Abstract
Background
Hypertension among young adults is common. However, the effect of isolated systolic hypertension (ISH), isolated diastolic hypertension (IDH), or systolic and diastolic hypertension (SDH) among young adults on chronic kidney disease (CKD) development is unknown.
Methods and Results
From a nationwide health screening database, we included 3 030 884 participants aged 20 to 39 years who were not taking antihypertensives at baseline examination in 2009 to 2010. Participants were categorized as having normal blood pressure (BP), elevated BP, stage 1 IDH, stage 1 ISH, stage 1 SDH, stage 2 IDH, stage 2 ISH, and stage 2 SDH. The primary outcome was incident CKD. A total of 5853 (0.19%) CKD events occurred. With normal BP as the reference, multivariable‐adjusted hazard ratios (HRs) (95% CIs) for CKD were 1.14 (95% CI, 1.04–1.26), elevated BP; 1.19 (95% CI, 1.10–1.28), stage 1 IDH; 1.24 (95% CI, 1.08–1.42), stage 1 ISH; 1.39 (95% CI, 1.28–1.51), stage 1 SDH; 1.88 (95% CI, 1.63–2.16), stage 2 IDH; 1.84 (95% CI, 1.54–2.19), stage 2 ISH; 2.70 (95% CI, 2.44–2.98), stage 2 SDH. The HRs for CKD were attenuated in the patients who were antihypertensive and began medication within 1 year of medical checkup than in those without antihypertensives.
Conclusions
Among Korean young adults, those with elevated BP, stage 1 IDH, stage 1 ISH, stage 1 SDH, stage 2 IDH, stage 2 ISH, and stage 2 SDH were associated with a higher CKD risk than those with normal BP. The CKD risk in ISH and IDH groups was similar but lower than that in the SDH group. Antihypertensives attenuated the risk of CKD in young adults with hypertension.
Keywords: blood pressure, chronic kidney disease, hypertension, isolated diastolic hypertension, isolated systolic hypertension, young adult
Subject Categories: High Blood Pressure, Hypertension
Nonstandard Abbreviations and Acronyms
- IDH
isolated diastolic hypertension
- ISH
isolated systolic hypertension
- SDH
systolic and diastolic hypertension
Clinical Perspective
What Is New?
This is the first study demonstrating the relationship between blood pressure and chronic kidney disease (CKD) development in young adults using a well‐established and validated longitudinal national database.
Blood pressure levels greatly influenced CKD development in young adults, including the elevated blood pressure group; the risk was greater in SDH than in the ISH or IDH group and for stage 2 than stage 1 hypertension.
Increased blood pressure was associated with increased CKD risk in young adults; antihypertensives reduced CKD risk.
What Are the Clinical Implications?
The 2017 American College of Cardiology/American Heart Association guidelines increased the prevalence of hypertension in young adults.
Antihypertensives reduced the risk of CKD in young adults.
Hypertension is the most important modifiable risk factor globally for overall mortality and morbidity. 1 Hypertension also plays a crucial role in the development and progression of kidney failure. 2 , 3 Blood pressure (BP) rises with a declining kidney function, which in turn aggravates the hypertension. Moreover, as chronic kidney disease (CKD) worsens, BP becomes more difficult to control. Thus, it becomes a vicious cycle. Therefore, early diagnosis and prompt treatment are crucial. However, hypertension is usually diagnosed late in young adults and this interdependence complicates the management of both diseases. In 2017, the American College of Cardiology/American Heart Association released an updated guideline with new criteria for hypertension defining stage 1 hypertension as a systolic BP (SBP) value of 130 mm Hg through 139 mm Hg or a diastolic BP (DBP) value of 80 mm Hg through 89 mm Hg. 4 Most of the study populations according to this guideline comprised middle‐aged and elderly adults, leaving a relative lack of evidence for young adults aged 20 through 39 years.
Hypertension among young people is common and affects 1 in 8 adults aged between 20 and 40 years. 1 However, the effect of isolated systolic hypertension (ISH), isolated diastolic hypertension (IDH), or systolic and diastolic hypertension (SDH) among young adults on the development of CKD is unknown. Moreover, hypertension can have harmful health effects even at a young age. In the short term, it is associated with higher rates of left ventricular hypertrophy 5 and alterations in the brain volume, suggesting that hypertension in young adults may affect cardiovascular and brain health. 6 , 7
Although previous cohort studies have investigated the association of BP with cardiovascular disease among young adults, 8 , 9 they have not studied the association of BP with CKD. Moreover, the new definition led to an increase in the prevalence rate of hypertension, particularly among young adults. 10 , 11 However, the awareness and level of treatment for hypertension remains poor. 12
This nationwide sample‐based study aimed to investigate the association between the BP categories according to the 2017 American College of Cardiology/American Heart Association guidelines and the risk of CKD among young adults using the Korean National Health Insurance Service database.
Methods
Data Availability
Because of the confidentiality of data used in this study and the strict privacy policy of the data holder stating that these data can be kept only among the designated research personnel, these data cannot be made available to others, whether or not they are made anonymous.
Study Design and Database
The Korean National Health Insurance Service database comprises the complete health information pertaining to 50 million Koreans including an eligibility database, a medical treatment database, a health examination database, and a medical care institution database. 13 , 14 , 15 The National Health Insurance Corporation, managed by the Korean government, is the single insurer to which ≈97% of Koreans subscribe. Enrollees of the National Health Insurance Corporation are recommended to undergo a standardized medical examination at least once every 2 years. Among 4 944 387 young adults who enrolled from 2009 to 2010 (index year), 3 233 386 participants who were available for follow up from 2013 to 2016 were selected. To avoid confounders due to preexisting diseases and minimize the potential effects of reverse causality, those who had a history of CKD before the index year were also excluded (n=111 394). Ultimately, the study sample consisted of 3 030 884 subjects (Figure 1).
Figure 1. Flow diagram of the study.
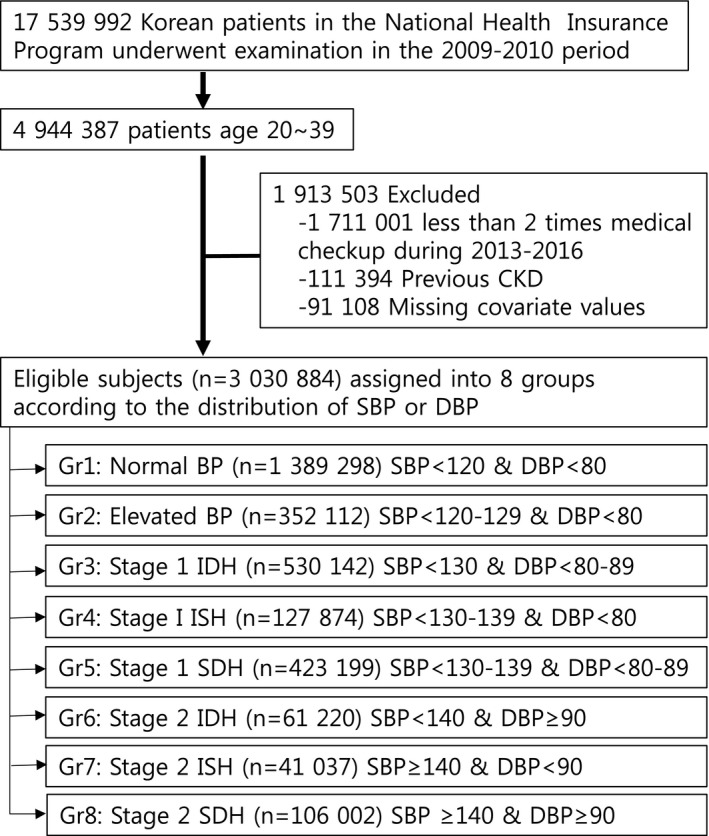
BP indicates blood pressure; CKD, chronic kidney disease; DBP, diastolic blood pressure; Gr, group; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; SBP, systolic blood pressure; and SDH, systolic and diastolic hypertension.
This study was approved by the Chonnam National University Hospital (study approval number: CNUH‐EXP‐2020‐228) and National Health Insurance Service, and it was conducted according to the principles of the Declaration of Helsinki. The need for written informed consent was waived by our institutional review board.
Measurements and Definitions
BP measurements were performed during the health checkup by trained medical staff using the auscultatory or oscillometric methods. The BP measurement protocol recommending at least 5 minutes of rest in a seated position followed by 2 repeated measurements at 5‐minute intervals was followed. 16 Participants were categorized into 8 mutually exclusive groups: (1) normal BP (untreated SBP <120/DBP <80 mm Hg; n=1 389 298); (2) elevated BP (SBP 120–129/DBP <80 mm Hg; n=352 112); (3) stage 1 IDH (SBP <130/DBP 80–89 mm Hg; n=530 142); (4) stage 1 ISH (SBP 130–139/DBP <80 mm Hg; n=124 874); (5) stage 1 SDH (SBP 130–139/DBP 80–89 mm Hg; n=423 199); (6) stage 2 IDH (SBP <140/DBP ≥90 mm Hg; n=61 220); (7) stage 2 ISH (SBP ≥140/DBP <90 mm Hg; n=41 037); (8) stage 2 SDH (SBP ≥140/DBP ≥90 mm Hg; n=106 002). Body mass index was calculated as the participant's weight in kilograms divided by the square of the participant's height in meters. Information on the current smoking and alcohol consumption habits was obtained by a questionnaire. Heavy alcohol consumption was defined as alcohol consumption more than 30 g per day. Regular exercise was defined as physical activity that was performed at least 5 times per week. The income level was dichotomized at the lower 25%. Blood samples for the measurement of serum glucose and total cholesterol levels were collected after overnight fasting. Proteinuria was tested by the dipstick method and defined as negative, trace, and 1+ to 4+. Comorbidities were identified using information gathered in the 1 year preceding the index date. Hypertension was defined as a previous diagnosis of hypertension as per the International Classification of Diseases, Tenth Revision (ICD‐10) codes (I10–13, I15) and a history of using at least 1 antihypertensive drug, or a recorded SBP of ≥140 mm Hg, or a DBPe of ≥90 mm Hg in the health examination database. Diabetes mellitus (DM) was identified using the appropriate diagnostic codes (E11–14) and a medical history of DM or a recorded fasting serum glucose concentration of ≥126 mg/dL in the health examination database. Dyslipidemia was identified using the appropriate diagnostic code (E78) and a history of lipid‐lowering drug use or a total serum cholesterol concentration of ≥240 mg/dL in the health examination database. CKD was defined as an estimated glomerular filtration rate of <60 mL/min per 1.73 m2 calculated using the CKD Epidemiology Collaboration equation. The participants' blood glucose (mg/dL) and total cholesterol (mg/dL) concentrations were measured in a fasting state. The quality of the laboratory tests was warranted by the Korean Association for Laboratory Medicine, and the hospitals participating in the National Health Insurance health checkup programs are certified by the National Health Insurance Service.
Study Outcomes and Follow‐Up
The study sample was followed up from the baseline period up to the date of CKD diagnosis or until December 31, 2016. The primary end point was incident CKD; it was defined using a combination of ICD‐10 codes (N18‐19) and an estimated glomerular filtration rate of <60 mL/min per 1.73 m2 calculated using the CKD Epidemiology Collaboration equation on more than 2 occasions during the medical checkup from 2013 to 2016.
Statistical Analysis
We report the mean±SD with intervals for continuous variables and the numbers (with percentages) for categorical variables. To identify the risk of CKD using the SBP and DBP levels, we calculated the hazard ratios (HRs) with 95% CIs and analyzed these data using the Cox proportional hazard regression model. We analyzed the associations between BP levels and CKD development using 5 models—Model 1: nonadjusted model; Model 2: adjusted for age and sex; Model 3: adjusted for model 2 plus smoking, alcohol drinking, physical activity, body mass index, and low income; Model 4 adjusted for Model 3 plus dyslipidemia, DM, and estimated glomerular filtration rate. Model 5 adjusted for Model 4 and the Charlson Comorbidity Index. 17 , 18 We also performed subgroup analyses for clinically important variables. A P<0.05 was considered to reflect statistical significance. SAS version 9.3 software and SAS survey procedures (SAS Institute, Inc., Cary, NC, USA) were used for all statistical analyses.
Results
Baseline Characteristics
Table 1 shows the baseline characteristics of the participants with respect to the development of CKD. Of the total, 5853 (0.19%) subjects developed CKD. The mean age of those who developed CKD was higher than that of those who did not. The proportion of men (72.29%), obesity (body mass index ≥25), and abdominal obesity (waist circumference ≥90 in men, ≥85 in women) was higher in the incident CKD than in the non‐CKD group. Comorbidities such as DM, hypertension, dyslipidemia, CKD, and proteinuria were more prevalent in the CKD group than in the non‐CKD group. The estimated glomerular filtration rate was lower and BP, total cholesterol, and glucose levels were higher in the CKD than in the non‐CKD group (Table 1).
Table 1.
Baseline Characteristics of Subjects According to the Incident CKD
| Group | None CKD (N=3 025 031) | CKD (N=5853) | P Value |
|---|---|---|---|
| Age, y | 31.82±4.81 | 35.02±3.82 | <0.0001 |
| Sex, male (%) | 2 026 248 (66.98) | 4231 (72.29) | <0.0001 |
| Current smoker | 1 116 411 (36.91) | 1905 (32.55) | <0.0001 |
| Heavy drinker* | 275 023 (9.09) | 453 (7.74) | 0.0003 |
| Physical activity‐regular | 430 539 (14.23) | 1084 (18.52) | <0.0001 |
| Income‐low † | 449 356 (14.85) | 855 (14.61) | 0.596 |
| BMI, kg/m2 | 23.22±3.45 | 24.63±3.69 | <0.0001 |
| Obesity (BMI ≥25) | 843 632 (27.89) | 2585 (44.17) | <0.0001 |
| BMI 5 level | <0.0001 | ||
| <18.5 | 190 167 (6.29) | 179 (3.06) | |
| 18.5–23 | 1 358 376 (44.9) | 1854 (31.68) | |
| 23–25 | 632 856 (20.92) | 1235 (21.1) | |
| 25–30 | 727 403 (24.05) | 2107 (36) | |
| ≥30 | 116 229 (3.84) | 478 (8.17) | |
| Waist circumference, cm‡ | 78.41±9.56 | 81.62±9.91 | <0.0001 |
| Abdominal obesity | 401 559 (13.27) | 1324 (22.62) | <0.0001 |
| Waist circumference 5 level | <0.0001 | ||
| M:<70/F:<65 | 277 050 (9.16) | 305 (5.21) | |
| M:70–79/F:65–74 | 1 226 510 (40.55) | 1785 (30.5) | |
| M:80–89/F:75–84 | 1 119 912 (37.02) | 2439 (41.67) | |
| M:90–99/F:85–94 | 337 567 (11.16) | 1089 (18.61) | |
| M:≥100/F:≥95 | 63 992 (2.12) | 235 (4.02) | |
| Diabetes mellitus | 57 867 (1.91) | 445 (7.6) | <0.0001 |
| Hypertension | 266 654 (8.81) | 1664 (28.43) | <0.0001 |
| Dyslipidemia | 219 937 (7.27) | 1029 (17.58) | <0.0001 |
| CCI score | 0.32±0.66 | 0.64±1.1 | <0.0001 |
| CCI grade | <0.0001 | ||
| 0 | 2 299 590 (76.02) | 3826 (65.37) | |
| 1 | 560 852 (18.54) | 1049 (17.92) | |
| 2 | 120 310 (3.98) | 550 (9.4) | |
| ≥3 | 44 279 (1.46) | 428 (7.31) | |
| Proteinuria | <0.0001 | ||
| Negative | 2 917 819 (96.7) | 4865 (83.3) | |
| Trace | 56 642 (1.88) | 191 (3.27) | |
| 1+ | 31 342 (1.04) | 321 (5.5) | |
| 2+ | 9589 (0.32) | 305 (5.22) | |
| 3+ | 1738 (0.06) | 134 (2.29) | |
| 4+ | 284 (0.01) | 24 (0.41) | |
| Height, cm | 169.13±8.17 | 169.63±7.86 | <0.0001 |
| Weight, cm | 66.82±13.06 | 71.3±13.85 | <0.0001 |
| Fasting blood glucose, mg/dL | 91.05±15.43 | 97.71±33.39 | <0.0001 |
| Systolic blood pressure, mm Hg | 118.5±12.87 | 123.85±15.68 | <0.0001 |
| Diastolic blood pressure, mm Hg | 74.38±9.27 | 78.29±11.13 | <0.0001 |
| Pulse pressure, mm Hg | 44.12±8.23 | 45.55±9.16 | <0.0001 |
| Total cholesterol, mg/dL | 186.4±33.74 | 197.74±37.97 | <0.0001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 96.31±51.36 | 80.26±51.93 | <0.0001 |
Data are presented as mean±SD or frequency (%). BMI indicates body mass index; CCI, Charlson Comorbidity Index; and CKD, chronic kidney disease.
Alcohol consumptions ≥30 g/day.
Low income 25%, abdominal obesity: waist circumference ≥90 in men, ≥85 in women.
Of the total number of participants, 530 142 (17.5%) had stage 1 IDH, 127 874 (4.2%) had stage 1 ISH, 423 199 (14.0%) had stage 1 SDH, 61 220 (2.0%) had stage 2 IDH, 41 037 (1.4%) had stage 2 ISH, and 106 002 (3.5%) had stage 2 SDH. Participants across all stages and subtypes of hypertension had higher body mass index, waist circumference, fasting glucose, and triglycerides levels; more severe DM and dyslipidemia; and were more likely to be male, current smokers, and frequent alcohol users than those in the normal BP group (Table 2).
Table 2.
Baseline Characteristics of Study Population by Blood Pressure Group
| Characteristics |
Normal BP (N=1 389 298) 45.8% |
Elevated BP (N=352 112) 11.6% |
Stage 1 Hypertension | Stage 2 Hypertension | ||||
|---|---|---|---|---|---|---|---|---|
|
Stage 1 IDH (N=530 142) 17.5% |
Stage 1 ISH (N=127 874) 4.2% |
Stage 1 SDH (N=423 199) 14.0% |
Stage 2 IDH (N=61 220) 2.0% |
Stage 2 ISH (N=41 037) 1.4% |
Stage 2 SDH (N=106 002) 3.5% |
|||
| Systolic BP, mm Hg | 108.01±7.25 | 122.65±2.99 | 119.63±4.89 | 132.6±2.89 | 132.22±3.07 | 129.35±5.77 | 144.66±5.82 | 148.64±9.41 |
| Diastolic BP, mm Hg | 67.6±5.94 | 71.58±4.26 | 80.94±2.1 | 73.21±4.09 | 82.39±3.09 | 91.88±3.53 | 81.66±4.9 | 96.52±6.99 |
| Age, y | 31.36±4.96 | 31.88±4.73 | 32.05±4.73 | 31.82±4.63 | 32.31±4.56 | 33.36±4.27 | 32.78±4.39 | 33.47±4.2 |
| Sex (male) | 692 596 (49.85) | 261 205 (74.18) | 400 704 (75.58) | 110 465 (86.39) | 376 524 (88.97) | 53 459 (87.32) | 37 632 (91.7) | 97 894 (92.35) |
| Smoking | ||||||||
| None | 868 263 (62.5) | 165 406 (46.98) | 238 497 (44.99) | 48 718 (38.1) | 146 305 (34.57) | 21 780 (35.58) | 13 651 (33.27) | 32 562 (30.72) |
| Ex‐ | 134 694 (9.7) | 47 560 (13.51) | 72 193 (13.62) | 19 683 (15.39) | 67 599 (15.97) | 10 326 (16.87) | 6941 (16.91) | 18 390 (17.35) |
| Current | 386 341 (27.81) | 139 146 (39.52) | 219 452 (41.39) | 59 473 (46.51) | 209 295 (49.46) | 29 114 (47.56) | 20 445 (49.82) | 55 050 (51.93) |
| Drinking | ||||||||
| None | 604 227 (43.49) | 120 207 (34.14) | 171 915 (32.43) | 37 207 (29.1) | 108 476 (25.63) | 15 748 (25.72) | 10 485 (25.55) | 22 780 (21.49) |
| Mild | 705 938 (50.81) | 200 170 (56.85) | 303 697 (57.29) | 75 515 (59.05) | 255 511 (60.38) | 36 187 (59.11) | 24 034 (58.57) | 63 311 (59.73) |
| Heavy | 79 133 (5.7) | 31 735 (9.01) | 54 530 (10.29) | 15 152 (11.85) | 59 212 (13.99) | 9285 (15.17) | 6518 (15.88) | 19 911 (18.78) |
| Exercise* | 178 062 (12.82) | 52 651 (14.95) | 79 894 (15.07) | 20 899 (16.34) | 67 876 (16.04) | 9549 (15.6) | 6604 (16.09) | 16 088 (15.18) |
| Income † | 213 877 (15.39) | 55 522 (15.77) | 82 512 (15.56) | 18 771 (14.68) | 55 067 (13.01) | 7730 (12.63) | 5387 (13.13) | 11 345 (10.7) |
| Diabetes mellitus | 14 286 (1.03) | 6018 (1.71) | 10 808 (2.04) | 3058 (2.39) | 13 389 (3.16) | 2373 (3.88) | 1954 (4.76) | 6426 (6.06) |
| Hypertension | 21 682 (1.56) | 7017 (1.99) | 12 495 (2.36) | 3321 (2.6) | 15 544 (3.67) | 61 220 (100) | 41 037 (100) | 106 002 (100) |
| Dyslipidemia | 66 584 (4.79) | 23 737 (6.74) | 42 695 (8.05) | 10 775 (8.43) | 46 006 (10.87) | 8229 (13.44) | 5526 (13.47) | 17 414 (16.43) |
| Weight, kg | 61.67±11.57 | 68.09±11.8 | 68.67±12.25 | 72.15±11.85 | 73.6±12.17 | 74.86±12.82 | 77.24±12.87 | 78.8±13.33 |
| Height, cm | 166.78±8.26 | 170.23±7.87 | 170.25±7.78 | 172.02±7.25 | 172.15±7.02 | 171.69±7.14 | 172.66±6.89 | 172.42±6.86 |
| Waist circumference, cm | 74.88±8.77 | 79.27±8.7 | 79.69±8.97 | 81.85±8.56 | 82.95±8.75 | 84.54±9.29 | 85.68±9.13 | 87.04±9.36 |
| Body mass index, kg/m2 | 22.04±3 | 23.4±3.15 | 23.59±3.31 | 24.31±3.28 | 24.77±3.46 | 25.32±3.63 | 25.85±3.69 | <0.0001 |
| Glucose, mg/dL | 88.93±12.86 | 91.19±14.47 | 91.44±15.86 | 92.96±15.84 | 93.94±18.06 | 95.02±19.98 | 96.94±21.04 | 98.46±23.61 |
| Total cholesterol, mg/dL | 180.99±31.91 | 185.9±33.11 | 188.73±33.78 | 189.21±34.07 | 194.2±34.82 | 197.79±35.66 | 197.65±35.95 | 202.53±36.63 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 97.22±52.97 | 98.25±62.55 | 94.86±46.11 | 96.87±52.56 | 93.6±39.82 | 95.17±49.71 | 98.46±68.25 | 94.25±45.57 |
| Pulse pressure, mm Hg | 40.41±5.93 | 51.07±4.42 | 38.69±4.75 | 59.4±4.28 | 49.83±3.01 | 37.48±6.04 | 62.99±7.05 | 52.12±8.58 |
| Charlson Comorbidity Index | 0.32±0.66 | 0.31±0.66 | 0.32±0.67 | 0.3±0.66 | 0.3±0.67 | 0.35±0.72 | 0.33±0.71 | 0.33±0.73 |
| 0 | 1 046 126 (75.3) | 268 295 (76.2) | 403 979 (76.2) | 99 179 (77.56) | 327 356 (77.35) | 45 956 (75.07) | 31 402 (76.52) | 81 123 (76.53) |
| 1 | 267 269 (19.24) | 65 179 (18.51) | 97 252 (18.34) | 22 133 (17.31) | 73 242 (17.31) | 11 321 (18.49) | 7182 (17.5) | 18 323 (17.29) |
| 2 | 57 030 (4.1) | 13 782 (3.91) | 20 718 (3.91) | 4745 (3.71) | 16 012 (3.78) | 2682 (4.38) | 1630 (3.97) | 4261 (4.02) |
| ≥3 | 18 873 (1.36) | 4856 (1.38) | 8193 (1.55) | 1817 (1.42) | 6589 (1.56) | 1261 (2.06) | 823 (2.01) | 2295 (2.17) |
| Chronic kidney disease event | 1940 (0.14) | 597 (0.17) | 1041 (0.2) | 240 (0.19) | 1037 (0.25) | 236 (0.39) | 140 (0.34) | 622 (0.59) |
Data are presented as mean±SD, or frequency (%).BP indicates blood pressure; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; and SDH, systolic and diastolic hypertension.
Regular exercise: midterm exercise ≥5 days or vigorous exercise ≥3 days in a week.
Low income 25%. P value for all categories <0.0001.
Isolated SBP or DBP and the Risk of CKD
With normal BP as the reference, the multivariable‐adjusted hazard ratios (95% CIs) for CKD as the outcome were 1.14 for elevated BP, 1.19 for stage 1 IDH, 1.24 for stage 1 ISH, 1.39 for stage 1 SDH, 1.88 for stage 2 IDH, 1.84 for stage 2 ISH, and 2.70 for stage 2 SDH groups (Table 3). We also evaluated the effect of antihypertensive medications on the CKD event. Antihypertensive medications within 1 year of medical checkup reduced the CKD risk in all groups significantly (Table 3). In order to excluding the effect of baseline proteinuria between BP and CKD risk, the analysis was performed only in patients without baseline proteinuria. The result were similar to those with baseline proteinuria (Table S1).
Table 3.
Multivariable Cox Analysis for Incident CKD by Isolated Systolic or Diastolic Hypertension
| BP Group | Total (n) | CKD (n) | % | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||
| Total population | ||||||||
| Normal | 1 389 298 | 1940 | 0.14 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Elevated BP | 352 112 | 597 | 0.17 | 1.22 (1.11–1.33) | 1.17 (1.07–1.29) | 1.10 (1.00–1.21) | 1.14 (1.04–1.25) | 1.14 (1.04–1.26) |
| Stage 1 IDH | 530 142 | 1041 | 0.20 | 1.41 (1.31–1.52) | 1.33 (1.23–1.43) | 1.24 (1.15–1.34) | 1.19 (1.10–1.28) | 1.19 (1.10–1.28) |
| Stage 1 ISH | 127 874 | 240 | 0.19 | 1.35 (1.17–1.54) | 1.33 (1.16–1.53) | 1.20 (1.05–1.38) | 1.23 (1.07–1.41) | 1.24 (1.08–1.42) |
| Stage 1 SDH | 423 199 | 1037 | 0.25 | 1.76 (1.63–1.89) | 1.63 (1.51–1.77) | 1.46 (1.35–1.58) | 1.38 (1.27–1.50) | 1.39 (1.28–1.51) |
| Stage 2 IDH | 61 220 | 236 | 0.39 | 2.77 (2.42–3.17) | 2.25 (1.96–2.58) | 1.94 (1.69–2.23) | 1.88 (1.64–2.16) | 1.88 (1.63–2.16) |
| Stage 2 ISH | 41 037 | 140 | 0.34 | 2.45 (2.06–2.91) | 2.15 (1.81–2.56) | 1.80 (1.51–2.14) | 1.82 (1.53–2.17) | 1.84 (1.54–2.19) |
| Stage 2 SDH | 106 002 | 622 | 0.59 | 4.22 (3.86–4.62) | 3.41 (3.10–3.75) | 2.81 (2.55–3.10) | 2.66 (2.41–2.93) | 2.70 (2.44–2.98) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Population without antihypertensive medication within 1‐yr after medical checkup | ||||||||
| Normal | 1 367 616 | 1739 | 0.13 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Elevated BP | 345 095 | 516 | 0.15 | 1.18 (1.07–1.30) | 1.15 (1.04–1.27) | 1.08 (0.98–1.20) | 1.12 (1.02–1.24) | 1.13 (1.02–1.24) |
| Stage 1 IDH | 517 647 | 884 | 0.17 | 1.34 (1.24–1.46) | 1.29 (1.18–1.40) | 1.21 (1.11–1.31) | 1.15 (1.06–1.25) | 1.15 (1.06–1.25) |
| Stage 1 ISH | 124 553 | 201 | 0.16 | 1.27 (1.10–1.47) | 1.29 (1.11–1.49) | 1.16 (1.00–1.35) | 1.20 (1.03–1.39) | 1.20 (1.03–1.39) |
| Stage 1 SDH | 407 655 | 849 | 0.21 | 1.64 (1.51–1.78) | 1.57 (1.44–1.71) | 1.40 (1.28–1.53) | 1.34 (1.23–1.46) | 1.34 (1.23–1.47) |
| Stage 2 IDH | 58 011 | 188 | 0.32 | 2.55 (2.20–2.97) | 2.13 (1.83–2.49) | 1.85 (1.59–2.16) | 1.82 (1.56–2.13) | 1.83 (1.57–2.13) |
| Stage 2 ISH | 38 444 | 102 | 0.27 | 2.09 (1.71–2.55) | 1.90 (1.55–2.32) | 1.59 (1.30–1.95) | 1.65 (1.35–2.03) | 1.67 (1.36–2.04) |
| Stage 2 SDH | 96 678 | 480 | 0.5 | 3.92 (3.54–4.34) | 3.27 (2.94–3.63) | 2.71 (2.43–3.02) | 2.62 (2.35–2.93) | 2.65 (2.38–2.96) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Population with antihypertensive medication within 1‐yr after medical checkup | ||||||||
| Normal | 21 682 | 201 | 0.93 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Elevated BP | 7017 | 81 | 1.15 | 1.25 (0.96–1.62) | 1.14 (0.88–1.48) | 1.14 (0.87–1.48) | 1.17 (0.90–1.52) | 1.16 (0.89–1.51) |
| Stage 1 IDH | 12 495 | 157 | 1.26 | 1.36 (1.10–1.68) | 1.22 (0.98–1.51) | 1.22 (0.99–1.52) | 1.18 (0.95–1.47) | 1.19 (0.96–1.48) |
| Stage 1 ISH | 3321 | 39 | 1.17 | 1.27 (0.90–1.79) | 1.13 (0.79–1.60) | 1.12 (0.79–1.59) | 1.16 (0.82–1.65) | 1.19 (0.83–1.69) |
| Stage 1 SDH | 15 544 | 188 | 1.21 | 1.31 (1.07–1.60) | 1.13 (0.92–1.39) | 1.15 (0.93–1.42) | 1.12 (0.91–1.39) | 1.17 (0.94–1.44) |
| Stage 2 IDH | 3209 | 48 | 1.50 | 1.62 (1.18–2.23) | 1.36 (0.98–1.88) | 1.37 (0.99–1.89) | 1.34 (0.96–1.85) | 1.37 (0.99–1.90) |
| Stage 2 ISH | 2593 | 38 | 1.47 | 1.59 (1.12–2.25) | 1.36 (0.96–1.94) | 1.37 (0.95–1.96) | 1.40 (0.98–2.01) | 1.51 (1.05–2.17) |
| Stage 2 SDH | 9324 | 142 | 1.52 | 1.65 (1.33–2.05) | 1.41 (1.13–1.77) | 1.44 (1.14–1.82) | 1.44 (1.14–1.81) | 1.55 (1.23–1.95) |
| P for trend | <0.0001 | 0.0037 | 0.007 | 0.0082 | 0.0007 | |||
Model 1: nonadjusted model. Model 2: adjusted for age, sex. Model 3: adjusted for Model 2 plus smoking, alcohol drinking, physical activity, body mass index, low income. Model 4 adjusted for Model 3 plus dyslipidemia, diabetes mellitus, and estimated glomerular filtration rate. Model 5 adjusted for Model 4 plus and Charlson Comorbidity Index. BP indicates blood pressure; CKD, chronic kidney disease; HR, hazard ratio; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; and SDH, systolic and diastolic hypertension.
BP and the Risk of CKD
We also analyzed the risk of developing CKD according to the SBP or DBP level. The risk of CKD increased with an increase in the SBP or DBP level. Patients taking antihypertensive medication within 1 year of medical checkup showed a lower risk of CKD than those not taking antihypertensive medication (Table 4, Figure 2).
Table 4.
Multivariable Cox Analysis for Incident CKD by Systolic or Diastolic Blood Pressure Level
| BP Group | Total (n) | CKD (n) | % | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||
| Systolic BP, mm Hg | ||||||||
| Total population | ||||||||
| <100 | 122 509 | 123 | 0.10 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 100–119 | 1 362 150 | 2009 | 0.15 | 1.47 (1.23–1.76) | 1.46 (1.21–1.75) | 1.35 (1.13–1.63) | 1.30 (1.09–1.57) | 1.31 (1.09–1.58) |
| 120–139 | 1 399 186 | 2959 | 0.21 | 2.11 (1.76–2.53) | 1.98 (1.64–2.38) | 1.69 (1.41–2.04) | 1.60 (1.33–1.93) | 1.62 (1.35–1.95) |
| 140–159 | 128 216 | 536 | 0.42 | 4.18 (3.43–5.08) | 3.45 (2.82–4.21) | 2.67 (2.18–3.27) | 2.51 (2.04–3.08) | 2.55 (2.08–3.13) |
| ≥160 | 18 823 | 226 | 1.20 | 12.09 (9.70–15.07) | 9.42 (7.52–11.79) | 6.98 (5.55–8.77) | 6.35 (5.05–7.98) | 6.57 (5.22–8.26) |
| Population without antihypertensive medication within 1 y after medical checkup | ||||||||
| <100 | 120 834 | 115 | 0.10 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 100–119 | 1 340 208 | 1788 | 0.13 | 1.40 (1.16–1.69) | 1.42 (1.17–1.71) | 1.32 (1.09–1.59) | 1.27 (1.05–1.53) | 1.27 (1.05–1.53) |
| 120–139 | 1 359 535 | 2474 | 0.18 | 1.91 (1.59–2.31) | 1.86 (1.54–2.25) | 1.60 (1.32–1.94) | 1.52 (1.25–1.84) | 1.53 (1.26–1.85) |
| 140–159 | 118 627 | 401 | 0.34 | 3.56 (2.89–4.38) | 3.09 (2.50–3.82) | 2.41 (1.94–2.99) | 2.31 (1.86–2.87) | 2.34 (1.88–2.90) |
| ≥160 | 16 495 | 181 | 1.10 | 11.65 (9.21–14.72) | 9.50 (7.48–12.07) | 7.10 (5.57–9.06) | 6.65 (5.21–8.49) | 6.80 (5.32–8.67) |
| Population with antihypertensive medication within 1 y after medical checkup | ||||||||
| <100 | 1675 | 8 | 0.48 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 100–119 | 21 942 | 221 | 1.01 | 2.12 (1.05–4.30) | 1.87 (0.92–3.80) | 1.85 (0.91–3.77) | 1.79 (0.88–3.64) | 1.78 (0.87–3.63) |
| 120–139 | 39 651 | 485 | 1.22 | 2.58 (1.30–5.19) | 2.04 (1.00–4.13) | 2.03 (0.10–4.13) | 1.96 (0.96–3.99) | 1.99 (0.98–4.06) |
| 140–159 | 9589 | 135 | 1.41 | 2.97 (1.46–6.08) | 2.27 (1.10–4.69) | 2.28 (1.10–4.73) | 2.24 (1.08–4.64) | 2.39 (1.15–4.96) |
| ≥160 | 2328 | 45 | 1.93 | 4.10 (1.93–8.73) | 3.19 (1.49–6.84) | 3.26 (1.51–7.03) | 3.18 (1.47–6.86) | 3.47 (1.60–7.50) |
| Diastolic BP, mm Hg | ||||||||
| Total population | ||||||||
| <70 | 670 954 | 845 | 0.13 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 70–79 | 1 205 401 | 1952 | 0.16 | 1.29 (1.19–1.39) | 1.27 (1.17–1.38) | 1.21 (1.12–1.31) | 1.12 (1.03–1.22) | 1.13 (1.04–1.22) |
| 80–89 | 987 307 | 2198 | 0.22 | 1.77 (1.63–1.92) | 1.65 (1.51–1.79) | 1.49 (1.37–1.62) | 1.33 (1.22–1.45) | 1.34 (1.23–1.45) |
| 90–99 | 117 199 | 473 | 0.4 | 3.21 (2.87–3.60) | 2.62 (2.33–2.94) | 2.20 (1.95–2.48) | 1.98 (1.76–2.23) | 2.00 (1.77–2.25) |
| ≥100 | 50 023 | 385 | 0.77 | 6.15 (5.45–6.94) | 4.81 (4.25–5.45) | 3.88 (3.41–4.41) | 3.37 (2.96–3.83) | 3.43 (3.01–3.90) |
| Population without antihypertensive medication within 1 y after medical checkup | ||||||||
| <70 | 661 231 | 771 | 0.12 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 70–79 | 1 182 732 | 1701 | 0.14 | 1.23 (1.13–1.34) | 1.23 (1.13–1.34) | 1.18 (1.08–1.29) | 1.09 (1.00–1.19) | 1.09 (1.00–1.19) |
| 80–89 | 957 047 | 1819 | 0.19 | 1.63 (1.50–1.78) | 1.56 (1.43–1.70) | 1.41 (1.29–1.55) | 1.27 (1.16–1.39) | 1.27 (1.16–1.39) |
| 90–99 | 109 457 | 364 | 0.33 | 2.86 (2.52–3.24) | 2.41 (2.12–2.74) | 2.04 (1.79–2.33) | 1.87 (1.64–2.14) | 1.88 (1.65–2.15) |
| ≥100 | 45 232 | 304 | 0.67 | 5.80 (5.08–6.62) | 4.70 (4.10–5.39) | 3.82 (3.31–4.40) | 3.39 (2.94–3.91) | 3.43 (2.98–3.95) |
| Population with antihypertensive medication within 1 y after medical checkup | ||||||||
| <70 | 9723 | 74 | 0.76 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 70–79 | 22 669 | 251 | 1.11 | 1.46 (1.13–1.89) | 1.33 (1.02–1.73) | 1.34 (1.03–1.74) | 1.29 (0.99–1.69) | 1.31 (1.00–1.71) |
| 80–89 | 30 260 | 379 | 1.25 | 1.65 (1.29–2.12) | 1.41 (1.09–1.83) | 1.43 (1.10–1.86) | 1.35 (1.04–1.75) | 1.40 (1.07–1.82) |
| 90–99 | 7742 | 109 | 1.41 | 1.86 (1.38–2.51) | 1.54 (1.13–2.09) | 1.57 (1.15–2.14) | 1.50 (1.10–2.05) | 1.58 (1.16–2.16) |
| ≥100 | 4791 | 81 | 1.69 | 2.24 (1.63–3.08) | 1.86 (1.34–2.58) | 1.93 (1.38–2.69) | 1.82 (1.31–2.55) | 2.01 (1.43–2.81) |
Model 1: nonadjusted model. Model 2: adjusted for age, sex. Model 3: adjusted for Model 2 plus smoking, alcohol drinking, physical activity, body mass index, low income. Model 4 adjusted for Model 3 plus dyslipidemia, diabetes mellitus, and estimated glomerular filtration rate. Model 5 adjusted for Model 4 plus and Charlson Comorbidity Index. BP indicates blood pressure; CKD, chronic kidney disease; and HR, hazard ratio.
Figure 2. Hazard ratios for chronic kidney disease according to index systolic blood pressure, diastolic blood pressure and pulse pressure in young adult without anti‐hypertensive medication within 1 y after medical checkup (A) and with anti hypertensive medication within 1 y after medical checkup (B).
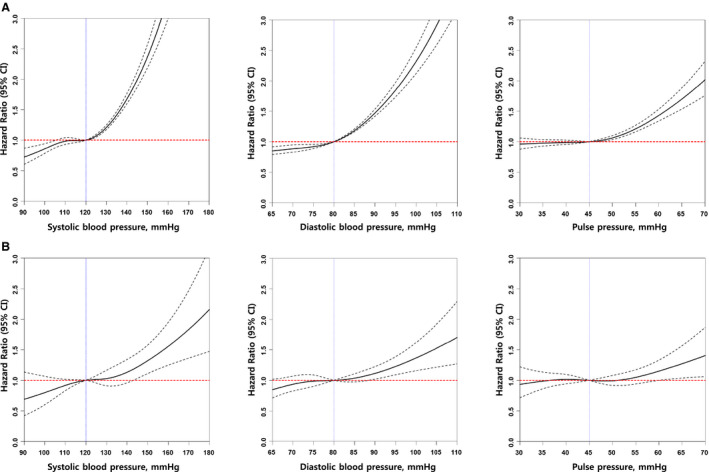
Adjusted for age, sex, income‐low 25%, diabetes mellitus, hypertension, dyslipidemia, current smoker, alcohol consumption, regular exercise, and estimated glomerular filtration rate.
Pulse Pressure and the Risk of CKD
Highest pulse pressure (Q5, pulse pressure quintile‐highest) increased the risk of developing CKD. The risk of CKD was attenuated by antihypertensive medication use within 1 year of medical checkup in pulse pressure quintile (Table 5, Figure 2).
Table 5.
Multivariable Cox Analysis for Incident CKD by Pulse Pressure Quintile
| Pulse Pressure Quintile | Total (n) | CKD (n) | % | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||
| Systolic blood pressure | ||||||||
| Total population | ||||||||
| Q1 | 440 377 | 753 | 0.17 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Q2 | 679 067 | 1202 | 0.18 | 1.04 (0.95–1.13) | 1.02 (0.93–1.11) | 0.99 (0.90–1.09) | 0.95 (0.86–1.04) | 0.95 (0.87–1.05) |
| Q3 | 687 095 | 1210 | 0.18 | 1.03 (0.94–1.13) | 1.07 (0.98–1.17) | 1.02 (0.93–1.12) | 1.03 (0.94–1.13) | 1.03 (0.94–1.13) |
| Q4 | 633 211 | 1280 | 0.20 | 1.18 (1.08–1.29) | 1.18 (1.08–1.29) | 1.09 (0.99–1.19) | 1.07 (0.98–1.18) | 1.09 (0.99–1.19) |
| Q5 | 591 134 | 1408 | 0.24 | 1.39 (1.28–152) | 1.38 (1.27–1.51) | 1.22 (1.12–1.34) | 1.27 (1.16–1.39) | 1.29 (1.18–1.41) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Population without antihypertensive medication within 1 y after medical checkup | ||||||||
| Q1 | 431 789 | 659 | 0.15 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Q2 | 664 736 | 1025 | 0.15 | 1.01 (0.92–1.11) | 1.02 (0.92–1.12) | 0.99 (0.90–1.10) | 0.95 (0.86–1.05) | 0.95 (0.86–1.05) |
| Q3 | 671 842 | 1018 | 0.15 | 0.99 (0.9–1.10) | 1.03 (0.94–1.14) | 0.99 (0.90–1.09) | 0.99 (0.90–1.09) | 0.99 (0.90–1.10) |
| Q4 | 615 220 | 1087 | 0.18 | 1.16 (1.05–1.28) | 1.17 (1.06–1.29) | 1.08 (0.98–1.19) | 1.08 (0.98–1.19) | 1.08 (0.98–1.19) |
| Q5 | 572 112 | 1170 | 0.20 | 1.34 (1.22–1.48) | 1.35 (1.22–1.48) | 1.20 (1.09–1.32) | 1.26 (1.14–1.38) | 1.26 (1.15–1.39) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Population with antihypertensive medication within 1 yr after medical checkup | ||||||||
| Q1 | 9196 | 99 | 1.08 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Q2 | 23 061 | 277 | 1.20 | 1.12 (0.89–1.41) | 1.07 (0.85–1.35) | 1.07 (0.85–1.35) | 1.04 (0.83–1.32) | 1.05 (0.83–1.33) |
| Q3 | 8073 | 100 | 1.24 | 1.15 (0.87–1.52) | 1.04 (0.78–1.38) | 1.03 (0.77–1.36) | 1.05 (0.79–1.40) | 1.06 (0.79–1.40) |
| Q4 | 18 819 | 212 | 1.13 | 1.05 (0.82–1.33) | 0.95 (0.74–1.21) | 0.94 (0.73–1.19) | 0.93 (0.73–1.19) | 0.96 (0.75–1.23) |
| Q5 | 16 036 | 206 | 1.28 | 1.20 (0.94–1.52) | 1.14 (0.90–1.46) | 1.12 (0.88–1.43) | 1.15 (0.90–1.47) | 1.20 (0.94–1.53) |
| P for trend | 0.3673 | 0.6902 | 0.8583 | 0.5599 | 0.3338 | |||
Model 1: nonadjusted model. Model 2: adjusted for age, sex. Model 3: adjusted for Model 2 plus smoking, alcohol drinking, physical activity, body mass index, low income. Model 4 adjusted for Model 3 plus dyslipidemia, diabetes mellitus, and estimated glomerular filtration rate. Model 5 adjusted for Model 4 plus and Charlson Comorbidity Index. BP indicates blood pressure; and HR, hazard ratio.
Subgroup Analyses
Subgroup analysis for the ISH and IDH groups showed that the increased CKD risk was attenuated by antihypertensive medication use within 1 year of medical checkup (Figure 3A).
Figure 3. Subgroup analysis for chronic kidney disease risk.
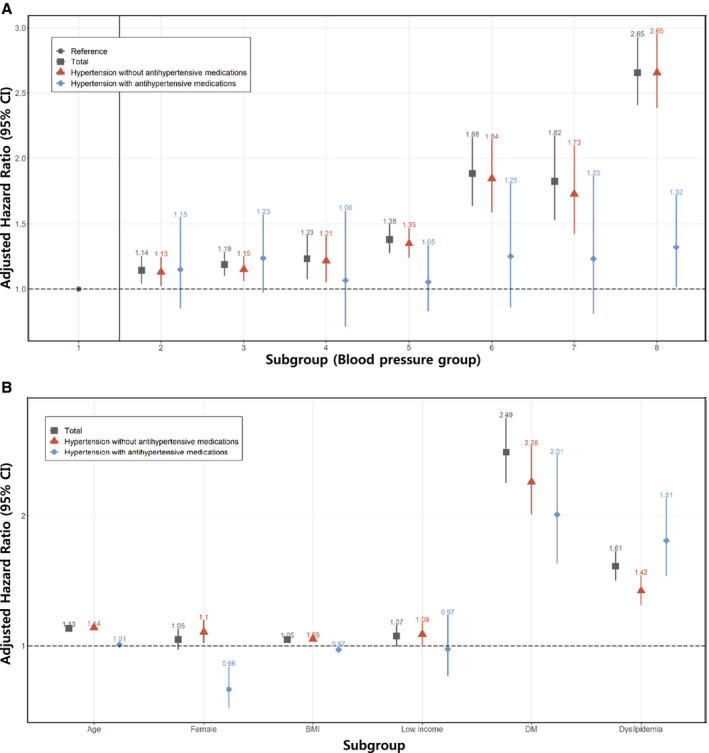
A, Blood pressure group. B, Age, sex, BMI, DM, dyslipidemia. BMI indicates body mass index; and DM, diabetes mellitus.
In the DM and dyslipidemia subgroup analysis, the HRs for incident CKD were higher in the DM and dyslipidemia groups than in the non‐DM and nondyslipidemia groups. The relative risks associated with high BP were higher in women than in men among young adults (Figure 3B).
Discussion
The present study demonstrated that increased levels of both SBP and DBP were associated with a higher risk of CKD in young adults aged 20 to 39 years. Not only the SBP but also the DBP level was associated with an increased risk of CKD. Moreover, patients not taking any antihypertensives within 1 year of medical checkup showed a higher CKD risk than those commencing antihypertensive medication within 1 year of medical checkup. Young adults with stage 1 ISH, IDH, or SDH all had a significantly higher risk for CKD events than those with normal BP. The risk of CKD associated with stage 1 ISH and stage 1 IDH was similar but lower than the risk of CKD associated with stage 1 SDH. As expected, the increase in the risk of CKD associated with ISH, IDH, and SDH was greater in participants with stage 2 than in those with stage 1 hypertension.
The presence of hypertension at a young age increases the risk of cardiovascular events at middle age. 19 It contributes to early‐onset coronary heart disease, heart failure, stroke, and transient ischemic attacks. 20 Although good national guidelines exist, the guidelines do not serve low‐risk young patients with hypertension as effectively as they do older patients. Furthermore, risk assessment is challenging in young patients because of the limited validity of and a greater focus on SBP that is less well correlated with cardiovascular disease outcomes. 19 , 21 Furthermore, the mechanisms underlying ISH and those underlying IDH among young adults may differ. A higher systemic vascular resistance is a major contributor to high DBP, whereas increased aortic stiffness and a reduced aortic diameter contribute to high SBP among young adults. 22 However, in the current study, the CKD risk associated with stage 1 ISH and IDH was comparable.
Whether ISH of the young is a benign condition caused by increased SBP amplification with increased brachial but normal central SBP 23 , 24 or results from increased arterial stiffness and a larger stroke volume that may evolve into sustained hypertension is debatable. 22 , 25 , 26 Consequently, it is still unclear whether BP‐lowering therapy would benefit young adults with ISH. Most young adults with stage 1 hypertension have a low absolute 10‐year atherosclerotic cardiovascular disease risk and thus would not be suitable candidates for pharmacological interventions. 10 , 11 However, after cumulative exposure to high BP levels, a decrease in BP levels later in life may not completely restore the risk of cardiovascular disease to normal levels in such individuals. 27 Therefore, young adults with stage 1 hypertension should be subjected to further risk stratification to identify those individuals who would benefit from pharmacological therapy in conjunction with lifestyle changes. In the current study, stage 1 hypertension group subjects were associated with a significantly higher risk of CKD than those with normal BP or elevated BP. Moreover, the risk of CKD posed by simultaneous elevation of the SBP and DBP (SDH group) was higher than that posed by isolated elevation of either. In addition, in our study antihypertensive therapy reduced the risk of CKD even in young adults with stage 1 ISH. These results may support the new 2017 American College of Cardiology/American Heart Association BP guideline regarding the CKD risk among young adults. This guideline was based on the fact that the SPRINT (Systolic Blood Pressure Intervention Trial) was prematurely ceased because ofthe benefit of being able to control the SBP levels of hypertensive patients to <120 mm Hg rather than <140 mm Hg, thus supporting the aim for a low SBP. 28
Previous studies have reported that compared with the BP pattern in men, women tend to show a steeper elevation in BP with age, starting from young adulthood and continuing throughout life. 29 The possible explanations include sex differences in the vascular biology, hormonal factors, and social determinants of health. 30 The relative risk of cardiovascular disease associated with high BP levels is also higher in women than in men among young to middle‐aged adults. 31 In this study, the subgroup analysis for sex also showed that the CKD risk was higher in women than in men, but the antihypertensive medication group did not reveal any differences between men and women. Our results warrant further testing in an independent cohort to verify if CKD outcomes associated with BP differ according to the sex.
The strengths of this study include the large nationwide longitudinal health screening database with high participation and outcome ascertainment rates owing to electronic linkages to universal health insurance records. This database covers a wide range of the Korean sample over a long follow‐up duration and, hence, allows inclusion of a sizable number of young adults. The events in these young participants are considered premature CKD, an important sample health outcome measure, yet one that has rarely been studied in a large sample size.
However, our study also has some limitations. First, although the 2017 American College of Cardiology/American Heart Association guidelines recommend that ≥2 BP readings be obtained before determining the stage of BP, in the current study, participants were classed based on their BP readings assessed during a single visit. The examination protocol recommends that the BP be measured twice and the average reading be used for the analysis. However, in a real‐world screening environment taking place on a nationwide scale, adherence to the protocol may be limited. Therefore, the BP measurements used for the classification might not have fully reflected an individual's BP phenotype. Second, possible residual confounding, including sodium intake and psychological factors, may affect the association between BP and CKD events. Third, our study was based on Korean adults subscribing to a universal health insurance and screening program; the results should be interpreted with caution when applied to different populations or healthcare systems. Fourth, because cause and effect are not established, there is a possibility that worsening hypertension was the result of CKD in some of the people in the database. Finally, the role of antihypertensives such as renin‐angiotensin‐aldosterone system blockers in delaying the progression of CKD is known, but the impact of the type of antihypertensive medication prescribed was not considered in this study.
Conclusions
In conclusion, among Korean young adults, those with elevated BP, stage 1 IDH, stage 1 ISH, stage 1 SDH, stage 2 IDH, stage 2 ISH, and stage 2 SDH were associated with a higher CKD risk than those with normal BP. The CKD risk associated with ISH and IDH was comparable but lower than the risk associated with SDH. Antihypertensive medications attenuated the risk of CKD in young adults with hypertension.
Perspectives
This is the first study describing the relationship between BP and the development of CKD in young adults using a well‐established and validated longitudinal national database. Our study demonstrated the enormous impact of BP on the development of CKD in young adults, even in those with elevated BP. The risk was greater in the SDH group than in the ISH or IDH group and more prominent for stage 2 hypertension than for stage 1 hypertension. Although antihypertensive therapy reduced the risk of CKD, we did not evaluate the impact of the class of the drug, and further studies are needed to clearly establish the effect of antihypertensive medication on the risk of CKD events in hypertension of the young.
Sources of Funding
This research was supported by the Bio & Medical Development Program of the National Research Foundation funded by the Korean Government (2017M3A9E8023001), grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0331, HR20C0021), and by a grant (BCRI20025) of Chonnam National University Hospital Biomedical Research Institute.
Disclosures
None.
Supporting information
Table S1
(J Am Heart Assoc. 2021;10:e019764. DOI: 10.1161/JAHA.120.019764.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019764
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Kyung‐Do Han, Email: hkd917@naver.com.
Soo Wan Kim, Email: skimw@chonnam.ac.kr.
References
- 1. Hinton TC, Adams ZH, Baker RP, Hope KA, Paton JFR, Hart EC, Nightingale AK. Investigation and treatment of high blood pressure in young people: too much medicine or appropriate risk reduction? Hypertension. 2020;75:16–22. DOI: 10.1161/HYPERTENSIONAHA.119.13820. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. DOI: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 3. Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, Cannon CP, de Lemos JA, Elliott WJ, Findeiss L, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. 2015;131:e435–e470. DOI: 10.1161/CIR.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. DOI: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 5. Drukteinis JS, Roman MJ, Fabsitz RR, Lee ET, Best LG, Russell M, Devereux RB. Cardiac and systemic hemodynamic characteristics of hypertension and prehypertension in adolescents and young adults: the Strong Heart Study. Circulation. 2007;115:221–227. DOI: 10.1161/CIRCULATIONAHA.106.668921. [DOI] [PubMed] [Google Scholar]
- 6. Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Parker TD, Malone IB, Lu K, James S‐N, Keshavan A, et al. Associations between blood pressure across adulthood and late‐life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol. 2019;18:942–952. DOI: 10.1016/S1474-4422(19)30228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williamson W, Lewandowski AJ, Forkert ND, Griffanti L, Okell TW, Betts J, Boardman H, Siepmann T, McKean D, Huckstep O, et al. Association of cardiovascular risk factors with MRI indices of cerebrovascular structure and function and white matter hyperintensities in young adults. JAMA. 2018;320:665–673. DOI: 10.1001/jama.2018.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCarron P, Smith GD, Okasha M, McEwen J. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet. 2000;355:1430–1431. DOI: 10.1016/S0140-6736(00)02146-2. [DOI] [PubMed] [Google Scholar]
- 9. Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25‐year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–1508. DOI: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 10. Kim HC, Jeon YW, Heo ST. Global impact of the 2017 American College of Cardiology/American Heart Association hypertension guidelines. Circulation. 2018;138:2312–2314. DOI: 10.1161/CIRCULATIONAHA.118.036312. [DOI] [PubMed] [Google Scholar]
- 11. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. DOI: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moon JY, Park KJ, Hwangbo Y, Lee MR, Yoo BI, Won JH, Park YH. A trend analysis of the prevalence, awareness, treatment, and control of hypertension by age group. J Prev Med Public Health. 2013;46:353–359. DOI: 10.3961/jpmph.2013.46.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service‐National Sample Cohort (NHIS‐NSC), South Korea. Int J Epidemiol. 2017;46:e15. DOI: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 14. Lee YH, Han K, Ko SH, Ko KS, Lee KU; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes A . Data analytic process of a nationwide population‐based study using national health information database established by National Health Insurance Service. Diabetes Metab J. 2016;40:79–82. DOI: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang HK, Han K, Kwon HS, Park YM, Cho JH, Yoon KH, Kang MI, Cha BY, Lee SH. Obesity, metabolic health, and mortality in adults: a nationwide population‐based study in Korea. Sci Rep. 2016;6:30329. DOI: 10.1038/srep30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SW, Lee HY, Ihm SH, Park SH, Kim TH, Kim HC. Status of hypertension screening in the Korea National General Health Screening Program: a questionnaire survey on 210 screening centers in two metropolitan areas. Clin Hypertens. 2017;23:23. DOI: 10.1186/s40885-017-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. DOI: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 18. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. DOI: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19. Sundstrom J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ. 2011;342:d643. DOI: 10.1136/bmj.d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, Gidding SS, Bress AP, Greenland P, Muntner P, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA. 2018;320:1774–1782. DOI: 10.1001/jama.2018.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies C . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. DOI: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 22. McEniery CM, Yasmin, Wallace S, Maki‐Petaja K, McDonnell B, Sharman JE, Retallick C, Franklin SS, Brown MJ, Lloyd RC, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. DOI: 10.1161/01.HYP.0000165310.84801.e0. [DOI] [PubMed] [Google Scholar]
- 23. Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens. 2003;16:229–232. DOI: 10.1016/S0895-7061(02)03255-7. [DOI] [PubMed] [Google Scholar]
- 24. O'Rourke MF, Adji A. Guidelines on guidelines: focus on isolated systolic hypertension in youth. J Hypertens. 2013;31:649–654. DOI: 10.1097/HJH.0b013e32835d8230. [DOI] [PubMed] [Google Scholar]
- 25. Franklin SS, Wilkinson IB, McEniery CM. Unusual hypertensive phenotypes: what is their significance? Hypertension. 2012;59:173–178. DOI: 10.1161/HYPERTENSIONAHA.111.182956. [DOI] [PubMed] [Google Scholar]
- 26. McEniery CM, Franklin SS, Wilkinson IB, Cockcroft JR. Isolated systolic hypertension in the young: a need for clarity. J Hypertens. 2013;31:1911–1913. DOI: 10.1097/HJH.0b013e3283635315. [DOI] [PubMed] [Google Scholar]
- 27. Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, et al. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels?: The Coronary Artery Risk Development in Young Adults (CARDIA) study and the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2015;4:e002275. DOI: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. DOI: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 29. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5:19–26. DOI: 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heise L, Greene ME, Opper N, Stavropoulou M, Harper C, Nascimento M, Zewdie D, Darmstadt GL, Greene ME, Hawkes S, et al. Gender inequality and restrictive gender norms: framing the challenges to health. Lancet. 2019;393:2440–2454. DOI: 10.1016/S0140-6736(19)30652-X. [DOI] [PubMed] [Google Scholar]
- 31. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. DOI: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Because of the confidentiality of data used in this study and the strict privacy policy of the data holder stating that these data can be kept only among the designated research personnel, these data cannot be made available to others, whether or not they are made anonymous.


