Abstract
Background
Cardio/kidney composite end points are clinically relevant but rarely analyzed in cardiovascular trials. This post hoc analysis of the EMPA‐REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial evaluated cardio/kidney composite end points by 2 statistical approaches.
Methods and Results
A total of 7020 patients with type 2 diabetes mellitus and established cardiovascular disease were treated with empagliflozin 10 or 25 mg (n=4687) or placebo (n=2333) on top of standard care. Cardio/kidney composite end points studied were: (1) cardiac or kidney death, kidney failure, hospitalization for heart failure, sustained decline in estimated glomerular filtration rate ≥40% from baseline, or sustained progression to macroalbuminuria; (2) cardiac or kidney death, kidney failure, hospitalization for heart failure, or sustained estimated glomerular filtration rate decline ≥40% from baseline; and (3) cardiac or kidney death, kidney failure, hospitalization for heart failure, or sustained doubling in serum creatinine from baseline. Cox regression using time‐to‐first‐event analysis and win ratio (WR) using hierarchical order of events were applied. Empagliflozin reduced the risk of all cardio/kidney composites. The results varied only slightly between Cox and WR (eg, composite 1: hazard ratio, 0.56 [95% CI, 0.49–0.64]; WR, 1.76 [95% CI, 1.53–2.02]. WR prioritizes events by clinical importance; in particular, all fatal events are evaluated, whereas Cox regression ignores deaths when preceded by nonfatal events. Of the 285 cardio/kidney deaths in the analysis, 44 to 56 (15%–20%), depending on the composite, occurred after a nonfatal event and were not evaluated in Cox regression but evaluated by the WR.
Conclusions
By considering the clinical relevance of different event types, the WR represents an appropriate method to complement the traditional time‐to‐first‐event analysis in cardio/kidney outcomes.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01131676.
Keywords: cardio/kidney composite end points, cardio‐renal, empagliflozin, hazard ratio, win ratio
Subject Categories: Cardiorenal Syndrome
Composite end points (ie, incorporating 1 or more nonfatal plus a fatal event, usually cardiovascular death) are currently the standard approach for the primary analysis of most randomized controlled clinical trials in the cardiovascular field. In randomized controlled clinical trials, the treatment effect is usually estimated using the time‐to‐first‐event model (eg, Cox model) producing a hazard ratio (HR) and respective 95% CI. 1 This approach is simple and familiar to most trialists and clinicians. However, in a time‐to‐first‐event model, the end point components are given equal importance, although they might differ considerably in severity (ie, hospitalization versus death). 2 To overcome these limitations, Schoenfeld and Finkelstein developed a model that is able to combine time‐to‐first‐event and longitudinal measures. 3 Later, Pocock and colleagues adapted this approach by introducing the win ratio (WR), which takes into account both the clinical relevance and timing of the components of the outcome, where the most important component, usually death, is given the highest priority in the analysis. 4
Commonly used combined cardiovascular end points usually include major adverse cardiac events comprising combinations of nonfatal stroke, nonfatal myocardial infarction, cardiovascular or hospitalization for heart failure (HHF), and cardiovascular death. 5 To date, kidney outcomes have infrequently been considered as part of composite end points in cardiovascular randomized controlled clinical trials. 6 However, cardiovascular and kidney disease share common risk factors (eg, age, diabetes mellitus, obesity, hypertension, and smoking), pathophysiology (eg, endothelial dysfunction, inflammation, and fibrosis), and have mutual clinical impact. 6 Therefore, such cardiovascular and kidney events can be combined in a cardio/kidney composite. 7 The use of clinically meaningful cardio/kidney composites will increase the number of observed outcome events, which, assuming the same treatment effect, will reduce the required sample size for reaching a target power and decrease recruitment challenges and costs. In the EMPA‐REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial, empagliflozin reduced cardiovascular death by 38%, hospitalization for heart failure by 35%, and incident or worsening nephropathy by 39%. 5 , 8
The aims of the current analyses were: (1) to integrate cardiovascular and kidney outcomes into clinically meaningful cardio/kidney composites and (2) to explore the WR as a potential method for the analysis of treatment effect, as a method that incorporates all fatal events, even those that occur after a nonfatal event.
The study design of the EMPA‐REG OUTCOME trial has been previously published. Briefly, 7020 patients with type 2 diabetes mellitus, established cardiovascular disease, and estimated glomerular filtration rate (eGFR; modification of diet in renal disease) ≥30 mL/min per 1.73 m2 were randomized and treated with empagliflozin 10 mg, empagliflozin 25 mg (n=4687, for the pooled doses), or placebo (n=2333), and were observed for a median of 3.1 years. 5 , 8
Methods
The study was approved by an institutional review board, and patients gave informed consent. The sponsor of the EMPA‐REG OUTCOME trial (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient‐level clinical study data. Researchers are invited to submit inquiries via the following website: https://trials.boehringer‐ingelheim.com/.
The investigation focused on 3 cardio/kidney composite end points that combined clinically relevant cardiac and kidney outcomes and reflected the event profile of high‐risk patients with cardiovascular disease. The studied composite outcomes, taking into account the order of event priority (ie, from highest to lowest) within each composite were: (1) cardiac or kidney death, kidney failure (KF; defined as sustained eGFR <15 mL/min per 1.73 m2 [equation developed by the Chronic Kidney Disease Epidemiology Collaboration], sustained initiation of continuous kidney replacement therapy including transplantation), HHF, sustained decline in eGFR ≥40% (Chronic Kidney Disease Epidemiology Collaboration) from baseline, or sustained progression to macroalbuminuria; (2) cardiac or kidney death, KF, HHF, or sustained decline in eGFR ≥40% (Chronic Kidney Disease Epidemiology Collaboration) from baseline; and (3) cardiac or kidney death, KF, HHF, or sustained doubling of serum creatinine from baseline. Kidney components of the composites were not independently adjudicated. Treatment effect of empagliflozin versus placebo on these outcomes was analyzed using time‐to‐first‐event Cox regression analysis and WR. The Cox models included terms for age, sex, geographical region, baseline glycated hemoglobin, baseline eGFR, baseline body mass index, as well as treatment. The WR incorporated all outcomes by hierarchical order of importance and relative timing of occurrence, in terms of an event/censoring appearing sooner or later, and is represented by the ratio of winners between the active and control groups. The WR can be defined as the odds that the patient on treatment did better than the patient on control for a specific outcome of interest on the basis of a pairwise comparison starting with the event with the highest clinical priority, usually death, to the lowest (eg, macroalbuminuria or creatinine changes). The unmatched WR approach compared each patient on empagliflozin with each patient on placebo. 4 , 9 , 10 The results of the 3 composite end points analyzed were compared by each strategy (ie, WR and HR based on the Cox model). A favorable treatment effect of empagliflozin versus placebo results in an HR <1 and a WR >1. To facilitate the comparison between the 2 methods, the results are also shown as 1/HR in the Table. Cox models were analyzed using SAS version 9.4 (SAS Institute), and the WR was analyzed using the genwr function in the R package WWR version 1.2.2 (https://cran.r‐project.org/web/packages/WWR/WWR.pdf).
Table 1.
Patients With Events Contributing to the Combined End Point in Hazard Ratio (First Event Only) Compared With Patients With Event Per Component
| Composite 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| End Point Components | Patients With First Event Considered in the Cox Regression Analysis, n (%) | Patients With ≥1 Event Per Component, n (%) | Deaths Not Evaluated in the Cox Regression Because of a Prior Nonfatal Event, n=56, n | Nonfatal Events (Only First Event Per Type) Preceding the 56 Cardiac or Kidney Deaths Not Evaluated in the Cox Regression, n=69, n | ||||
| Empagliflozin, N=4199 | Placebo, N=2118 | Empagliflozin, N=4687 | Placebo, N=2333 | Empagliflozin | Placebo | Empagliflozin | Placebo | |
| Cardiac or kidney death | 133 (3.2) | 96 (4.5) | 159 (3.4) | 126 (5.4) | 26 | 30 | … | … |
| KF | 3 (0.1) | 3 (0.1) | 14 (0.3)* | 11 (0.5)* | 2 | 1 | ||
| HHF | 107 (2.5) | 79 (3.7) | 126 (2.7) | 95 (4.1) | 17 | 23 | ||
| Sustained eGFR decline ≥40% | 82 (2.0) | 62 (2.9) | 97 (2.1)* | 78 (3.4)* | 4 | 7 | ||
| Sustained macroalbuminuria | 165 (3.9) | 164 (7.7) | 166 (4.1) † | 168 (8.3) † | 7 | 8 | ||
| Total events | 490 | 404 | 559 | 481 | 30 | 39 | ||
| Cardiac outcomes ‡ | 240 | 175 | 285 | 221 | 17 | 23 | ||
| Kidney outcomes § | 250 | 229 | 274 | 260 | 13 | 16 | ||
| Composite 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| End Point Components | Patients With First Event Considered in the Cox Regression Analysis, n (%) | Patients With ≥1 Event Per Component, n (%) | Deaths Not Evaluated in the Cox Regression Because of a Prior Nonfatal Event, n=46, n | Nonfatal Events (Only First Event Per Type) Preceding the 46 Cardiac or Kidney Deaths Not Evaluated in the Cox Regression, n=54, n | ||||
| Empagliflozin, N=4648 | Placebo, N=2325 | Empagliflozin, N=4687 | Placebo, N=2333 | Empagliflozin | Placebo | Empagliflozin | Placebo | |
| Cardiac or kidney death | 138 (3.0) | 101 (4.3) | See above | See above | 21 | 25 | … | … |
| KF | 4 (0.1) | 3 (0.1) | See above | See above | 2 | 1 | ||
| HHF | 115 (2.5) | 89 (3.8) | See above | See above | 17 | 23 | ||
| Sustained eGFR decline ≥40% | 87 (1.9) | 68 (2.9) | See above | See above | 4 | 7 | ||
| Total events | 344 | 261 | 393 | 313 | 23 | 31 | ||
| Cardiac outcomes ‡ | 253 | 190 | 285 | 221 | 17 | 23 | ||
| Kidney outcomes § | 91 | 71 | 108 | 92 | 6 | 8 | ||
| Composite 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| End Point Components | Patients With First Event Considered in the Cox Regression Analysis, n (%) | Patients With ≥1 Event Per Component, n (%) | Deaths Not Evaluated in the Cox Regression Because of a Prior Nonfatal Event, n=44, n | Nonfatal Events (Only First Event Per Type) Preceding the 44 Cardiac or Kidney Deaths Not Evaluated in the Cox Regression, n=48, n | ||||
| Empagliflozin, N=4648 | Placebo, N=2325 | Empagliflozin, N=4687 | Placebo, N=2333 | Empagliflozin | Placebo | Empagliflozin | Placebo | |
| Cardiac or kidney death | 139 (3.0) | 102 (4.4) | See above | See above | 20 | 24 | … | … |
| KF | 6 (0.1) | 6 (0.3) | See above | See above | 2 | 1 | ||
| HHF | 123 (2.6) | 91 (3.9) | See above | See above | 17 | 23 | ||
| Sustained doubling of serum creatinine | 12 (0.3) | 21 (0.9) | 17 (0.4) | 25 (1.1) | 2 | 3 | ||
| Total events | 280 | 220 | 313 | 260 | 21 | 27 | ||
| Cardiac outcomes ‡ | 262 | 193 | 285 | 221 | 17 | 23 | ||
| Kidney outcomes § | 18 | 27 | 28 | 39 | 4 | 4 | ||
Cardio/kidney composite 1: cardiac or kidney death or KF or HHF or sustained decline in eGFR ≥40% from baseline or sustained progression to macroalbuminuria. Cardio/kidney composite 2: cardiac or kidney death or KF or HHF or sustained decline in eGFR ≥40% from baseline. Cardio/kidney composite 3: cardiac or kidney death or KF or HHF or sustained doubling of serum creatinine from baseline. The time‐to‐first‐event model does not capture the totality of the fatal events and gives more weight to end points of lower clinical relevance such as macroalbuminuria, whereas the win ratio captures the totality of the fatal events. eGFR indicates estimated glomerular filtration rate, equation developed by Chronic Kidney Disease Epidemiology Collaboration; HHF, hospitalization for heart failure; and KF, kidney failure.
Number available for analysis=4645 for empagliflozin, 2323 for placebo.
Number available for analysis=4049 for empagliflozin, 2020 for placebo.
Cardiac outcomes: cardiac or kidney death (because a total of only 3 kidney deaths occurred)+HHF.
Kidney outcomes, excluding kidney death: KF+sustained eGFR (Chronic Kidney Disease Epidemiology Collaboration) decline ≥40%+sustained macroalbuminuria.
Results
Empagliflozin reduced the risk of all 3 cardio/kidney composites regardless of the applied method (eg, for composite 1 empagliflozin versus placebo: HR, 0.56 [95% CI, 0.49–0.64]; Figure 1; and WR, 1.76 [95% CI, 1.53–2.02]; Figure 2). Composite 1 incorporated macroalbuminuria and in consequence evaluated more events in both treatment arms and showed an increased treatment effect compared with composite 2 and 3 because of the additional effect on progression to macroalbuminuria.
Figure 1. End point hierarchy from highest to lowest priority of ordered cardio/kidney composite end point definition.
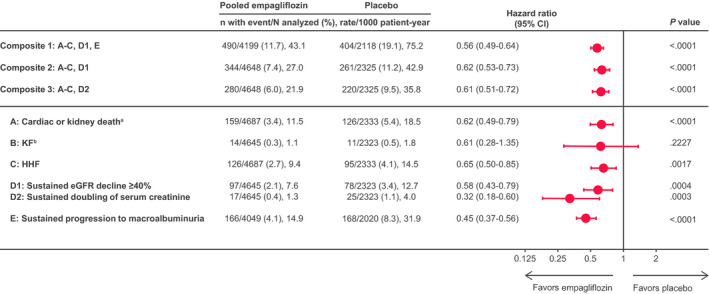
A: Cardiac (excluding fatal stroke) or kidney death; B: KF; C: HHF; D1: sustained eGFR decline of ≥40%; D2: doubling of serum creatinine; E: sustained progression to macroalbuminuria. aNumber of kidney deaths=3. bKF=sustained eGFR <15 mL/min per 1.73 m2 or sustained continuous kidney replacement therapy (including transplantation). eGFR indicates estimated glomerular filtration rate, equation developed by the Chronic Kidney Disease Epidemiology Collaboration; HHF, hospitalization for heart failure; and KF, kidney failure.
Figure 2. Unmatched win ratio analysis.
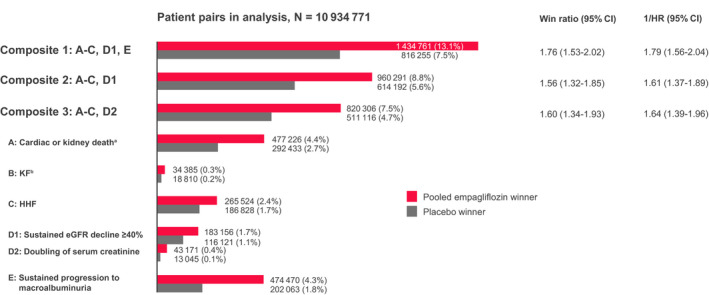
End point hierarchy from highest to lowest priority of ordered cardio/kidney composite end point definition. A: Cardiac (excluding fatal stroke) or kidney death; B: KF; C: HHF; D1: sustained eGFR decline of ≥40%; D2: doubling of serum creatinine; E: sustained progression to macroalbuminuria. Number (percent) of pairs winning for placebo (gray bar) or empagliflozin (red bar). Number of pairs in analysis: 10 934 771. Number of patient pairs (empagliflozin, placebo) for which no decision on winning can be made (ie, for all end points, either both patients are censored or 1 patient is censored before the other patient having an event: Composite 1: 8 683 755 (79.4%); Composite 2: 9 360 288 (85.6%); Composite 3: 9 603 349 (87.8%). Counting 1 event/participant and type. aNumber of kidney deaths=3. bKF=sustained eGFR <15 mL/min per 1.73 m2 or sustained continuous kidney replacement therapy (including transplantation). eGFR indicates estimated glomerular filtration rate, equation developed by Chronic Kidney Disease Epidemiology Collaboration; HHF, hospitalization for heart failure; HR, hazard ratio; and KF, kidney failure.
Although it is rational to formally recognize the potential of cardio/kidney composite end points, one must also consider the clinical relevance of the individual components. In this regard, after death, it seems reasonable to consider the onset of KF as more severe for a patient than occurrence of HHF, eGFR decline, or progression to macroalbuminuria. This hierarchy is elegantly reflected when using the WR approach as depicted for the individual components in Figure 2, whereas the time‐to‐first‐event analysis accounts for more soft outcomes such as albuminuria and eGFR decline, but overall incorporates fewer events for most components (Table). Of the 285 total cardiac or kidney deaths, 44 to 56 (15%–20%), depending on the composite, are not evaluated in the Cox regression because of a preceding nonfatal event in these patients but are included in the WR analysis (Table). Furthermore, in the design of cardio/kidney composite end points for future outcomes trials, it may be important to adequately reflect the affected organ systems (ie, event rates capturing cardiac and kidney outcomes). Interestingly, the suggested composites differed in this regard. In composite 1, most frequent first events were progression to macroalbuminuria and cardiac or kidney death, and event rates were balanced for cardiac and kidney components. However, in composite 2 and especially 3, the composite outcomes comprised more frequently hard cardiac than kidney events, because the trial population experienced few hard kidney outcomes such as KF (Table). This effect occurred both in time‐to‐first‐event and WR analyses, reflecting the low proportion of hard kidney outcomes in the EMPA‐REG OUTCOME trial.
Discussion
How is the WR applied in this and other studies? The WR is a useful method to evaluate composite outcomes comprising events of different clinical importance, even across distinct organ systems. Notwithstanding, it should be noted that the WR has limitations. In contrast to the Cox model, there are no model assumptions except for the hierarchy of the different components, and the hierarchy of the nonfatal outcomes may be debatable. For example, in our case, it could be argued that a doubling of serum creatinine, representing a 57% decline in eGFR, is as important as an HHF. One may also combine WR and recurrent event analysis, which is a topic of current research. This will then use information from the totality of events, but at the cost of increased complexity of analysis and interpretation. In our analyses, recurrent nonfatal events are ignored; only the first event per event type is evaluated. The WR performs separate comparisons per event type, starting with the event type of highest priority and only proceeding to the comparison of the event type of the next lower priority in case no clear decision could yet be taken. In contrast, the Cox model uses only the first event irrespective of importance of events. It should also be noted that, similar to a combined end point Cox model, descriptive time‐to‐event analyses for the single components should always be evaluated in addition to a WR analysis. This is because the WR does not evaluate the precise time‐to‐event but only whether the event occurred sooner in a patient compared with his/her comparator.
Of the 2 approaches to calculate the WR (matched‐pairs versus all‐pairs approach), we used the latter. We acknowledge that this leads to unfair comparisons of patients with high‐risk baseline variables to patients with low‐risk at baseline in both directions and, in turn, to a conservative estimate of treatment effect. However, the matched‐pairs approach heavily relies on an appropriately defined risk score for matching. This was considered challenging in our case because of the combination of both cardiovascular and kidney events, although risk factors for kidney and cardiovascular risk are well known. In particular, mimicking the approach as outlined by Pocock et al. 4 will likely not adequately reflect the risk for the combination of events as analyzed in the WR, because it would use the relative risk computed by a combined end point Cox regression with known kidney and cardiovascular baseline risk factors as covariates. It will, in any case, not be a straightforward approach, would require additional checking of proper risk balancing, and introduces another subjective element in the analysis, on top of the hierarchy of events. Also, the WR depends on the censoring distribution of the components, as tied pairs are simply discarded from the analysis, which is especially relevant for composites 2 (85.6% ties) and 3 (87.8% ties) compared with 79.4% ties in composite 1, whereas Cox regression uses censoring information from all patients. For a discussion of the application of the WR method across different settings in cardiovascular outcome trials, we refer to Pocock et al. 4 and Ferreira et al. 9
In conclusion, the WR considers the clinical relevance of different event types, and in particular, does not ignore fatal events that occur after an earlier nonfatal event. Thus, it represents an appropriate method to study cardio/kidney composite outcomes and may complement traditional time‐to‐first‐event analyses. Empagliflozin reduced the risk of cardio/kidney composite end points by applying either method.
Sources of Funding
This study was funded by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance.
Disclosures
Dr Ferreira receives modest consulting fees from Boehringer Ingelheim. Dr Kraus has received grant support from the IZKF Würzburg (Interdisziplinäres Zentrum für Klinische Forschung, project ZZ‐13) and honoraria from Boehringer Ingelheim. Drs Zwiener, Koitka‐Weber, George, and Ofstad are employees of Boehringer Ingelheim. Dr Fitchett has received honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck & Co., and Sanofi. Dr Zinman has received grant support from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi‐Aventis. Dr Lauer has received consulting fees from Hoffmann La Roche and Boehringer Ingelheim. Dr Wanner has received honoraria for consultancy and lecturing from AbbVie, Actelion, Amgen, Bayer, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen, Protalix, Sanofi Genzyme, and Shire. Dr Zannad has recently received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius.
Acknowledgments
The authors thank the investigators, coordinators, and patients who participated in this trial. Editorial assistance, supported financially by Boehringer Ingelheim, was provided by Paul Lidbury and Charlie Bellinger of Elevate Scientific Solutions.
Author contributions: Drs Zwiener and Lauer performed the statistical analyses, and Drs Ferreira and Kraus drafted the manuscript. All authors were involved at all stages of manuscript development, approved the final version, and agreed to be accountable for all aspects of the work.
(J Am Heart Assoc. 2021;10:e020053. DOI: 10.1161/JAHA.120.020053.)
For Sources of Funding and Disclosures, see page 6.
References
- 1. Cox DR. Regression models and life‐tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. DOI: 10.1111/j.2517-6161.1972.tb00899.x. [DOI] [Google Scholar]
- 2. Ferreira‐González I, Permanyer‐Miralda G, Domingo‐Salvany A, Busse JW, Heels‐Ansdell D, Montori VM, Akl EA, Bryant DM, Alonso‐Coello P, Alonso J, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ. 2007;334:786. DOI: 10.1136/bmj.39136.682083.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999;18:1341–1354. DOI: . [DOI] [PubMed] [Google Scholar]
- 4. Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176–182. DOI: 10.1093/eurheartj/ehr352. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. DOI: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 6. Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138:929–944. DOI: 10.1161/CIRCULATIONAHA.117.028814. [DOI] [PubMed] [Google Scholar]
- 7. Patel RB, Ter Maaten JM, Ferreira JP, McCausland FR, Shah SJ, Rossignol P, Solomon SD, Vaduganathan M, Packer M, Thompson A, et al. Challenges of cardio‐kidney composite outcomes in large‐scale clinical trials. Circulation. 2021;143:949–958. DOI: 10.1161/CIRCULATIONAHA.120.049514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. DOI: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 9. Ferreira JP, Jhund PS, Duarte K, Claggett BL, Solomon SD, Pocock S, Petrie MC, Zannad F, McMurray JJV. Use of the win ratio in cardiovascular trials. JACC Heart Fail. 2020;8:441–450. DOI: 10.1016/j.jchf.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 10. Redfors B, Gregson J, Crowley A, McAndrew T, Ben‐Yehuda O, Stone GW, Pocock SJ. The win ratio approach for composite endpoints: practical guidance based on previous experience. Eur Heart J. 2020;41:4391–4399. DOI: 10.1093/eurheartj/ehaa665. [DOI] [PubMed] [Google Scholar]


