Abstract
Mitral annular calcification with mitral valve disease is a challenging problem that could necessitate surgical mitral valve replacement (SMVR). Transcatheter mitral valve replacement (TMVR) is emerging as a feasible alternative in high‐risk patients with appropriate anatomy. PubMed, Embase, and Cochrane Central Register of Controlled Trials were searched from inception to December 25, 2019 for studies discussing SMVR or TMVR in patients with mitral annular calcification; 27 of 1539 articles were selected for final review. TMVR was used in 15 studies. Relevant data were available on 82 patients who underwent hybrid transatrial TMVR, and 354 patients who underwent transapical or transseptal TMVR. Outcomes on SMVR were generally reported as small case series (447 patients from 11 studies); however, 1 large study recently reported outcomes in 9551 patients. Patients who underwent TMVR had a shorter median follow‐up of 9 to 12 months (range, in‐hospital‒19 months) compared with patients with SMVR (54 months; range, in‐hospital‒120 months). Overall, those undergoing TMVR were older and had higher Society of Thoracic Surgeons risk scores. SMVR showed a wide range of early (0%–27%; median 6.3%) and long‐term mortality (0%–65%; median at 1 year, 15.8%; 5 years, 38.8%, 10 years, 62.4%). The median in‐hospital, 30‐day, and 1‐year mortality rates were 16.7%, 22.7%, and 43%, respectively, for transseptal/transapical TMVR, and 9.5%, 20.0%, and 40%, respectively, for transatrial TMVR. Mitral annular calcification is a complex disease and TMVR, with a versatile option of transatrial approach in patients with challenging anatomy, offers a promising alternative to SMVR in high‐risk patients. However, further studies are needed to improve technology, patient selection, operative expertise, and long‐term outcomes.
Keywords: mitral annulus calcification, mitral valve, mitral valve replacement, transcatheter
Subject Categories: Cardiovascular Surgery, Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- CTA
computed tomography angiography
- LVOT
left ventricular outflow tract
- LVOTO
left ventricular outflow tract obstruction
- MAC
mitral annular calcification
- MITRAL
Mitral Implantation of Transcatheter Valves
- PVL
paravalvular leak
- SMVR
surgical mitral valve replacement
- TMVR
transcatheter mitral valve replacement
Mitral annular calcification (MAC) is a degenerative process of the fibrous annulus of the mitral valve. 1 It is often an incidental finding, asymptomatic, and under‐reported. Prevalence ranges from 8% to 15%, with a higher incidence among patients with advanced age, atherosclerosis, chronic kidney disease, hypertension, and valvular heart disease. 2 , 3 , 4 , 5 , 6 , 7 , 8 Some studies have reported its prevalence to be as high as 40% in people aged >65 years. 9 MAC has been associated with systemic atherosclerotic disease 6 and is an independent predictor of poor outcomes. 4 In addition, extensive calcification of the mitral annulus can cause mitral stenosis and/or mitral regurgitation. 10 MAC has also been associated with an increased risk of stroke, myocardial infarction, arrhythmias, heart failure, and perioperative complications. 3 , 22 Not only are patients with MAC at high surgical risk given their comorbidities, MAC makes mitral valve surgery technically challenging. 23 In a case series by Feindel et al, the presence of MAC led to a 6‐fold increase in the operative mortality of patients undergoing isolated mitral valve surgery. 23 Others have reported early mortality with surgical mitral valve replacement (SMVR) in MAC to be as high as 28%. 24 , 25 In such high‐risk patients, transcatheter mitral valve replacement (TMVR) may provide a less invasive option. 26 , 27 Initial reports have been encouraging, and TMVR has emerged as a reasonable alternative, especially in patients at prohibitive surgical risk. 28 , 29 , 30 We sought to compile a list of surgical and transcatheter approaches to mitral valve replacement in patients with MAC and mitral valve disease and to critically assess the outcomes of such in a systematic fashion.
Methods
A database search was performed in PubMed, Embase, and Cochrane Central Register of Controlled Trials without any limitations to identify all studies up to December 25, 2019. The keywords "mitral annular calcification", "calcific mitral valve", "calcific mitral stenosis", and "calcific mitral annulus" were indexed in all combinations for original reports and clinical studies, including cross‐sectional studies, observational studies, clinical trial studies, and reviews. These reports were evaluated against a priori inclusion and exclusion criteria for eligibility. Throughout the design and implementation process, we followed the Cochrane methodology and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Studies were included if (1) patients with MAC were evaluated, (2) they underwent mitral valve replacement (either surgical or transcatheter), (3) the relevant design constituted case studies, registry reports, or prospective or retrospective analyses, and (4) outcomes such as in‐hospital, 30‐day, 1‐year, or long‐term all‐cause mortality were reported. Studies were excluded if (1) they reported results not pertinent to patients with MAC, (2) they were case reports, and (3) the procedure being assessed was mitral valve repair rather than replacement. If >1 study was published from a center with the same study population, only the study fulfilling all inclusion criteria with the largest number of patients was included in the analysis. Weighting by study size was not performed.
The primary end point was short‐term (in‐hospital and 30‐day) and long‐term mortality for SMVR and TMVR. Median of the study‐specific mortality rates were calculated. If available, the following secondary end points were included: procedural or technical success, and rates of complications such as major bleeding, left ventricular outflow tract obstruction (LVOTO), and paravalvular leak (PVL). Two independent investigators (A.M., S.A.) extracted all data, and any disagreements were resolved by mutual discussion. Details of the studies can be found in the Supplemental Material.
Results
Our search identified 1539 articles, of which 963 remained after de‐duplication. A detailed title and abstract screen to identify and select full‐text articles for complete review was then performed (Figure 1). After full‐text review, we determined 12 articles of SMVR had data relevant to patients with MAC. 24 , 40 Similarly, 15 studies were found to report outcomes of TMVR, including the hybrid transatrial approach for MAC. 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 One‐year data from the MITRAL (Mitral Implantation of Transcatheter Valves) trial were also added. 56 In total, 6 studies reported data for the transatrial approach. 42 , 45 , 46 , 47 , 55 , 56
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram for study selection.

MAC indicates mitral annular calcification; MVR, mitral valve replacement; PVL, paravalvular leak; SMVR, surgical mitral valve replacement; and TMVR, transcatheter mitral valve replacement.
Surgical Mitral Valve Replacement
Table S1 summarizes the characteristics of all the studies that have reported outcomes of SMVR in patients with MAC. Eleven studies evaluated a total of 447 patients, and 1 study contributed 9551 patients. Studies reporting SMVR outcomes generally provided fewer details on risk profiles. All reports were retrospective observational studies with a median follow‐up of 3.5 years (range, in‐hospital‒10 years). There were differences between in‐hospital and 30‐day mortality rates among the studies that reported 30‐day outcomes. Early mortality, defined as in‐hospital up to 30‐day mortality, ranged from 0% to 27.3% (median 6.3%), whereas long‐term mortality rates at 1, 5, and 10 years were 15.8% (range, 0%–17%), 38.8% (range, 0%–68.6%), and 62.4% (range, 60%–64.8%), respectively (Figure 2). 24 , 40
Figure 2. Mortality with surgical mitral valve replacement in patients with mitral annular calcification.

Transseptal and Transapical Transcatheter Mitral Valve Replacement
The baseline characteristics and outcomes of patients with MAC from 13 studies of transseptal and transapical TMVR are shown in Tables S2 and S3. Data on a total of 354 patients with MAC were available, with the most extensive studies reporting outcomes on 100 and 116 patients from the STS/ACC/TVT (Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy) Registry and the TMVR in MAC Global Registry, respectively. 42 , 54
All included studies were observational with outcome data collected retrospectively, except for the MITRAL trial, which was prospective. 56 Almost half of the studies were short case series of ≤12 patients. The patient population that underwent TMVR for MAC was older compared with patients with SMVR (median age, 75 versus 72 years); 68% were female, and the mean Society of Thoracic Surgeons‐Predicted Risk of Mortality score was 12%. These patients were at a significantly higher surgical risk, with a median of 92% of patients suffering from heart failure with New York Heart Association Class ≥III symptoms.
Currently, only short‐ and medium‐term follow‐up are available after TMVR with a median of 9 months (range, in‐hospital‒12 months). Two studies reported separate outcomes for the hybrid transatrial approach and transseptal/transapical approach. 42 , 56 Four studies reported combined outcomes of all strategies. These studies were primarily based on transseptal and transapical approaches, with the transatrial method constituting only 14 cases. 44 , 48 , 52 , 55 Results of the hybrid transatrial approach are described below.
Most patients underwent Sapien valve (Sapien/XT/3) (Edwards Lifesciences LLC, Irvine, CA) implantation via a transseptal approach. Median technical success for non‐transatrial TMVR was 75%, and the risk of left ventricular outflow tract obstruction (LVOTO) was 11.2%. The median incidence of at least moderate post‐procedural mitral regurgitation was 4.1%. The median risk of device embolism was 3.7%; 16.7% of patients required reintervention, and 5.2% suffered from significant major in‐hospital bleeding. Overall, the median in‐hospital, 30‐day, and 1‐year mortality rates for non‐transatrial TMVR in MAC were 16.7%, 22.7%, and 43%, respectively (Figure 3). 41 , 42 , 43 , 44 , 54 , 56
Figure 3. Mortality with transcatheter mitral valve replacement (transseptal and transapical) in patients with mitral annular calcification.
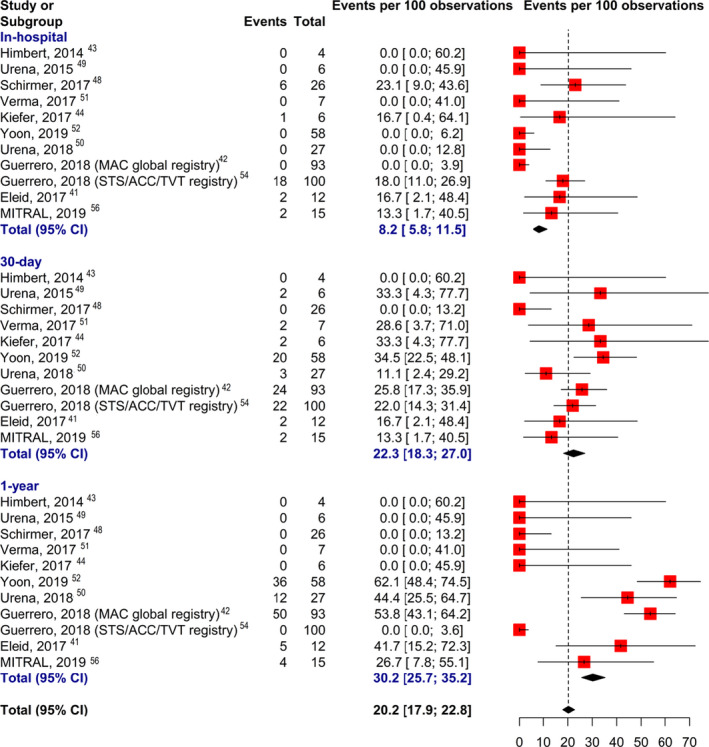
MAC indicates mitral annular calcification; and STS/ACC/TVT, Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy.
Hybrid Transatrial Transcatheter Mitral Valve Replacement
Eight‐two patients from 6 studies underwent transatrial TMVR, which is a hybrid strategy that involves cardiopulmonary bypass and direct implantation of a transcatheter valve within the calcified mitral annulus (Table S4). The mean Society of Thoracic Surgeons‐Predicted Risk of Mortality score was lower than that of the patients who underwent transseptal or transapical TMVR. 45 The only prospective trial (MITRAL) showed the overall median in‐hospital, 30‐day, and 1‐year mortality for transatrial TMVR in MAC to be 9.5%, 20.0%, and 40%, respectively, with an LVOTO rate of 6.7% and 80% technical success. Only 1.9% of patients required reintervention (Figure 4). 42 , 45 , 46 , 47 , 55 , 56
Figure 4. Mortality with transcatheter mitral valve replacement (transatrial) in patients with mitral annular calcification.

MAC indicates mitral annular calcification.
Discussion
Key findings of our systematic review are: (1) SMVR, consisting of lower surgical risk patients, can lead to favorable long‐term survival if patients can overcome the initial increased risk of surgical morbidity and mortality; (2) Percutaneous TMVR offers a promising alternative in higher‐risk patients, with a 1‐year survival >50%; (3) Hybrid transatrial TMVR with direct valve implantation may provide the most favorable short‐term procedural success and clinical benefit; and (4) technological and procedural improvements are necessary to reduce the mortality and morbidity in this challenging patient population.
Conventional SMVR remains the preferred intervention for symptomatic patients with MAC with acceptable surgical risk. 57 The calcification of the mitral annulus severely hampers suture anchoring of a prosthetic valve during replacement, resulting in an increased risk of PVL, injury to the left circumflex artery, and atrioventricular groove dissociation. To address this, 2 contrasting surgical approaches of “respect” or “resect” have been used. 58 The “respect” technique allows the implantation of the prosthetic valve on top of the calcium bar without removing it but can result in poor sealing and significant PVL. On the other hand, the “resect” technique, which would allow for a larger prosthesis and a better sealing with reduced PVL, risks weakening the mitral annulus and atrioventricular groove, leading to a potentially fatal disruption and high operative mortality. 59 Moreover, the “resect” technique requires advanced technical expertise and longer cross‐clamp and cardiopulmonary bypass times. 23 , 37 , 58 The study by Kaneko et al provided extensive data from 9551 patients undergoing SMVR, with estimated higher inpatient mortality of 5.8% among patients with MAC compared with patients without MAC (4.4%). 39 However, the analysis was based on the Society of Thoracic Surgeons Adult Cardiac Surgery Database that is prone to coding inadequacies and incompleteness, including a lack of information on the severity of MAC.
Following initial success, TMVR has expanded the treatment options for MAC, especially in patients with higher surgical risk. 26 , 27 , 30 , 60 , 61 These patients are complex given their advanced age, multiple comorbidities, and hostile cardiac anatomy. Currently, the majority of TMVR in MAC is performed with the Sapien 3 valve, designed originally for transcatheter aortic valve replacement. Because the mitral annulus is D‐shaped and the Sapien 3 valve is a balloon‐expandable valve, sufficient MAC is a prerequisite to anchor the valve securely; circularization of the mitral annulus is also necessary to avoid PVL. 62 Percutaneous TMVR has been associated with a higher risk of LVOTO, embolization, and perforation. However, intraprocedural complications have lessened with better patient selection and experience. 42 Unlike in SMVR, the anterior leaflet cannot be resected in percutaneous TMVR, thus conferring higher risk of LVOTO. 63 This risk can be alleviated by the techniques described below. Other complications such as left ventricular perforation and valve migration and embolization have been reported because of insufficient sizing, inability to predict anchoring, and technical errors. 10 , 63
In our review, the median incidence of LVOTO was 13.4%, similar to the 9.7% reported in the latest prospective trial (MITRAL). In the earlier registry by Yoon et al, it was reported to be as high as 40%. 52 More recently, the same group evaluated the predictors of LVOTO and found an LVOTO incidence of 54%. 64 This highlights the importance of comprehensive preoperative evaluation, including the use of multidetector computed tomography and transesophageal echocardiography to determine the risk of LVOTO and to evaluate the location and severity of MAC. Known predictors of LVOTO include septal hypertrophy, anterior mitral leaflet length, and aortomitral angle. 65 In addition to preoperative multidetector computed tomography, imaging software analysis and 3‐dimensional printing can be used to predict the risk of LVOTO. 10 , 64 , 66 , 67 Wang et al showed 100% sensitivity and 96.8% specificity for predicting LVOTO by using post‐processing tools and software to virtually overlay the transcatheter valve in the mitral position. 68
When anticipated, strategies to prevent LVOTO include preemptive alcohol septal ablation 69 , 70 or intraoperative resection of the anterior mitral leaflet +/− septal myectomy during transatrial implantation, and the LAMPOON (Laceration of the Anterior Mitral Leaflet to Prevent Left Ventricular Outflow Tract Obstruction) study technique as an adjunct to percutaneous TMVR. 71 Alcohol septal ablation can be used as a bailout during TMVR; however, it is preferable to perform at least 4 to 6 weeks before TMVR, thus allowing adequate septal thinning and remodeling for sufficient left ventricular outflow tract clearance. 72 Balloon‐expandable valves have been deployed via a percutaneous transseptal approach, whereas both balloon‐expandable and mechanically expanding valves have been deployed via a transapical approach. 73 , 74 Accurate sizing of these valves is vital as excessive oversizing may rupture the annulus or cause LVOTO. Undersizing may lead to significant PVL or valve embolization. With an improved patient selection, especially identifying patients who are at risk for LVOTO, the transseptal approach has shown improvement in procedural and early mortality; 54 7 of 15 patients from the prospective MITRAL trial underwent preemptive alcohol septal ablation based on the preoperative assessment that deemed them a higher risk of LVOTO. All patients were alive at 30‐day follow‐up, suggesting that preemptive alcohol septal ablation may be a viable strategy to reduce LVOTO risk in patients with appropriate anatomy. 53 Recently, the LAMPOON trial also showed promise with 93% 30‐day survival in 30 patients, although only 15 patients had MAC and 30‐day survival was 87% in those patients. 75 With growing experience, the 30‐day mortality rate of percutaneous TMVR has improved from 25% to 16.7%. 42 , 53 The TMVR in MAC Global Registry showed a much lower 30‐day mortality and conversion to open surgery rates in the second half of treated patients (31%–19% and 7.6%–0%, respectively). 76 This improvement in outcomes is indicative of a steep learning curve associated with this procedure.
TMVR in MAC with a dedicated transcatheter mitral valve is currently undergoing early feasibility and pilot studies. The Tendyne (Abbott Structural Heart, Santa Clara, CA) (NCT NCT03539458) TMVR device deployed transapically is presently being evaluated in patients with MAC with promising early results. 77 However, the screen failure rates for both Sapien 3 and dedicated TMVR devices because of unfavorable anatomy were at least 40%, because of the risk of LVOTO, non‐concentric calcium, inadequate calcium to anchor, interference with the TMVR device, and high‐risk features predisposing to PVL or embolization. Continuing technological and procedural advancements are necessary to improve both short‐ and longer‐term outcomes of these patients.
Among patients with severe MAC who are surgical candidates, an emerging option is direct TMVR via a hybrid transatrial approach. There are several benefits of the transatrial approach: (1) It allows for anterior leaflet resection or septal myectomy to avoid LVOTO. 47 (2) Commissural plication can be performed to better circularize the annulus to reduce the risk of PVL. (3) To improve sealing against the calcium bar and reduce the risk of PVL, ≥1 layers of Teflon felt stripe can be sewn circumferentially around the Sapien 3 valve frame. (4) Operators can place sutures directly at non‐calcified segments of the annulus, at the remnant of the anterior mitral leaflet, and at the left atrial wall, thus reducing the risk of device migration or embolization. This approach is currently being assessed in the prospective single‐arm SITRAL (Surgical Implantation of Transcatheter Valve in Native Mitral Annular Calcification, NCT 08230204) trial. The transatrial approach has resulted in superior mean technical success (91.8% versus 64.3%) compared with other approaches, with a lower risk of mean device embolization (0.7% versus 4.2%) and reintervention (3.6% versus 13.3%). However, since it involves surgery, it is associated with a higher risk of major bleeding (11.3% versus 4.7%) and higher 1‐year mortality of up to 40% in the MITRAL trial. Post‐procedural moderate mitral regurgitation has generally been lower with this approach (3% versus 6.8%), except for the study reported by El Sabbagh et al which was early on at a single center that has improved with experience. 55 Overall, with evolving trends, we can hope to see improved outcomes with innovative devices and standardized treatment algorithms. 78 , 79
Limitations
It must be noted that selection bias is inherent in this review, given the different anatomic inclusions for TMVR versus SMVR and the transatrial approach. Direct comparisons between procedures should be interpreted cautiously, as many patients who undergo TMVR are not surgical candidates, thus negating the validity of the comparative analysis. Furthermore, the long‐term safety and efficacy of TMVR in patients with MAC is uncertain and should be further evaluated.
Overall, the TMVR studies had limited, older study populations; were retrospective (with the exception of the MITRAL study); included higher risk patients; and had shorter follow‐up. On the other hand, the SMVR studies had limited details on risk factors and involved a combination of operative techniques. Di Stefano et al 32 avoided calcium debridement while Vander Salm 40 championed ultrasonic resection and Mihaljevic and Uchimuro promoted debridement. 34 , 37 Others, such as Nataf et al, had caveats; they removed only leaflets with extensive MAC. 38 The rest used a combination of “respect” and “resect” techniques depending on calcium burden.
Conclusions
Future directions for MAC management in mitral valve disease include optimizing patient selection, performing predictive modeling through multimodality imaging, improving device designs, and standardizing treatment strategies using an established algorithm (Figure 5). 79 Currently both conventional and transcatheter approaches are considered (Figure 6). SMVR is preferred in surgical candidates with favorable anatomy. TMVR can be considered in patients with high or prohibitive surgical risk and appropriate anatomy. In surgical candidates with severe MAC, the transatrial approach combines the advantages of versatility in conventional SMVR and simplicity in percutaneous TMVR and may be a superior alternative to either treatment strategy.
Figure 5. The Mount Sinai management algorithm of patients with mitral annular calcification (reprinted with permission).
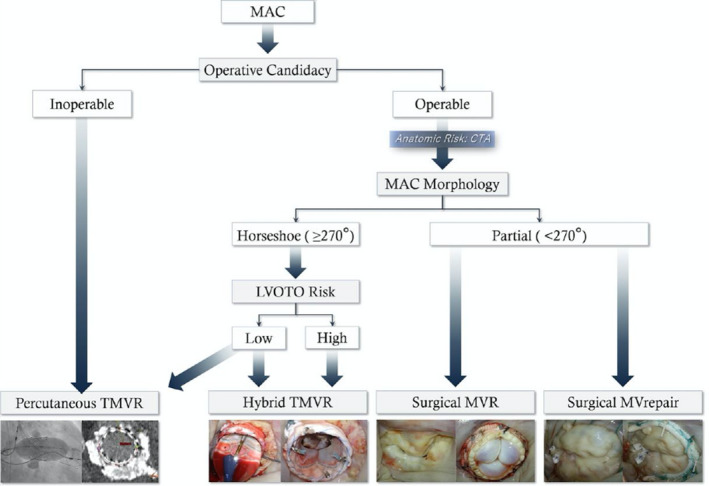
Current management algorithm at Mount Sinai of patients with mitral valve disease and mitral annular calcification. CTA, computed tomography angiography; LVOTO, left ventricular outflow tract obstruction; MAC, mitral annular calcification; MV, mitral valve; MVR, mitral valve replacement; TMVR, transcatheter mitral valve replacement. Reproduced with permission from El‐Eshmawi et al 79 ©2020, Wolters Kluwer Health, Inc.
Figure 6. Overview of treatment options in patients with mitral valve disease and mitral annular calcification.
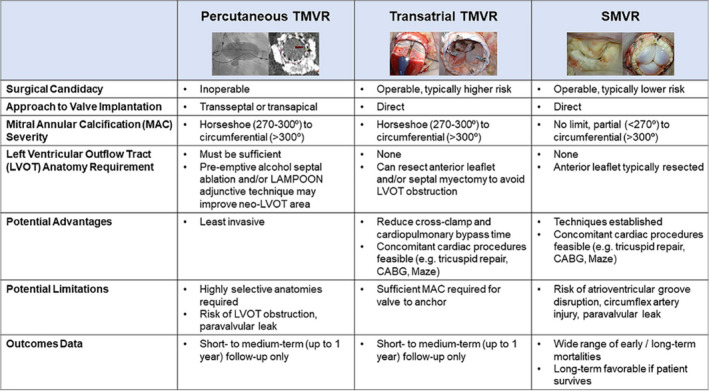
Summary of reviewed treatment options in patients with mitral valve disease and mitral annular calcification. In operable patients, both surgical mitral valve replacement and transatrial transcatheter mitral valve replacement can be considered depending on mitral annular calcification anatomy. In inoperable patients with favorable anatomy, percutaneous transcatheter mitral valve replacement can be considered. CABG indicates coronary artery bypass grafting; LAMPOON indicates Laceration of the Anterior Mitral Leaflet to Prevent Left Ventricular Outflow Tract Obstruction; LVOT, left ventricular outflow tract; MAC, mitral annular calcification; SMVR, surgical mitral valve replacement; and TMVR, transcatheter mitral valve replacement.
Sources of Funding
None.
Disclosures
Dr George has served as a consultant and has received speaker fee from Medtronic and Edwards Lifesciences. Dr Kodali is on the Steering Committee for Edwards Lifesciences, is a consultant for Medtronic and Claret Medical, and is on the scientific advisory board for Thubrikar Aortic Valve Inc. Dr Hahn reports speaker fees from Boston Scientific Corporation, Baylis Medical, Edwards Lifesciences and Medtronic, consulting for Abbott Structural, Edwards Lifesciences, W. L. Gore & Associates, Medtronic, Navigate, and Philips Healthcare, non‐financial support from 3mensio, Equity with Navigate, and is the Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry‐sponsored trials, for which she receives no direct industry compensation. Dr Khalique has served on the Speakers Bureau for Edwards Lifesciences and Boston Scientific and as a reader for a Core Laboratory that has contracts with Edwards Lifesciences. Dr Guerrero has received research grant support from Edwards Lifesciences. Dr Leon has served as a non‐paid member of the Scientific Advisory Board of Edwards Lifesciences and consultant for Abbott Vascular and Boston Scientific. Dr Adams has served as the national co‐principal investigator of the Medtronic APOLLO Pivotal Trial and the Medtronic CoreValve US Pivotal Trial. In addition, the Icahn School of Medicine at Mount Sinai receives royalty payments from Edwards Lifesciences and Medtronic for intellectual property related to the development of valve repair rings. Dr Bapat served as a consultant for Medtronic, Edwards Lifesciences, 4C, and Boston Scientific. Dr Tang has served as a physician proctor for Medtronic and a consultant for Abbott Structural Heart, Medtronic, and W. L. Gore & Associates. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
(J Am Heart Assoc. 2021;10:e018514. DOI: 10.1161/JAHA.120.018514.)
This work was presented at the Annual Virtual Meeting of the American College of Cardiology, November 13 to 14, 2020.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol. 2015;66:1934–1941. DOI: 10.1016/j.jacc.2015.08.872. [DOI] [PubMed] [Google Scholar]
- 2. Movahed MR, Saito Y, Ahmadi‐Kashani M, Ebrahimi R. Mitral annulus calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc Ultrasound. 2007;5:14. DOI: 10.1186/1476-7120-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fox E, Harkins D, Taylor H, McMullan M, Han H, Samdarshi T, Garrison R, Skelton T. Epidemiology of mitral annular calcification and its predictive value for coronary events in African Americans: the Jackson Cohort of the Atherosclerotic Risk in Communities Study. Am Heart J. 2004;148:979–984. DOI: 10.1016/j.ahj.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 4. Fox CS, Vasan RS, Parise H, Levy D, O’Donnell CJ, D’Agostino RB, Benjamin EJ. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. DOI: 10.1161/01.CIR.0000058168.26163.BC. [DOI] [PubMed] [Google Scholar]
- 5. Savage DD, Garrison RJ, Castelli WP, McNamara PM, Anderson SJ, Kannel WB, Feinleib M. Prevalence of submitral (anular) calcium and its correlates in a general population‐based sample (the Framingham Study). Am J Cardiol. 1983;51:1375–1378. DOI: 10.1016/0002-9149(83)90315-6. [DOI] [PubMed] [Google Scholar]
- 6. Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–866. DOI: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 7. Hamirani YS, Nasir K, Blumenthal RS, Takasu J, Shavelle D, Kronmal R, Budoff M. Relation of mitral annular calcium and coronary calcium (from the Multi‐Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2011;107:1291–1294. DOI: 10.1016/j.amjcard.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 8. Potpara TS, Vasiljevic ZM, Vujisic‐Tesic BD, Marinkovic JM, Polovina MM, Stepanovic JM, Stankovic GR, Ostojic MC, Lip GY. Mitral annular calcification predicts cardiovascular morbidity and mortality in middle‐aged patients with atrial fibrillation: the Belgrade Atrial Fibrillation Study. Chest. 2011;140:902–910. DOI: 10.1378/chest.10-2963. [DOI] [PubMed] [Google Scholar]
- 9. Barasch E, Gottdiener JS, Larsen EK, Chaves PH, Newman AB, Manolio TA. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. the Cardiovascular Health Study (CHS). Am Heart J. 2006;151:39–47. DOI: 10.1016/j.ahj.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 10. Eleid MF, Foley TA, Said SM, Pislaru SV, Rihal CS. Severe mitral annular calcification: multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc Imaging. 2016;9:1318–1337. DOI: 10.1016/j.jcmg.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 11. Kohsaka S, Jin Z, Rundek T, Boden‐Albala B, Homma S, Sacco RL, Di Tullio MR. Impact of mitral annular calcification on cardiovascular events in a multiethnic community: the Northern Manhattan Study. JACC Cardiovasc Imaging. 2008;1:617–623. DOI: 10.1016/j.jcmg.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamenský G, Lisy L, Polak E, Piknova E, Plevova N. Mitral annular calcifications and aortic plaques as predictors of increased cardiovascular mortality. J Cardiol. 2001;37(suppl 1):21–26. [PubMed] [Google Scholar]
- 13. Benjamin EJ, Plehn JF, D'Agostino RB, Belanger AJ, Comai K, Fuller DL, Wolf PA, Levy D. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–379. DOI: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 14. Kizer JR, Wiebers DO, Whisnant JP, Galloway JM, Welty TK, Lee ET, Best LG, Resnick HE, Roman MJ, Devereux RB. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36:2533–2537. DOI: 10.1161/01.STR.0000190005.09442.ad. [DOI] [PubMed] [Google Scholar]
- 15. Nestico PF, Depace NL, Morganroth J, Kotler MN, Ross J. Mitral annular calcification: clinical, pathophysiology, and echocardiographic review. Am Heart J. 1984;107:989–996. DOI: 10.1016/0002-8703(84)90840-8. [DOI] [PubMed] [Google Scholar]
- 16. Korn D, Desanctis RW, Sell S. Massive calcification of the mitral annulus. A clinicopathological study of fourteen cases. N Engl J Med. 1962;267:900–909. DOI: 10.1056/NEJM196211012671802. [DOI] [PubMed] [Google Scholar]
- 17. Fulkerson PK, Beaver BM, Auseon JC, Graber HL. Calcification of the mitral annulus. Etiology, clinical associations, complications and therapy. Am J Med. 1979;66:967–977. DOI: 10.1016/0002-9343(79)90452-2. [DOI] [PubMed] [Google Scholar]
- 18. Nair CK, Runco V, Everson GT, Boghairi A, Mooss AN, Mohiuddin SM, Sketch MH. Conduction defects and mitral annulus calcification. Br Heart J. 1980;44:162–167. DOI: 10.1136/hrt.44.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Neal WT, Efird JT, Nazarian S, Alonso A, Heckbert SR, Soliman EZ. Mitral annular calcification and incident atrial fibrillation in the Multi‐Ethnic Study of Atherosclerosis. Europace. 2015;17:358–363. DOI: 10.1093/europace/euu265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox CS, Parise H, Vasan RS, Levy D, O’Donnell CJ, D’Agostino RB, Plehn JF, Benjamin EJ. Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis. 2004;173:291–294. DOI: 10.1016/j.atherosclerosis.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 21. Boerlage‐van dijk K, Kooiman KM, Yong ZY, Wiegerinck EM, Damman P, Bouma BJ, Tijssen JG, Piek JJ, Knops RE, Baan J, et al. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin Electrophysiol. 2014;37:1520–1529. DOI: 10.1111/pace.12460. [DOI] [PubMed] [Google Scholar]
- 22. Durst R, Avelar E, McCarty D, Poh KK, Fernandez Friera L, Llano MF, Chu J, Reddy Anumandla AK, Rodriguez LL, Mack MJ, et al. Outcome and improvement predictors of mitral regurgitation after transcatheter aortic valve implantation. J Heart Valve Dis. 2011;20:272–281. [PubMed] [Google Scholar]
- 23. Feindel CM, Tufail Z, David TE, Ivanov J, Armstrong S. Mitral valve surgery in patients with extensive calcification of the mitral annulus. J Thorac Cardiovasc Surg. 2003;126:777–782. DOI: 10.1016/S0022-5223(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 24. d’Alessandro C, Vistarini N, Aubert S, Jault F, Acar C, Pavie A, Gandjbakhch I. Mitral annulus calcification: determinants of repair feasibility, early and late surgical outcome. Eur J Cardiothorac Surg. 2007;32:596–603. DOI: 10.1016/j.ejcts.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 25. Cammack PL, Edie RN, Edmunds LH Jr. Bar calcification of the mitral anulus. A risk factor in mitral valve operations. J Thorac Cardiovasc Surg. 1987;94:399–404. DOI: 10.1016/S0022-5223(19)36254-3. [DOI] [PubMed] [Google Scholar]
- 26. Hasan R, Mahadevan VS, Schneider H, Clarke B. First in human transapical implantation of an inverted transcatheter aortic valve prosthesis to treat native mitral valve stenosis. Circulation. 2013;128:e74–e76. DOI: 10.1161/CIRCULATIONAHA.113.001466. [DOI] [PubMed] [Google Scholar]
- 27. Sinning J‐M, Mellert F, Schiller W, Welz A, Nickenig G, Hammerstingl C. Transcatheter mitral valve replacement using a balloon‐expandable prosthesis in a patient with calcified native mitral valve stenosis. Eur Heart J. 2013;34:2609. DOI: 10.1093/eurheartj/eht254. [DOI] [PubMed] [Google Scholar]
- 28. Puri R, Abdul‐Jawad Altisent O, del Trigo M, Campelo‐Parada F, Regueiro A, Barbosa Ribeiro H, DeLarochellière R, Paradis JM, Dumont E, Rodés‐Cabau J. Transcatheter mitral valve implantation for inoperable severely calcified native mitral valve disease: a systematic review. Catheter Cardiovasc Interv. 2016;87:540–548. DOI: 10.1002/ccd.26262. [DOI] [PubMed] [Google Scholar]
- 29. Urena M, Himbert D, Brochet E, Carrasco JL, Iung B, Nataf P, Vahanian A. Transseptal transcatheter mitral valve replacement using balloon‐expandable transcatheter heart valves: a step‐by‐step approach. JACC Cardiovasc Interv. 2017;10:1905–1919. DOI: 10.1016/j.jcin.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 30. Guerrero M, Greenbaum A, O'Neill W. First in human percutaneous implantation of a balloon expandable transcatheter heart valve in a severely stenosed native mitral valve. Catheter Cardiovasc Interv. 2014;83:E287–E291. DOI: 10.1002/ccd.25441. [DOI] [PubMed] [Google Scholar]
- 31. Ben‐Avi R, Orlov B, Sternik L, Kogan A, Kuperstien R, Shalabi A, Ram E, Lipey A, Raanani E. Short‐ and long‐term results after prosthetic mitral valve implantation in patients with severe mitral annulus calcification. Interact Cardiovasc Thorac Surg. 2017;24:876–881. DOI: 10.1093/icvts/ivx043. [DOI] [PubMed] [Google Scholar]
- 32. Di Stefano S, Lopez J, Florez S, Rey J, Arevalo A, San RA. Building a new annulus: a technique for mitral valve replacement in heavily calcified annulus. Ann Thorac Surg. 2009;87:1625–1627. DOI: 10.1016/j.athoracsur.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 33. Ishida M, Toda K, Nakamura T, Miyagawa S, Yoshikawa Y, Fukushima S, Saito S, Ueno T, Kuratani T, Sawa Y. Reinforced mitral valve replacement using a xenopericardium collared prosthetic valve for a heavily calcified or disrupted mitral annulus: a simple “Dumpling technique”. Surg Today. 2017;47:895–898. DOI: 10.1007/s00595-016-1447-9. [DOI] [PubMed] [Google Scholar]
- 34. Mihaljevic T, Koprivanac M, Kelava M, Smedira NG, Lytle BW, Blackstone EH. Mitral valve replacement in patients with severely calcified mitral valve annulus: surgical technique. J Thorac Cardiovasc Surg. 2013;146:233–235. DOI: 10.1016/j.jtcvs.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 35. Salhiyyah K, Kattach H, Ashoub A, Patrick D, Miskolczi S, Tsang G, Ohri SK, Barlow CW, Velissaris T, Livesey S. Mitral valve replacement in severely calcified mitral valve annulus: a 10‐year experience. Eur J Cardiothorac Surg. 2017;52:440–444. DOI: 10.1093/ejcts/ezx086. [DOI] [PubMed] [Google Scholar]
- 36. Saran N, Greason KL, Schaff HV, Cicek SM, Daly RC, Maltais S, Stulak JM, Pochettino A, King KS, Dearani JA, et al. Does mitral valve calcium in patients undergoing mitral valve replacement portend worse survival? Ann Thorac Surg. 2019;107:444–452. DOI: 10.1016/j.athoracsur.2018.07.098. [DOI] [PubMed] [Google Scholar]
- 37. Uchimuro T, Fukui T, Shimizu A, Takanashi S. Mitral valve surgery in patients with severe mitral annular calcification. Ann Thorac Surg. 2016;101:889–895. DOI: 10.1016/j.athoracsur.2015.08.071. [DOI] [PubMed] [Google Scholar]
- 38. Nataf P, Pavie A, Jault F, Bors V, Cabrol C, Gandjbakhch I. Intraatrial insertion of a mitral prosthesis in a destroyed or calcified mitral annulus. Ann Thorac Surg. 1994;58:163–167. DOI: 10.1016/0003-4975(94)91092-8. [DOI] [PubMed] [Google Scholar]
- 39. Kaneko T, Hirji S, Percy E, Aranki S, McGurk S, Body S, Heydarpour M, Mallidi H, Singh S, Pelletier M, et al. Characterizing risks associated with mitral annular calcification in mitral valve replacement. Ann Thorac Surg. 2019;108:1761–1767. DOI: 10.1016/j.athoracsur.2019.04.080. [DOI] [PubMed] [Google Scholar]
- 40. Vander Salm TJ, Perras M. As originally published in 1989: mitral annular calcification: a new technique for valve replacement. Updated in 1997. Ann Thorac Surg. 1997;63:1819–1820. [DOI] [PubMed] [Google Scholar]
- 41. Eleid MF, Whisenant BK, Cabalka AK, Williams MR, Nejjari M, Attias D, Fam N, Amoroso N, Foley TA, Pollak PM, et al. Early outcomes of percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2017;10:1932–1942. DOI: 10.1016/j.jcin.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 42. Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, et al. 1‐year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. 2018;71:1841–1853. DOI: 10.1016/j.jacc.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 43. Himbert D, Bouleti C, Iung B, Nejjari M, Brochet E, Depoix JP, Ghodbane W, Fassa AA, Nataf P, Vahanian A. Transcatheter valve replacement in patients with severe mitral valve disease and annular calcification. J Am Coll Cardiol. 2014;64:2557–2558. DOI: 10.1016/j.jacc.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 44. Kiefer P, Noack T, Seeburger J, Hoyer A, Linke A, Mangner N, Lehmkuhl L, Mohr FW, Holzhey D. Transapical mitral valve implantation for native mitral valve stenosis using a balloon‐expandable prosthesis. Ann Thorac Surg. 2017;104:2030–2036. DOI: 10.1016/j.athoracsur.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 45. Langhammer B, Huber C, Windecker S, Carrel T. Surgical antegrade transcatheter mitral valve implantation for symptomatic mitral valve disease and heavily calcified annulus. Eur J Cardiothorac Surg. 2017;51:382–384. DOI: 10.1093/ejcts/ezw265. [DOI] [PubMed] [Google Scholar]
- 46. Praz F, Khalique OK, Lee R, Veeragandham R, Russell H, Guerrero M, Islam AM, Deaton DW, Kaneko T, Kodali SK, et al. Transatrial implantation of a transcatheter heart valve for severe mitral annular calcification. J Thorac Cardiovasc Surg. 2018;156:132–142. DOI: 10.1016/j.jtcvs.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 47. Russell HM, Guerrero ME, Salinger MH, Manzuk MA, Pursnani AK, Wang D, Nemeh H, Sakhuja R, Melnitchouk S, Pershad A, et al. Open atrial transcatheter mitral valve replacement in patients with mitral annular calcification. J Am Coll Cardiol. 2018;72:1437–1448. DOI: 10.1016/j.jacc.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 48. Schirmer J, Holzhey DM, Sinning JM, Schroefel H, Oertel F, Bleiziffer S, Unbehaun A, Lauten A, Frerker C, Blankenberg S, et al. Implantation of commercially available transcatheter heart valves (THV) for treatment of native calcific mitral stenosis: results from the First German THV Mitral Stenosis Registry. J Am Coll Cardiol. 2017;70:B254. [Google Scholar]
- 49. Urena M, Himbert D, Bouleti C, Brochet E, Iung B, Ou P, Dilly MP, Ghodhbane W, Nataf P, Vahanian A. Initial results of fully percutaneous transcatheter mitral valve implantation for native mitral valve disease in patients with extensive annular calcification. Eur Heart J. 2015;36:888.26052604 [Google Scholar]
- 50. Urena M, Brochet E, Lecomte M, Kerneis C, Carrasco JL, Ghodbane W, Abtan J, Alkhoder S, Raffoul R, Iung B, et al. Clinical and haemodynamic outcomes of balloon‐expandable transcatheter mitral valve implantation: a 7‐year experience. Eur Heart J. 2018;39:2679–2689. DOI: 10.1093/eurheartj/ehy271. [DOI] [PubMed] [Google Scholar]
- 51. Verma DR, Pershad A, Morse M, Morris M, Gellert G, Okoh A, Cohen M, Lotun K, Dhoble A, Smalling R, et al. A US multicenter registry for percutaneous transcatheter mitral valve replacement using balloon expandable valve in patients who are at prohibitive risk for surgery. J Am Coll Cardiol. 2017;70:B192. [Google Scholar]
- 52. Yoon S‐H, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, Schofer N, Eschenbach L, Bansal E, Murdoch DJ, Ancona M, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40:441–451. DOI: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- 53. Guerrero M. 30‐day outcomes of transcatheter mv replacement in patients with severe mitral valve disease secondary to mitral annular calcification or failed annuloplasty rings. Presented at TCT. 2017.
- 54. Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, Sandhu G, Salinger M, Russell H, Greenbaum A, et al. Thirty‐day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (valve‐in‐valve), failed surgical rings (valve‐in‐ring), and native valve with severe mitral annular calcification (valve‐in‐mitral annular calcification) in the United States: data from the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13:e008425. DOI: 10.1161/CIRCINTERVENTIONS.119.008425. [DOI] [PubMed] [Google Scholar]
- 55. El Sabbagh A, Eleid MF, Foley TA, Al‐Hijji MA, Daly RC, Rihal CS, Said SM. Direct transatrial implantation of balloon‐expandable valve for mitral stenosis with severe annular calcifications: early experience and lessons learned. Eur J Cardiothorac Surg. 2018;53:162–169. DOI: 10.1093/ejcts/ezx262. [DOI] [PubMed] [Google Scholar]
- 56. Guerrero M, Wang D, Pursnani A, Eleid M, Salinger M, Russell H, Kodal S, George I, Greenbaum A, Kar S, et al. One‐year outcomes of transcatheter mitral valve‐in‐valve, valve‐in‐ring and valve‐in‐mitral annular calcification: results from the MITRAL trial. Presented at EuroPCR. 2019.
- 57. Sud K, Agarwal S, Parashar A, Raza MQ, Patel K, Min D, Rodriguez LL, Krishnaswamy A, Mick SL, Gillinov AM, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation. 2016;133:1594–1604. DOI: 10.1161/CIRCULATIONAHA.115.020185. [DOI] [PubMed] [Google Scholar]
- 58. Bedeir K, Kaneko T, Aranki S. Current and evolving strategies in the management of severe mitral annular calcification. J Thorac Cardiovasc Surg. 2019;157:555–566. DOI: 10.1016/j.jtcvs.2018.05.099. [DOI] [PubMed] [Google Scholar]
- 59. Okada Y. Surgical management of mitral annular calcification. Gen Thorac Cardiovasc Surg. 2013;61:619–625. DOI: 10.1007/s11748-013-0207-7. [DOI] [PubMed] [Google Scholar]
- 60. Astarci P, Glineur D, De Kerchove L, El Khoury G. Transcatheter valve used in a bailout technique during complicated open mitral valve surgery. Interact Cardiovasc Thorac Surg. 2013;17:745–747. DOI: 10.1093/icvts/ivt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ribeiro HB, Doyle D, Urena M, Allende R, Amat‐Santos I, Pasian S, Bilodeau S, Mohammadi S, Paradis J‐M, DeLarochellière R, et al. Transapical mitral implantation of a balloon‐expandable valve in native mitral valve stenosis in a patient with previous transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014;7:e137–e139. DOI: 10.1016/j.jcin.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 62. Van Mieghem NM, Piazza N, Anderson RH, Tzikas A, Nieman K, De Laat LE, McGhie JS, Geleijnse ML, Feldman T, Serruys PW, et al. Anatomy of the mitral valvular complex and its implications for transcatheter interventions for mitral regurgitation. J Am Coll Cardiol. 2010;56:617–626. DOI: 10.1016/j.jacc.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 63. Regueiro A, Granada JF, Dagenais F, Rodés‐Cabau J. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol. 2017;69:2175–2192. DOI: 10.1016/j.jacc.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 64. Yoon S‐H, Bleiziffer S, Latib A, Eschenbach L, Ancona M, Vincent F, Kim W‐K, Unbehaum A, Asami M, Dhoble A, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2019;12:182–193. DOI: 10.1016/j.jcin.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 65. Guerrero M, Urena M, Pursnani A, Wang DD, Vahanian A, O'Neill W, Feldman T, Himbert D. Balloon expandable transcatheter heart valves for native mitral valve disease with severe mitral annular calcification. J Cardiovasc Surg. 2016;57:401–409. [PubMed] [Google Scholar]
- 66. El Sabbagh A, Eleid M, Said S, Nkomo V, Rihal C, Foley T. 3D printing for procedural simulation of transcatheter mitral valve replacement in patients with mitral annular calcification. J Am Coll Cardiol. 2017;69:1142. DOI: 10.1016/S0735-1097(17)34531-X. [DOI] [PubMed] [Google Scholar]
- 67. Alexis S, El‐Eshmawi A, Kini A, Sharma S, Dangas G, Adams D, Tang G. Novel technique for quantifying MAC in the era of transcatheter mitral techniques. J Am Coll Cardiol. 2019;73:1047. [Google Scholar]
- 68. Wang D, Guerrero M, Pantelic M, Song T, Myers E, Forbes M, Nelson C, Paone G, Greenbaum A, O'Neill W. Predicting risk of left ventricular outflow tract obstruction after transcatheter mitral valve replacement (TMVR): using multidetector computed tomography (CT), 3D computer‐aided design (CAD), and 3D Print to identify those at greatest risk. Catheter Cardiovasc Interv. 2015;85:S61–S62. [Google Scholar]
- 69. Wang DD, Guerrero M, Eng MH, Eleid MF, Meduri CU, Rajagopal V, Yadav PK, Fifer MA, Palacios IF, Rihal CS, et al. Alcohol septal ablation to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: first‐in‐man study. JACC Cardiovasc Interv. 2019;12:1268–1279. [DOI] [PubMed] [Google Scholar]
- 70. Yadav P, Wang DD, Eng M, Greenbaum A, O'Neill W. Alcohol septal ablation in patients with left ventricular outflow tract obstruction after transcatheter mitral valve replacement for mitral annular calcification (TMVR in MAC). J Am Coll Cardiol. 2016;68:B329. [Google Scholar]
- 71. Babaliaros VC, Greenbaum AB, Khan JM, Rogers T, Wang DD, Eng MH, O’Neill WW, Paone G, Thourani VH, Lerakis S, et al. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement: first‐in‐human experience. JACC Cardiovasc Interv. 2017;10:798–809. DOI: 10.1016/j.jcin.2017.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guerrero M, Wang DD, Himbert D, Urena M, Pursnani A, Kaddissi G, Iyer V, Salinger M, Chakravarty T, Greenbaum A, et al. Short‐term results of alcohol septal ablation as a bail‐out strategy to treat severe left ventricular outflow tract obstruction after transcatheter mitral valve replacement in patients with severe mitral annular calcification. Catheter Cardiovasc Interv. 2017;90:1220–1226. DOI: 10.1002/ccd.26975. [DOI] [PubMed] [Google Scholar]
- 73. Wyler von Ballmoos MC, Kalra A, Reardon MJ. Complexities of transcatheter mitral valve replacement (TMVR) and why it is not transcatheter aortic valve replacement (TAVR). Ann Cardiothorac Surg. 2018;7:724–730. DOI: 10.21037/acs.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lim ZY, Boix R, Prendergast B, Rajani R, Redwood S, Hancock J, Young C, Bapat V. First reported case of transcatheter mitral valve implantation in mitral annular calcification with a fully repositionable and self‐expanding valve. Circ Cardiovasc Interv. 2015;8:e003031. DOI: 10.1161/CIRCINTERVENTIONS.115.003031. [DOI] [PubMed] [Google Scholar]
- 75. Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, Paone G, Eng MH, Leshnower BG, Gleason PT, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73:2521–2534. DOI: 10.1016/j.jacc.2019.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guerrero M, Dvir D, Himbert D, Urena M, Eleid M, Wang DD, Greenbaum A, Mahadevan VS, Holzhey D, O’Hair D, et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first multicenter global registry. JACC Cardiovasc Interv. 2016;9:1361–1371. DOI: 10.1016/j.jcin.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 77. Sorajja P, Babaliaros V, Badhwar V, Chehab B, Thourani V. Transcatheter mitral valve implantation in patients with severe mitral annular calcification: early results from the Tendyne MAC Study. Presented at EuroPCR. 2019.
- 78. Sorajja P, Gössl M, Bae R, Tindell L, Lesser JR, Askew J, Farivar RS. Severe mitral annular calcification: first experience with transcatheter therapy using a dedicated mitral prosthesis. JACC Cardiovasc Interv. 2017;10:1178–1179. DOI: 10.1016/j.jcin.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 79. El‐Eshmawi A, Alexis SL, Sengupta A, Pandis D, Rimsukcharoenchai C, Adams DH, Tang GH. Surgical management of mitral annular calcification. Curr Opin Cardiol. 2020;35:107–115. DOI: 10.1097/HCO.0000000000000718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


