dossett C, Choi L, Farley G, Biaggioni I. Acarbose, an alpha‐glucosidase inhibitor, attenuates postprandial hypotension in autonomic failure. Hypertension. 2007;50:54–61. [DOI] [PubMed] [Google Scholar]
- 39. Deschamps A, Magder S. Effects of heat stress on vascular capacitance. Am J Physiol. 1994;266:H2122–H2129. DOI: 10.1152/ajpheart.1994.266.5.H2122. [DOI] [PubMed] [Google Scholar]
- 40. Kregel KC, Gisolfi CV. Circulatory responses to heat after celiac ganglionectomy or adrenal demedullation. J Appl Physiol (1985). 1989;66:1359–1363. DOI: 10.1152/jappl.1989.66.3.1359. [DOI] [PubMed] [Google Scholar]
- 41. Choi MS, Lee HJ, Lee JH. Early intervention for low‐temperature burns: comparison between early and late hospital visit patients. Arch Plast Surg. 2015;42:173–178. DOI: 10.5999/aps.2015.42.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ten Harkel AD, Van Lieshout JJ, Wieling W. Treatment of orthostatic hypotension with sleeping in the head‐up tilt position, alone and in combination with fludrocortisone. J Intern Med. 1992;232:139–145. DOI: 10.1111/j.1365-2796.1992.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 43. Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all‐cause mortality events. JAMA Intern Med. 2015;175:542–548. DOI: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 44. Li Z, Jiang W, Chen Y, Wang G, Yan F, Zeng T, Fan H. Acute and short‐term efficacy of sauna treatment on cardiovascular function: a meta‐analysis. Eur J Cardiovasc Nurs. 2020;1474515120944584. DOI: 10.1177/1474515120944584. [DOI] [PubMed] [Google Scholar]
- 45. Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, Biaggioni I. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–2715. DOI: 10.1161/01.CIR.101.23.2710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


