Abstract
Background
Only one third of patients recommended intensified treatment by the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for high blood pressure would have been eligible for the clinical trials on which recommendations were largely based. We sought to identify characteristics of adults who would have been trial‐ineligible in order to inform clinical practice and research priorities.
Methods and Results
We examined the proportion of adults diagnosed with hypertension who met trial inclusion and exclusion criteria, stratified by age, diabetes mellitus status, and guideline recommendations in a cross‐sectional study of the National Health and Nutrition Examination Survey, 2013–2016. Of the 107.7 million adults (95% CI, 99.3–116.0 million) classified as having hypertension by the ACC/AHA guideline, 23.1% (95% CI, 20.8%–25.5%) were below the target blood pressure of 130/80 mm Hg, 22.2% (95% CI, 20.1%–24.4%) would be recommended nonpharmacologic treatment, and 54.6% (95% CI, 52.5%–56.7%) would be recommended additional pharmacotherapy. Only 20.6% (95% CI, 18.8%–22.4%) of adults with hypertension would be trial‐eligible. The majority of adults <50 years were excluded because of low cardiovascular risk and lack of access to primary care. The majority of adults aged ≥70 years were excluded because of multimorbidity and limited life expectancy. Reasons for trial exclusion were similar for patients with and without diabetes mellitus.
Conclusions
Intensive blood pressure treatment trials were not representative of many younger adults with low cardiovascular risk and older adults with multimorbidity who are now recommended more intensive blood pressure goals.
Keywords: clinical trials, guidelines, hypertension
Subject Categories: Hypertension, Statements and Guidelines, Health Services, High Blood Pressure, Clinical Studies
Nonstandard Abbreviations and Acronyms
- ACC
American College of Cardiology
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- AHA
American Heart Association
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- SBP
systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Clinical Perspective
What Is New?
Clinical trials of more versus less intensive blood pressure treatment targets have led national guidelines to recommend additional pharmacotherapy for millions of adults with hypertension.
In this study of the National Health and Nutrition Examination Survey, the authors find that the majority of US adults aged <50 years would have been excluded from intensive blood pressure treatment trials because of low cardiovascular risk and/or lack of access to primary care, and the majority of adults aged ≥70 years would have been excluded because of multimorbidity, limited life expectancy, or both.
What Are the Clinical Implications?
Clinical trials studying the outcomes of intensive blood pressure treatment in younger adults at low cardiovascular risk and in older adults with multimorbidity are urgently needed, and, until these data are available, a patient‐centered approach tailoring treatments and targets by degree of blood pressure elevation, comorbidity, and likelihood of benefit is likely preferable to universal adoption of intensive treatment targets.
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure (BP) in Adults marked a significant change in the recommended management of hypertension. 1 Compared with prior guidelines, 2 , 3 the 2017 ACC/AHA guideline decreased the thresholds for diagnosing and treating hypertension to a systolic BP (SBP) of 130 mm Hg and diastolic BP of 80 mm Hg, significantly expanding the population recommended pharmacologic treatment. 4
This recommendation was primarily based on evidence generated from 2 randomized clinical trials comparing intensive and standard BP treatment goals. 5 SPRINT (Systolic Blood Pressure Intervention Trial) studied adults without diabetes mellitus and demonstrated a risk reduction in major cardiovascular events and death with intensive treatment, 6 whereas, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial studied adults with diabetes mellitus and did not demonstrate a significant reduction in major cardiovascular events or death with intensive treatment. 7
Understanding the representativeness of these trials to the populations that are now recommended additional pharmacologic treatment by the 2017 ACC/AHA guideline is crucial to inform both clinical decision‐making for individual patients and population health efforts. For individuals excluded from the trials, the harm to benefit ratio for intensive BP control may differ from trial results. Patients at lower cardiovascular risk and those with barriers to adherence may face less opportunity for benefit from intensive BP control while still encountering increased costs and risks of adverse events. Patients with limited life expectancy may not survive to experience long‐term risk reduction from intensive control and may face a higher burden of polypharmacy.
We recently reported that of all adults recommended intensified pharmacotherapy by the 2017 ACC/AHA guideline, less than one third met trial eligibility criteria. 8 Our prior research highlighted the need for clinicians to understand current evidence gaps and personalize antihypertensive prescribing decisions to individual patient characteristics. In the present study, we sought to describe the characteristics of patient groups excluded from the trials underlying the 2017 ACC/AHA guideline in order to help guide clinical practice. We used National Health and Nutrition Examination Survey (NHANES) data to identify adults who would meet additional antihypertensive treatment criteria by the 2017 ACC/AHA guideline and those who would be excluded for SPRINT and the ACCORD trial because of low cardiovascular risk, comorbidities, limited likelihood of benefit, or factors likely to limit adherence to treatment.
Methods
Data Source and Study Population
We analyzed NHANES questionnaire, laboratory, and physical examination data from the 2013 to 2014 and 2015 to 2016 cycles. NHANES is conducted every 2 years by the National Center for Health Statistics (NCHS) using a stratified, multistage probability sampling design to generate nationally representative estimates of the noninstitutionalized US population. 9 Following NHANES recommendations, we pooled data across cycles to improve stability for subgroup estimates. The NCHS institutional review board reviewed each NHANES cycle and all participants provided written informed consent. The data that support the findings of this study are publicly available from the NCHS, 9 and the statistical code used in the analysis is available from the corresponding author upon reasonable request.
We included all adult participants aged >18 years. We examined BP and antihypertensive use at the time of survey administration as well as demographic and clinical characteristics used to determine hypertension clinical trial eligibility. Missing data were imputed using the fully conditional specification method and 20 imputation sets. 10 Certain survey questions were only asked to population subsets (eg, only participants aged ≥60 years completed cognitive function surveys); for these questions, missing data were imputed only for the target population specified by NHANES.
BP Measurement and Hypertension Definition
NHANES protocol required BP measurement in seated participants by a trained physician following 5 minutes of rest. Appropriate cuff sizes and a mercury sphygmomanometer were used to collect 3 measurements of which we examined the mean. Hypertension was defined as BP ≥130/80 mm Hg or any self‐reported antihypertensive medication use.
Trial Eligibility
As previously described, 8 published SPRINT and ACCORD protocols were used to determine trial eligibility in NHANES participants without and with diabetes mellitus, respectively. 6 , 7 , 11 Diabetes mellitus was defined by self‐reported history or a glycated hemoglobin level ≥6.5%.
Inclusion criteria for both trials were elevated SBP (≥130 mm Hg) with or without prior antihypertensive use and increased cardiovascular risk. For patients without diabetes mellitus, we defined increased cardiovascular risk based on SPRINT criteria as self‐reported history of myocardial infarction, coronary heart disease, angina, estimated glomerular filtration rate between 20 and 59 mL/min per 1.73m2 by the Modification of Diet in Renal Disease equation, 10‐year Framingham Risk Score ≥15%, or age >75 years. 6 We defined increased cardiovascular risk for patients with diabetes mellitus based on ACCORD criteria as self‐reported history of myocardial infarction, coronary heart disease, angina, or stroke, or the presence of >2 of the following risk factors: elevated low‐density lipoprotein cholesterol (>130 mg/dL), low high‐density lipoprotein cholesterol (<40 mg/dL for men and <50 mg/dL for women), SBP >140 mm Hg or diastolic BP >95 mm Hg, current cigarette smoking, and body mass index >32 kg/m2. 7 , 11
Exclusion criteria for both trials included SBP >180 mm Hg, end‐stage renal disease, symptomatic heart failure, cancer (excluding nonmelanomatous skin cancer) within the preceding 2 years, estimated life expectancy <3 years, and presence of factors likely to limit medication adherence. The ACCORD trial additionally excluded individuals whose age was <40 or >79 years, body mass index was >45 kg/m2, and those with laboratory evidence of transaminitis or chronic kidney disease. SPRINT additionally excluded individuals with a history of stroke and those whose age was <50 years.
To estimate life expectancy, we used the Lee index, which estimates likelihood of survival at different time intervals based on an individual's age, presence of chronic conditions, smoking status, body mass index, and measures of functional status. 12 Patients with a Lee index score of ≥14 were excluded, as a score of 14 is associated with a median predicted life expectancy of 3.1 years and higher scores are associated with shorter life expectancies. 13
While both trials excluded individuals with factors likely to limit medication adherence, only SPRINT specified these factors, thus SPRINT exclusions were applied to both populations. Factors for which related NHANES questions were available included alcohol abuse within the prior 12 months, lack of support from a primary care provider, and impaired cognition. Alcohol abuse was estimated from self‐reported history of consuming ≥5 drinks at least twice per week in the past year (or >104 days per year). Lack of primary care support was defined by self‐report of not having a routine place to go for health care other than the emergency department. Impaired cognition was assessed only for respondents aged ≥60 years and defined by a score of ≤14 on Animal Fluency testing. 14 , 15 The Animal Fluency test examines categorical verbal fluency and scores have been shown to discriminate between persons with normal cognitive functioning compared with those with mild cognitive impairment and more severe forms of cognitive impairment, such as Alzheimer disease. 16 , 17 As cognitive function testing was only administered for the 2013 to 2014 NHANES cycle, Animal Fluency test scores were imputed for individuals in the 2015 to 2016 cycle based on the available cognitive function data and covariate data from both NHANES cycles.
Statistical Analysis
Sampling weights were used to provide nationally representative estimates with 95% CIs. We first calculated the number and proportion of adults classified as having hypertension, at goal BP, and recommended treatment according to the 2017 ACC/AHA guideline.
We then calculated the number and proportion of individuals with hypertension who met each trial inclusion and exclusion criteria. We categorized individuals with hypertension into 1 of 4 categories: (1) trial‐eligible (meeting all trial eligibility criteria); (2) excluded because of low cardiovascular risk (not meeting inclusion criteria because of young age or lack of cardiovascular risk factors); (3) excluded because of other criteria (meeting at least 1 exclusion criteria beside young age or low cardiovascular disease risk); or (4) excluded because of both low cardiovascular risk and other criteria. We examined these categories overall and stratified by age decade.
We next examined individuals recommended additional antihypertensive pharmacologic therapy (either to initiate pharmacologic treatment or to intensify existing treatment), again classifying individuals into the 4 trial eligibility categories, calculating population estimates stratified overall, by age, and by diabetes mellitus status. We also identified the total number of exclusion criteria met for each individual and the prevalence of individual exclusion criteria in the overall population and by age decade.
All analyses were conducted using Stata 14.1 (StataCorp LLC).
Results
We estimated that 107.7 million US adults would be diagnosed as having hypertension by the 2017 ACC/AHA guideline definition (Table 1). The minority of these individuals (23.1%; 95% CI, 20.8%–25.5%) were below the target BP of 130/80 mm Hg, 22.2% would be recommended nonpharmacologic treatment (95% CI, 20.1%–24.4%), and 54.6% would be recommended additional pharmacotherapy (95% CI, 52.5%–56.7%). Among adults aged <40 years, 17.8 million (21.2%) would be given a diagnosis of hypertension, of which only 8.4% would be at goal. While most of these younger adults would be recommended nonpharmacological interventions to achieve target BP, 5.9 million (33.1%) would be recommended antihypertensives by the 2017 ACC/AHA guideline. In contrast, the majority of adults aged >60 years would be recommended pharmacotherapy rather than nonpharmacologic treatment (1.1 million versus 32.6 million).
Table 1.
Prevalence of Controlled and Uncontrolled Hypertension in US Adults According to ACC/AHA Guideline, by Age
| No., Millions | Overall | Age 18–29 y | Age 30–39 y | Age 40–49 y | Age 50–59 y | Age 60–69 y | Age 70–79 y | Age 80+ y |
|---|---|---|---|---|---|---|---|---|
| Adult population | 231.8 (214.1–249.6) | 43.3 (39.7–46.9) | 40.6 (36.5–44.7) | 41.7 (37.2–46.1) | 42.6 (37.9–47.3) | 34.1 (29.9–38.2) | 18.8 (16.5–21.1) | 10.5 (8.6–12.3) |
| At goal | 24.9 (22.0–27.7) | 0.2 (0.1–0.4) | 1.3 (0.8–1.8) | 3.5 (2.9–4.1) | 6.3 (4.7–7.9) | 7.3 (6.1–8.5) | 3.7 (3.1–4.4) | 2.2 (1.6–2.8) |
| Hypertensive | 107.7 (99.3–116.0) | 7.0 (5.9–8.1) | 10.8 (9.2–12.5) | 17.7 (15.5–19.9) | 24.6 (22.0–27.3) | 24.1 (21.1–27.1) | 14.3 (12.6–16.0) | 8.8 (7.2–10.4) |
| Hypertensive currently taking pharmacotherapy | 56.2 (52.0–60.5) | 0.4 (0.2–0.7) | 3.1 (2.3–3.9) | 6.7 (5.9–7.6) | 12.5 (10.6–14.4) | 16.5 (14.7–18.3) | 10.6 (9.4–11.9) | 6.3 (5.0–7.6) |
| Recommended additional nonpharmacologic treatment* | 23.9 (20.6–27.1) | 5.2 (4.2–6.2) | 5.0 (4.1–6) | 6.6 (5.2–8.0) | 5.7 (4.7–6.8) | 1.1 (0.6–1.7) | 0 | 0 |
| Recommended additional pharmacotherapy* | 58.8 (53.7–63.8) | 1.5 (1.1–1.9) | 4.4 (3.5–5.3) | 7.5 (6.4–8.7) | 12.5 (10.9–14) | 15.5 (13.1–17.9) | 10.5 (9.0–12.0) | 6.6 (5.3–7.9) |
Values are population estimates in millions with 95% CIs unless. The American College of Cardiology/American Heart Association (ACC/AHA) guideline defined hypertension as a systolic blood pressure (SBP) ≥130 mm Hg or a diastolic blood pressure ≥80 mm Hg for the entire population. National Health and Nutrition Examination Survey participants were classified as hypertensive if the mean of 3 measured blood pressures (BPs) met guideline criteria or they reported currently taking antihypertensives regardless of recorded BP.
The ACC/AHA guideline recommends pharmacologic therapy to patients whose BP is >140/90 mm Hg and whose BP is between 130 to 139/80 to 89 mm Hg if they have a history of diabetes mellitus, age >65 years, or a 10‐year cardiovascular disease risk of >10% by pooled cohort risk equations. For all other patients not currently taking medications whose BP is between 130 to 139/80 to 89 mm Hg, nonpharmacological therapy is recommended.
Among all US adults with hypertension as classified by the 2017 ACC/AHA guideline, only 20.6% (95% CI, 18.8%–22.4%) would meet the eligibility criteria for the ACCORD trial or SPRINT. The representativeness of these trials in both the overall population with hypertension (Figure 1A) and those recommended additional antihypertensives (Figure 1B) varied greatly by age. Of the 58.8 million adults with hypertension recommended additional antihypertensive medications, trials were most representative of patients between the ages of 50 and 79 years. The majority of adults aged >50 years with hypertension and recommended additional treatment would have been excluded from intensive treatment trials becasuse of low cardiovascular risk; the majority of older adults would meet the inclusion criteria for increased cardiovascular risk but would be excluded because of other criteria.
Figure 1. Representativeness of intensive blood pressure (BP) treatment trials in US adults with hypertension as defined by the American College of Cardiology/American Heart Association (ACC/AHA) guideline, by age category.
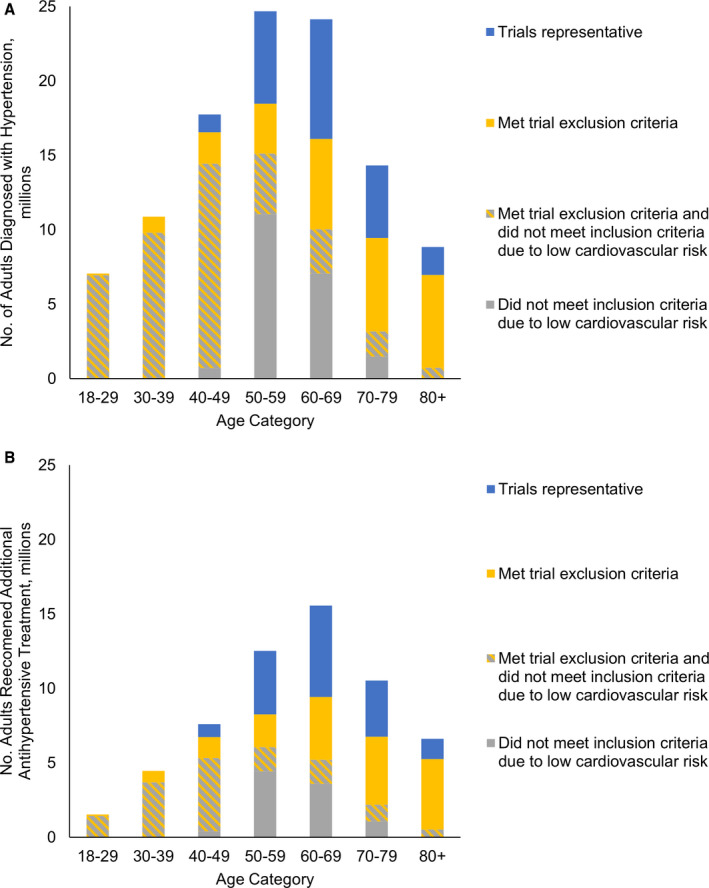
A, Adults diagnosed with hypertension; (B) adults recommended additional antihypertensive pharmacologic treatment. The 2017 ACC/AHA guideline defines hypertension based on BP >130/80 mm Hg. National Health and Nutrition Examination Survey analysis includes individuals with BP >130/80 mm Hg and those reporting use of BP medications regardless of measured BP. BP indicates blood pressure.
Of adults with hypertension recommended additional antihypertensive medications, 15.3 million (95% CI, 13.5–17.2 million) had diabetes mellitus and 43.5 million (95% CI, 39.2–47.8 million) did not. Among patients without diabetes mellitus, 26.9% would have met SPRINT eligibility criteria (95% CI, 24.0%–29.7%). SPRINT did not enroll patients aged <50 years and the majority of patients in older age groups would not have been eligible because of a combination of low cardiovascular risk and presence of exclusion criteria (Figure 2A). Among patients with diabetes mellitus, 30.7% would have met ACCORD eligibility criteria (95% CI, 25.7%–35.7%). The ACCORD trial was most representative of patients between the ages of 40 and 69 years (Figure 2B).
Figure 2. Trial eligibility of adults recommended additional antihypertensive pharmacologic treatment by the American College of Cardiology/American Heart Association (ACC/AHA) guideline, by diabetes mellitus status and age.
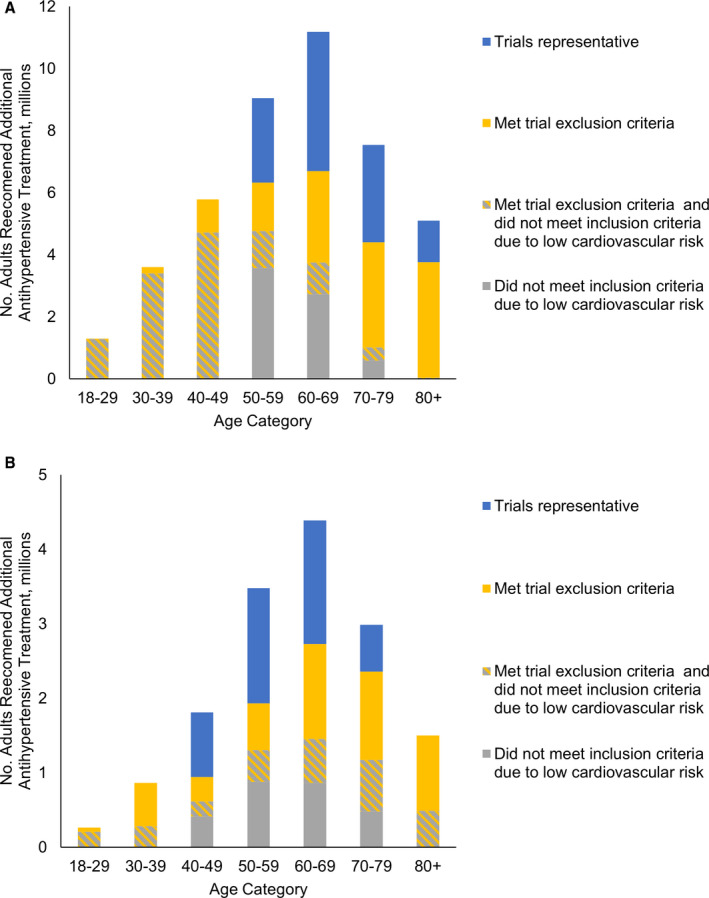
A, Adults without diabetes mellitus; (B) adults with diabetes mellitus. Individuals recommended additional antihypertensive pharmacologic treatment include those not previously taking any antihypertensives and those currently taking antihypertensives but above the ACC/AHA guideline goal of 130/80 mm Hg. Striped section refers to individuals whose estimated cardiovascular risk was lower than required for trial inclusion and who met at least 1 exclusion criteria.
Figure 3A depicts the prevalence of exclusion criteria among patients recommended additional antihypertensive medications stratified by age group. All adults aged <40 years were excluded because of trial age requirements, and most younger adults met at least 1 additional exclusion criteria. Following age, the most common exclusion criteria in the overall population were impaired cognition, lack of access to regular care, and medical comorbidities (Figure 3B). Table 2 depicts the individual reasons for exclusion, which differed greatly by age. In younger adults, exclusions related to comorbidities were uncommon, and the most common exclusion criteria were lack of access to regular medical care and alcohol abuse. In older adult populations, competing chronic conditions such as cancer, limited life expectancy, and impaired cognition were the primary reasons for trial exclusion. A small proportion of the hypertensive population met exclusion criteria because of stroke (3.1%), proteinuria (2.7%), chronic kidney disease (2.1%), or heart failure (1.8%), conditions that are often sequala of long‐standing uncontrolled hypertension.
Figure 3. Prevalence of hypertension trial exclusion criteria in the population recommended additional antihypertensive pharmacologic treatment by the American College of Cardiology/American Heart Association guideline.
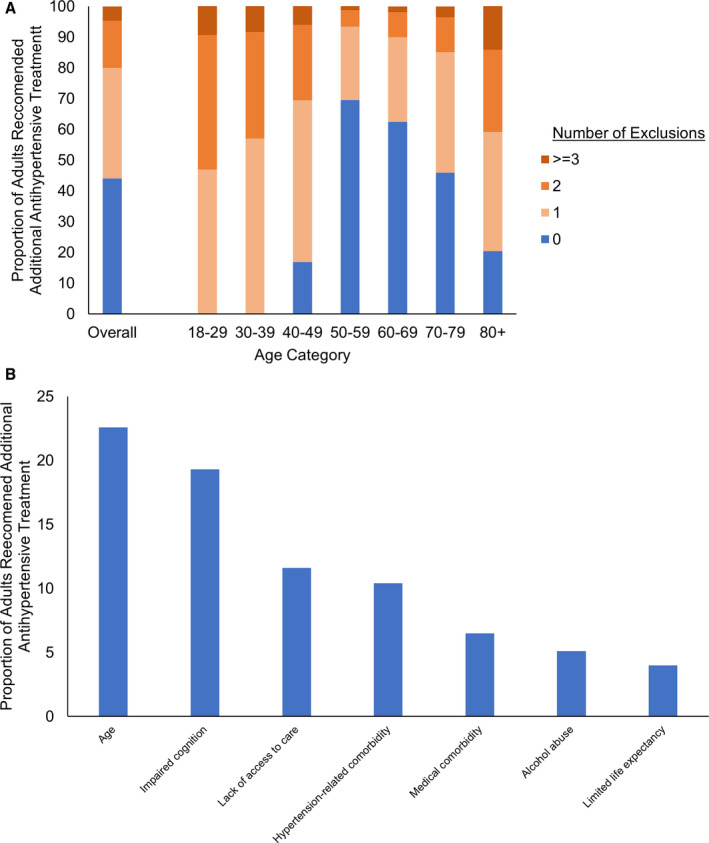
A, Number of exclusion criteria; (B) exclusion criteria. Medical comorbidity exclusions consist of recent cancer, liver disease, and elevated body mass index. Hypertension‐related comorbidity consisted of stroke, heart failure, chronic kidney disease, proteinuria, and systolic blood pressure >180 mm Hg.
Table 2.
Proportion of US Adults With Elevated BP Meeting Trial Inclusion and Exclusion Criteria
| Overall | Age 18–29 y | Age 30–39 y | Age 40–49 y | Age 50–59 y | Age 60–69 y | Age 70–79 y | Age 80+ y | |
|---|---|---|---|---|---|---|---|---|
| Population recommended additional pharmacotherapy, n (millions) (95% CI) | 58.8 (53.7–63.8) | 1.5 (1.1–1.9) | 4.4 (3.5–5.3) | 7.5 (6.4–8.7) | 12.5 (10.9–14.0) | 15.5 (13.1–17.9) | 10.5 (9.0–12.0) | 6.6 (5.3–7.9) |
| Increased cardiovascular risk, % (95% CI) | ||||||||
| Increased cardiovascular risk | 60.5 (57.8–63.2) | 11.4 (3.5–19.3) | 21.2 (14.1–28.3) | 34 (28.0–39.9) | 53.9 (47.7–60.1) | 68.3 (63.3–73.3) | 79.9 (75.2–84.6) | 92.6 (89.3–95.9) |
| History of cardiovascular condition | 11.4 (10.1–12.8) | 1.6 (0–5.0) | 4.3 (1.0–7.5) | 5.7 (3.3–8.2) | 6.0 (3.1–8.8) | 12.7 (9.2–16.2) | 16.1 (12.8–19.4) | 24.9 (20.6–29.2) |
| >1 Risk factor | 57.3 (54.8–59.8) | 11.1 (3.4–18.8) | 18.1 (11.4–24.8) | 30.5 (24.6–36.5) | 51.6 (45.5–57.6) | 65.6 (60.2–70.9) | 75.8 (70.8–80.8) | 87.2 (83.5–91) |
| Exclusion criteria, % excluded (95% CI) | ||||||||
| Age | 22.6 (20.6–24.6) | 100 | 100 | 76.2 (70.3–82.1) | 0.0 | 0.0 | 0.0 | 22.7 (18.1–27.3) |
| Proteinuria | 2.7 (2.0–3.4) | 3.2 (0.0–7.3) | 1.8 (0.0–4.3) | 3.3 (1.4–5.2) | 2.4 (0.9–3.9) | 2.2 (0.9–3.4) | 2.7 (1.0–4.4) | 4.1 (2.0–6.3) |
| BMI | 3.0 (2.2–3.7) | 12.0 (5.4–18.7) | 8.6 (3.4–13.8) | 5.4 (2.7–8.1) | 2.5 (0.5–4.4) | 2.3 (0.6–4.1) | 0.8 (0.0–1.6) | 0.2 (0.0–1.5) |
| Chronic kidney disease | 2.1 (1.5–2.7) | 1.1 (0.0–3.8) | 0.2 (0.0–1.3) | 1.2 (0.3–2.0) | 2.0 (0.0–4.1) | 1.8 (0.9–2.7) | 2.9 (1.3–4.4) | 4.5 (2.4–6.5) |
| Liver disease | 1.3 (0.8–1.8) | 0.4 (0.0–2.7) | 1.7 (0.0–3.5) | 1.0 (0.0–2.2) | 1.9 (0.1–3.6) | 1.2 (0.0–2.4) | 1.3 (0.0–3.1) | 0.5 (0.0–1.1) |
| Heart failure | 1.8 (1.2–2.3) | 0.0 | 0.5 (0.0–1.3) | 0.8 (0.0–1.6) | 0.8 (0.4–1.3) | 1.4 (0.8–2.0) | 3.5 (1.7–5.3) | 4.4 (1.8–6.9) |
| Stroke | 3.1 (2.3–3.9) | 0.0 | 0.4 (0.0–1.3) | 1.8 (0.2–3.9) | 2.7 (0.8–4.5) | 3.2 (1.4–5.1) | 3.3 (1.7–4.9) | 7.1 (4.2–10.1) |
| Cancer diagnosed in prior 2 y | 2.2 (1.5–2.9) | 0.0 | 0.6 (0.0–1.9) | 1.1 (0.0–2.8) | 1.8 (0.4–3.2) | 1.7 (0.7–2.7) | 3.2 (1.1–5.2) | 5.7 (2.9–8.5) |
| Alcohol abuse | 5.1 (3.9–6.2) | 12.1 (3.0–21.2) | 11.8 (5.6–18.1) | 9.9 (5.0–14.7) | 7.9 (4.6–11.2) | 2.6 (1.0–4.3) | 1.1 (0.3–1.9) | 0.1 (0.0–0.7) |
| Lack of access to regular care | 11.6 (9.8–13.4) | 32.8 (20.1–45.5) | 24.9 (16.6–33.3) | 17.7 (13.2–22.2) | 13.6 (9.3–17.8) | 8.6 (5.2–12) | 5.6 (3.9–7.4) | 3.4 (1.4–5.4) |
| Impaired cognition | 19.3 (16.6–22.1) | 0.0 | 0.0 | 0.0 | 0.0 | 19.4 (14.0–24.8) | 41.6 (33.1–50.2) | 60.1 (52.2–68) |
| Limited life expectancy | 4.0 (3.2–4.8) | 0.0 | 0.6 (0.0–1.6) | 0.2 (0.0–0.7) | 0.9 (0.0–1.9) | 2.2 (0.7–3.6) | 5.3 (3.4–7.1) | 19.8 (14.9–24.6) |
| Severely elevated BP | 2.9 (2.1–3.6) | 0.0 | 1.0 (0.0–2.2) | 1.6 (0.1–3) | 2.0 (0.8–3.3) | 3.1 (1.4–4.8) | 1.7 (0.4–3) | 8.9 (5.7–12.2) |
| Any exclusion | 56.0 (52.5–59.4) | 100 | 100 | 83.1 (77.7–88.5) | 30.4 (25.2–35.6) | 37.5 (31.9–43.1) | 54.1 (45.8–62.3) | 79.6 (73.8–85.4) |
| >2 exclusion | 19.9 (17.8–22) | 53.1 (39.0–67.2) | 43.0 (34.0–52.1) | 30.6 (23.6–37.5) | 6.7 (4.3–9.1) | 10.0 (6.9–13.0) | 14.8 (10.8–18.9) | 40.9 (34.8–47) |
BMI indicates body mass index; and BP, blood pressure.
Discussion
In this study of US adults, we estimated that over 107 million adults would be diagnosed with hypertension by the 2017 ACC/AHA guideline, the majority of whom would be recommended additional pharmacotherapy. Fewer than one quarter of adults with hypertension would meet the eligibility criteria for the major trials on which updated guideline recommendations are based, with an even smaller proportion of adults aged <40 years or >70 years meeting trial eligibility criteria. These results extend prior estimates of the representatives of the trial populations underlying the 2017 ACC/AHA guideline, 8 , 18 by describing the characteristics of patients excluded from intensive BP treatment trials. These results provide clinicians and health systems with important context on the populations for which evidence for intensive treatment is less robust and for whom tailoring of antihypertensive targets to individual patients is particularly salient. Our findings have key implications for the care of 3 large patient populations: adults aged <50 years, adults aged ≥70 years, and adults with diabetes mellitus.
Both SPRINT and the ACCORD trial were well‐designed, multisite, explanatory clinical trials comparing intensive and standard BP treatment targets. However, clinical trials are often not representative of the larger population receiving care in clinical practice. 19 In focusing exclusively on individuals with increased cardiovascular risk, SPRINT and the ACCORD trial leave a knowledge gap on the effectiveness of intensive BP treatment targets in the majority of adults aged <50 years, who largely have low cardiovascular risk. Prior clinical trials have not demonstrated cardiovascular benefit from treating mild hypertension in low‐risk populations 20 , 21 , 22 , 23 and well‐designed observational studies suggest a possible increased risk of adverse events. 24 Beyond low cardiovascular risk, SPRINT and the ACCORD trial excluded patients with lack of access to regular care because of concerns about reduced adherence to therapy. We found that over one quarter of adults aged 18 to 39 years reported a lack of routine source of care, suggesting a potential need to reach patients outside of traditional care settings. 25
SPRINT and the ACCORD trial also excluded patients with recent cancer, impaired cognition, and limited life expectancy, all conditions that primarily affect older adults, a population often underrepresented in clinical trials. 26 Older adults may have both a reduced likelihood of benefiting from intensive treatment because of competing risks of noncardiovascular death and an increased risk of adverse events related to polypharmacy, drug‐drug interactions, and medication confusion. 27 The relationship between BP lowering and risk of future cognitive impairment remains uncertain 28 , 29 , 30 , 31 ; however, no prior trials have examined BP lowering in populations with existing cognitive impairment. Thus, additional clinical trial research aimed at elucidating the balance of benefit and harms from intensive BP treatment is particularly crucial for this population given their elevated risk of both cardiovascular events and medication‐related harms.
Given the differences in trial design and outcomes of SPRINT and the ACCORD trial, understanding the limits of trial representativeness is particularly important for patients with diabetes mellitus. While patients with diabetes mellitus have increased cardiovascular risk and strong evidence exists for targeting SBP goals <140 mm Hg, 32 unlike SPRINT, the ACCORD trial did not demonstrate a significant benefit in mortality or cardiovascular events with more intensive BP lowering 7 and systematic review evidence of benefit for treatment targets of <140 mm Hg is mixed, even among trial‐eligible patients. 5 , 33 , 34 Given less clear benefits of intensive BP lowering, additional caution is needed in applying intensive treatment targets to patients with diabetes mellitus with multimorbidity or increased risk for medication adverse events.
Our findings have important implications for patients, clinicians, and health systems. For younger adults, clinicians should be aware that the risk benefit profile of treating mild hypertension in this population is not known and that guideline recommendations are based on expert consensus and extrapolation of results from higher risk populations. For the majority of younger adults with mildly elevated BP, the value added of diagnosing a chronic condition for which the recommended treatment of weight loss and exercise is already standard is unclear. For older adults, patient‐centered decision‐making that considers both likely benefits and risk associated with treatments, rather than a uniform adoption of strict BP thresholds, may be the best path forward, particularly for older adults with multimorbidity and limited life expectancy. Notably, controversy on treatment targets remains among older adults, with the American College of Physicians and American Academy of Family Physicians recommending an SBP treatment target of 150 mm Hg for most older adults. 35 , 36
From a population health perspective, full implementation of the 2017 ACC/AHA guideline could prevent as many as 3 million cardiovascular disease events over 10 years. 37 Additionally, intensive SBP control has been shown to be cost‐effective in SPRINT‐eligible individuals. 38 However, these results may not extend to younger low‐risk populations, older multimorbid populations, or patients with diabetes mellitus. Thus, caution is necessary in constructing population health initiatives that set performance metrics and financial incentives based on achieving guideline‐directed goal BPs. Benchmarks focused on standardized adoption of strict BP thresholds may discourage clinician efforts to engage in patient‐centered decision‐making recommended by guidelines. Furthermore, as the marginal cardiovascular risk reduction is greater for lowering BP with severely elevated BP than for lowering BP from 140 mm Hg to 130 mm Hg, efforts to reach high‐risk populations with barriers to adherence or access to care 39 , 40 may be more beneficial than focusing further lowering previously treated patients who are near goal.
This study has several limitations. BP was measured at a single visit in NHANES, which contrasts with 2017 ACC/AHA guideline recommendations to diagnose hypertension based on measurements obtained from multiple visits, although both approaches may be poor approximations of routine clinical practice to which the guidelines are set to apply. Consistent with other NHANES studies, our definition of controlled hypertension required individuals to report taking BP medications and thus may underestimate the total number of individuals with hypertension by omitting those whose BP is controlled by lifestyle modifications only; however, this would not change our study findings as these individuals would not be recommended additional pharmacotherapy. NHANES comorbidity questions relied primarily on self‐report, and NHANES did not collect information on all trial inclusion and exclusion criteria; specifically, the survey lacked information on subclinical cardiovascular disease (ie, coronary calcium score, ankle brachial index, left ventricular hypertrophy, or carotid stenosis) and history of poor adherence with medical care. As a result of NHANES reporting age top‐coded at 80 years, our estimate of life expectancy is likely to be conservative. Cognitive function testing was assessed using a single validated screening instrument rather than comprehensive cognitive testing. Finally, patients residing in nursing homes were excluded from both clinical trials and NHANES, thus this population of >1 million older adults is not represented in this study, although similar generalizability concerns exist.
Conclusions
The majority of younger adults and older adults diagnosed with hypertension and recommended additional antihypertensive treatment by the 2017 ACC/AHA guideline would not have been eligible from the clinical trials underlying more intensive BP treatment thresholds. Clinical trials studying the outcomes of intensive BP treatment in younger adults at low cardiovascular risk and in older adults with multimorbidity are urgently needed, and, until these data are available, a patient‐centered approach that tailors treatments and targets by degree of BP elevation, comorbidity, and likelihood of benefit is likely preferable to universal adoption of intensive treatment targets.
Sources of Funding
Dr Anderson was supported by the National Institute on Aging (L30AG060493 and R03AG064373). Dr Bellows was supported by the National Heart, Lung, and Blood Institute (K01HL140170). Dr Bibbins‐Domingo was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK103992). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or approval of the article; and decision to submit the article for publication. Dr Bibbins‐Domingo is the former chair of the US Preventative Services Task Force (USPSTF). This article reflects her own work and not the official positions of the USPSTF.
Disclosures
Dr Odden reports personal fees from Cricket Health outside the submitted work. The remaining authors have no disclosures to report.
Acknowledgments
Dr Anderson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Anderson, Odden, Penko, Bibbins‐Domingo. Acquisition, analysis, or interpretation of data: all authors. Drafting of the article: Anderson. Critical revision of the article for important intellectual content: all authors. Statistical analysis: Anderson. Obtained funding: not applicable. Administrative, technical, or material support: Penko. Study supervision: Bibbins‐Domingo.
(J Am Heart Assoc. 2021;10:e019707. DOI: 10.1161/JAHA.120.019707.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 2. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. DOI: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al; for the National High Blood Pressure Education Program Coordinating Committee . The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2571. [DOI] [PubMed] [Google Scholar]
- 4. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. DOI: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EP III, Polonsky T, Thompson‐Paul AM, Vupputuri S. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138:e595–e616. DOI: 10.1016/j.jacc.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 6. SPRINT Research Group , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A Randomized Trial of Intensive versus Standard Blood‐Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ACCORD Study Group , Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson TS, Odden M, Penko J, Kazi DS, Bellows BK, Bibbins‐Domingo K. Generalizability of clinical trials supporting the 2017 American College of Cardiology/American Heart Association blood pressure guideline. JAMA Intern Med. 2020;180:795–797. DOI: 10.1001/jamainternmed.2020.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . National Center for Health Statistics. National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed April 15, 2019.
- 10. Langkamp DL, Lehman A, Lemeshow S. Techniques for handling missing data in secondary analyses of large surveys. Acad Pediatr. 2010;10:205–210. DOI: 10.1016/j.acap.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ACCORD Study Group , Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. [DOI] [PubMed] [Google Scholar]
- 12. Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4‐year mortality in older adults. JAMA. 2006;295:801–808. DOI: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 13. Lee SJ, Boscardin WJ, Kirby KA, Covinsky KE. Individualizing life expectancy estimates for older adults using the Gompertz Law of Human Mortality. PLoS One. 2014;9:e108540. DOI: 10.1371/journal.pone.0108540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henry JP, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta‐analysis. Neuropsychologia. 2004;42:1212–1222. DOI: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15. Canning SD, Leach L, Stuss D, Ngo L, Black SE. Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology. 2004;62:556–562. DOI: 10.1212/WNL.62.4.556. [DOI] [PubMed] [Google Scholar]
- 16. Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR Jr, Galasko DR, Doody R, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. DOI: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 17. Clark LJ, Gatz M, Zheng L, Chen YL, McCleary C, Mack WJ. Longitudinal verbal fluency in normal aging, preclinical and prevalent Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2009;24:461–468. DOI: 10.1177/1533317509345154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of SPRINT results to the US adult population. J Am Coll Cardiol. 2016;67:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kennedy‐Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. DOI: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helgeland A. Treatment of mild hypertension: a five year controlled drug trial: the Oslo study. Am J Med. 1980;69:725–732. DOI: 10.1016/0002-9343(80)90438-6. [DOI] [PubMed] [Google Scholar]
- 21. Neaton JD, Grimm RH Jr, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, Cutler JA, Flack JM, Schoenberger JA, McDonald R, et al. Treatment of mild hypertension study research group. Treatment of mild hypertension study: final results. JAMA. 1993;270:713–724. DOI: 10.1001/jama.1993.03510060059034. [DOI] [PubMed] [Google Scholar]
- 22. Lonn EM, Bosch J, López‐Jaramillo P, Zhu J, Liu L, Pais P, Diaz R, Xavier D, Sliwa K, Dans A, et al; HOPE‐3 Investigators . Blood‐pressure lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–2020. DOI: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 23. Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev. 2012;8:CD006742. DOI: 10.1002/14651858.CD006742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheppard JP, Stevens S, Stevens R, Martin U, Mant J, Hobbs FD, McManus RJ. Benefits and harms of antihypertensive treatment in low‐risk patients with mild hypertension. JAMA Intern Med. 2018;178:1626–1634. DOI: 10.1001/jamainternmed.2018.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx‐Drew D, et al. A cluster‐randomized trial of blood‐pressure reduction in black barbershops. N Engl J Med. 2018;378:1291–1301. DOI: 10.1056/NEJMoa1717250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lockett J, Sauma S, Radziszewska B, Bernard MA. Adequacy of inclusion of older adults in NIH‐funded phase III clinical trials. J Am Geriatr Soc. 2019;67:218–222. DOI: 10.1111/jgs.15786. [DOI] [PubMed] [Google Scholar]
- 27. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. DOI: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 28. Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, Bossini A, Fagard R, Gil‐Extremera B, Laks T, et al; Systolic Hypertension in Europe Investigators . The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst‐Eur) study. Arch Intern Med. 2002;162:2046–2052. [DOI] [PubMed] [Google Scholar]
- 29. Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, et al; HYVET Investigators . Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET‐COG): a double‐blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. DOI: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 30. Williamson JD, Launer LJ, Bryan RN, Coker LH, Lazar RM, Gerstein HC, Murray AM, Sullivan MD, Horowitz KR, Ding J, et al; Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Investigators . Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324–333. DOI: 10.1001/jamainternmed.2013.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. SPRINT MIND Investigators for the SPRINT Research Group , Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2015;313:603–615. DOI: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 33. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta‐analyses. BMJ. 2016;352:i717. DOI: 10.1136/bmj.i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. DOI: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 35. Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA; Clinical Guidelines Committee of the American College of Physicians and the Commission on Health of the Public and Science of the American Academy of Family Physicians . Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166:430–437. DOI: 10.7326/M16-1785. [DOI] [PubMed] [Google Scholar]
- 36. Weiss J, Freeman M, Low A, Fu R, Kerfoot A, Paynter R, Motu'apuaka M, Kondo K, Kansagara D. Benefits and harms of intensive blood pressure treatment in adults aged 60 years or older: a systematic review and meta‐analysis. Ann Intern Med. 2017;166:419–429. DOI: 10.7326/M16-1754. [DOI] [PubMed] [Google Scholar]
- 37. Bress AP, Colantonio LD, Cooper RS, Kramer H, Booth JN, Odden MC, Bibbins‐Domingo K, Shimbo D, Whelton PK, Levitan EB, et al. Potential cardiovascular disease events prevented with adoption of the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline. Circulation. 2019;139:24–36. DOI: 10.1161/CIRCULATIONAHA.118.035640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, Berlowitz DR, Conroy MB, Fine L, Oparil S, et al. Cost‐effectiveness of intensive versus standard blood‐pressure control. N Engl J Med. 2017;377:745–755. DOI: 10.1056/NEJMsa1616035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fontil V, Bibbins‐Domingo K, Kazi DS, Sidney S, Coxson PG, Khanna R, Victor RG, Pletcher MJ. Simulating strategies for improving control of hypertension among patients with usual source of care in the United States: the blood pressure control model. J Gen Intern Med. 2015;30:1147–1155. DOI: 10.1007/s11606-015-3231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fontil V, Gupta R, Moise N, Chen E, Guzman D, McCulloch CE, Bibbins‐Domingo K. Adapting and evaluating a health system intervention from Kaiser Permanente to improve hypertension management and control in a large network of safety‐net clinics. Circ Cardiovasc Qual Outcomes. 2018;11:e004386. DOI: 10.1161/CIRCOUTCOMES.117.004386. [DOI] [PMC free article] [PubMed] [Google Scholar]


