Abstract
Background
Better cardiovascular health (CVH) scores are associated with lower risk of cardiovascular disease (CVD). However, estimates of the potential population‐level impact of improving CVH on US CVD event rates are not currently available.
Methods and Results
Using data from the National Health and Nutrition Examination Survey 2011 to 2016 (n=11 696), we estimated the proportions of US adults in CVH groups. Levels of 7 American Heart Association CVH metrics were scored as ideal (2 points), intermediate (1 point), or poor (0 points), and summed to define overall CVH (low, 0–8 points; moderate, 9–11 points; or high, 12–14 points). Using individual‐level data from 7 US community‐based cohort studies (n=30 447), we estimated annual incidence rates of major CVD events by levels of CVH. Using the combined data sources, we estimated population attributable fractions of CVD and the number of CVD events that could be prevented annually if all US adults achieved high CVH. High CVH was identified in 7.3% (95% CI, 6.3%–8.3%) of US adults. We estimated that 70.0% (95% CI, 56.5%–79.9%) of CVD events were attributable to low and moderate CVH. If all US adults attained high CVH, we estimated that 2.0 (95% CI, 1.6–2.3) million CVD events could be prevented annually. If all US adults with low CVH attained moderate CVH, we estimated that 1.2 (95% CI, 1.0–1.4) million CVD events could be prevented annually.
Conclusions
The potential benefits of achieving high CVH in all US adults are considerable, and even a partial improvement in CVH scores would be highly beneficial.
Keywords: cardiovascular disease, epidemiology, health status disparities, prevention, risk factors
Subject Categories: Cardiovascular Disease, Epidemiology, Primary Prevention, Race and Ethnicity, Women
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- CVH
cardiovascular health
- NHANES
National Health and Nutrition Examination Survey
- PAF
population attributable fraction
- PIF
potential impact fraction
Clinical Perspective
What Is New?
This is the first analysis to estimate the potential impact of improving population‐level cardiovascular health (CVH) on reductions in cardiovascular disease (CVD) events.
We estimated that only 7.3% of US adults had a high CVH score, and 70.0% of CVD events in the Unites States were attributable to low and moderate CVH.
If all US adults attained high CVH, we estimated that 2.0 million CVD events could be prevented annually in the United States; even partial improvements would be beneficial; if all US adults with low CVH attained moderate CVH, we estimated that 1.2 million CVD events could be prevented annually.
What Are the Clinical Implications?
The American Heart Association CVH score integrates 7 of the most important metrics for CVD prevention: smoking status, body mass index, physical activity, diet quality, total cholesterol, blood pressure, and fasting glucose.
Higher CVH scores are associated with lower risk of CVD, and discussions with individuals about improving their CVH score may contribute to population‐level reductions in CVD burden.
Future research should identify multipronged approaches and policy initiatives targeting CVH in conjunction with the social determinants of health throughout the life course to improve CVH in the United States.
Cardiovascular disease (CVD) is a major cause of morbidity and the leading cause of death in the United States and globally. 1 , 2 Epidemiologic and clinical trial evidence has established the causal associations of high blood pressure (BP), dyslipidemia, diabetes mellitus, and cigarette smoking with increased risk of CVD. 3 However, despite the availability of effective interventions targeting these risk factors for primary prevention, previously declining CVD death rates are plateauing. 4 In an effort to shift attention toward the preservation of ideal levels of risk factors (ie, primordial prevention), the American Heart Association (AHA) developed the cardiovascular health (CVH) concept, which integrates 7 modifiable factors: smoking status, body mass index (BMI), physical activity, diet quality, total cholesterol, BP, and fasting glucose. 5
Several studies have documented the associations of CVH with various CVD outcomes 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 and have described the US population prevalence of CVH over time. 7 , 15 , 16 In 2012, Yang et al reported the associations of CVH metrics with population health indicators, including population attributable fractions (PAFs) of mortality. 7 However, PAFs for incident nonfatal as well as fatal CVD events have not been estimated for individual CVH metrics or by level of the total CVH score. Furthermore, the potential impact of achieving high CVH among all US adults on CVD events is unknown. This information could aid researchers, clinicians, and policy makers in developing and implementing strategies to reduce the overall burden of CVD in US adults.
Using a comprehensive analytic framework and nationally representative data, the objectives of this study were 2‐fold: to quantify the prevalence and PAFs of CVH levels in US adults overall and in subgroups defined by age, sex, and race/ethnicity; and to estimate the number of CVD events that could be prevented annually if US adults had improved CVH levels.
Methods
The data and materials that support the findings of this study can be requested from the corresponding author and the National Heart, Lung, and Blood Institute BioLINCC.
Estimating the Proportions of US Adults in 3 CVH Score Groups
The National Health and Nutrition Examination Survey (NHANES) uses a complex, stratified, multistage probability cluster sampling design to select representative samples of the US civilian, noninstitutionalized population. 17 We combined 3 survey cycles, conducted between 2011 and 2016, which included 17 048 participants aged ≥20 years. The analysis was limited to nonpregnant, nonlactating participants with complete information for characterization of all 7 CVH metrics, yielding a final analysis sample size of 11 696 adults. Smoking status and the frequency, duration, and intensity of leisure‐time physical activities in a typical week were ascertained via questionnaires. BMI was calculated as the weight in kilograms divided by height in meters squared. Dietary intake was assessed via 2 interviewer‐administered 24‐hour recalls, and the average was used in analyses, yielding a healthy diet score ranging from 0 to 5 based on AHA criteria. 5 Total cholesterol and BP were measured according to standard NHANES protocols. Because fasting plasma glucose values were only available for a subsample of NHANES participants, we used glycated hemoglobin values as a proxy for fasting glucose levels, as suggested by the American Diabetes Association. 7 , 18
On the basis of AHA definitions (Table 1), CVH was scored for each individual metric as ideal (2 points), intermediate (1 point), or poor (0 points). 5 A composite CVH score (range, 0–14 points), based on the sum of individual CVH metrics, was calculated for each participant. We stratified participants, a priori, into high (12–14 points), moderate (9–11 points), and low (0–8 points) CVH groups, which is supported by prior reports 10 , 13 , 19 and hazard ratios (HRs) of CVD events for CVH scores in the current study (Figure S1).
Table 1.
Proportions of US Adults Meeting Recommended Levels of CVH Metrics: NHANES 2011 to 2016
| Health Metric | Definition | Proportion of US Adults in CVH Categories, % (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| All Adults | Sex | Race | |||||
| Men | Women | White/Other § | Black | ||||
| Smoking | Ideal | Never or quit >12 mo | 78.6 (77.2–80.0) | 76.3 (74.3–78.3) | 80.8 (79.0–82.6) | 79.2 (77.7–80.8) | 73.9 (71.0–76.8) |
| Intermediate | Former, quit ≤12 mo | 2.8 (2.3–3.4) | 3.4 (2.6–4.3) | 2.2 (1.5–2.9) | 2.9 (2.3–3.5) | 2.3 (1.5–3.1) | |
| Poor | Current | 18.6 (17.2–19.9) | 20.2 (18.3–22.1) | 17.0 (15.4–18.6) | 17.9 (16.4–19.4) | 23.8 (21.2–26.4) | |
| Body mass index, kg/m2 | Ideal | <25 | 29.3 (27.7–31.0) | 26.0 (24.3–27.6) | 32.4 (30.1–34.7) | 30.1 (28.3–31.9) | 23.0 (21.0–25.0) |
| Intermediate | 25–29.99 | 33.0 (31.4–34.6) | 38.4 (36.3–40.4) | 27.9 (25.8–30.1) | 33.9 (32.2–35.7) | 25.3 (22.9–27.7) | |
| Poor | ≥30 | 37.7 (35.9–39.5) | 35.7 (33.1–38.2) | 39.6 (37.7–41.5) | 36.0 (34.0–37.9) | 51.7 (48.9–54.5) | |
| Physical activity | Ideal | ≥150 min/wk moderate or ≥75 min/wk vigorous or ≥150 combination | 39.3 (37.3–41.3) | 42.1 (40.0–44.2) | 36.7 (33.7–39.6) | 39.7 (37.5–42.0) | 35.7 (32.9–38.5) |
| Intermediate | 1–149 min/wk moderate or 1–74 min/wk vigorous or 1–149 min/wk combination | 16.9 (15.6–18.2) | 15.6 (13.9–17.3) | 18.2 (16.6–19.8) | 17.1 (15.6–18.5) | 15.9 (14.2–17.6) | |
| Poor | None | 43.8 (41.3–46.3) | 42.3 (39.4–45.2) | 45.1 (42.0–48.2) | 43.2 (40.4–45.9) | 48.4 (45.5–51.3) | |
| Healthy diet score* | Ideal | 4–5 Components | 0.7 (0.5–0.9) | 0.3 (0.2–0.5) | 1.0 (0.6–1.3) | 0.6 (0.4–0.8) | 1.1 (0.6–1.5) |
| Intermediate | 2–3 Components | 24.7 (22.8–26.7) | 20.4 (18.5–22.3) | 28.8 (26.1–31.5) | 25.5 (23.3–27.7) | 18.9 (16.2–21.5) | |
| Poor | 0–1 Component | 74.6 (72.6–76.6) | 79.2 (77.3–81.1) | 70.2 (67.6–72.9) | 73.9 (71.7–76.1) | 80.1 (77.3–82.9) | |
| Total cholesterol, mg/dL | Ideal | <200 (untreated) | 46.3 (44.3–48.3) | 46.8 (44.3–49.2) | 45.8 (43.2–48.4) | 45.1 (43.1–47.2) | 55.3 (52.7–57.8) |
| Intermediate | 200–239 or treated to goal | 41.5 (39.5–43.5) | 41.9 (39.4–44.3) | 41.1 (38.7–43.6) | 42.3 (40.1–44.4) | 35.2 (32.7–37.8) | |
| Poor | ≥240 | 12.2 (11.1–13.4) | 11.4 (10.0–12.7) | 13.1 (11.4–14.7) | 12.6 (11.4–13.8) | 9.5 (7.9–11.2) | |
| Blood pressure, mm Hg | Ideal | <120/<80 (untreated) | 41.2 (39.6–42.7) | 35.4 (33.2–37.7) | 46.5 (44.6–48.4) | 42.2 (40.4–44.0) | 32.9 (30.4–35.3) |
| Intermediate | 120–139/80–89 or treated to goal | 43.8 (42.3–45.3) | 48.5 (46.5–50.5) | 39.4 (37.7–41.1) | 43.4 (41.9–45.0) | 46.4 (44.3–48.5) | |
| Poor | ≥140/90 | 15.1 (13.8–16.3) | 16.1 (14.2–17.9) | 14.1 (12.9–15.4) | 14.4 (12.9–15.8) | 20.7 (18.7–22.8) | |
| HbA1c, % † | Ideal | <5.7 (Untreated) | 66.8 (65.3–68.3) | 66.4 (64.4–68.3) | 67.2 (65.6–68.8) | 68.7 (67.1–70.3) | 51.8 (49.0–54.5) |
| Intermediate | 5.7–6.5 or treated to goal | 25.0 (23.8–26.3) | 24.5 (22.9–26.1) | 25.5 (24.0–27.1) | 23.7 (22.3–25.0) | 35.8 (33.3–38.3) | |
| Poor | ≥6.5 | 8.2 (7.4–9.0) | 9.2 (7.9–10.4) | 7.3 (6.5–8.0) | 7.6 (6.7–8.6) | 12.4 (11.0–13.8) | |
| Total CVH score ‡ | High | 12–14 Points | 7.3 (6.3–8.3) | 6.1 (5.1–7.1) | 8.4 (6.7–10.1) | 7.8 (6.6–8.9) | 3.5 (2.3–4.8) |
| Moderate | 9–11 Points | 34.2 (32.4–36.0) | 33.6 (31.3–35.9) | 34.7 (32.4–37.0) | 35.0 (33.1–37.0) | 27.5 (24.8–30.1) | |
| Low | 0–8 Points | 58.5 (56.1–60.9) | 60.3 (57.7–62.9) | 56.9 (54.0–59.8) | 57.2 (54.6–59.8) | 69.0 (66.0–71.9) | |
CVH indicates cardiovascular health; HbA1c, glycated hemoglobin; and NHANES, National Health and Nutrition Examination Survey.
*The 5 dietary score components include ≥4.5 cups/day of fruits/vegetables, ≥2 servings of fish per week (3.5 oz), ≥3 servings of whole grains per day (1 oz), <1500 mg/d of sodium, and <450 kcal/wk of sugar‐sweetened beverages. Dietary values are scaled to a 2000‐kcal/d diet.
†Given that fasting plasma glucose values were only available for a subsample of NHANES participants, we used HbA1c values as a proxy for fasting glucose levels (fasting plasma glucose ≥126 mg/dL=HbA1c ≥6.5%; fasting plasma glucose 100–125 mg/dL=HbA1c 5.7%–6.4%; fasting plasma glucose <100 mg/dL untreated=HbA1c <5.7% untreated), as suggested by the American Diabetes Association.
‡The total CVH score represents the sum of individual metric point values and ranges from 0 to 14. Each individual metric was scored as ideal (2 points), intermediate (1 point), or poor (0 points). We defined overall CVH as high (12–14 points), moderate (9–11 points), or low (0–8 points).
§Includes Mexican American, other Hispanic, non‐Hispanic Asian, and other races.
We used survey analysis procedures to estimate the proportion of US adults in CVH score groups by subgroups of age (20–39, 40–59, and ≥60 years), sex, and race/ethnicity (White/other [including Mexican American, other Hispanic, non‐Hispanic Asian, and other races] and Black race/ethnicity). Original NHANES sampling weights were recalibrated on the basis of the proportion of participants excluded because of missing CVH data by age, sex, and race/ethnicity within each NHANES cycle, yielding US nationally representative estimates. 20
Estimating the Incidence and HRs of Major CVD Events
We pooled individual‐level data from 30 447 participants free of CVD at baseline and with complete information for characterization of all 7 CVH metrics, from 7 US cohort studies included in the Lifetime Risk Pooling Project 19 , 21 : the ARIC (Atherosclerosis Risk in Communities) Study, 22 CARDIA (Coronary Artery Risk Development in Young Adults Study), 23 CHS (Cardiovascular Health Study), 24 MESA (Multi‐Ethnic Study of Atherosclerosis), 25 FHS (Framingham Heart Study), 26 Framingham Offspring Study, 27 and the WHI (Women's Health Initiative) Observational Study. 28 Detailed data ascertainment methods are available in the original design publications from each study, and the data were harmonized for comparability. 19 , 21 , 29 Because individual studies used different instruments for assessing physical activity and diet, we used modified definitions for data harmonization (Table S1), which have been detailed previously. 14 , 19 , 29 , 30
We used Poisson regression models to estimate incidence of major CVD events (composite of nonfatal myocardial infarction, stroke, heart failure, or CVD death), which were adjudicated using similar methods in each cohort. 19 , 21 The estimates were calibrated to reflect an annual occurrence of 2.85 million major CVD events (795 000 stroke, 1 055 000 coronary heart disease, and 1 000 000 heart failure) in the US adult population in 2019, as reported by the AHA. 31 We used Cox proportional hazards regression to estimate HRs of CVD events for the overall CVH score and its individual components, stratified by age group and sex. HRs were not further stratified by race/ethnicity because few CVD events occurred among Black participants with a high CVH score. However, there was no evidence of a CVH‐by‐race/ethnicity interaction (P=0.82), which is supported by prior reports. 6 , 13 The earliest initial data collection was March 25, 1985, and follow‐up time was censored at the time of a first CVD event, death from non‐CVD causes, or end of follow‐up through August 31, 2016.
Estimating the PAFs and Number of Events Prevented
A more detailed description of the PAF methods is available in Data S1. We calculated PAFs to estimate the proportion of CVD events that could hypothetically be prevented (or postponed), assuming a causal relationship, if the entire US adult population (or subgroups) had high CVH. 32 Within each age‐, sex‐, and race/ethnicity‐specific stratum, we multiplied the PAFs by the expected number of annual CVD events to estimate the absolute number of events that could be prevented, which were summed to produce adjusted estimates overall and by subgroup. 33 , 34 We calculated PAFs in 2 separate analyses based on different assumptions: (1) all adults with a low or moderate CVH score would achieve a high CVH score; and (2) all adults with a low CVH score would achieve a moderate CVH score, while all adults with a moderate or high CVH score would remain in these groups. We repeated this general approach for each individual component of CVH.
Two sensitivity analyses were conducted. First, we estimated the number of CVD events prevented assuming only fractions of US adults achieved a high or moderate CVH score. Second, we assessed continuous CVH scores by estimating potential impact fractions (PIFs). 32 Specifically, we estimated the number of CVD events prevented assuming the mean CVH score in the US population increased by 1, 2, or 3 points. We additionally estimated the PIFs and number of CVD events prevented assuming all US adults achieved the maximum CVH score.
We accounted for uncertainty in our estimation of the proportions of CVH score groups, incidence rates, and HRs of CVD using Monte Carlo simulation. 35 , 36 For each age‐, sex‐, and race/ethnicity‐specific PAF and PIF calculation, 10 000 simulations were conducted, with the 2.5th and 97.5th percentiles forming 95% CIs. All analyses were conducted using SAS version 9.4 and R version 3.6.2. This study was approved by the institutional review board at Northwestern University. Written informed consent was obtained from all participants for initial data collection in each original cohort and NHANES.
Results
The mean CVH score among US adults from NHANES was 7.9 (95% CI, 7.8–8.0), and scores were approximately normally distributed (Figure S2). High CVH was identified in 7.3% (95% CI, 6.3%–8.3%), moderate CVH in 34.2% (95% CI, 32.4%–36.0%), and low CVH in 58.5% (95% CI, 56.1%–60.9%) of US adults (Table 1). The prevalence of high CVH was greater among younger compared with older adults, women compared with men, and White/other [including Mexican American, other Hispanic, non‐Hispanic Asian, and other races] compared with Black adults (Table 1 and Table S2). More detailed characteristics of US adults in NHANES are presented in Table S3.
A total of 30 447 participants were included from the pooled cohorts (mean [SD] age at baseline, 55.0 [13.9] years; 60.6% women; 31.8% Black race), and CVH scores were approximately normally distributed (Figure S2). High CVH was identified in 7.5%, moderate CVH in 35.9%, and low CVH in 56.6% of participants (Table S1). More detailed baseline characteristics of the participants are presented in Table S4.
A total of 6546 incident CVD events (1430 stroke, 2764 coronary heart disease, and 2352 heart failure) occurred in the pooled cohort participants over 538 477 person‐years of follow‐up (mean [SD] follow‐up, 16.2 [7.2] years). Incidence rates were higher among older compared with younger participants, men compared with women, and Black compared with White/other [including Mexican American, other Hispanic, non‐Hispanic Asian, and other races] participants (Table 2). Among men aged 40 to 59 years, compared with low CVH, HRs (95% CIs) were 0.38 (0.33–0.44) for moderate CVH and 0.19 (0.11–0.33) for high CVH. Corresponding HRs (95% CIs) among women aged 40 to 59 years were 0.37 (0.31–0.43) and 0.12 (0.07–0.20). Patterns were similar among participants aged 20 to 39 years and ≥60 years. HRs for individual CVH metrics are presented in Table S5.
Table 2.
Associations Between CVH Score Categories and Incident CVD Events: The Lifetime Risk Pooling Project
| Subgroups | Low CVH | Moderate CVH | High CVH | Per 1‐Unit Higher CVH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events/Participants | Events per 1000 PYs | HR (95% CI)* | Events/Participants | Events per 1000 PYs | HR (95% CI)* | Events/Participants | Events per 1000 PYs | HR (95% CI)* | HR (95% CI)* | |
| Aged 20–39 y | ||||||||||
| White/other † men | 26/207 | 4.93 | 1 (Reference) | 29/578 | 1.82 | 0.41 (0.28–0.59) | 2/297 | 0.23 | 0.07 (0.03–0.20) | 0.69 (0.63–0.76) |
| Black men | 29/211 | 5.28 | 28/463 | 2.15 | 2/93 | 0.77 | ||||
| White/other † women | 10/164 | 2.23 | 1 (Reference) | 13/588 | 0.78 | 0.43 (0.28–0.68) | 7/481 | 0.51 | 0.24 (0.11–0.51) | 0.75 (0.68–0.84) |
| Black women | 28/361 | 2.77 | 25/672 | 1.28 | 2/121 | 0.57 | ||||
| Aged 40–59 y | ||||||||||
| White/other † men | 721/2772 | 16.1 | 1 (Reference) | 174/1622 | 6.13 | 0.38 (0.33–0.44) | 12/227 | 2.99 | 0.19 (0.11–0.33) | 0.77 (0.75–0.79) |
| Black men | 293/870 | 22.2 | 32/278 | 7.37 | 1/30 | 2.10 | ||||
| White/other † women | 489/2607 | 10.8 | 1 (Reference) | 161/2396 | 3.71 | 0.37 (0.31–0.43) | 13/565 | 1.29 | 0.12 (0.07–0.20) | 0.75 (0.73–0.77) |
| Black women | 398/2123 | 12.7 | 53/842 | 4.28 | 0/100 | 0.00 | ||||
| Aged ≥60 y | ||||||||||
| White/other † men | 1155/2373 | 42.0 | 1 (Reference) | 382/1169 | 24.6 | 0.55 (0.49–0.61) | 20/100 | 14.6 | 0.34 (0.22–0.51) | 0.83 (0.81–0.85) |
| Black men | 183/536 | 29.8 | 34/166 | 18.4 | 3/16 | 16.0 | ||||
| White/other † women | 1269/2987 | 31.3 | 1 (Reference) | 390/1437 | 18.4 | 0.58 (0.52–0.64) | 25/183 | 9.22 | 0.29 (0.20–0.42) | 0.83 (0.81–0.84) |
| Black women | 449/2029 | 19.4 | 86/726 | 10.2 | 2/57 | 2.77 | ||||
CVD indicates cardiovascular disease; CVH, cardiovascular health; HR, hazard ratio; and PY, person‐year.
*HRs for CVD events are adjusted for age (continuous) and race/ethnicity. HRs for moderate and high CVH were estimated using low CVH as the reference category.
†Includes Mexican American, other Hispanic, non‐Hispanic Asian, and other races.
An estimated 2.85 million CVD events occurred among US adults in 2019 (Table S6). 31 We estimated that 70.0% (95% CI, 56.5%–79.9%) of CVD events were attributable to low and moderate levels of CVH, and 2.0 (95% CI, 1.6–2.3) million CVD events could potentially be prevented annually if all US adults attained a high CVH score (Table 3). We estimated that 42.0% (95% CI, 35.3%–48.2%) of all CVD events could be attributed to low levels of CVH alone, and that 1.2 (95% CI, 1.0–1.4) million CVD events could be prevented annually if these individuals attained moderate CVH. PAFs for CVD events were higher among younger compared with older adults, women compared with men, and Black compared with White/other [including Mexican American, other Hispanic, non‐Hispanic Asian, and other races] adults.
Table 3.
CVH Category Prevalence and Potential Impact of Improving CVH Among US Adults
| Subgroups | Proportion of US Adults in CVH Categories, % (95% CI) |
Achievement of Moderate CVH Among US Adults With Low CVH |
Achievement of High CVH Among All US Adults |
||||
|---|---|---|---|---|---|---|---|
| Low | Moderate | High | Population Attributable Fraction, % (95% CI) | CVD Events Prevented, No. in Thousands (95% CI)* | Population Attributable Fraction, % (95% CI) | CVD Events Prevented, No. in Thousands (95% CI) † | |
| Aged 20–39 y | |||||||
| White/other ‡ men | 41.7 (37.7–45.7) | 46.8 (43.6–50.0) | 11.5 (9.2–13.7) | 37.7 (21.9–51.9) | 30 (17–41) | 88.2 (76.1–94.9) | 70 (60–75) |
| Black men | 46.5 (39.9–53.2) | 45.1 (39.8–50.4) | 8.4 (4.0–12.7) | 40.3 (23.8–54.8) | 6 (4–8) | 88.9 (77.3–95.4) | 13 (12–14) |
| White/other ‡ women | 32.4 (28.7–36.1) | 50.0 (46.7–53.2) | 17.6 (14.2–21.1) | 29.7 (12.8–46.0) | 10 (4–16) | 59.4 (33.7–77.0) | 21 (12–27) |
| Black women | 54.7 (47.7–61.7) | 40.0 (33.6–46.3) | 5.3 (2.6–8.1) | 41.7 (20.0–59.6) | 4 (2–6) | 67.8 (42.9–83.2) | 7 (5–9) |
| Total | 38.9 (36.0–41.7) | 47.6 (45.3–49.9) | 13.5 (11.7–15.4) | 36.7 (19.9–51.9) | 51 (28–72) | 80.4 (63.8–90.6) | 112 (89–126) |
| Aged 40–59 y | |||||||
| White/other ‡ men | 65.9 (61.2–70.6) | 29.8 (25.1–34.5) | 4.3 (2.7–5.9) | 51.7 (45.0–57.7) | 232 (202–259) | 75.5 (61.7–85.2) | 340 (277–383) |
| Black men | 81.8 (77.0–86.7) | 17.3 (12.5–22.1) | 0.8 (0.0–1.8) | 57.0 (50.4–62.7) | 45 (40–50) | 78.4 (64.2–87.1) | 62 (51–69) |
| White/other ‡ women | 60.4 (56.4–64.3) | 32.7 (29.3–36.2) | 6.9 (4.9–8.9) | 51.2 (44.7–57.0) | 146 (128–163) | 84.2 (75.5–90.3) | 241 (216–258) |
| Black women | 76.6 (70.7–82.4) | 21.3 (15.7–26.9) | 2.1 (0.8–3.4) | 57.1 (50.5–62.9) | 35 (31–39) | 86.3 (77.9–91.9) | 53 (48–56) |
| Total | 64.9 (61.7–68.1) | 30.0 (27.0–32.9) | 5.2 (3.9–6.4) | 53.1 (46.4–59.1) | 459 (401–511) | 80.4 (68.5–88.7) | 696 (592–767) |
| Aged ≥60 y | |||||||
| White/other ‡ men | 75.7 (72.0–79.5) | 22.3 (18.9–25.8) | 1.9 (0.9–3.0) | 38.8 (32.5–44.5) | 355 (298–408) | 62.1 (46.2–73.8) | 569 (424–676) |
| Black men | 81.9 (76.6–87.2) | 17.0 (12.0–21.9) | 1.1 (0.0–2.3) | 40.6 (34.2–46.5) | 25 (21–29) | 63.3 (47.2–75.1) | 39 (29–46) |
| White/other ‡ women | 75.0 (70.5–79.5) | 23.1 (18.6–27.5) | 1.9 (0.9–3.0) | 35.7 (29.8–41.2) | 285 (238–330) | 67.9 (55.8–77.1) | 543 (446–617) |
| Black women | 89.8 (87.0–92.7) | 9.5 (6.6–12.4) | 0.7 (0.0–1.3) | 39.9 (34.0–45.5) | 22 (19–25) | 70.1 (57.5–79.3) | 39 (32–44) |
| Total | 76.3 (73.1–79.4) | 21.9 (18.9–24.8) | 1.8 (1.2–2.5) | 37.2 (31.2–42.9) | 688 (577–791) | 64.4 (50.4–74.9) | 1189 (931–1383) |
| Men | 60.3 (57.7–62.9) | 33.6 (31.3–35.9) | 6.1 (5.1–7.1) | 43.8 (36.8–50.2) | 694 (583–795) | 69.0 (53.9–79.9) | 1093 (854–1265) |
| Women | 56.9 (54.0–59.8) | 34.7 (32.4–37.0) | 8.4 (6.7–10.1) | 39.8 (33.4–45.7) | 504 (423–579) | 71.3 (59.9–79.9) | 903 (758–1011) |
| White/other ‡ adults | 57.2 (54.6–59.8) | 35.0 (33.1–37.0) | 7.8 (6.6–8.9) | 41.4 (34.7–47.6) | 1060 (889–1217) | 69.7 (56.1–79.6) | 1783 (1436–2037) |
| Black adults | 69.0 (66.0–71.9) | 27.5 (24.8–30.1) | 3.5 (2.3–4.8) | 47.4 (40.0–53.8) | 138 (117–157) | 73.4 (60.4–82.0) | 214 (176–239) |
| All adults | 58.5 (56.1–60.9) | 34.2 (32.4–36.0) | 7.3 (6.3–8.3) | 42.0 (35.3–48.2) | 1198 (1005–1374) | 70.0 (56.5–79.9) | 1996 (1611–2276) |
CVD indicates cardiovascular disease; and CVH, cardiovascular health.
* Number of CVD events prevented annually if (1) all adults with low CVH had moderate CVH; and (2) all adults with moderate CVH or high CVH remained in these groups.
† Number of CVD events prevented annually if all adults with low CVH or moderate CVH had high CVH.
‡Includes Mexican American, other Hispanic, non‐Hispanic Asian, and other races.
We estimated that 42.4% (95% CI, 33.3%–50.1%) of CVD events were attributable to poor and intermediate levels of diet, whereas 4.8% (95% CI, 2.4%–7.1%) were attributable to poor and intermediate levels of total cholesterol. Thus, if all US adults achieved ideal levels of diet or total cholesterol, 42.4% or 4.8% of CVD events, respectively, could be prevented annually (Figure 1, blue bars). Corresponding PAFs for other metrics ranged from 12.8% (smoking) to 39.7% (BP). This pattern was similar when evaluating the proportion of CVD events attributable to poor levels alone (Figure 1, orange bars). However, the PAFs for smoking, BMI, physical activity, BP, and glycated hemoglobin were similar in magnitude (range, 9.1%–10.7%). Compared with older adults, PAFs among younger adults were higher for BMI, diet, and total cholesterol; and lower for BP (Table S7). Compared with women, PAFs among men were higher for total cholesterol and lower for BMI (Table S8). Compared with White/other [including Mexican American, other Hispanic, non‐Hispanic Asian, and other races] adults, PAFs among Black adults were higher for all metrics, except physical activity and diet (Table S9).
Figure 1. Population attributable fractions of cardiovascular disease (CVD) events for individual cardiovascular health (CVH) score metrics.
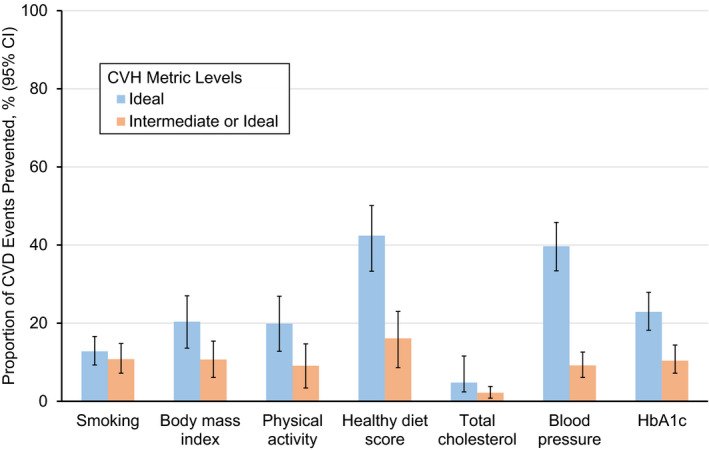
The blue bars represent the estimated population attributable fractions if adults with a poor or intermediate level of a given CVH metric achieved an ideal level. The orange bars represent the estimated population attributable fractions if (1) adults with a poor level of a given CVH metric achieved an intermediate level; and (2) all adults with an intermediate or ideal level of a given CVH metric remained in the same score groups. Error bars indicate 95% CIs. HbA1c indicates glycated hemoglobin.
The estimated numbers of CVD events prevented for fractions of US adults achieving high or moderate CVH are presented in Figure 2. For example, if the proportion of US adults with high CVH increased from 7.3% to 30.0%, an estimated 489 000 (95% CI, 394 000–558 000) CVD events could be prevented annually. A similar number of CVD events could be prevented if the proportion of US adults with moderate or high CVH increased from 41.5% to 65.4%.
Figure 2. Estimated numbers of cardiovascular disease (CVD) events prevented annually with US population improvement of cardiovascular health (CVH) scores.
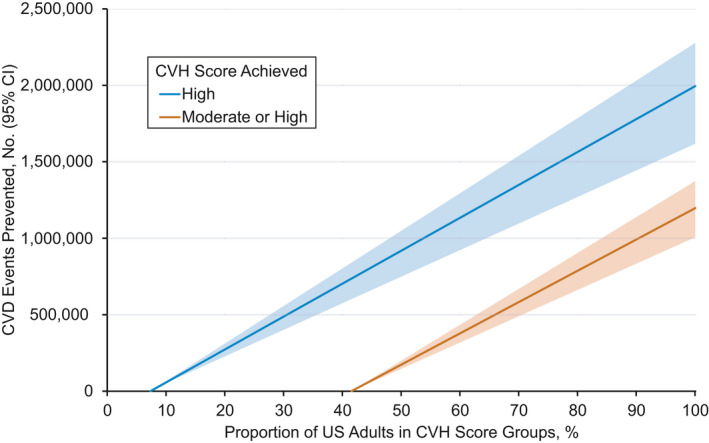
The blue line represents the estimated number of CVD events prevented annually if adults with a low or moderate CVH score achieved a high CVH score. The orange line represents the estimated number of CVD events prevented annually if (1) adults with a low CVH score achieved a moderate CVH score; and (2) all adults with a moderate or high CVH score remained in the same score groups. Tinted regions indicate 95% CIs.
The estimated PIF for a 1‐point greater mean CVH score among US adults was 19.7% (95% CI, 17.4%–21.9%), which could prevent an estimated 559 000 (95% CI, 497 000–618 000) CVD events annually (Table S10). If all US adults achieved a maximum CVH score of 14, the corresponding PIF and number of CVD events prevented annually were 76.2% (95% CI, 71.5%–80.0%) and 2.2 (95% CI, 2.0–2.3) million, respectively.
Discussion
In this analysis, which combined US nationally representative survey data and CVD incidence data from >30 000 adults from 7 community‐based US cohort studies, we found that a high CVH score was uncommon among US adults but associated with a substantially lower risk of CVD compared with a low or moderate CVH score. If all individuals with either a low or moderate CVH score achieved a high CVH score, we estimated that nearly 2 million CVD events could be prevented annually, or ≈70% of all CVD events among US adults in 2019. Even if only a small fraction of the US population had higher CVH, we estimated that there would still be a large number of CVD events prevented. The current findings revealed a low prevalence of high CVH among US adults and highlight the potential impact of preventive efforts targeting future achievement of high CVH among all US adults.
Higher CVH scores are associated with lower risks of various CVD (and non‐CVD) outcomes. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 In 2012, Yang et al examined time trends of CVH metrics among US adults and estimated associated PAFs in relation to all‐cause and CVD mortality. 7 However, PAFs for incident CVD events, including those that are not fatal, were not estimated because of a lack of CVD incidence data in NHANES. Fatal and nonfatal CVD events cost the United States an estimated $351.2 billion annually 31 and are leading causes of disability‐adjusted life years. 1 The current study estimated PAFs for incident CVD events by combining nationally representative CVH prevalence estimates with incidence data from a large, diverse pooled cohort of US adults. In addition, the current study estimated the potential impact of population‐level achievement of high CVH on the number of CVD events prevented. On the basis of our analyses, only 7.3% of US adults had a high CVH score. We estimated that among all US adults, 70.0% of CVD events were attributable to a low or moderate CVH score, with 41.3% attributable to a low CVH score alone. Our estimate of 2 million CVD events prevented by achievement of a high CVH score in all US adults represents a theoretical limit for event reduction, assuming a causal relationship between the CVH score and CVD events. In addition, it may not be realistic to fully achieve this goal in the short‐term, as interventions to improve and maintain high CVH may not have an immediate effect on reducing the burden of CVD. 37 However, the estimated benefits of even a partial improvement in CVH scores were considerable. Therefore, CVH is a potential target for comprehensive prevention policy initiatives to reduce the future burden of CVD. 38 , 39
Persistent sex differences and racial disparities in CVH are well documented. 15 , 16 Men typically have higher rates of CVD mortality compared with women throughout the life course, until old age. 40 The current findings suggest that, on average, women had better CVH scores, although low levels of CVH became more common in older age, contributing to a higher PAF for CVD among women compared with men. Black adults had higher PAFs for CVD compared with White/other [including Mexican American, other Hispanic, non‐Hispanic Asian, and other races] adults in all age groups, reflecting persistently worse CVH scores, which have been identified in previous analyses among US adults. 15 , 16 Consequently, improvement of CVH specifically in Black adults could prevent a relatively large number of CVD events. Overall, our estimates suggest that targeting improvement of CVH in the US population may contribute to reducing sex‐ and race/ethnicity‐associated differences in CVD burden. Multilevel interventions that can be effectively translated, disseminated, and implemented should be developed to address the maintenance of high CVH as early as possible in all adults, 41 , 42 which could have a greater theoretical impact on CVD prevention compared with treating existing risk factors.
The current quantitative estimates of potential population‐level improvements may simultaneously aid individuals, clinicians, policy makers, and researchers as they work to address plateaus in the declining burden of CVD. 4 The CVH score provides a simple and useful composite measure of an individual's health status, which can be tracked over time and targeted for preservation across the life course. 37 Discussions with individuals about their CVH score may be beneficial, and primordial prevention is explicitly endorsed by the 2019 American College of Cardiology/AHA Guideline on the Primary Prevention of Cardiovascular Disease. 43 Among younger adults in our study, PAFs were greater for BMI, healthy diet, and total cholesterol, whereas the PAFs for BP were greater among older adults. Maintaining ideal levels of upstream risk factors, like healthy body weight, physical activity, and diet, could translate to future CVD risk reductions. Lifestyle modifications are effective for supporting weight loss or maintenance and improving levels of primary cardiovascular risk factors and CVD events. 44 , 45 Ultimately, achievement of high CVH among all US adults will require multipronged approaches and policy initiatives targeting CVH in conjunction with social determinants of health throughout the life course. 43 , 46 , 47 Recent US nationally representative data from the Medical Expenditure Panel Survey 2006 to 2015 suggested CVH levels in the United States are worsening. 48 Therefore, it is imperative that clinicians, policy makers, researchers, and individuals work together in making primordial prevention of CVD a priority so that the estimated benefits reported herein can be realized.
Limitations
First, HRs and PAFs were derived from pooled observational cohort data. Thus, our data cannot predict event reductions based on individual therapeutic targeting of CVH, although prior analyses suggest improving CVH among individuals is associated with more favorable subclinical and clinical CVD outcomes. 14 , 39 Second, we were unable to adjust our estimates for covariables other than age, sex, and race because of insufficient sample size for further stratification in the PAF weighting approach. 33 , 34 Thus, residual confounding is possible, attributable to factors like socioeconomic status or health insurance status, which are associated with both CVH and CVD. 47 Furthermore, our estimates assume a causal relationship between CVH and CVD, and it is possible that factors beyond the 7 CVH metrics could be contributing to estimated associations between CVH and CVD. Third, although our pooled data were harmonized, 19 , 21 , 29 different measurement methods were used in individual cohorts, which may have resulted in reduced precision for quantification of CVD risk among different total CVH score and individual metric levels, particularly for self‐reported lifestyle components of CVH, like diet and physical activity. Fourth, data from studies included in the pooled cohorts were collected over a period of >30 years. Thus, the potential for secular trends in CVD event rates and risk factor associations is unavoidable, and the estimated HRs of CVD may not be representative of all contemporary US adults. However, both the distribution of CVH scores and participant characteristics in the 2 included data sources were comparable, and magnitudes of association for the components of the CVH score have been consistent in many different populations over time. 21 , 49 Finally, data presented in this study are solely from the United States, and we were able to report estimates only for White/other [including Mexican American, other Hispanic, non‐Hispanic Asian, and other races] and Black adults because of insufficient data for disaggregation of other race/ethnicity groups.
Conclusions
The potential impact of achieving a high CVH score in all US adults is considerable, and even a partial improvement in CVH scores would be highly beneficial. Population‐ and individual‐level strategies to maintain or restore high CVH are urgently needed to reduce the prevailing high burden of CVD in US adults.
Sources of Funding
The Lifetime Risk Pooling Project was supported in its inception by the National Institutes of Health/National Heart, Lung, and Blood Institute (R21HL085375) and is currently supported by funds from the Northwestern University Feinberg School of Medicine. Dr Bundy was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development career development grant (K12HD043451). Dr Wilkins is supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (K23HL136601 and R01HL146844). Drs Whelton and He were supported by a Center for Biomedical Research Excellence grant from the National Institute of General Medical Sciences (P20GM109036).
Disclosures
None.
Supporting information
Data S1
Tables S1–S10
Figures S1–S2
(J Am Heart Assoc. 2021;10:e019681. DOI: 10.1161/JAHA.120.019681.)
For Sources of Funding and Disclosures, see page 10.
See Editorial by Michos and Khan
References
- 1. Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A, Khan AR, Ahmadi A, Ferrari AJ, et al. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–1472. DOI: 10.1001/jama.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. DOI: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. DOI: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 4. Shah NS, Lloyd‐Jones DM, O’Flaherty M, Capewell S, Kershaw K, Carnethon M, Khan SS. Trends in cardiometabolic mortality in the United States, 1999–2017. JAMA. 2019;322:780–782. DOI: 10.1001/jama.2019.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. DOI: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. DOI: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Q, Cogswell ME, Dana Flanders W, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. DOI: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. DOI: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, Mcclellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. DOI: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai CS, Ning H, Liu K, Reis JP, Gidding SS, Armstrong A, Lima JAC, Lloyd‐Jones DM. Cardiovascular health in young adulthood and association with left ventricular structure and function later in life: the Coronary Artery Risk Development in Young Adults Study. J Am Soc Echocardiogr. 2015;28:1452–1461. DOI: 10.1016/j.echo.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ommerborn MJ, Blackshear CT, Hickson DA, Griswold ME, Kwatra J, Djoussé L, Clark CR. Ideal cardiovascular health and incident cardiovascular events. Am J Prev Med. 2016;51:502–506. DOI: 10.1016/j.amepre.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo L, Zhang S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: a meta‐analysis of prospective studies. Clin Cardiol. 2017;40:1339–1346. DOI: 10.1002/clc.22836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polonsky TS, Ning H, Daviglus ML, Liu K, Burke GL, Cushman M, Eng J, Folsom AR, Lutsey PL, Nettleton JA, et al. Association of cardiovascular health with subclinical disease and incident events: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2017;6:e004894. DOI: 10.1161/JAHA.116.004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enserro DM, Vasan RS, Xanthakis V. Twenty‐year trends in the American Heart Association cardiovascular health score and impact on subclinical and clinical cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc. 2018;7:e008741. DOI: 10.1161/JAHA.118.008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pool LR, Ning H, Lloyd‐Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J Am Heart Assoc. 2017;6:e006027. DOI: 10.1161/JAHA.117.006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown AF, Liang LJ, Vassar SD, Escarce JJ, Merkin SS, Cheng E, Richards A, Seeman T, Longstreth WT. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168:541–549. DOI: 10.7326/M17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;56:1–37. [PubMed] [Google Scholar]
- 18. American Diabetes Association . 2: Classification and diagnosis of diabetes: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S13–S28. DOI: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 19. Bundy JD, Ning H, Zhong VW, Paluch AE, Lloyd‐Jones DM, Wilkins JT, Allen NB. Cardiovascular health score and lifetime risk of cardiovascular disease: the cardiovascular lifetime risk pooling project. Circ Cardiovasc Qual Outcomes. 2020;13:e006450. DOI: 10.1161/CIRCOUTCOMES.119.006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the association of the 2017 and 2014 hypertension guidelines with cardiovascular events and deaths in US adults. JAMA Cardiol. 2018;3:572–581. DOI: 10.1001/jamacardio.2018.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilkins JT, Karmali KN, Huffman MD, Allen NB, Ning H, Berry JD, Garside DB, Dyer A, Lloyd‐Jones DM. Data resource profile: the cardiovascular disease lifetime risk pooling project. Int J Epidemiol. 2015;44:1557–1564. DOI: 10.1093/ije/dyv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Atherosclerosis Risk in Communities (ARIC) study, design and objectives: the ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 23. Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E Jr, Jacobs DR, Liu K, Orden S, Pirie P, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) study. Control Clin Trials. 1987;8:68–73. DOI: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 24. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. DOI: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 25. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Kronmal R, Liu K, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. DOI: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 26. Dawber TR, Kannel WB, Lye LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. DOI: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 27. Feinleib M, Kannel W, Garrison R, McNamara P, Castelli W. The Framingham Offspring Study: design and preliminary data. Prev Med. 1975;4:518–525. DOI: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 28. Design of the Women’s Health Initiative clinical trial and observational study: the Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 29. Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, et al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321:1081–1095. DOI: 10.1001/jama.2019.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 32. Murray CJL, Ezzati M, Lopez AD, Rodgers A, Vander HS. Comparative quantification of health risks conceptual framework and methodological issues. Popul Health Metr. 2003;1:1. DOI: 10.1186/1478-7954-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10:195–216. DOI: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 34. Flegal KM, Graubard BI, Williamson DF. Methods of calculating deaths attributable to obesity. Am J Epidemiol. 2004;160:331–338. DOI: 10.1093/aje/kwh222. [DOI] [PubMed] [Google Scholar]
- 35. Fishman G. Monte Carlo: Concepts, Algorithms, and Applications. New York, NY: Springer; 1996. [Google Scholar]
- 36. Greenland S. Interval estimation by simulation as an alternative to and extension of confidence intervals. Int J Epidemiol. 2004;33:1389–1397. DOI: 10.1093/ije/dyh276. [DOI] [PubMed] [Google Scholar]
- 37. Labarthe D, Lloyd‐Jones DM. 50×50×50: Cardiovascular health and the cardiovascular disease endgame. Circulation. 2018;138:968–970. DOI: 10.1161/CIRCULATIONAHA.118.035985. [DOI] [PubMed] [Google Scholar]
- 38. Stamler J, Neaton JD, Cohen JD, Cutler J, Eberly L, Grandits G, Kuller LH, Ockene J, Prineas R; MRFIT Research Group . Multiple risk factor intervention trial revisited: a new perspective based on nonfatal and fatal composite endpoints, coronary and cardiovascular, during the trial. J Am Heart Assoc. 2012;1:e003640. DOI: 10.1161/JAHA.112.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130:10–17. DOI: 10.1161/CIRCULATIONAHA.113.005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2:e000298.– 10.1136/bmjgh-2017-000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. DOI: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 42. Mensah GA, Cooper RS, Siega‐Riz AM, Cooper LA, Smith JD, Brown CH, Westfall JM, Ofili EO, Price LN, Arteaga S, et al. Reducing cardiovascular disparities through community‐engaged implementation research: a National Heart, Lung, and Blood Institute workshop report. Circ Res. 2018;122:213–230. DOI: 10.1161/CIRCRESAHA.117.312243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu E, Malik VS, Hu FB. Cardiovascular disease prevention by diet modification: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:914–926. DOI: 10.1016/j.jacc.2018.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1622–1639. DOI: 10.1016/j.jacc.2018.08.2141. [DOI] [PubMed] [Google Scholar]
- 46. Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. DOI: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. DOI: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tibuakuu M, Okunrintemi V, Savji N, Stone NJ, Virani SS, Blankstein R, Thamman R, Blumenthal RS, Michos ED. Nondietary cardiovascular health metrics with patient experience and loss of productivity among US adults without cardiovascular disease: the Medical Expenditure Panel Survey 2006 to 2015. J Am Heart Assoc. 2020;9:e016744. DOI: 10.1161/JAHA.120.016744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. DOI: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S10
Figures S1–S2


