Abstract
Background
Prior studies have shown that women have worse 3‐month survival after receiving a left ventricular assist device compared with men. Currently used prognostic scores, including the Heartmate II Risk Score, do not account for the increased residual risk in women. We used the IMACS (International Society for Heart and Lung Transplantation Mechanically Assisted Circulatory Support) registry to create and validate a sex‐specific risk score for early mortality in left ventricular assist device recipients.
Methods and Results
Adult patients with a continuous‐flow LVAD from the IMACS registry were randomly divided into a derivation cohort (DC; n=9113; 21% female) and a validation cohort (VC; n=6074; 21% female). The IMACS Risk Score was developed in the DC to predict 3‐month mortality, from preoperative candidate predictors selected using the Akaike information criterion, or significant sex × variable interaction. In the DC, age, cardiogenic shock at implantation, body mass index, blood urea nitrogen, bilirubin, hemoglobin, albumin, platelet count, left ventricular end‐diastolic diameter, tricuspid regurgitation, dialysis, and major infection before implantation were retained as significant predictors of 3‐month mortality. There was significant ischemic heart failure × sex and platelet count × sex interaction. For each quartile increase in IMACS risk score, men (odds ratio [OR], 1.86; 95% CI, 1.74–2.00; P<0.0001), and women (OR, 1.93; 95% CI, 1.47–2.59; P<0.0001) had higher odds of 3‐month mortality. The IMACS risk score represented a significant improvement over Heartmate II Risk Score (IMACS risk score area under the receiver operating characteristic curve: men: DC, 0.71; 95% CI, 0.69–0.73; VC, 0.69; 95% CI, 0.66–0.72; women: DC, 0.73; 95% CI, 0.70–0.77; VC, 0.71 [95% CI, 0.66–0.76; P<0.01 for improvement in receiver operating characteristic) and provided excellent risk calibration in both sexes. Removal of sex‐specific interaction terms resulted in significant loss of model fit.
Conclusions
A sex‐specific risk score provides excellent risk prediction in LVAD recipients.
Keywords: left ventricular assist device, mortality, prognosis, risk score, sex disparity
Subject Categories: Heart Failure, Cardiovascular Surgery, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- AIC
Akaike Information Criterion
- DC
derivation cohort
- DTRS
Destination Therapy Risk Score
- HMRS
Heartmate II Risk Score
- ICM
ischemic cardiomyopathy
- IMACS
ISHLT Mechanically Assisted Circulatory Support Registry
- IMACS‐RS
ISHLT Mechanically Assisted Circulatory Support Registry Risk Score
- INTERMACS profile
Interagency Registry for Mechanical Circulatory Support profile
- ISHLT
International Society for Heart and Lung Transplantation
- LVEDD
left ventricular end diastolic diameter
- TR
tricuspid regurgitation
- VC
validation cohort
Clinical Perspective
What Is New?
Women have worse 3‐month survival after receiving a left ventricular assist device (LVAD) compared with men; unique sex‐specific determinants of this increased residual risk exist but are not accounted for in currently used prognostic scores including the Heartmate II Risk Score.
In this study, we used the IMACS (International Society for Heart and Lung Transplantation Mechanically Assisted Circulatory Support) registry to create and validate a sex‐specific risk score (IMACS Risk Score) for early mortality in LVAD recipients.
The IMACS Risk Score includes 2 variables with sex‐specific effects: ischemic heart failure etiology and platelet count; the IMACS Risk Score has significantly better risk discrimination than the Heartmate II Risk Score and the model for end‐stage liver disease score and demonstrates excellent risk calibration.
What Are the Clinical Implications?
The sex‐specific IMACS Risk Score, which includes 2 variables with sex‐specific effects: ischemic HF etiology and platelet count, provides excellent risk prediction for 3‐month mortality in LVAD recipients.
An online IMACS Risk Score calculator has been developed for easy application (http://www.eccri.emory.edu/lvad‐risk/index.html); a similar approach is necessary to improve prognostication and bridge sex disparities across the heart failure spectrum.
This is the first report to describe sex‐specific effects of heart failure etiology and platelet count in LVAD recipients. It is possible that sex‐specific antiplatelet regimens are necessary to optimally balance the risk of hemorrhagic with thrombotic events in LVAD recipients.
Women represent over half of the 6.2 million patients with heart failure (HF) in the United States and account for 58% of annual HF‐related deaths. 1 , 2 , 3 , 4 Risk factors, epidemiology, and clinical outcomes associated with HF are unique to sex. Yet evidence‐based HF therapies are based on landmark trials conducted with 20% to 25% female representation, with sex‐specific analyses not showing a benefit of several of these therapies in women. 5 Commonly used HF prognostic risk scores including the Seattle Heart Failure Model (SHFM), 6 and the Meta‐Analysis Global Group in Chronic Heart Failure score 7 were derived and validated in predominantly male cohorts, without testing for potential interaction effects with sex.
Similarly, there is a paucity of data on sex‐specific risk factors and correlates of mortality in the advanced HF population. Women are less likely to undergo LVAD implantation for reasons that are poorly understood 8 and are therefore underrepresented in all large LVAD clinical trials. 9 , 10 , 11 , 12 Prior analyses of INTERMACS (Interagency Registry for Mechanical Circulatory Support), 13 and the IMACS (International Society for Heart and Lung Transplantation [ISHLT] Mechanically Assisted Circulatory Support) registry 14 have identified a distinctly higher period of postoperative mortality for women in the first 3 to 4 months after implantation. While the HeartMate II Risk Score (HMRS) was developed for the prediction of 3‐month mortality, it was derived and validated in a clinical trial cohort of 1122 patients consisting of <25% women, 15 and may not fully account for the increased residual risk of postoperative mortality seen in women.
We have previously shown that sex‐specific differences in left ventricular (LV) size and valvular dysfunction mediate >20% of the increased risk of postoperative mortality seen in women. 14 Building upon our prior findings, we used the IMACS database to explore novel risk factors, and sex‐specific effects to construct a user‐friendly score that quantifies sex‐specific mortality risk in LVAD recipients. Further, we compared the performance of this risk score to the HMRS, since the HMRS has consistently performed better than other prognostic scores for LVAD recipients in external validation studies. 16 , 17
Methods
Database
Deidentified patient‐level data were obtained from the IMACS registry, 18 which collects data from patients undergoing durable LVAD support in 35 countries across the globe. Data are uploaded yearly and merged into the registry for analysis. Single‐country, single‐collective, device brand, and race data are not available for analysis. This paper was reviewed and approved by the IMACS Steering Committee and considered exempt from review by the Emory University Institutional Review Board.
Patient Population
Adults (≥18 years) who received continuous‐flow LVAD from January 9, 2013, to September 30, 2017, were included in the study. Alive subjects with <3 months of follow‐up and those undergoing transplantation or explant for recovery in <3 months after implantation were excluded (n=311), leaving 15 187 patients in the final analytic cohort (Figure).
Figure 1. Flowchart of derivation and validation of IMACS‐RS.
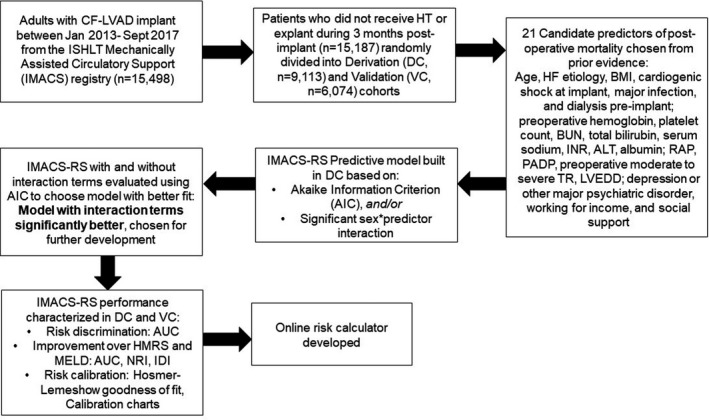
AIC indicates Akaike information criterion; ALT, alanine aminotransferase; AUC, area under the receiver operating characteristic curve; BMI, body mass index; BUN, blood urea nitrogen; DC, derivation cohort; IDI, integrated discrimination improvement; IMACS, ISHLT Mechanically Assisted Circulatory Support Registry; IMACS‐RS, ISHLT Mechanically Assisted Circulatory Support Registry‐Risk Score; INR, international normalized ratio; ISHLT, International Society for Heart and Lung Transplantation; HF, heart failure; HT, heart transplantation; LVEDD, left ventricular end‐diastolic diameter; MELD, model for end‐stage liver disease; NRI, Net Reclassification Index; PADP, pulmonary artery diastolic pressure; RAP, right atrial pressure; TR, tricuspid regurgitation; and VC, validation cohort.
Outcomes
The primary outcome of interest was 3‐month mortality. This outcome was chosen on the basis of our prior data, which demonstrates that female LVAD recipients have a higher risk of mortality only during the first 3 to 4 months after implantation but not after. 14 The last date of follow‐up was October 31, 2017.
Statistical Analysis
Data are presented as mean±SD, median (interquartile range), or as number (%) of patients. Baseline characteristics between men and women, and the derivation (DC) and validation (VC) cohorts were compared using a 2‐sample t test for normally distributed continuous variables, Wilcoxon signed‐rank test for nonnormally distributed continuous variables, and chi‐square test for categorical variables. Association of female sex with 3‐month mortality was determined using multivariable binary logistic regression, adjusting for all baseline covariates that were significantly different between men and women.
Derivation and Validation of the IMACS Risk Score
Patients were randomly divided into DC (60%, n=9113) and VC (40%; n=6074) cohorts. The IMACS Risk Score (IMACS‐RS) was developed in the DC for prediction of 3‐month postoperative mortality. Clinically relevant preimplant covariates with ≤20% missing data were considered for inclusion in the IMACS‐RS. The following covariates had 5% to 10% missingness: HF etiology, albumin, alanine aminotransferase, social support (married versus single/divorced/widowed). For pulmonary artery diastolic pressure, right atrial pressure, LV end‐diastolic diameter (LVEDD) and working for income status, there were 10% to 20% missing data. All other covariates had <5% missingness. Missing data were imputed to the median for women and men, in the DC and VC. 19 Based on prior literature, candidate predictors considered for inclusion in the IMACS‐RS were age; ischemic HF etiology (ischemic cardiomyopathy [ICM]); body mass index, cardiogenic shock at implantation (INTERMACS [Interagency Registry for Mechanical Circulatory Support] profile 1–2 versus 3–7), major infection, and dialysis before implantation; preoperative hemoglobin, platelet count, blood urea nitrogen, total bilirubin, serum sodium, international normalized ratio, alanine aminotransferase, and albumin; right atrial pressure, pulmonary artery diastolic pressure, preoperative moderate to severe tricuspid regurgitation (TR), and LVEDD; depression or other major psychiatric disorder; working for income; and social support (married versus single/divorced/widowed). Nonnormally distributed predictors were log‐transformed for further analysis. All characteristics and preimplant adverse events were defined per INTERMACS. 20 The Akaike information criterion (AIC) 21 was employed to select predictors for inclusion in IMACS‐RS. In addition, predictors with significant female sex × predictor interaction in the multivariable logistic regression models were included in the IMACS‐RS. Multicollinearity of variables were assessed with variance inflation factor analysis to confirm independence of variables included in the risk score. 22 To test whether the inclusion of sex‐specific interactions improves model fit, AIC 21 was determined for the IMACS‐RS with and without the inclusion of interaction terms (smaller AIC with Δ AIC >10 is a criterion for model selection). 21 This method was chosen as the AIC model penalizes overfitting, preferring the more parsimonious model as long as the other models do not provide a substantially better fit. 21
Model Performance
Risk Discrimination
Model discrimination 23 was evaluated with the area under the receiver operating characteristic curve (AUC) in women and men, in the DC and VC. To minimize risk of bias attributable to imputation of missing data, a sensitivity complete‐case analysis was performed to evaluate AUC in the subset of VC patients that had no missing data for any IMACS‐RS predictors. Subgroup analysis by pump type (centrifugal versus axial flow pump), and continent (Americas versus Asia‐Pacific versus Europe) was performed. In addition, IMACS‐RS risk discrimination for outcome of 1‐year mortality was evaluated.
IMACS‐RS discrimination was compared with the HMRS (calculated for each patient as previously described, without modification for center volume) 15 by computing corresponding AUCs, continuous Net Reclassification Index and Integrated Discrimination Improvement index. 15 , 24 , 25 , 26 , 27 A supplemental analysis comparing IMACS‐RS to the Model for End‐Stage Liver Disease score (MELD, calculated as previously described), 28 which has also been shown to predict 3‐month mortality in LVAD recipients, 17 was performed.
Risk Calibration
Model calibration 23 was evaluated in men and women in the overall cohort with the Hosmer–Lemeshow goodness‐of‐fit test to determine if there were statistically significant differences in observed versus predicted risk (smaller chi‐square value with a nonsignificant P value indicates better model calibration). 29 Continuous IMACS‐RS was grouped according to quartiles of risk, and corresponding calibration charts of predicted versus observed risk were constructed.
A flowchart summarizing the creation and validation of the IMACS‐RS is depicted in the Figure. P<0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
Baseline characteristics of the entire cohort (n=15 187) are described in Table 1. Women were younger, more likely to have nonischemic cardiomyopathy, and be in cardiogenic shock (INTERMACS profiles 1–2) at implantation. They were more likely to carry a diagnosis of depression or another major psychiatric disorder and to be single, divorced, or widowed at implantation. They had a smaller LVEDD and more TR. Additionally, they had a lower hemoglobin, but also less evidence of hepatic and renal dysfunction.
Table 1.
Baseline Characteristics for Male Versus Female Continuous‐Flow LVAD Recipients in the IMACS Registry Cohort (n=15 187)
| Men (n=12 040) | Women (n=3147) | P Value | |
|---|---|---|---|
| Age at implant, y | 59 (49–66) | 56 (45–64) | <0.001 |
| Body mass index, kg/m2 | 27.2 (23.6–31.6) | 27.3 (22.7–32.7) | 0.77 |
| Ischemic cardiomyopathy | 4896 (40.7) | 709 (22.5) | <0.001 |
| Cardiogenic shock (INTERMACS 1 and 2) | 1804 (15.0) | 519 (16.5) | 0.04 |
| Dialysis, preimplantation | 353 (2.9) | 87 (2.8) | 0.67 |
| Major infection during index hospitalization, preimplantation | 668 (5.5) | 202 (6.4) | 0.07 |
| Blood urea nitrogen, mg/dL | 26 (18–39) | 22 (15–32) | <0.001 |
| Total bilirubin, mg/dL | 1.00 (0.70–1.61) | 0.80 (0.50–1.40) | <0.001 |
| ALT, U/L | 29 (19–49) | 25 (17–43) | <0.001 |
| Serum sodium, meq/L | 136 (132–138) | 136 (133–139) | <0.001 |
| INR | 1.20 (1.10–1.40) | 1.20 (1.10–1.40) | <0.001 |
| Hemoglobin, g/dL | 11.5 (9.90–13.0) | 10.6 (9.30–12.0) | <0.001 |
| Platelet count | 184 (138–236) | 198 (143–259) | <0.001 |
| Albumin, g/dL | 3.5 (3.0–3.9) | 3.5 (3.0–3.8) | 0.37 |
| Pulmonary artery diastolic pressure, mm Hg | 25 (19–31) | 24 (18–30) | <0.001 |
| Right atrial pressure, mm Hg | 11 (7–16) | 11 (7–16) | 0.32 |
| Echocardiographic characteristics | |||
| Moderate to severe TR | 4357 (36.2) | 1420 (45.1) | <0.001 |
| LVEDD, cm | 6.9 (6.2–7.6) | 6.4 (5.8–7.2) | <0.001 |
| Working for income | 2068 (17.2) | 424 (13.5) | <0.001 |
| Major depression or other psychiatric disorder | 358 (3.0) | 174 (5.5) | <0.001 |
| Single, divorced, or widowed | 3544 (29.4) | 1357 (43.1) | <0.001 |
Data are presented as N (%), mean (SD), or median (interquartile range). ALT indicates alanine aminotransferase; INR, international normalized ratio; INTERMACS, Interagency Registry for Mechanical Circulatory Support; LVEDD, left ventricular end diastolic diameter; and TR, tricuspid regurgitation.
Overall survival for the cohort was 90.3% at 90 days, 83.0% at 1 year, and 75.2% at 3 years. Overall survival at 90 days and 3 years was 89.7% and 74.6%, respectively, for women and 90.5% and 75.3%, respectively, for males. After adjusting for the aforementioned covariates, women had a worse short‐term outcome after LVAD with 25% higher odds of death in the first 3 months after implantation compared with men (adjusted odds ratio [OR], 1.25; 95% CI, 1.08–1.44; P=0.002).
Characteristics of the DC and VC
After randomization, there were no significant differences in baseline characteristics between the DC (n=9113; 20.7% women) and VC (n=6074; 20.8% women) (Table S1). There were 876 (9.6%) deaths in the DC, and 590 (9.7%) deaths in the VC in the first 3 months after implantation (P=0.84 for difference).
Development of IMACS‐RS
Figure S1 shows the forest plot for correlates of mortality at 90 days overall (Figure S1) and on the basis of patient sex (Figure S2) in the DC. In the DC (n=9113), age, cardiogenic shock at implantation (INTERMACS profiles 1–2), body mass index, blood urea nitrogen, bilirubin, hemoglobin, albumin, platelet count, LVEDD, TR, dialysis, and major infection before implantation were retained as significant predictors of 3‐month postoperative mortality. There was a significant female sex × ICM (P=0.02) and female sex × platelet count (P=0.006) interaction. ICM was associated with increased mortality in women (adjusted OR, 1.52; 95% CI, 1.05–2.24; P=0.03), but not in men (adjusted OR, 0.90; 95% CI, 0.76–1.07; P=0.24). Higher platelet counts were associated with decreased mortality in women (adjusted OR, 0.67; 95% CI, 0.54–0.86 per 1 Log [Platelet count × 103/μL] increase; P=0.001) but not in males (adjusted OR, 0.96; 95% CI, 0.83–1.12; P=0.62).
Model fit was examined using AIC for IMACS‐RS with and without the inclusion of interaction terms. Removal of the interaction terms resulted in loss of model fit (ΔAICwithout interaction−with interaction:14.15). Based on these results, IMACS‐RS with inclusion of interaction terms was further developed and validated. Predictors comprising the IMACS‐RS, sex‐specific weights, and formula for IMACS‐RS calculation are depicted in Table 2. An online calculator was created for easier use and application of the formula (http://www.eccri.emory.edu/lvad‐risk/index.html).
Table 2.
Weights for IMACS‐RS Predictors in Men Versus Women
| Predictors in IMACS‐RS* | Women | Men |
|---|---|---|
| Intercept | −2.75 | −2.75 |
| Age, y | 0.04 | 0.04 |
| Body mass index | 0.02 | 0.02 |
| Cardiogenic shock at implant (INTERMACS 1 or 2) | 0.34 | 0.34 |
| Dialysis, preimplantation | 0.75 | 0.75 |
| Major infection during index hospitalization, preimplantation | 0.35 | 0.35 |
| LVEDD, cm | −1.10 | −1.10 |
| Moderate to severe TR | 0.18 | 0.18 |
| Blood urea nitrogen, mg/dL | 0.40 | 0.40 |
| Total bilirubin, mg/dL | 0.57 | 0.57 |
| Hemoglobin, g/dL | −0.31 | −0.31 |
| Albumin, g/dL | −0.33 | −0.33 |
| Female sex | 1.74 | 0 |
| Ischemic HF etiology | 0.35 | −0.11 |
| Platelet count, ×103/μL | −0.40 | −0.05 |
Multicollinearity among independent variables was checked: variance inflation factor ranged from 1.04 (major infection, preimplantation) to 1.56 (platelet count), indicating that the IMACS‐RS predictors were independent of each other. HF indicates heart failure; IMACS‐RS, ISHLT Mechanical Assisted Circulatory Support Registry‐Risk Score; INTERMACS, Interagency Registry for Mechanical Circulatory Support; LVEDD, left ventricular end‐diastolic diameter; and TR, tricuspid regurgitation.
Calculation of IMACS‐RS: (−2.75+(0.04×[age in years])+(0.02×[BMI in kg/m2])+0.34 (if cardiogenic shock at implantation)+0.75 (if dialysis preimplantation)+0.35 (if major infection preimplantation)−(1.10×[Log(LVEDD in cm)])+0.18 (if moderate to severe TR)+(0.40×[Log(blood urea nitrogen in mg/dL)])+(0.57×[Log(total bilirubin in mg/dL+1)])−(0.31×[log(hemoglobin in g/dL)])−(0.33x[albumin in g/dL])−0.11 (if ischemic HF etiology)−(0.05×[Log(platelet count, ×103/μL])+1.74 (if female)+0.46 (if ischemic HF etiology, if female)−(0.35×(Log[platelet count, ×103/μL]), if female))×100.
IMACS‐RS and Outcomes
The median IMACS‐RS was 7.77 (interquartile range, 4.70–12.12) in the DC, and 7.85 (interquartile range, 4.84–12.23) in the VC. The IMACS‐RS provided moderately good discrimination in both men (AUC DC, 0.71; 95% CI, 0.69–0.73; AUC VC, 0.69; 95% CI, 0.66–0.72) and women (AUC DC, 0.73; 95% CI, 0.70–0.77; AUC VC, 0.71; 95% CI, 0.66–0.76) (Table 3). In a complete‐case sensitivity analysis of patients with no missing data in the VC (n=3694), good discrimination was retained in both men (AUC, 0.71; 95% CI, 0.68–0.74) and women (AUC, 0.76; 95% CI, 0.69–0.82). Subgroup analysis by pump type demonstrated that IMACS‐RS risk discrimination did not vary by pump type (centrifugal [n=5385]: AUC, 0.71; 95% CI, 0.68–0.73), axial flow (n=9802: AUC, 0.71; 95% CI, 0.69–0.73). Subgroup analysis by continent demonstrated that IMACS‐RS risk discrimination did not vary by continent of implantation (Americas [n=12 584]: AUC, 0.70; 95% CI, 0.69–0.72); Asia‐Pacific [n=732]: AUC, 0.72; 95% CI, 0.65–0.79), Europe [n=1871]: AUC, 0.70; 95% CI, 0.66–0.74).
Table 3.
Risk Discrimination of IMACS‐RS Versus HMRS in the Derivation and Validation Cohorts
| Women | Men | |||||
|---|---|---|---|---|---|---|
| HMRS | IMACS‐RS | P Value | HMRS | IMACS‐RS | P Value | |
| Derivation cohort (n=9113) | ||||||
| AUC | 0.66 (0.62–0.69) | 0.73 (0.70–0.77) | <0.0001 | 0.64 (0.62–0.67) | 0.71 (0.69–0.73) | <0.0001 |
| NRI, % | 64.38 (51.91–76.85) | <0.0001 | 45.72 (37.88–53.56) | <0.0001 | ||
| IDI | 0.061 (0.044–0.079) | <0.0001 | 0.037 (0.030–0.045) | <0.0001 | ||
| Validation cohort (n=6074) | ||||||
| AUC | 0.65 (0.60–0.70) | 0.71 (0.66–0.76) | 0.007 | 0.62 (0.60–0.65) | 0.69 (0.66–0.72) | <0.0001 |
| NRI, % | 52.74 (36.14–69.34) | <0.0001 | 48.39 (38.97–57.81) | <0.0001 | ||
| IDI | 0.055 (0.035–0.075) | <0.0001 | 0.034 (0.025–0.043) | <0.0001 | ||
AUC indicates area under the receiver operating characteristic curve; HMRS, HeartMate II Risk Score; IDI, Integrated Discrimination Improvement index; IMACS‐RS, ISHLT Mechanical Assisted Circulatory Support Registry‐Risk Score; and NRI, Net Reclassification Index.
The IMACS‐RS retained modest discrimination for the outcome of 1‐year mortality (n=2576) in both men (AUC, 0.68; 95% CI, 0.67–0.69) and women (AUC, 0.68; 95% CI, 0.66–0.71).
IMACS‐RS Versus HMRS and MELD
The IMACS‐RS provided significant improvement in AUC compared with the HMRS in both men (HMRS: AUC DC, 0.64; 95% CI, 0.62–0.67; AUC VC, 0.62; 95% CI, 0.60–0.65; P value for improvement in AUC <0.0001) and women (HMRS: AUC DC, 0.66; 95% CI, 0.62–0.69; AUC VC, 0.65; 95% CI, 0.60–0.70; P value for improvement in AUC <0.01). Similarly, IMACS‐RS provided significant improvement in continuous Net Reclassification Index and Integrated Discrimination Improvement index over HMRS in both men (P<0.0001 in both DC and VC) and women (P<0.0001 in both DC and VC) (Table 3). The IMACS‐RS provided a similar significant improvement over MELD in both men and women in the DC and VC (P<0.0001 for all comparisons) (Table S2). Receiver operating characteristic curves demonstrating improvement in risk discrimination over HMRS and MELD are depicted in Figure S3.
Risk Calibration
The Hosmer–Lemeshow goodness‐of‐fit test chi‐square was 6.75 (P=0.56) in men and 2.78 (P=0.95) in women, suggesting no significant difference between observed and predicted risk and overall excellent calibration. For each quartile increase in IMACS‐RS, men had 86% increased odds (OR, 1.86; 95% CI, 1.74–2.00; P<0.0001), and women had 93% increased odds (OR, 1.93; 95% CI, 1.47–2.59; P<0.0001) of 3‐month mortality. Calibration charts by quartile for men and women are shown in Figures S4 and S5.
Conclusions
In this study, we used the largest contemporary multinational registry of continuous‐flow LVAD implants to construct and validate a sex‐specific risk score of 3‐month postoperative mortality in LVAD recipients. The IMACS‐RS includes 2 variables with sex‐specific effects—ischemic HF etiology and platelet count—since this resulted in a substantially better model fit. The IMACS‐RS has significantly better risk discrimination than the HMRS and MELD and demonstrates excellent risk calibration with no significant difference between observed and predicted risk in both sexes. This study is novel and adds to the existing literature because it is the first to create a sex‐specific risk score for prognostication in the advanced HF population.
Striking sex differences exist across the spectrum of HF. 30 , 31 After the age of 65, HF incidence triples in women but only doubles in men. 31 Women are more likely to present with HF with preserved ejection fraction from diabetes mellitus and hypertension, while men are more likely to present with reduced ejection fraction in the setting of ischemic heart disease. Certain nonischemic cardiomyopathy etiologies such as peripartum cardiomyopathy and dilated cardiomyopathy from adjuvant breast cancer chemotherapy are unique to women. 30 Signs and symptoms associated with HF differ between sexes, with women more likely to present with nonspecific tiredness and fatigue. 30 Although no sex‐specific cutoffs exist, baseline levels of HF biomarkers, such as natriuretic peptides and cardiac troponins, are different in women and men. 32 Once diagnosed, women are less likely to be prescribed guideline‐directed devices and medical therapy 33 or be referred for advanced HF therapies. 34 Psychosocial and socioeconomic determinants of cardiovascular heath have a greater impact on women than men. 35 , 36 Although there have been several calls to action for sex‐disaggregated research on prognostication in HF, 5 , 30 a paucity of data of exists. Neither the Seattle Heart Failure Model 6 nor the Meta‐Analysis Global Group in Chronic Heart Failure score 7 incorporate sex‐specific interaction effects. Vishram‐Nielsen and colleagues studied the sex‐specific performance of these widely used prognostic scores to show that they markedly overestimated 3‐year mortality in women. 37
The lack of sex‐specific prognostic data is even more pronounced in the field of mechanical circulatory support, compounded by the underrepresentation of women in all seminal LVAD clinical trials. 9 , 10 , 11 , 12 Although conflicting data exist on sex differences in long‐term outcomes after LVAD, the 8th annual INTERMACS report 13 and our prior analysis of the IMACS registry demonstrate a higher postoperative 3‐month mortality risk in women. 14 Currently used risk scores of post‐LVAD mortality including the HMRS, 15 MELD score, 17 and the Destination Therapy Risk Score 38 were derived and validated in predominantly male cohorts, and were therefore inadequately powered to detect sex‐specific correlates of risk 16 (Table S3). Additionally, these risk scores have consistently performed modestly in external validation studies. 16 Accurate risk estimation that accounts for the higher residual risk of postoperative mortality in women is important for patient selection, as well as shared decision making before implantation. 16
In this study, we have developed and validated a sex‐specific mortality risk score, with excellent risk calibration in both women and men. Of 21 candidate predictors, 13 were retained in the IMACS‐RS (2 with sex‐specific effects; Table 2). Of these, age and albumin have been previously associated with 90‐day mortality in the HMRS. 15 Additionally, the MELD score incorporates bilirubin, 17 and binary cutoffs for low platelet count, albumin, hematocrit, and high blood urea nitrogen are incorporated into the Destination Therapy Risk Score. 38 INTERMACS profile is associated with both short and long‐term survival after LVAD. 39 In addition to these aforementioned predictors, the IMACS‐RS incorporates body mass index, dialysis and a major infection before implantation, LVEDD and moderate to severe TR. The 8th annual INTERMACS report has previously identified body mass index and preimplant dialysis as important predictors of higher postoperative 3‐month mortality. 13 A major infection during the preimplant LVAD hospitalization could conceivably be associated with worse postoperative outcomes attributable to the associated systemic inflammatory response. 40 , 41 We have previously demonstrated that a smaller LV and more TR mediates >20% of the increased hazard of early mortality in females after LVAD implantation. 14 In addition to making implant surgery more technically challenging, LV–LVAD size mismatch increases the risk of “suction” events by shifting the interventricular septum to the left, worsening right ventricular failure and further diminishing LV cavity size. 42 The presence of moderate to severe TR before implantation also portends worse survival after LVAD implantation, likely attributable to more severe right ventricular dysfunction. 43 Our study confirms the collective findings of these reports and for the first time incorporates these covariates as independent risk factors in a composite risk score for post‐LVAD mortality.
An interesting and novel finding in our study is the demonstration of sex‐specific prognostic effects for platelet count and HF etiology; with a lower platelet count and ICM conferring increased risk only in women. Prior studies have demonstrated that lower preimplant platelet counts are associated with worse outcomes after LVAD, including prolonged mechanical ventilation, 44 early bleeding events, 45 and intensive care unit mortality 46 ; however, none have demonstrated a sex‐specific effect. This finding is particularly important in the setting of the acquired von Willebrand syndrome and alterations in platelet function that are known to occur after LVAD implantation, 47 and the higher major bleeding risk for women after LVAD implantation. 48 , 49 Platelet counts, aggregation, reactivity, and response to antiplatelet therapy are sex dependent. 50 It is possible that sex‐specific antiplatelet regimens are necessary to optimally balance the risk of hemorrhagic with thrombotic events in LVAD recipients. There is a paucity of data on the impact of HF etiology on post‐LVAD outcomes. A recent study of 3511 patients from the Nationwide Inpatient Sample database found that while patients with ICM did not have increased mortality compared with their nonischemic cardiomyopathy counterparts, they had higher vascular complications requiring surgery, hemorrhage, and postoperative myocardial infarction. 51 However, this study examined only in‐hospital mortality, included ≈23% women, and did not report sex‐specific interactions with mortality. Although women are less likely to be diagnosed with ischemic heart disease, they are more likely to die once diagnosed. 52 To our knowledge, this is the first report to describe sex‐specific effects of HF etiology and platelet count in LVAD recipients.
Limitations
Even though we used a multinational “real‐world” registry cohort for the construction of IMACS‐RS, participation in the IMACS database is voluntary, and whether the data are truly representative of all LVAD implanting sites is unknown. Therefore, the IMACS‐RS should be externally validated at the level of a single center. IMACS relies on accurate data entry by participating hospitals, and other variables that might influence outcomes, such as country, implant center, and race/ethnicity, are not available in the IMACS registry. The IMACS registry does not include HeartMate 3 LVAD recipients. Therefore, performance of the IMACS‐RS in patients undergoing HeartMate 3 LVAD implantation is unknown. In addition, although testing for interaction effects with sex allowed identification of unique risk factors for mortality in women, the same issue of underrepresentation of women persists with the IMACS registry, reflective of established data that demonstrate lower LVAD implantation in women. 53
In conclusion, we used the multinational IMACS registry to create and validate a novel sex‐specific mortality risk score for prognostication in patients being evaluated for LVAD placement. An online IMACS‐RS calculator was developed for easy application (http://www.eccri.emory.edu/lvad‐risk/index.html). We report good risk discrimination and calibration in both men and women, driven by inclusion of sex‐specific interaction terms. A similar approach is necessary to bridge sex disparities across the HF spectrum.
Sources of Funding
Dr Morris is supported by funding from the National Institutes of Health/National Heart, Lung, and Blood Institute (K23 HL124287, R03 HL146874) and the Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program). Dr Mehta is supported by American Heart Association grant 19POST34400057 and the Abraham J. & Phyllis Katz Foundation. J. Pennington is supported by the Society of Thoracic Surgeons and World Society for Pediatric and Congenital Heart Surgery. Dr Simon is supported by NIH grants 1R01AG058659, 2P01HL103455, and UL1 TR001857.
Disclosures
Dr. Cowger is a speaker for Abbott and Medtronic, consultant for Medtronic, and a member of the IMACS steering committee. Dr Kirklin is the director of the Data Coordinating Center for Society of Thoracic Surgeons INTERMACS. Dr Simon reports research support from Novartis, Aadi, and consultancy fees from Complexa, Actelion, and United Therapeutics. Dr Kormos is an Abbott employee. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S5
Acknowledgments
Support for this analysis was provided by the ISHLT in the form of data provided by the IMACS Registry. The interpretation and reporting of these data are the responsibility of the author(s). These analyses are based on IMACS Registry data as of October 31, 2017.
(J Am Heart Assoc. 2021;10:e020019. DOI: 10.1161/JAHA.120.020019.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020019
This manuscript was sent to John S. Ikonomidis, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, De Ferranti S, Després J‐P, Fullerton HJ, Howard VJ, et al. Executive summary: heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:434–441. DOI: 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 2. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP; ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. DOI: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3. Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, et al. Influence of a performance‐improvement initiative on quality of care for patients hospitalized with heart failure: results of the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF). Arch Intern Med. 2007;167:1493–1502. DOI: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 4. Cook JL, Grady KL, Colvin M, Joseph SM, Brisco MA, Walsh MN; genVAD Working Group . Sex differences in the care of patients with advanced heart failure. Circ Cardiovasc Qual Outcomes. 2015;8:S56–S59. DOI: 10.1161/CIRCOUTCOMES.115.001730. [DOI] [PubMed] [Google Scholar]
- 5. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J. 2019;40:3859–3868c. DOI: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 6. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. DOI: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 7. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. DOI: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 8. McIlvennan CK, Lindenfeld J, Kao DP. Sex differences and in‐hospital outcomes in patients undergoing mechanical circulatory support implantation. J Heart Lung Transplant. 2017;36:82–90. DOI: 10.1016/j.healun.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, et al. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001;345:1435–1443. DOI: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 10. Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, Howard Frazier O, Icenogle T, Najjar SS, Boyce SW, et al. Heartware ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–683. DOI: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM III, Long JW, et al. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. DOI: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 12. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, et al. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med. 2019;380:1618–1627. DOI: 10.1056/NEJMoa1900486. [DOI] [PubMed] [Google Scholar]
- 13. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. DOI: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 14. Nayak A, Hu Y, Ko YA, Mehta A, Liu C, Pennington J, Xie R, Cowger J, Kirklin JK, Kormos RL, et al. Gender differences in mortality after left ventricular assist device implant: a causal mediation analysis approach. ASAIO J. 2020. [epub ahead of print]. DOI: 10.1097/MAT.0000000000001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–321. DOI: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 16. Ravichandran AK, Cowger J. Left ventricular assist device patient selection: do risk scores help? J Thorac Dis. 2015;7:2080. DOI: 10.3978/j.issn.2072-1439.2015.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matthews JC, Pagani FD, Haft JW, Koelling TM, Naftel DC, Aaronson KD. Model for end‐stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation. 2010;121:214–220. DOI: 10.1161/CIRCULATIONAHA.108.838656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirklin JK, Mehra MR. The dawn of the ISHLT Mechanical Assisted Circulatory Support (IMACS) Registry: fulfilling our mission. J Heart Lung Transplant. 2012;31:115–116. DOI: 10.1016/j.healun.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Z. Missing data imputation: focusing on single imputation. Ann Transl Med. 2016;4:9.DOI: 10.3978/j.issn.2305-5839.2015.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. INTERMACS Executive Committee . Intermacs adverse event definitions: adult and pediatric patients. INTERMACS. Date published: May 30, 2014. Available at: http://www.uab.edu/medicine/intermacs/appendices‐4‐0. Accessed June 30, 2020.
- 21. Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33:261–304. DOI: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 22. Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc. 1992;87:178–183. DOI: 10.1080/01621459.1992.10475190. [DOI] [Google Scholar]
- 23. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, McGinn T, Guyatt G. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA. 2017;318:1377–1384. DOI: 10.1001/jama.2017.12126. [DOI] [PubMed] [Google Scholar]
- 24. Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25:114–121. DOI: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. DOI: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207–112. DOI: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 27. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. DOI: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 28. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33:464–470. DOI: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 29. Agresti A, Kateri M. Categorical Data Analysis. Hoboken, NJ: John Wiley & Sons. Inc. 2014. [Google Scholar]
- 30. Maas AH, van der Schouw YT, Regitz‐Zagrosek V, Swahn E, Appelman YE, Pasterkamp G, Ten Cate H, Nilsson PM, Huisman MV, Stam HC, et al. Red alert for women's heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J. 2011;32:1362–1368. DOI: 10.1093/eurheartj/ehr048. [DOI] [PubMed] [Google Scholar]
- 31. Motiejūnaitė J, Akiyama E, Cohen‐Solal A, Maggioni AP, Mueller C, Choi D‐J, Kavoliūnienė A, Čelutkienė J, Parenica J, Lassus J, et al. The association of long‐term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur Heart J. 2020;41:1357–1364. DOI: 10.1093/eurheartj/ehaa071. [DOI] [PubMed] [Google Scholar]
- 32. Suthahar N, Meems LMG, Ho JE, de Boer RA. Sex‐related differences in contemporary biomarkers for heart failure: a review. Eur J Heart Fail. 2020;22:775–788. DOI: 10.1002/ejhf.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsich EM. Sex differences in advanced heart failure therapies. Circulation. 2019;139:1080–1093. DOI: 10.1161/CIRCULATIONAHA.118.037369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steinberg RS, Nayak A, O'Connell C, Burford S, Pekarek A, Chesnut N, Cole RT, Gupta D, Laskar SR, Bhatt K, et al. Sex differences in eligibility for advanced heart failure therapies. Clin Transplant. 2020;34:e13839. DOI: 10.1111/ctr.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw LJ, Pepine CJ, Xie J, Mehta PK, Morris AA, Dickert NW, Ferdinand KC, Gulati M, Reynolds H, Hayes SN, et al. Quality and equitable health care gaps for women: attributions to sex differences in cardiovascular medicine. J Am Coll Cardiol. 2017;70:373–388. DOI: 10.1016/j.jacc.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 36. Williams SA, Kasl SV, Heiat A, Abramson JL, Krumholz HM, Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community‐based study. Psychosom Med. 2002;64:6–12. DOI: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 37. Vishram‐Nielsen JKK, Foroutan F, Ross HJ, Gustafsson F, Alba AC. Performance of prognostic risk scores in heart failure patients: do sex differences exist? Can J Cardiol. 2020;36:45–53. DOI: 10.1016/j.cjca.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 38. Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post‐rematch era: implications for patient selection. Circulation. 2007;116:497–505. DOI: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 39. Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, Gelijns AC, Hong KN, Teuteberg JJ. Clinical outcomes for continuous‐flow left ventricular assist device patients stratified by pre‐operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–407. DOI: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 40. Caruso R, Botta L, Verde A, Milazzo F, Vecchi I, Trivella MG, Martinelli L, Paino R, Frigerio M, Parodi O. Relationship between pre‐implant interleukin‐6 levels, inflammatory response, and early outcome in patients supported by left ventricular assist device: a prospective study. PLoS One. 2014;9:e90802. DOI: 10.1371/journal.pone.0090802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang PC, Haft JW, Romano MA, Bitar A, Hasan R, Palardy M, Aaronson KD, Pagani FD. Right ventricular failure following left ventricular assist device implantation is associated with a preoperative pro‐inflammatory response. J Cardiothorac Surg. 2019;14:80. DOI: 10.1186/s13019-019-0895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Topilsky Y, Oh JK, Shah DK, Boilson BA, Schirger JA, Kushwaha SS, Pereira NL, Park SJ. Echocardiographic predictors of adverse outcomes after continuous left ventricular assist device implantation. JACC Cardiovasc Imaging. 2011;4:211–222. DOI: 10.1016/j.jcmg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 43. Song HK, Gelow JM, Mudd J, Chien C, Tibayan FA, Hollifield K, Naftel D, Kirklin J. Limited utility of tricuspid valve repair at the time of left ventricular assist device implantation. Ann Thorac Surg. 2016;101:2168–2174. DOI: 10.1016/j.athoracsur.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Papathanasiou M, Mincu RI, Lortz J, Horacek M, Koch A, Pizanis N, Kamler M, Rassaf T, Luedike P. Prolonged mechanical ventilation after left ventricular assist device implantation: risk factors and clinical implications. ESC Heart Fail. 2019;6:545–551. DOI: 10.1002/ehf2.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muslem R, Caliskan K, van Thiel R, Kashif U, Akin S, Birim O, Constantinescu AA, Brugts JJ, Bunge JJH, Bekkers JA, et al. Incidence, predictors and clinical outcome of early bleeding events in patients undergoing a left ventricular assist device implant. Eur J Cardiothorac Surg. 2018;54:176–182. DOI: 10.1093/ejcts/ezy044. [DOI] [PubMed] [Google Scholar]
- 46. Piffard M, Nubret‐Le Coniat K, Simon O, Leuillet S, Remy A, Barandon L, Ouattara A. Independent risk factors for ICU mortality after left ventricular assist device implantation. Artif Organs. 2020;44:153–161. DOI: 10.1111/aor.13540. [DOI] [PubMed] [Google Scholar]
- 47. Gallo M, Trivedi JR, Mondal NK, Birks EJ, Slaughter MS. Management of antiplatelet therapy during continuous‐flow left ventricular assist device support after thrombotic hemorrhagic events. ASAIO J. 2019;65:683–689. DOI: 10.1097/MAT.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 48. Magnussen C, Bernhardt AM, Ojeda FM, Wagner FM, Gummert J, de By TMMH, Krabatsch T, Mohacsi P, Rybczynski M, Knappe D, et al. Gender differences and outcomes in left ventricular assist device support: the European Registry for Patients with Mechanical Circulatory Support. J Heart Lung Transplant. 2018;37:61–70. DOI: 10.1016/j.healun.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 49. Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD; HeartMate II Clinical Investigators . Pre‐operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014;63:880–888. DOI: 10.1016/j.jacc.2013.08.1656. [DOI] [PubMed] [Google Scholar]
- 50. Patti G, De Caterina R, Abbate R, Andreotti F, Biasucci LM, Calabrò P, Cioni G, Davì G, Di Sciascio G, Golia E, et al. Platelet function and long‐term antiplatelet therapy in women: is there a gender‐specificity? A “state‐of‐the‐art” paper. Eur Heart J. 2014;35:2213–2223. DOI: 10.1093/eurheartj/ehu279. [DOI] [PubMed] [Google Scholar]
- 51. Abubakar H, Subahi A, Adegbala O, Yassin AS, Akintoye E, Abdulrahman A, Ahmed A, Alade A, Pahuja M, Afonso L. Comparison of in‐hospital outcomes of patients with‐versus‐without ischemic cardiomyopathy undergoing left ventricular assist device placement. Am J Cardiol. 2019;123:414–418. DOI: 10.1016/j.amjcard.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 52. McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, Fletcher GF, Gulati M, Mehta LS, Pettey C, et al. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation. 2016;133:1302–1331. DOI: 10.1161/CIR.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. DeFilippis EM, Truby LK, Garan AR, Givens RC, Takeda K, Takayama H, Naka Y, Haythe JH, Farr MA, Topkara VK. Sex‐related differences in use and outcomes of left ventricular assist devices as bridge to transplantation. JACC Heart Fail. 2019;7:250–257. DOI: 10.1016/j.jchf.2019.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S5


