Abstract
Background
Obese patients have lower NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels. The prognostic implications of achieving NT‐proBNP levels ≤1000 pg/mL in obese patients with heart failure (HF) receiving biomarker‐guided therapy are not completely known. We evaluated the prognostic implications of obesity and having NT‐proBNP levels (≤1000 pg/mL) in the GUIDE‐IT (Guiding Evidence‐Based Therapy Using Biomarker‐Intensified Treatment in HF) trial participants.
Methods and Results
The risk of adverse cardiovascular events (HF hospitalization or cardiovascular mortality) was assessed using multivariable‐adjusted Cox proportional hazard models based on having NT‐proBNP ≤1000 pg/mL (taken as a time‐varying covariate), stratified by obesity status. The study outcome was also assessed on the basis of the body mass index at baseline. The predictive ability of NT‐proBNP for adverse cardiovascular events was assessed using the likelihood ratio test. Compared with nonobese patients, obese patients were mostly younger, Black race, and more likely to be women. NT‐proBNP levels were 59.0% (95% CI, 39.5%–83.5%) lower among obese individuals. The risk of adverse cardiovascular events was lower in obese (hazard ratio [HR], 0.48; 95% CI, 0.29–0.59) and nonobese (HR, 0.32; 95% CI, 0.19–0.57) patients with HF who had NT‐proBNP levels ≤1000 pg/mL, compared with those who did not. There was no interaction between obesity and having NT‐proBNP ≤1000 pg/mL on the study outcome (P>0.10). Obese patients had a greater risk of developing adverse cardiovascular events (HR, 1.39; 95% CI, 1.01–1.90) compared with nonobese patients. NT‐proBNP was the strongest predictor of adverse cardiovascular event risk in both obese and nonobese patients.
Conclusions
On‐treatment NT‐proBNP level ≤1000 pg/mL has favorable prognostic implications, irrespective of obesity status. NT‐proBNP levels were the strongest predictor of cardiovascular events in both obese and nonobese individuals in this trial.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01685840.
Keywords: cardiovascular outcomes, heart failure, mortality, natriuretic peptides, obesity
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- GDMT
guideline‐directed medical therapy
- GUIDE‐IT
Guiding Evidence‐Based Therapy Using Biomarker‐Intensified Treatment in Heart Failure
- HFrEF
heart failure with reduced ejection fraction
- NP
natriuretic peptide
- NYHA
New York Heart Association
Clinical Perspective
What Is New?
NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels, a strong prognostic biomarker, are ≈59% lower in obese patients with heart failure with reduced ejection fraction.
Increasing body mass index and obesity in patients with heart failure with reduced ejection fraction is associated with a higher risk of an adverse cardiovascular event, irrespective of whether the biomarker‐based strategy guided clinical care was used or not.
What Are the Clinical Implications?
NT‐proBNP levels are the strongest prognostic markers for adverse cardiovascular events, irrespective of the obesity status.
Compared with those with NT‐proBNP levels of >1000 pg/mL, patients with heart failure with reduced ejection fraction with on‐treatment NT‐proBNP levels of ≤1000 pg/mL have a lower risk of heart failure hospitalization or death.
Obesity is a growing epidemic in the United States and a major risk factor for the development of heart failure (HF). 1 , 2 , 3 , 4 , 5 , 6 , 7 However, once HF develops, overweight and obese patients have paradoxically been reported to have a better prognosis compared with those who are normal or underweight. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Natriuretic peptides (NPs) (specifically, B‐type NP and NT‐proBNP [N‐terminal pro‐B‐type NP]) are the gold standard biomarkers for HF risk stratification, diagnosis, and prognostication. They are also unexpectedly lower in obese individuals. 7 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
Despite having lower NP levels than nonobese patients, prior data indicate that NT‐proBNP levels may retain diagnostic and prognostic significance among obese patients presenting with acute dyspnea or advanced HF. 7 , 19 , 20 , 21 , 24 , 25 The results from the multicenter, randomized GUIDE‐IT (Guiding Evidence‐Based Therapy Using Biomarker‐Intensified Treatment in HF) trial, evaluating the role of NT‐proBNP levels to guide HF management, were neutral. 26 Secondary analyses from the GUIDE‐IT trial and prior studies indicate that those who achieved the NT‐proBNP levels of ≤1000 pg/mL had a lower risk of adverse cardiovascular events. 17 , 27 , 28 , 29 , 30 However, the prognostic implications of having on‐treatment NT‐proBNP ≤1000 pg/mL in obese patients with HF receiving biomarker‐guided HF therapy are not known. Moreover, the implications of obesity, a known NP deficient state, in the setting of a guided HF trial, are not known.
We hypothesize that obese patients with HF have a greater risk of adverse cardiovascular events than nonobese patients, which is predicted by NT‐proBNP levels and improves with the attainment of NT‐proBNP levels of ≤1000 pg/mL. We examined the participants of the GUIDE‐IT trial to characterize HF with reduced ejection fraction (HFrEF) in obese patients by evaluating the prognostic implications of body mass index (BMI), baseline NT‐proBNP, and having NT‐proBNP levels of ≤1000 pg/mL in obese patients with HF receiving biomarker‐guided HF therapy. We also analyzed the optimization of guideline‐directed medical therapy (GDMT) during the study period, stratified by obesity status.
Methods
Anonymized study data are available at the National Heart, Lung, and Blood Institute BioLINCC data repository and can be accessed at https://biolincc.nhlbi.nih.gov/home/. The GUIDE‐IT trial was a multicenter, controlled, unblinded trial that randomized 894 patients with HFrEF to biomarker‐guided and standard evidence‐based medical care for the management of HF. 26 , 31 The National Heart, Lung, and Blood Institute–sponsored study was conducted at 45 clinical sites in the United States and Canada, between January 16, 2013, and September 20, 2016. The local institutional review boards at the respective trial sites approved the study, and all participants provided written informed consent. 26 , 31
Inclusion and Exclusion Criteria
The study rationale, design, inclusion and exclusion criteria, and results of the trial have been previously described. 26 , 31 In brief, the trial enrolled patients with recently documented systolic HF (left ventricular ejection fraction ≤40% within 12 months before randomization), having had an HF event (HF hospitalization, emergency department visit for HF, or outpatient HF management with intravenous diuretics) in the preceding 12 months and elevated NP levels (B‐type NP >400 pg/mL or NT‐proBNP >2000 pg/mL) in the 30 days before randomization. We included 873 participants with baseline BMI and NT‐proBNP data available.
Study Procedures
The GUIDE‐IT trial participants underwent extensive baseline testing and physical examination before simple, unrestricted randomization to respective HF management arms. Subjects were followed up at 2 weeks, 6 weeks, 3 months, and then every 3 months for the remaining study period. Each follow‐up visit entailed the collection of blood for NT‐proBNP assessment locally (in biomarker‐guided arm only) and additional sample collection for centralized NT‐proBNP testing (in both arms) at the biomarker core laboratory in Duke Clinical Research Institute, Durham, NC. 31 The results from core laboratory assessments were not transmitted back to the investigators. The follow‐up visits also involved focused history collection, physical examination, and medication adjustment with the documentation of the rationale behind the adjustments. Although the specific medication adjustments were at the discretion of the treating physicians, prioritization of the neurohormonal antagonist titration was encouraged over diuretics in the absence of volume overload or congestion. 26 , 31 The prespecified primary outcome for the GUIDE‐IT trial was the time‐to‐first HF hospitalization or cardiovascular cause of death, which was adjudicated by a blinded clinical events committee. The trial was discontinued by the National Heart, Lung, and Blood Institute–appointed data and safety monitoring board at 81% of planned enrollment after 50% of planned primary end point events had occurred, as the study met the prespecified inefficiency criteria. 26
Measures and Outcomes
The GUIDE‐IT trial participants were stratified on the basis of baseline BMI into quartiles and into obese (BMI ≥30 kg/m2) and nonobese groups (BMI <30 kg/m2). The medical therapy use was evaluated at each study visit and computed as the GDMT score. 17 Having NT‐proBNP levels of ≤1000 pg/mL and the GDMT score were treated as time‐varying covariates, computed at each visit. The change in New York Heart Association (NYHA) class from baseline was taken as a time‐varying covariate in secondary analysis. Achievement of NT‐proBNP levels ≤1000 pg/mL among patients with HF has been shown to be associated with a lower risk of adverse cardiovascular events. 17 , 27 , 28 , 29 , 30 Furthermore, the biomarker‐guided treatment arm of the GUIDE‐IT trial required titration of the medical therapy to an NT‐proBNP target of <1000 pg/mL. 26 Hence, given the high clinical significance, the NT‐proBNP levels were dichotomized at 1000 pg/mL in this study. The outcome of interest was the development of adverse cardiovascular events (composite of HF hospitalization and death attributable to cardiovascular causes).
Statistical Analysis
The baseline characteristics were compared using descriptive statistics. The continuous variables were summarized as median and the interquartile range (25th–75th percentile), and compared using the Wilcoxon rank‐sum or Kruskal‐Wallis tests. The categorical variables were summarized as counts and percentages and compared using the χ2 or Fisher exact tests. NT‐proBNP was log transformed because of its skewed distribution, and log‐transformed values were used in all analyses. The percentage difference in NT‐proBNP levels between obese and nonobese individuals was computed, as previously described (Data S1, Table S1). 16 , 17 , 30 , 32 , 33 , 34 The NT‐proBNP levels at each study visit were compared between the obese and nonobese groups.
The hazard for the development of adverse cardiovascular outcome in obese individuals, nonobese individuals, and across quartiles of baseline BMI was determined using multivariable‐adjusted Cox proportional hazard models. The model was adjusted for known clinical correlates of NPs, 16 , 17 , 30 , 32 , 33 , 34 such as age, sex, race (Black race versus others), blood urea nitrogen, left ventricular ejection fraction, diabetes mellitus, GDMT score (time varying), systolic and diastolic blood pressure, NYHA class, ischemic heart disease, serum potassium, history of cancer in past 5 years, sleep apnea, log‐transformed NT‐proBNP as a time‐varying covariate, and treatment strategy (biomarker‐guided versus usual care arm). The covariate description and model fit statistics are provided in Data S1. The proportional hazard assumption was assessed using Schoenfeld residuals for all covariates. Potential interaction by treatment strategy for the study outcome was assessed using a multiplicative interaction term (obese status×treatment group). In a sensitivity analysis, we performed nonlinear (restricted cubic spline) survival analyses, as described previously, 33 to assess the relationship of baseline BMI on the study outcome in the Cox regression model, adjusted for the above‐mentioned covariates. The relative strength of association between the risk of adverse cardiovascular outcomes and various baseline characteristics, stratified by obesity status, was evaluated in multivariable‐adjusted Cox models and compared using the likelihood ratio test, using previously described methods. 16 , 35 , 36 In brief, multivariate regression models with and without respective covariates were used to calculate the χ2 values for each covariate.
The prognostic importance (risk of adverse cardiovascular events) of having NT‐proBNP ≤1000 pg/mL, overall and in both obese and nonobese individuals, was assessed using Cox regression models, taking NT‐proBNP ≤1000 pg/mL as a time‐varying covariate. The model was adjusted for baseline log‐transformed NT‐proBNP levels and BMI, along with other above‐mentioned covariates. The interaction between obesity and NT‐proBNP ≤1000 pg/mL levels on the study outcome was done using a multiplicative interaction term (obese status × having NT‐proBNP ≤1000 pg/mL) in Cox regression analysis. Descriptive reporting of the reasons for the lack of titration of medications and inadequate medication adjustment in the biomarker‐guided treatment arm (defined as having NT‐proBNP ≤1000 pg/mL with no change in dosage of angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, β‐blocker, or mineralocorticoid receptor antagonist) was assessed and stratified by obesity status. In the secondary analyses, we assessed the prognostic ability of per unit increase in log NT‐proBNP baseline and on‐treatment log NT‐proBNP levels in obese and nonobese individuals using multivariable‐adjusted Cox regression. In an additional analysis, we also evaluated the prognostic ability of change in NYHA class (from baseline) for risk of adverse cardiovascular outcomes in obese and nonobese individuals using multivariable‐adjusted Cox regression. The models were adjusted for the above‐mentioned covariates. All statistical analyses were done using Stata SE 16.0 (StataCorp, College Station, TX), with a 2‐sided type I error of 0.05 considered statistically significant.
Results
Among 873 patients with HFrEF from the GUIDE‐IT trial, 42% were obese. Obese patients were younger (58 [25th–75th percentile, 49–66] years versus 66 [25th–75th percentile, 57–75] years; P<0.001) and more likely to be women (38.1% versus 27.7%; P<0.001) and Black race (43.9% versus 30.9%; P<0.001) than nonobese patients. Obese patients also had a higher prevalence of hypertension (83.4% versus 76.1%; P=0.01) and diabetes mellitus (54.2% versus 40%; P<0.001), with a higher GDMT score (7 [25th–75th percentile, 4–9]; P<0.001), than nonobese patients (Table 1). The median baseline NT‐proBNP values were 59.0% (95% CI, 39.5%–83.5%) lower among obese individuals (1964 [25th–75th percentile, 1145–3643] versus 3412 [25th–75th percentile, 1847–6771] pg/mL; adjusted P<0.001) than nonobese individuals. The baseline characteristics of the study population across the BMI quartiles are described in Table 2.
Table 1.
Baseline Characteristics of Study Participants: Stratified by Obesity Status
| Characteristics | Nonobese (N=506) | Obese (N=367) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y | 66 (57–75) | 58 (49–66) | <0.001 |
| Black race | 163 (30.9) | 161 (43.9) | <0.001 |
| Men | 381 (72.3) | 227 (61.9) | 0.001 |
| Medical history | |||
| Alcohol abuse | 69 (13.1) | 33 (9.0) | 0.69 |
| Atrial fibrillation | 82 (15.8) | 63 (17.4) | 0.60 |
| Cancer | 34 (6.5) | 10 (2.7) | 0.01 |
| CKD | 190 (36.1) | 140 (38.2) | 0.57 |
| COPD | 107 (20.3) | 86 (23.4) | 0.28 |
| Depression treated with medication | 79 (15) | 62 (16.9) | 0.46 |
| Diabetes mellitus | 211 (40.0) | 199 (54.2) | <0.001 |
| Duration of HF, mo | 11.0 (1.0–60.0) | 21.5 (1.0–81.5) | 0.07 |
| Dyslipidemia | 302 (57.4) | 222 (60.5) | 0.37 |
| History of IHD | 295 (56.1) | 152 (41.4) | <0.001 |
| History of smoking | 188 (35.7) | 116 (31.6) | 0.22 |
| Hypertension | 400 (76.1) | 306 (83.4) | 0.01 |
| ICD or pacemaker | 238 (45.3) | 157 (42.8) | 0.49 |
| Peripheral arterial disease | 62 (11.8) | 32 (8.7) | 0.15 |
| Prior MI | 174 (33.1) | 77 (21) | <0.001 |
| Sleep apnea | 54 (10.3) | 147 (40.1) | <0.001 |
| Stroke | 57 (10.8) | 38 (10.4) | 0.91 |
| Ventricular tachycardia/fibrillation | 99 (18.8) | 61 (16.7) | 0.42 |
| Clinical assessment | |||
| 6‐min Walk test distance, m | 300.0 (199.0–375.0) | 281.9 (165.0–365.7) | 0.03 |
| Ascites | 30 (5.7) | 16 (4.4) | 0.50 |
| BMI, kg/m2 | 25.2 (22.8–27.5) | 35.1 (32.2–39.7) | <0.001 |
| DBP, mm Hg | 68 (60–78) | 72 (63–80) | <0.001 |
| Heart rate, beats/min | 75 (66–85) | 78 (68–88) | 0.01 |
| JVD | 144 (28.9) | 99 (29.2) | 0.79 |
| LVEF, % | 23.0 (20.0–30.0) | 24.0 (20.0–30.0) | 0.63 |
| NYHA class | 0.006 | ||
| I | 39 (7.4) | 39 (7.4) | |
| II | 279 (53.2) | 279 (53.2) | |
| III | 193 (36.8) | 193 (36.8) | |
| IV | 7 (1.3) | 7 (1.3) | |
| Orthopnea | 0.27 | ||
| None | 158 (30.2) | 158 (30.2) | |
| Mild | 156 (29.8) | 156 (29.8) | |
| Moderate | 133 (25.4) | 133 (25.4) | |
| Severe | 52 (9.9) | 52 (9.9) | |
| Percent oxygen saturation (SpO2) | 98 (96–99) | 97 (95–98) | 0.003 |
| Peripheral edema | 199 (51.8) | 185 (48.2) | 0.47 |
| Rales | 63 (12.5) | 37 (10.1) | 0.06 |
| S3 gallop | 51 (9.7) | 30 (8.2) | 0.63 |
| SBP, mm Hg | 112 (100–126) | 116 (103–130) | 0.02 |
| Medications at baseline | |||
| ACE inhibitor | 333 (63.6) | 232 (63.2) | 0.94 |
| ARBs | 93 (17.8) | 56 (15.3) | 0.36 |
| β‐Blocker | 499 (95.2) | 346 (94.3) | 0.47 |
| CCB | 29 (5.5) | 39 (10.6) | 0.01 |
| GDMT score | 6 (4–8) | 7 (4–9) | <0.001 |
| Loop diuretic | 491 (93.7) | 358 (97.6) | 0.01 |
| Mineralocorticoid receptor antagonist | 256 (48.9) | 188 (51.2) | 0.50 |
| Statin | 309 (59) | 221 (60.2) | 0.87 |
| Laboratory data | |||
| BUN, mg/dL | 22.0 (13.0–33.0) | 23.0 (16.0–35.0) | 0.04 |
| Creatinine, mg/dL | 1.29 (1.0–1.7) | 1.3 (1.1–1.7) | 0.48 |
| NT‐proBNP, pg/mL | 3412.0 (1847.0–6771.0) | 1964.0 (1145.0–3643.0) | <0.001 |
| Serum potassium, mmol/L | 4.4 (4.1–4.8) | 4.2 (3.9–4.6) | <0.001 |
| Serum sodium, mmol/L | 138.0 (136.0–141.0) | 139.0 (136.0–141.0) | 0.40 |
| Biomarker‐guided therapy arm | 266 (50.5) | 180 (49.1) | 0.68 |
Data represented as count (percentage) and median (25th–75th percentile). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CCB, calcium channel blocker; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; GDMT, guideline‐directed medical therapy; HF, heart failure; ICD, implantable cardioverter‐defibrillator; IHD, ischemic heart disease; JVD, jugular venous distension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; and SBP, systolic blood pressure.
Table 2.
Baseline Characteristics of Study Participants: Stratified by BMI Quartiles
| Characteristics | Quartile 1 (<24.6 kg/m2) (N=218) |
Quartile 2 (24.6–28.8 kg/m2) (N=218) |
Quartile 3 (28.8–33.8 kg/m2) (N=219) |
Quartile 4 (>33.8 kg/m2) (N=218) | P Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 68 (58–77) | 65 (56–74) | 63 (54–71) | 56 (46–65) | <0.001 |
| Men | 157 (72.0) | 160 (73.4) | 155 (70.8) | 125 (57.3) | 0.001 |
| Black race | 64 (29.4) | 69 (31.7) | 74 (33.8) | 111 (50.9) | <0.001 |
| Medical history | |||||
| Alcohol abuse | 40 (18.4) | 24 (11) | 19 (8.7) | 18 (8.3) | 0.005 |
| Atrial fibrillation | 34 (15.7) | 38 (17.8) | 41 (18.8) | 31 (14.5) | 0.62 |
| Cancer | 19 (8.7) | 13 (6) | 7 (3.2) | 5 (2.3) | 0.01 |
| CKD | 68 (31.2) | 79 (36.2) | 85 (38.8) | 92 (42.2) | 0.11 |
| COPD | 48 (22) | 42 (19.3) | 47 (21.5) | 52 (23.9) | 0.71 |
| Depression treated with medication | 32 (14.7) | 34 (15.6) | 38 (17.4) | 35 (16.1) | 0.90 |
| Diabetes mellitus | 74 (33.9) | 93 (42.7) | 116 (53) | 120 (55.1) | <0.001 |
| Duration of HF, mo | 5.0 (1.0–44.0) | 13.0 (1.0–51.0) | 24.0 (1.0–84.0) | 24.0 (1.0–84.0) | 0.003 |
| Dyslipidemia | 108 (49.5) | 136 (62.4) | 146 (66.7) | 122 (56) | 0.002 |
| History of IHD | 123 (56.4) | 122 (56) | 114 (52.1) | 78 (35.8) | <0.001 |
| History of smoking | 85 (39) | 76 (34.9) | 68 (31.1) | 69 (31.7) | 0.28 |
| Hypertension | 152 (69.7) | 175 (80.3) | 174 (79.5) | 189 (86.7) | <0.001 |
| ICD or pacemaker | 82 (37.6) | 106 (48.6) | 99 (45.2) | 99 (45.4) | 0.12 |
| Peripheral arterial disease | 27 (12.4) | 29 (13.3) | 24 (11.0) | 14 (6.4) | 0.08 |
| Prior MI | 80 (36.7) | 66 (30.3) | 61 (27.9) | 41 (18.8) | <0.001 |
| Sleep apnea | 18 (8.3) | 23 (10.6) | 59 (26.9) | 99 (45.4) | <0.001 |
| Stroke | 29 (13.3) | 21 (9.6) | 20 (9.1) | 21 (9.6) | 0.47 |
| Ventricular tachycardia/fibrillation | 35 (16.1) | 43 (19.7) | 37 (16.9) | 41 (18.9) | 0.73 |
| Clinical assessment | |||||
| 6‐min Walk test distance, m | 300.0 (195.6–366.5) | 297.0 (196.5–372.0) | 305.0 (204.0–381.2) | 257.5 (148.2–356.8) | 0.02 |
| Ascites | 10 (4.6) | 16 (7.3) | 9 (4.1) | 9 (4.1) | 0.60 |
| BMI, kg/m2 | 22.4 (21.0–23.7) | 26.5 (25.7–27.6) | 30.9 (29.8–32.3) | 38.8 (36–43.9) | <0.001 |
| DBP, mm Hg | 68 (60–76) | 69 (60–79) | 70 (60–82) | 72 (64–80) | <0.001 |
| Heart rate, beats/min | 75 (65.5–85) | 77 (67–86) | 75 (68–86) | 80 (68–88) | 0.07 |
| JVD | 60 (28.8) | 59 (28.6) | 52 (25.1) | 65 (32.7) | 0.64 |
| LVEF, % | 20.0 (17.0–30.0) | 25.0 (20.0–31.9) | 24.0 (20.0–30.0) | 25.0 (20.0–30.0) | 0.08 |
| NYHA class | 0.002 | ||||
| I | 16 (7.3) | 17 (7.8) | 13 (5.9) | 12 (5.5) | |
| II | 111 (50.9) | 125 (57.3) | 112 (51.1) | 92 (42.2) | |
| III | 87 (39.9) | 68 (31.2) | 88 (40.2) | 106 (48.6) | |
| IV | 2 (0.9) | 3 (1.4) | 3 (1.4) | 7 (3.2) | |
| Orthopnea | 0.55 | ||||
| None | 73 (33.5) | 64 (29.4) | 62 (28.3) | 64 (29.4) | |
| Mild | 58 (26.6) | 73 (33.5) | 63 (28.8) | 53 (24.3) | |
| Moderate | 56 (25.7) | 51 (23.4) | 58 (26.5) | 55 (25.2) | |
| Severe | 23 (10.6) | 19 (8.7) | 27 (12.3) | 33 (15.1) | |
| Percent oxygen saturation (SpO2) | 98 (96–99) | 98 (96–99) | 97 (96–98) | 97 (95–98) | 0.005 |
| Peripheral edema | 68 (18.3) | 94 (25.1) | 96 (25.6) | 117 (31.2) | 0.51 |
| Rales | 31 (31.6) | 21 (21.4) | 23 (23.5) | 23 (23.5) | 0.33 |
| S3 gallop | 21 (9.6) | 20 (9.2) | 19 (8.7) | 17 (7.8) | 0.35 |
| SBP, mm Hg | 109 (98–122) | 115 (103–129) | 116 (101–130) | 116 (104–131.5) | 0.002 |
| Medications at baseline | |||||
| ACE inhibitor | 150 (68.8) | 131 (60.1) | 136 (62.1) | 138 (63.3) | 0.27 |
| ARBs | 33 (15.1) | 46 (21.1) | 30 (13.7) | 36 (16.5) | 0.19 |
| β‐Blocker | 205 (94) | 211 (96.8) | 210 (95.9) | 202 (92.7) | 0.27 |
| CCB | 7 (3.2) | 16 (7.3) | 15 (6.9) | 27 (12.4) | 0.004 |
| GDMT score | 5 (3–8) | 6 (4–9) | 7 (4–9) | 7 (5–9) | <0.001 |
| Loop diuretic | 205 (94) | 204 (93.6) | 211 (96.4) | 213 (97.7) | 0.12 |
| Mineralocorticoid receptor antagonist | 103 (47.3) | 108 (49.5) | 110 (50.2) | 114 (52.3) | 0.80 |
| Statin | 120 (55.1) | 128 (58.7) | 140 (63.9) | 129 (59.2) | 0.31 |
| Laboratory data | |||||
| BUN, mg/dL | 19 (11–29) | 24 (16–34) | 22 (16–33) | 24 (17–38.5) | <0.001 |
| Creatinine, mg/dL | 1.23 (1.0–1.7) | 1.3 (1.1–1.7) | 1.3 (1.1–1.7) | 1.3 (1.1–1.8) | 0.13 |
| NT‐proBNP, pg/mL | 3592.0 (1769.0–6774.0) | 3299.0 (1780.0–7165.0) | 2274.0 (1435.0–4065.0) | 1935.0 (987.7–3846.0) | <0.001 |
| Serum potassium, mmol/L | 4.5 (4.1–4.8) | 4.4 (4.1–4.8) | 4.3 (4–4.7) | 4.2 (3.9–4.5) | <0.001 |
| Sodium, mmol/L | 138 (136–140) | 138 (136–141) | 139 (136–141) | 139 (137–141) | 0.66 |
| Biomarker‐guided therapy arm | 109 (50) | 108 (49.5) | 110 (50.2) | 106 (48.6) | 0.98 |
Data represented as count (percentage) and median (25th–75th percentile). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CCB, calcium channel blocker; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; GDMT, guideline‐directed medical therapy; HF, heart failure; ICD, implantable cardioverter‐defibrillator; IHD, ischemic heart disease; JVD, jugular venous distension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; and SBP, systolic blood pressure.
NT‐proBNP Levels: Stratified by Obesity Status
The NT‐proBNP levels were lower among obese individuals, both at baseline and through the entire trial period at the various study visits (P<0.05 at visits except month 24) (Figure 1).
Figure 1. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels during study period: stratified by obesity.
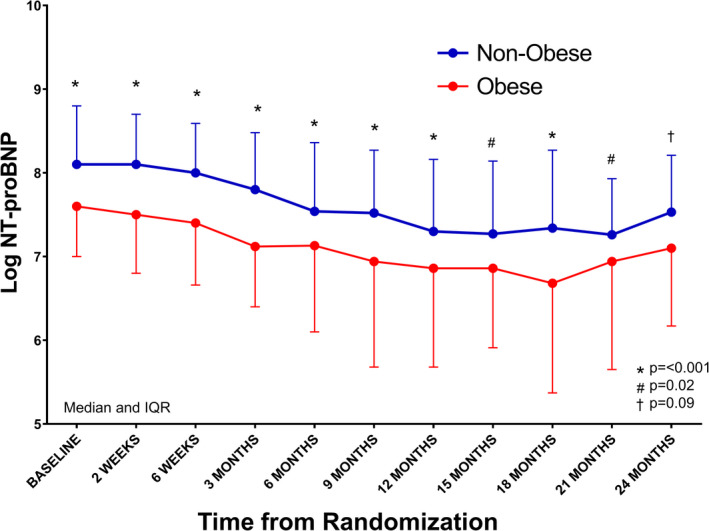
The data are represented as median (point) and interquartile range (error bars). Red represents obese, and blue represents nonobese. Adjusted for age, sex, race, blood urea nitrogen, left ventricular ejection fraction, diabetes mellitus, guideline‐directed medical therapy score, systolic and diastolic blood pressure, New York Heart Association class, ischemic heart disease history, history of cancer in past 5 years, sleep apnea, and treatment arm. IQR indicates interquartile range.
Risk of Adverse Cardiovascular Events: Stratified by Baseline BMI
In the multivariable‐adjusted models, obese individuals had a greater risk of developing adverse cardiovascular events (hazard ratio [HR], 1.39; 95% CI, 1.01–1.90; P=0.04) compared with their nonobese counterparts (Figure 2A). Taking those in the lowest BMI quartile as a reference, individuals in the third (HR, 1.42; 95% CI, 0.97–2.08; P=0.07) and fourth BMI quartiles (HR, 1.91; 95% CI, 1.24–2.96; P=0.003) had a greater risk of development of adverse cardiovascular events (Figure 2B). There was no interaction between treatment strategy and obesity status on the study outcome (P>0.10). In sensitivity analyses, we evaluated the association of BMI with the study outcome using restricted cubic splines. This demonstrated that a higher BMI was associated with a worse prognosis, whereas those with a lower BMI had a relatively lower risk (Figure 2C). The absolute event rates for outcome analyses are depicted in Table S2.
Figure 2. Obesity and risk of adverse cardiovascular events.
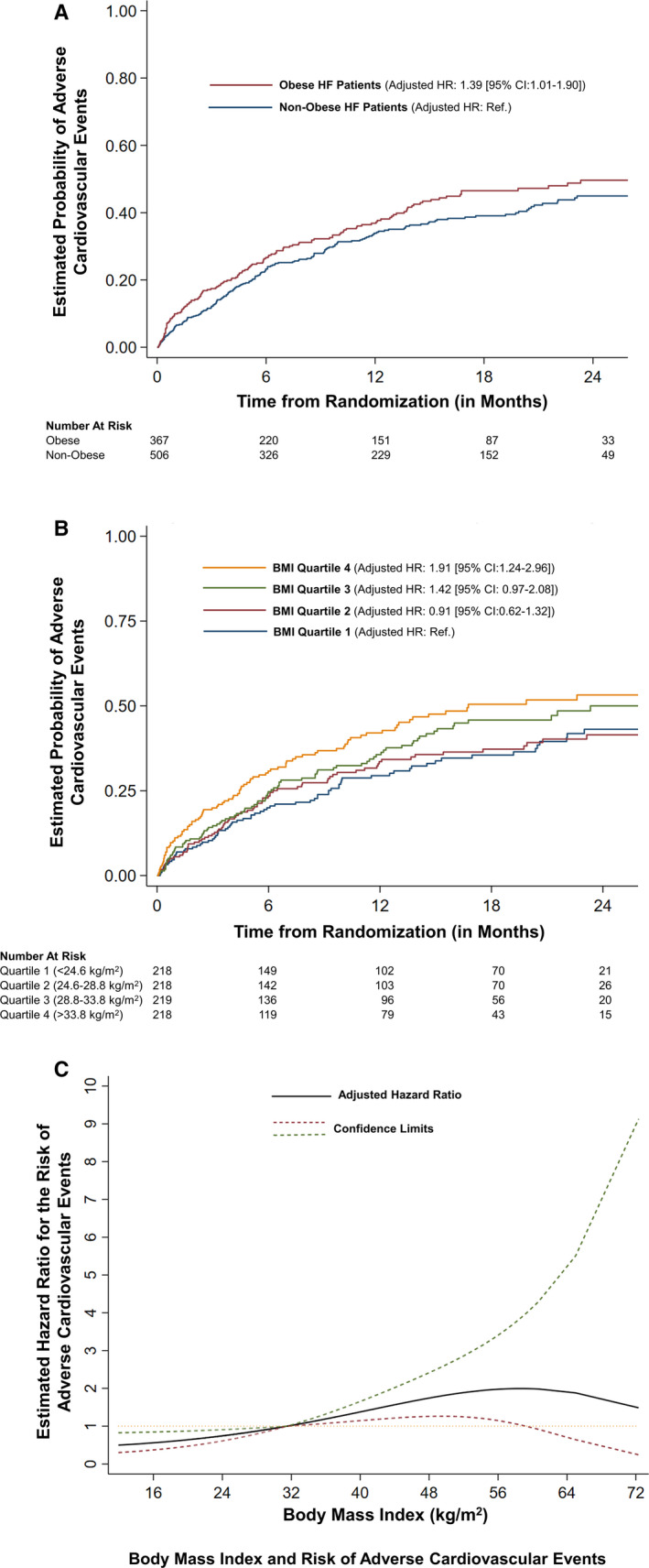
The figure shows the risk of adverse cardiovascular events based on baseline body mass index (BMI), stratified on obesity status (A) and quartiles (B). C, The relationship of the risk of adverse cardiovascular events with baseline BMI. Adjusted for age, sex, race, blood urea nitrogen, left ventricular ejection fraction, diabetes mellitus, guideline‐directed medical therapy score, systolic and diastolic blood pressure, log‐transformed NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) (time varying), New York Heart Association class, ischemic heart disease history, history of cancer in past 5 years, sleep apnea, and treatment arm. HF indicates heart failure; HR, hazard ratio; and Ref., reference.
Ranking of the Relative Strength of Association of Risk Factors With Adverse Cardiovascular Events
Obesity status–stratified clinical and demographic correlates of the study outcome, ranked by their χ2 values, are depicted in Figure 3. The NT‐proBNP level was the strongest correlate for the development of HF hospitalization or cardiovascular death in both obese and nonobese patients with HF. Among obese individuals, NYHA class, age, blood pressure, race, and GDMT score were strong correlates of the study outcome. In contrast, race, ischemic heart disease, NYHA class, and GDMT score were strong clinical correlates for adverse cardiovascular outcomes among nonobese individuals.
Figure 3. Ranking of strength of association of risk factors with adverse cardiovascular events: stratified by obesity.
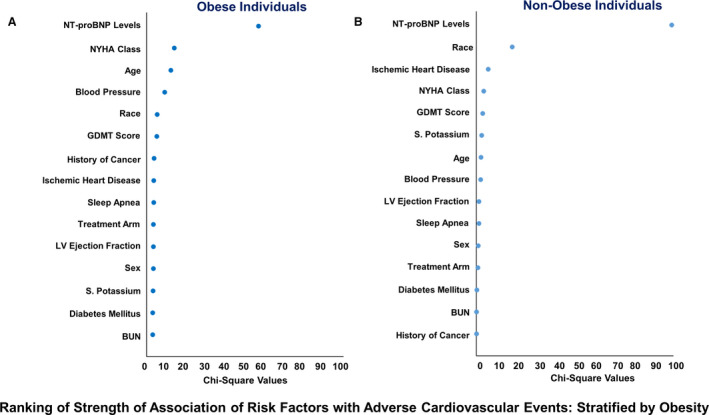
The figure depicts the relative contribution of each variable to the risk of adverse cardiovascular events in terms of χ2 values in obese (A) and nonobese (B) individuals. To ensure comparison on the same scale, the covariates were corrected for the degree of freedom. BUN indicates blood urea nitrogen; GDMT, guideline‐directed medical therapy; LV, left ventricular; NYHA, New York Heart Association; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and S., serum.
Risk of Adverse Cardiovascular Outcomes With NT‐proBNP ≤1000 pg/mL
In multivariable‐adjusted models, having NT‐proBNP levels of ≤1000 pg/mL was associated with a 58% lower risk of adverse cardiovascular outcomes (HR, 0.42; 95% CI, 0.29–0.59; P<0.001) (Figure 4A). Among obese individuals, those with NT‐proBNP ≤1000 pg/mL had a lower risk for adverse cardiovascular outcomes (HR, 0.48; 95% CI, 0.29–0.78; P=0.003). Similarly, among nonobese individuals, those with NT‐proBNP levels ≤1000 pg/mL had a significantly lower risk for adverse cardiovascular outcomes (HR, 0.32; 95% CI, 0.19–0.57; P<0.001). There was no interaction between obesity and having NT‐proBNP levels ≤1000 pg/mL on the study outcome (P>0.10). Per unit increase in on‐treatment log NT‐proBNP levels had a higher hazard for the prediction of the study outcome compared with per unit increase in baseline log NT‐proBNP levels in both obese and nonobese individuals (Table S3).
Figure 4. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) ≤1000 mg/dL and risk of adverse cardiovascular events.
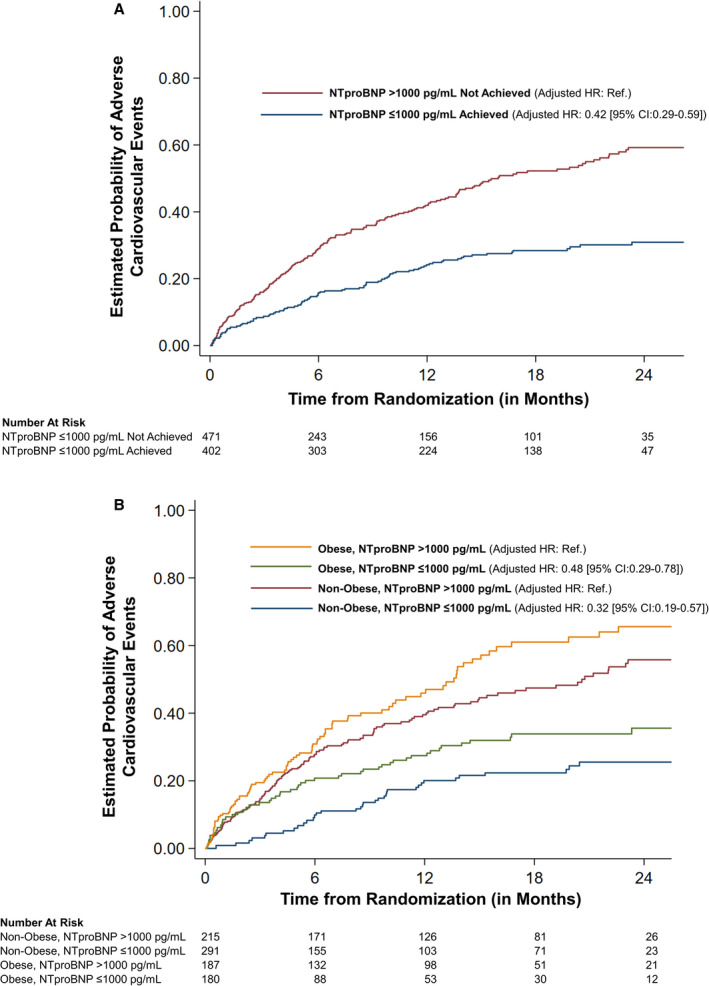
A, The risk of adverse cardiovascular events stratified by achieved NT‐proBNP levels. B, The risk of adverse cardiovascular events stratified by obesity and having NT‐proBNP ≤1000 mg/dL. Adjusted for age, sex, race, blood urea nitrogen, left ventricular ejection fraction, diabetes mellitus, guideline‐directed medical therapy score, systolic and diastolic blood pressure, body mass index, log‐transformed baseline NT‐proBNP, New York Heart Association class, ischemic heart disease history, history of cancer in past 5 years, sleep apnea, and treatment arm. HR indicates hazard ratio; and Ref., reference.
The reduction in NYHA class from baseline indicated a 28% lower risk of study outcome in obese individuals and a 25% lower risk of the study outcome in nonobese individuals (Table S4).
Optimization of GDMT in GUIDE‐IT Trial: Stratified by Obesity Status
The GDMT score through the study period is depicted in Figure S1. The most common reason for not titrating medication was the clinician's decision in both obese and nonobese patients. Being at maximally tolerated therapy was more prevalent in nonobese individuals compared with obese individuals. In the biomarker‐guided arm, the prevalence of inappropriate adjustment of medication during the visit was higher in nonobese individuals (51.4%) compared with obese individuals (41.6%) (P<0.001) (Figure S2).
Discussion
In this study, we observed that obese patients with HFrEF had 59% lower NT‐proBNP levels than nonobese patients at baseline, which remained consistent during the study period, indicating a similar reduction in NT‐proBNP levels on HF treatment in both groups. Obesity or higher BMI was associated with worse functional status (NYHA class and 6‐minute walk test) and a higher incidence of adverse cardiovascular events, irrespective of whether clinical care was guided by the biomarker‐based strategy or not. The GDMT score remained similar through the study period, suggesting similar up‐titration of HF medications, with clinician decision being the most common reason for lack of medication up‐titration. Baseline NT‐proBNP level was a strong clinical predictor for the risk of adverse cardiovascular outcomes in both obese and nonobese individuals. Thus, having on‐treatment NT‐proBNP levels of ≤1000 pg/mL has favorable prognostic implications, irrespective of obesity status, with risk for HF hospitalization or death being 68% lower in nonobese and 52% lower in obese patients with HFrEF with NT‐proBNP levels of ≤1000 pg/mL compared with those with NT‐proBNP levels of >1000 pg/mL.
Obesity in the setting of HFrEF has often been attributed to being paradoxically "protective." The evidence for this is primarily from observational epidemiological studies, with limited data from a robust, well‐phenotyped population of HFrEF in clinical trials. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Notwithstanding the prior investigations, the evidence in support of the mechanistic hypotheses postulated to support the obesity paradox in HF is lacking. Obesity adversely impacts the cardiac and vascular function, and remodeling, which is mediated by systemic microvascular endothelial inflammation. 37 , 38 Moreover, data from HF with preserved ejection fraction trials indicate that obesity, which is considered a unique HF with preserved ejection fraction disease phenotype, is associated with worse clinical outcomes. 39 , 40 , 41 Previous investigations have looked into the impact of obesity on adverse outcomes, mostly in the setting of acute decompensated HF, or advanced HF, and may be limited because of potential information bias, with limited phenotyping of the baseline characteristics, inadequate accounting for the impact of unmeasured confounders, and infrequent follow‐up. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Obese patients presenting earlier with noncardiac edema and dyspnea may be more quickly diagnosed with HFrEF, which introduces potential misclassification or lead‐time bias in these investigations. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 42 In the current investigation, a closely followed population with HFrEF in the setting of a multicenter, randomized clinical trial, we found that obese individuals have a higher risk of adverse cardiovascular events, despite being younger, having lower NT‐proBNP levels, having similar ejection fraction, having greater GDMT scores, and having less frequent and inadequate medication up‐titration. Further studies to shed light on the obesity paradox association are warranted.
The ascertainment of the clinical condition of obese patients with HF using physical examination may be challenging. This resultant inadequate characterization of the clinical state may, therefore, preclude adequate up‐titration of medications among obese individuals with HFrEF. This consequently increases the reliance on surrogate biomarkers of clinical condition, such as NT‐proBNP levels. 7 , 19 , 21 There are limited data assessing the value of NT‐proBNP levels of ≤1000 pg/mL in treated obese patients with HFrEF, who represent a known NP deficient population. In our study, NT‐proBNP levels were observed to be consistently lower in obese individuals. Numerous mechanisms have been suggested for this relative deficiency of NPs in obese individuals, 9 , 43 , 44 , 45 such as increased clearance or impaired processing NPs. 7 , 45 , 46 , 47 Recent data indicate that increased glycosylation of the precursor peptide in obese individuals may cause impairment of the posttranslational processing of the precursor peptide, resulting in low circulating NP levels identified by the clinical assays. 46 , 47 Lower circulating bioactive NP molecules reduce the beneficial effect of NPs on cardiovascular physiological features, which are mediated through natriuresis, 48 , 49 vasodilation, 50 and direct inhibition of the renin‐angiotensin‐aldosterone system. 51 , 52 , 53 , 54 This is of paramount importance in the state of cardiac dysfunction and congestion. This is consistent with our study findings, where NT‐proBNP was observed to be a strong prognostic marker in obese patients with HFrEF, despite lower circulating NP levels. We advance the findings of the GUIDE‐IT trial by demonstrating that having on‐treatment NT‐proBNP levels of ≤1000 mg/dL holds important prognostic implications in both obese and nonobese patients, irrespective of the treatment strategy. Furthermore, the interaction seen in the primary trial may have been attributable to the lesser inadequate up‐titration of medication among obese individuals in the biomarker‐guided arm, as noted in our analyses.
Our study has important clinical implications. The obesity burden is burgeoning in the United States and globally, and this may contribute to an increase in the incidence and prevalence of HF. Our study highlights that once these patients develop HFrEF, they are likely to have greater morbidity and mortality with higher BMI, despite a similar response to HF treatment. The lack of a treatment target is HFrEF, especially in the setting of limited physical examination, such as in obesity, leaves the titration of HF treatment to the physician's discretion. Even with the high prevalence of inadequate medication up‐titration, reaching NT‐proBNP levels of ≤1000 pg/mL holds prognostic importance.
Furthermore, NT‐proBNP levels are frequently part of the inclusion criteria for clinical trials and have also been used as a surrogate therapeutic end point in HFrEF clinical trials. Since obesity is an natriuretic peptideNP deficient state, there may be a selection bias introduced in the enrollment into HFrEF clinical trials. 55 The data from our study indicate that careful consideration of NP deficiency states in the inclusion criteria of HFrEF trials may warrant different NT‐proBNP inclusion thresholds for these NP deficiency states. 30 , 55
Study Limitations
The use of BMI to categorize obesity may not be ideal, and other measures, such as waist/hip ratio, body fat proportion, and fat mass, have been suggested. 44 Despite this, BMI is a validated surrogate clinical measure of adiposity. 11 Although the dichotomization of variables, such as BMI and NT‐proBNP levels, introduces bias and has several limitations, it allows for easier clinical interpretation of the data. However, we also evaluated the association of BMI as a continuous variable in our sensitivity analyses that demonstrated similar results as the categorical division of BMI. The GDMT score was developed to provide a comprehensive understanding of the HF medications being taken by the patients, and the medication used is likely to change through the course of the study period. 17 Therefore, we used the GDMT score as a continuous time‐varying variable to account for the changing medication use throughout the study period. Because of the small study population, our study was underpowered to detect a significant interaction between obesity and NT‐proBNP ≤1000 pg/mL on the study outcome. Our study does not provide information about how frequently the assessment of NT‐proBNP levels must be performed in patients with HFrEF. Lowering of NT‐proBNP beyond 1000 pg/mL is prognostically important, even in the NP deficient population of obese individuals with HFrEF. Reduction in NT‐proBNP concentration along with aggressive, goal‐oriented up‐titration of medications can be part of the optimal management of HFrEF, irrespective of obesity status. 17
Conclusions
NT‐proBNP concentration is a strong prognostic marker in HFrEF, irrespective of obesity status. On‐treatment NT‐proBNP level ≤1000 pg/mL, achieved through adequate GDMT, is associated with a more favorable prognosis in HFrEF, irrespective of obesity status.
Sources of Funding
This work is supported by the National Institutes of Health Mentored‐Patient Oriented Research Award (5K23 HL146887‐02) to Dr Pankaj Arora.
Disclosures
Dr Wang has taken personal fees from Novartis outside of submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S4
Figures S1–S2
(J Am Heart Assoc. 2021;10:e018689. DOI: 10.1161/JAHA.120.018689.)
For Sources of Funding and Disclosures, see page 13.
References
- 1. Kalra R, Parcha V, Patel N, Bhargava A, Booker KS, Arora G, Arora P. Increased awareness, inadequate treatment, and poor control of cardiovascular risk factors in American young adults: 2005–2016. Eur J Prev Cardiol. 2020:2047487320905190. DOI: 10.1177/2047487320905190. [DOI] [PubMed] [Google Scholar]
- 2. Patel N, Kalra R, Bhargava A, Arora G, Arora P. Ideal cardiovascular health among American adults after the economic recession of 2008–2009: insights from NHANES. Am J Med. 2019;132:1182–1190.e5. DOI: 10.1016/j.amjmed.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Deswal A, Folsom AR, Heiss G. The potentially modifiable burden of incident heart failure due to obesity: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2010;172:781–789. DOI: 10.1093/aje/kwq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebong IA, Goff DC Jr, Rodriguez CJ, Chen H, Bluemke DA, Szklo M, Bertoni AG. The relationship between measures of obesity and incident heart failure: the Multi‐Ethnic Study of Atherosclerosis. Obesity (Silver Spring). 2013;21:1915–1922. DOI: 10.1002/oby.20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, Selvin E, Ballantyne CM, Nambi V. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–607. DOI: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. DOI: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 7. Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT‐proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176:611–617. DOI: 10.1016/j.ijcard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. DOI: 10.1016/S0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 9. Abdulla J, Kober L, Abildstrom SZ, Christensen E, James WP, Torp‐Pedersen C. Impact of obesity as a mortality predictor in high‐risk patients with myocardial infarction or chronic heart failure: a pooled analysis of five registries. Eur Heart J. 2008;29:594–601. DOI: 10.1093/eurheartj/ehn010. [DOI] [PubMed] [Google Scholar]
- 10. Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M; ADHERE Scientific Advisory Committee and Investigators . An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. DOI: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 11. Oreopoulos A, Padwal R, Kalantar‐Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta‐analysis. Am Heart J. 2008;156:13–22. DOI: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 12. Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez‐Jimenez F, Arbab‐Zadeh A, Mukherjee D, Lazar JM. Meta‐analysis of the relation of body mass index to all‐cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428–1434. DOI: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 13. Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–894. DOI: 10.1016/S0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 14. Shah R, Gayat E, Januzzi JL Jr, Sato N, Cohen‐Solal A, diSomma S, Fairman E, Harjola V‐P, Ishihara S, Lassus J, et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–785. DOI: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 15. Khalid U, Ather S, Bavishi C, Chan W, Loehr LR, Wruck LM, Rosamond WD, Chang PP, Coresh J, Virani SS, et al. Pre‐morbid body mass index and mortality after incident heart failure: the ARIC Study. J Am Coll Cardiol. 2014;64:2743–2749. DOI: 10.1016/j.jacc.2014.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel N, Cushman M, Gutiérrez OM, Howard G, Safford MM, Muntner P, Durant RW, Prabhu SD, Arora G, Levitan EB, et al. Racial differences in the association of NT‐proBNP with risk of incident heart failure in REGARDS. JCI Insight. 2019;5:e129979. DOI: 10.1172/jci.insight.129979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Januzzi JL Jr, Ahmad T, Mulder H, Coles A, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston‐Miller N, Mark DB, et al. Natriuretic peptide response and outcomes in chronic heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;74:1205–1217. DOI: 10.1016/j.jacc.2019.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AHB, et al. Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. DOI: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 19. Daniels LB, Clopton P, Bhalla V, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, et al. How obesity affects the cut‐points for B‐type natriuretic peptide in the diagnosis of acute heart failure: results from the Breathing Not Properly Multinational Study. Am Heart J. 2006;151:999–1005. DOI: 10.1016/j.ahj.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 20. Horwich TB, Hamilton MA, Fonarow GC. B‐type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. DOI: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 21. Bayes‐Genis A, Lloyd‐Jones DM, van Kimmenade RR, Lainchbury JG, Richards AM, Ordonez‐Llanos J, Santalo M, Pinto YM, Januzzi JL Jr. Effect of body mass index on diagnostic and prognostic usefulness of amino‐terminal pro‐brain natriuretic peptide in patients with acute dyspnea. Arch Intern Med. 2007;167:400–407. DOI: 10.1001/archinte.167.4.400. [DOI] [PubMed] [Google Scholar]
- 22. Bajaj NS, Patel N, Prabhu SD, Arora G, Wang TJ, Arora P. Effect of NT‐proBNP‐guided therapy on all‐cause mortality in chronic heart failure with reduced ejection fraction. J Am Coll Cardiol. 2018;71:951–952. DOI: 10.1016/j.jacc.2017.11.070. [DOI] [PubMed] [Google Scholar]
- 23. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. DOI: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 24. Nadruz W Jr, Claggett BL, McMurray JJ, Packer M, Zile MR, Rouleau JL, Desai AS, Swedberg K, Lefkowitz M, Shi VC, et al. Impact of body mass index on the accuracy of N‐terminal pro‐brain natriuretic peptide and brain natriuretic peptide for predicting outcomes in patients with chronic heart failure and reduced ejection fraction: insights from the PARADIGM‐HF Study (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial). Circulation. 2016;134:1785–1787. DOI: 10.1161/CIRCULATIONAHA.116.024976. [DOI] [PubMed] [Google Scholar]
- 25. Khalid U, Wruck LM, Quibrera PM, Bozkurt B, Nambi V, Virani SS, Jneid H, Agarwal S, Chang PP, Loehr L, et al. BNP and obesity in acute decompensated heart failure with preserved vs. reduced ejection fraction: the Atherosclerosis Risk in Communities Surveillance Study. Int J Cardiol. 2017;233:61–66. DOI: 10.1016/j.ijcard.2017.01.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston‐Miller N, Januzzi JL Jr, Mark DB, Piña IL, Passmore G, et al. Effect of natriuretic peptide‐guided therapy on hospitalization or cardiovascular mortality in high‐risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318:713–720. DOI: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaggin HK, Mohammed AA, Bhardwaj A, Rehman SU, Gregory SA, Weiner RB, Baggish AL, Moore SA, Semigran MJ, Januzzi JL Jr. Heart failure outcomes and benefits of NT‐proBNP‐guided management in the elderly: results from the prospective, randomized ProBNP outpatient tailored chronic heart failure therapy (PROTECT) study. J Card Fail. 2012;18:626–634. DOI: 10.1016/j.cardfail.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 28. Januzzi JL Jr, Rehman SU, Mohammed AA, Bhardwaj A, Barajas L, Barajas J, Kim H‐N, Baggish AL, Weiner RB, Chen‐Tournoux A, et al. Use of amino‐terminal pro‐B‐type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881–1889. DOI: 10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 29. Januzzi JL Jr, Sakhuja R, O’Donoghue M, Baggish AL, Anwaruddin S, Chae CU, Cameron R, Krauser DG, Tung R, Camargo CA Jr, et al. Utility of amino‐terminal pro‐brain natriuretic peptide testing for prediction of 1‐year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166:315–320. DOI: 10.1001/archinte.166.3.315. [DOI] [PubMed] [Google Scholar]
- 30. Parcha V, Patel N, Kalra R, Arora G, Januzzi JL Jr, Felker GM, Wang TJ, Arora P. Racial differences in serial NT‐proBNP levels in heart failure management: insights from the GUIDE‐IT trial. Circulation. 2020;142:1018–1020. DOI: 10.1161/CIRCULATIONAHA.120.046374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Felker GM, Ahmad T, Anstrom KJ, Adams KF, Cooper LS, Ezekowitz JA, Fiuzat M, Houston‐Miller N, Januzzi JL, Leifer ES, et al. Rationale and design of the GUIDE‐IT study: guiding evidence based therapy using biomarker intensified treatment in heart failure. JACC Heart Fail. 2014;2:457–465. DOI: 10.1016/j.jchf.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bajaj NS, Gutiérrez OM, Arora G, Judd SE, Patel N, Bennett A, Prabhu SD, Howard G, Howard VJ, Cushman M, et al. Racial differences in plasma levels of n‐terminal pro‐B‐type natriuretic peptide and outcomes: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Cardiol. 2018;3:11–17. DOI: 10.1001/jamacardio.2017.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel N, Gutierrez OM, Arora G, Howard G, Howard VJ, Judd SE, Prabhu SD, Levitan EB, Cushman M, Arora P. Race‐based demographic, anthropometric and clinical correlates of N‐terminal‐pro B‐type natriuretic peptide. Int J Cardiol. 2019;286:145–151. DOI: 10.1016/j.ijcard.2019.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel N, Russell GK, Musunuru K, Gutierrez OM, Halade G, Kain V, Lv W, Prabhu SD, Margulies KB, Cappola TP, et al. Race, natriuretic peptides, and high‐carbohydrate challenge: a clinical trial. Circ Res. 2019;125:957–968. DOI: 10.1161/CIRCRESAHA.119.315026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. York MK, Gupta DK, Reynolds CF, Farber‐Eger E, Wells QS, Bachmann KN, Xu M, Harrell FE Jr, Wang TJ. B‐type natriuretic peptide levels and mortality in patients with and without heart failure. J Am Coll Cardiol. 2018;71:2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parcha V, Patel N, Kalra R, Kim J, Gutierrez OM, Arora G, Arora P. Incidence and implications of atrial fibrillation/flutter in hypertension: insights from the SPRINT trial. Hypertension. 2020;75:1483–1490. DOI: 10.1161/HYPERTENSIONAHA.120.14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. DOI: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 38. Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging. 2013;6:142–152. DOI: 10.1161/CIRCIMAGING.111.964627. [DOI] [PubMed] [Google Scholar]
- 39. Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all‐cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70:2739–2749. DOI: 10.1016/j.jacc.2017.09.1111. [DOI] [PubMed] [Google Scholar]
- 40. Pandey A, Berry JD, Drazner MH, Fang JC, Tang WHW, Grodin JL. Body mass index, natriuretic peptides, and risk of adverse outcomes in patients with heart failure and preserved ejection fraction: analysis from the TOPCAT Trial. J Am Heart Assoc. 2018;7:e009664. DOI: 10.1161/JAHA.118.009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reddy YNV, Lewis GD, Shah SJ, Obokata M, Abou‐Ezzedine OF, Fudim M, Sun J‐L, Chakraborty H, McNulty S, LeWinter MM, et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX Trial Ancillary Study. Mayo Clin Proc. 2019;94:1199–1209. DOI: 10.1016/j.mayocp.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 42. Wang TJ. The obesity paradox in heart failure: weighing the evidence. J Am Coll Cardiol. 2014;64:2750–2752. DOI: 10.1016/j.jacc.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 43. Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH Jr, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. DOI: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 44. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich‐Horvat P, Liu C‐Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. DOI: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 45. Arora P, Reingold J, Baggish A, Guanaga DP, Wu C, Ghorbani A, Song Y, Chen‐Tournaux A, Khan AM, Tainsh LT, et al. Weight loss, saline loading, and the natriuretic peptide system. J Am Heart Assoc. 2015;4:e001265. DOI: 10.1161/JAHA.114.001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parcha V, Arora P. Glycosylation of natriuretic peptides in obese heart failure: mechanistic insights. Ann Transl Med. 2019;7:611. DOI: 10.21037/atm.2019.10.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lewis LK, Raudsepp SD, Prickett TCR, Yandle TG, Doughty RN, Frampton CM, Pemberton CJ, Richards AM. ProBNP that is not glycosylated at threonine 71 is decreased with obesity in patients with heart failure. Clin Chem. 2019;65:1115–1124. DOI: 10.1373/clinchem.2019.302547. [DOI] [PubMed] [Google Scholar]
- 48. Melo LG, Steinhelper ME, Pang SC, Tse Y, Ackermann U. ANP in regulation of arterial pressure and fluid‐electrolyte balance: lessons from genetic mouse models. Physiol Genomics. 2000;3:45–58. DOI: 10.1152/physiolgenomics.2000.3.1.45. [DOI] [PubMed] [Google Scholar]
- 49. de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. DOI: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 50. Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. DOI: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 51. Epstein FH, Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. DOI: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 52. Johnston CI, Hodsman PG, Kohzuki M, Casley DJ, Fabris B, Phillips PA. Interaction between atrial natriuretic peptide and the renin angiotensin aldosterone system: endogenous antagonists. Am J Med. 1989;87:24S–28S. DOI: 10.1016/S0002-9343(89)80924-6. [DOI] [PubMed] [Google Scholar]
- 53. Cody RJ, Atlas SA, Laragh JH, Kubo SH, Covit AB, Ryman KS, Shaknovich A, Pondolfino K, Clark M, Camargo MJ, et al. Atrial natriuretic factor in normal subjects and heart failure patients: plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion. J Clin Invest. 1986;78:1362–1374. DOI: 10.1172/JCI112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hunt PJ, Espiner EA, Nicholls MG, Richards AM, Yandle TG. Differing biological effects of equimolar atrial and brain natriuretic peptide infusions in normal man. J Clin Endocrinol Metab. 1996;81:3871–3876. [DOI] [PubMed] [Google Scholar]
- 55. Ibrahim NE, Burnett JC Jr, Butler J, Camacho A, Felker GM, Fiuzat M, O’Connor C, Solomon SD, Vaduganathan M, Zile MR. Natriuretic peptides as inclusion criteria in clinical trials: a JACC Heart Failure Position Paper. JACC Heart Fail. 2020;8:347–358. DOI: 10.1016/j.jchf.2019.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S4
Figures S1–S2


