Abstract
Background
Less than 40% of acute stroke patients have computed tomography (CT) imaging performed within 25 minutes of hospital arrival. We aimed to examine the race‐ethnic and sex differences in door‐to‐CT (DTCT) ≤25 minutes in the FSR (Florida Stroke Registry).
Methods and Results
Data were collected from 2010 to 2018 for 63 265 patients with acute ischemic stroke from the FSR and secondary analysis was performed on 15 877 patients with intravenous tissue plasminogen activator‐treated ischemic stroke. Generalized estimating equation models were used to determine predictors of DTCT ≤25. DTCT ≤25 was achieved in 56% of cases of suspected acute stroke, improving from 36% in 2010 to 72% in 2018. Women (odds ratio [OR], 0.90; 95% CI, 0.87–0.93) and Black (OR, 0.88; CI, 0.84–0.94) patients who had strokes were less likely, and Hispanic patients more likely (OR, 1.07; CI, 1.01–1.14), to achieve DTCT ≤25. In a secondary analysis among intravenous tissue plasminogen activator‐treated patients, 81% of patients achieved DTCT ≤25. In this subgroup, women were less likely to receive DTCT ≤25 (0.85, 0.77–0.94) whereas no significant differences were observed by race or ethnicity.
Conclusions
In the FSR, there was considerable improvement in acute stroke care metric DTCT ≤25 in 2018 in comparison to 2010. However, sex and race‐ethnic disparities persist and require further efforts to improve performance and reduce these disparities.
Keywords: disparities, ethnicity, ischemic stroke, race, sex
Subject Categories: Computerized Tomography (CT), Epidemiology, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- DTCT ≤25
door‐to‐computed tomography time within 25 minutes
- DTN
door‐to‐needle time
Clinical Perspective
What Is New?
Female and Black patients with acute ischemic stroke were less likely to achieve door‐to‐computed tomography time within 25 minutes (DTCT ≤25) relative to male and White patients, respectively.
Hispanic patients, however, are more likely to achieve DTCT ≤25 in comparison to White patients.
In the subgroup of patients with acute ischemic stroke who receive intravenous tissue plasminogen activator, sex but not race‐ethnic disparity in DTCT ≤25 was present; all sex and race‐ethnic groups have seen improvements from 2010 to 2018.
What Are the Clinical Implications?
Sex and race‐ethnic disparities in DTCT after acute ischemic stroke continue to persist, despite improvements in the proportion of patients achieving the American Heart Association/American Stroke Association recommended guideline of DTCT ≤25.
The causes of sex disparities in DTCT may differ from causes of race‐ethnic disparities, and further research is needed to identify them and reduce observed disparities.
In the United States, on average, a stroke occurs every ≈40 seconds and an individual dies of stroke every 4 minutes, making stroke a leading contributor of morbidity and mortality. 1 Treatment with intravenous tissue plasminogen activator (IV tPA) is the gold standard for treatment of acute ischemic strokes (AIS). 2 The limited time window in which IV tPA is effective has led the American Heart Association/American Stroke Association (AHA/ASA) to establish the GWTG‐S (Get With The Guidelines‐Stroke) quality improvement program. GWTG‐S was designed to improve rapid diagnosis of AIS and acute stroke performance metrics, by implementing evidence‐based care. 3 GWTG‐S has also provided crucial insights into trends and disparities in the quality and delivery of stroke care. 4 , 5 , 6 , 7 , 8
The FSR (Florida Stroke Registry) was established both to identify stroke disparities in the diverse patient population of Florida and to develop effective programs to reduce these disparities. The Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities has previously reported significant stroke care disparities in providing all appropriate interventions to patients (ie, "defect‐free care") among different sex and race‐ethnic groups. 9 , 10 , 11 However, other acute stroke reporting measures, which can have a direct effect on primary stroke outcomes, have still not been explored in the FSR nor the Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities.
A door‐to‐computed tomography time (DTCT), that is, from arrival at hospital to brain CT imaging, of ≤25 minutes is the established AHA/ASA target goal for patients with suspected strokes. 12 Failure to meet this goal can result in longer door‐to‐needle (DTN) times, delayed therapeutic interventions, and worse outcomes. 13 , 14 , 15 An analysis of 1193 patients from 25 Michigan hospitals found that although median door‐to‐imaging time from 2009 to 2012 decreased from 21 to 18 minutes, 31.6% of their patients still experienced times above 25 minutes, with significant variation between hospitals and resultant increases in DTN. 15 Findings such as these necessitate the continual need to assess and address such disparities both longitudinally and across various populations.
In the process of stroke care from onset, emergency services response, arrival to a hospital setting, and interventions, CT imaging occurs after arrival and before intervention. Though delays in CT imaging occur, disparities in timing of imaging are not frequently studied. However, time from door to CT is an actionable step for improvement within the hospital process of stroke care. Disparities in DTCT can be eliminated by implementing protocols that improve timing of DTCT by rapid transport from the emergency department to the CT suite and by improving proximity of the CT to the emergency department.
Prior work has shown that participation in GWTG‐S, including duration of participation, improves stroke outcomes through systematic data collecting that standardizes the hospital protocols and improves systems of care, provides more complete data extraction, improves data quality, and reduces disparities by subsequent analysis and education. 16 , 17 , 18 , 19 Therefore, the main factors that affect DTCT time are mostly dependent on hospital characteristics (eg, size, type) and readiness to treat patients with strokes, especially during OFF hours, as well as on prehospital factors (eg, mode of arrival) and individual level factors (eg, insurance). Longer participation in the GWTG‐S program may mitigate some of these disparities.
The known disparities in stroke interventions (ie, DTN) and outcomes occur independently and add to disparities that persist from prehospital systems of care. 4 , 27 Although there were few race‐ethnic disparities identified in DTN ≤60 or ≤45 minutes in this study population, female patients were significantly less likely than male patients to receive thrombolytic therapy in accordance with AHA/ASA Target: Stroke Phase II recommendations. 10 , 12 It is currently not well established whether there are sex and/or race‐ethnic disparities in achieving DTCT ≤25.
The primary aim of this study is to assess whether sex and/or race‐ethnic disparities in DTCT exist in a diverse AIS Florida population and, if such disparities are identified, to assess whether they depend on prehospital factors (eg, mode of arrival, insurance, etc), hospital characteristics, and/or individual‐level factors. The secondary aim is to explore whether there are similar disparities in patients who received IV tPA within 4.5 hours of stroke onset, as prior work in this patient population identified sex and race‐ethnic differences in thrombolytic therapy. 9 , 10
Methods
The study data and materials were obtained from hospitals via data use agreements that prohibit them being shared. Instead, we offer the opportunity to propose studies with the data, with methods and analyses performed by the FSR Biostatistics Core. The FSR includes 93 Florida hospitals (out of 124 hospitals that treat stroke in Florida) that participate in GWTG‐S. The FSR includes both prospective and retrospective hospital‐collected GWTG‐S data on patients with a primary diagnosis of ischemic stroke, transient ischemic attack, subarachnoid hemorrhage, intracerebral hemorrhage, and stroke not otherwise specified. A total of 164 173 patients with AIS from Florida were enrolled from January 2010 through December 2018. Exclusion criteria from GWTG‐S Patient Management Tool for % DTCT ≤25 was used: age under 18 years, stroke occurred as inpatient, arrival at the hospital over 24 hours from stroke onset, symptoms resolved upon arrival, any date/time fields missing or unknown, negative calculated time difference, initial National Institute of Health Stroke total score of zero, current participation in a clinical trial, and uncompleted elective carotid intervention or brain imaging. The total sample included 63 265 AIS records.
Data Source
Data were originally collected as part of the AHA/ASA GWTG‐S program, a voluntary national registry with the purpose of improving stroke care. For participation in the FSR, each participating center received institutional approval to enroll cases in the FSR with an amendment made to the GWTG‐S agreement granting the University of Miami Miller School of Medicine access to limited GWTG‐S data. Complete data collections methods have been previously reported. 9 Data collection was approved by each participating institutional review board—without requiring individual patient consent under the common rule or a waiver of authorization and exemption—and this study was approved by the University of Miami Miller School of Medicine institutional review board.
Outcome Measure
The main outcome measure examined in this study is DTCT ≤25 minutes upon arrival to hospital for all patients suspected of AIS. Our analysis includes all diagnosed AISs. Race‐ethnicity (Non‐Hispanic White, Non‐Hispanic Black, and Hispanic) was self‐reported. The term "Hispanic" was specified by the GWTG‐S Patient Management and Data Collection Tool and represents "Hispanic or Latino" patients. 28 Additional analyses were performed by arrival time after stroke onset (0–6 hours versus 6–24 hours) and by "ON" (ie, working) and "OFF" (ie, nonworking) hours with OFF hours being Monday through Friday from 6 pm to 7 am, along with full days on Saturdays, Sundays, and government holidays.
Statistical Analysis
Univariable descriptive and multivariable analyses were conducted using SAS, version 9.3. In the univariable analysis of patient‐ and hospital‐level characteristics, continuous variables were summarized as means with SD and categorical variables were presented as frequencies with percentages. The Student t test and the Pearson χ2 test were used to compare the differences for continuous and categorical variables, respectively. To account for within‐hospital clustering, multilevel multivariable logistic regression with generalized estimating equations approach was performed to examine the factors associated with faster DTCT time and the race/ethnicity and sex disparities in DTCT time. For generalized estimating equations, the exponential family of distribution, logit link function, and compound symmetry covariance structure were used. To account for the contribution of the patient‐level and hospital‐level characteristics in explaining the aforementioned disparities, 3 sequential models were constructed to include patient‐level and hospital‐level covariates. These covariates are prespecified, and models sequentially follow from our primary interest (sex and race‐ethnic disparity)—to first determine disparities independent of individual levels of stroke risk—and progress to include hospital characteristics and systems of care. Model 1 was adjusted for age and either race/ethnicity or sex. Model 2 was adjusted for covariates included in Model 1 and health insurance status, mode of arrival and hospital‐level factors including number of beds, years in GWTG‐S, and academic status. Model 3 included Model 2 with additional adjustments for vascular risk factors (current smoker, hypertension, diabetes mellitus, dyslipidemia), and past medical history of atrial fibrillation, coronary artery disease/prior MI, and previous stroke/TIA. The effect modifications of working/nonworking hours and arrival time after onset of stroke (within 0–6 hours/6–24 hours) were tested in Model 3 and stratified analyses were performed.
Results
The baseline patient and hospital characteristics are detailed in Table 1. The median DTCT time was 22 minutes (interquartile range [IQR]=34) and DTCT ≤25 was achieved in 56% of all patients. Overall median DTCT time was 23 minutes (IQR=36) in women and 21 minutes (IQR=33) in men. DTCT time was lowest in White patients, 21 minutes (IQR=32); followed by Hispanic patients, 22 minutes (IQR=32); and Black patients, 23 minutes (IQR=37).
Table 1.
Patient‐ and Hospital‐Level Characteristics of All Acute Ischemic Stroke Patients Within the Florida Stroke Registry Cohort (2010–2018)
| Patient Characteristics | All (N=63 265) | Door to CT ≤25 min (N=35 312) | Door to CT >25 min (N=27 953) |
|---|---|---|---|
| Age (y), mean±SD | 72±14 | 73±14 | 71±15 |
| Sex, n (%) | |||
| Men | 31 794 (50) | 18 070 (51) | 13 724 (49) |
| Women | 31 471 (50) | 17 242 (49) | 14 229 (51) |
| Race/ethnicity, n (%) | |||
| White | 42 704 (68) | 24 100 (68) | 18 604 (67) |
| Black | 11 186 (18) | 5833 (17) | 5353 (19) |
| Hispanic | 9375 (15) | 5379 (15) | 3996 (14) |
| Region, n (%) | |||
| South FL | 26 092 (41) | 16 093 (46) | 9999 (36) |
| West Central FL | 18 610 (29) | 9420 (27) | 9190 (33) |
| East Central FL | 11 537 (18) | 6051 (17) | 5486 (20) |
| North FL and Panhandle | 7026 (11) | 3748 (11) | 3278 (12) |
| Vascular risk factor, n (%) | |||
| Current smoker | 9839 (16) | 5251 (15) | 4588 (16) |
| Hypertension | 44 274 (70) | 24 511 (69) | 19 763 (71) |
| Diabetes mellitus | 18 355 (29) | 9649 (27) | 8706 (31) |
| Dyslipidemia | 26 182 (41) | 14 475 (41) | 11 707 (42) |
| Medical history, n (%) | |||
| Atrial fibrillation/flutter | 14 850 (23) | 9134 (26) | 5716 (20) |
| Coronary artery disease/prior myocardial infarction | 14 577 (23) | 7991 (23) | 6586 (24) |
| Previous stroke/transient ischemic attack | 18 104 (29) | 9773 (28) | 8331 (30) |
| Insurance status, n (%) | |||
| Private* | 22 187 (35) | 12 472 (35) | 9715 (35) |
| Medicare | 21 804 (34) | 11 641 (33) | 10 163 (36) |
| Uninsured † | 5967 (9) | 2915 (8) | 3052 (11) |
| Unknown | 13 307 (21) | 8284 (23) | 5023 (18) |
| National Institute of Health Stroke Score, n (%) | |||
| ≤5 | 26 100 (41) | 13 361 (38) | 12 739 (46) |
| 6–15 | 18 346 (29) | 12 429 (35) | 5917 (21) |
| ≥16 | 11 435 (18) | 8150 (23) | 3285 (12) |
| Missing | 7384 (12) | 1372 (4) | 6012 (22) |
| Arrival mode, n (%) | |||
| EMS | 44 633 (71) | 27 680 (78) | 16 953 (61) |
| Non‐EMS | 16 040 (25) | 6426 (18) | 9614 (34) |
| Missing/unknown | 2592 (4) | 1206 (3) | 1386 (5) |
| Ambulation status, n (%) | |||
| Independent | 22 525 (36) | 12 289 (35) | 10 236 (37) |
| Unable/with assistance | 24 256 (38) | 13 949 (40) | 10 307 (37) |
| Missing/ND | 16 484 (26) | 9074 (26) | 7410 (27) |
| Hospital characteristics | |||
| Hospital size ‡ , n (%) | |||
| Low (<250 beds) | 20 668 (33) | 12 229 (35) | 8439 (30) |
| Medium (250–450 beds) | 20 905 (33) | 12 079 (34) | 8826 (32) |
| High (>450 beds) | 21 692 (34) | 11 004 (31) | 10 688 (38) |
| Academic status, n (%) | |||
| Teaching hospital | 21 789 (34) | 11 645 (33) | 10 144 (36) |
| Nonteaching hospital | 41 476 (66) | 23 667 (67) | 17 809 (64) |
| Years in Get With The Guidelines‐Stroke § , n (%) | |||
| Low | 18 867 (30) | 10 910 (31) | 7957 (28) |
| Medium | 22 038 (35) | 11 418 (32) | 10 620 (38) |
| High | 22 360 (35) | 12 984 (37) | 9376 (34) |
All P values are <0.01. AF atrial fibrillation; CAD, coronary artery disease; EMS indicates emergency medical services; FL, Florida; and ND, not determined.
Includes private insurance, Veterans Affairs, and other.
Includes Medicaid, self‐pay, and no insurance.
Determined by licensed number of beds.
Determined by tercile. Each terciles' mean (SD) value is: low=6.4 (2.7), medium=11.7 (0.5), high=13.9 (1.2).
In unadjusted analysis of acute suspected strokes (Table 1), patients with DTCT ≤25 were significantly older (mean age, 73 versus 71) and more likely to be men (51% versus 49%) than those with DTCT >25 minutes. Black patients with stroke had a lower percentage achieving DTCT ≤25, 52%, versus 56% for White patients and 57% for Hispanic patients. By geographic region, South Florida had the highest likelihood of DTCT ≤25, 62% versus 52% for all other Florida regions. Significant factors associated with DTCT >25 minutes were diabetes mellitus and a history of coronary artery disease or myocardial infarction (Table S1). Patients who received DTCT ≤25 had a larger proportion of severe strokes (National Institute of Health Stroke Score >5) and were more likely to arrive by emergency medical services (EMS). Arrival by EMS resulted in 61% of patients receiving DTCT ≤25 in comparison to 40% of patients not arriving by EMS.
Table 2 shows adjusted odds ratios for receiving DTCT ≤25 by sex, race/ethnicity, ON/OFF hours, and arrival time from onset of symptoms. Overall, women were less likely to receive DTCT ≤25 (55% compared with 57% of men). In fully adjusted models (ie, adjusting for both patient‐ and hospital‐level characteristics), women were also less likely to receive DTCT ≤25 when compared with men (odds ratio [OR], 0.90; 95% CI, 0.87–0.93).
Table 2.
Adjusted Odds Ratio and 95% CI for Race‐Ethnic and Sex Differences in Door‐to‐CT Time Within 25 Minutes
| White | Black | Hispanic | Male | Female | |
|---|---|---|---|---|---|
| No. eligible patients | 42 704 | 11 186 | 9375 | 31 794 | 31 471 |
| % DTCT ≤25 min | 56.4 | 52.2 | 57.4 | 56.8 | 54.8 |
| Model 1 | [REF] | 0.83 (0.79–0.89) | 1.01 (0.95–1.08) | [REF] | 0.92 (0.89–0.95) |
| Model 2 | [REF] | 0.86 (0.81–0.91) | 1.05 (0.98–1.12) | [REF] | 0.91 (0.88–0.93) |
| Model 3 | [REF] | 0.88 (0.84–0.94) | 1.07 (1.01–1.14) | [REF] | 0.90 (0.87–0.93) |
| ON hours* | [REF] | 0.89 (0.83–0.96) | 1.05 (0.94–1.17) | [REF] | 0.92 (0.88–0.96) |
| OFF hours* | [REF] | 0.88 (0.82–0.95) | 1.09 (1.01–1.19) | [REF] | 0.88 (0.84–0.92) |
| Arrival Time from Onset: 0–6 h* | [REF] | 0.94 (0.87–1.01) | 1.04 (0.96–1.11) | [REF] | 0.91 (0.87–0.95) |
| Arrival Time from Onset: 6–24 h* | [REF] | 0.88 (0.80–0.97) | 1.13 (1.02–1.26) | [REF] | 0.88 (0.84–0.93) |
Model 1: adjusted for age plus either race/ethnicity or sex. Model 2: includes Model 1 with additional adjustments for health insurance status, mode of arrival, and hospital‐level factors including number of beds, years in Get With The Guidelines‐Stroke, and academic status. Model 3: includes Model 2 with additional adjustments for vascular risk factors (current smoker, hypertension, diabetes mellitus, dyslipidemia), and past medical history (atrial fibrillation, coronary artery disease/prior myocardial infarction, and previous stroke/transient ischemic attack). DTCT indicates door‐to‐computed tomography time.
Adjusted for Model 3.
In the fully adjusted model (Table 2), Black patients were less likely to achieve DTCT ≤25 than White patients (OR, 0.88; 95% CI, 0.84–0.94), whereas Hispanic patients showed increased odds (OR, 1.07; 95% CI, 1.01–1.14). Stratification by admission time revealed that Black patients, when compared with White patients, experienced lower odds of DTCT ≤25 on both ON (OR, 0.89; 95% CI, 0.83–0.96) and OFF (OR, 0.88; 95% CI, 0.82–0.95) hours whereas Hispanic patients experienced higher odds only during OFF (OR, 1.09; 95% CI, 1.01–1.19) hours. When stratifying patients by arrival time at hospital after onset of stroke symptoms, women showed decreased odds of DTCT ≤25 in both the 0 to 6 hours (OR, 0.91; 95% CI, 0.87–0.95) and 6 to 24 hours (OR, 0.88; 95% CI, 0.84–0.93) subgroups (Table 2). Although there were no differences in odds of DTCT ≤25 by race‐ethnic group when patients arrived within 6 hours of symptom onset, arrival 6 to 24 hours after symptom onset led to Black patients experiencing lower odds (OR, 0.88; 95% CI, 0.80–0.97) and Hispanic patients experiencing higher odds (OR, 1.13; 95% CI, 1.02–1.26) of DTCT ≤25 compared with White patients. No significant effect modification between sex and race/ethnicity in Model 3 was noted (P=0.13) (Table S2).
Examination of temporal trends from 2010 to 2018 revealed median DTCT time has improved from 35 to 13 minutes and DTCT ≤25 improved from 36 to 72%. Proportion of DTCT ≤25 showed a statistically significant temporal increase (P<0.0001) for both men and women and across all race‐ethnic groups (Figure 1A and 1B). Despite this improvement, women were consistently less likely to get brain imaging performed within 25 minutes after hospital arrival. The greatest improvement in DTCT ≤25 was observed in Hispanic patients (33%–75%) and the least improvement was observed in Black patients (34%–69%); White patients improved from 38% to 73%. Examination of geographic trends in DTCT ≤25 showed all regions steadily improved over time (Figure 1C). Improvement was greatest in the East Central region (25–70%), followed by South Florida (42%–77%), North Florida and the Panhandle (38%–71%), and West Central Florida (34%–66%).
Figure 1. % DTCT ≤25 minutes over time across sex, race‐ethnicity, and region.
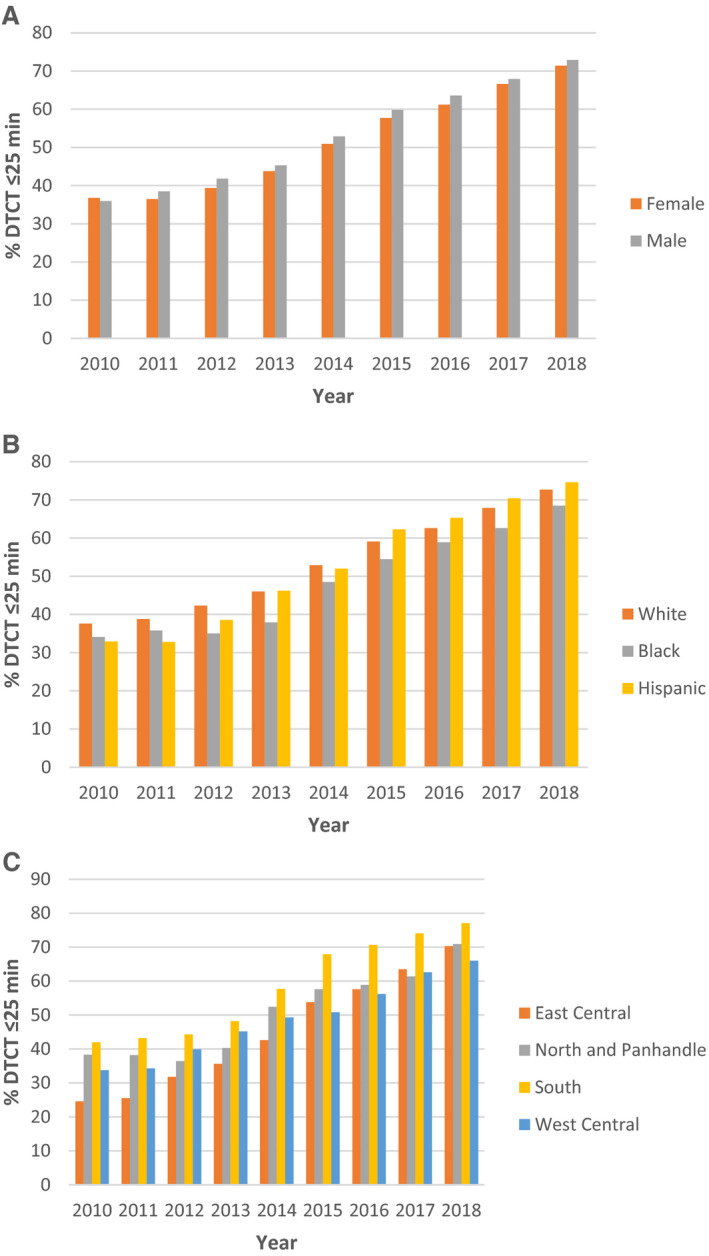
The percentage of patients with acute ischemic stroke achieving DTCT ≤25 minutes increased over time in all (A) sex, (B) race‐ethnic, and (C) regional subgroups from 2010 to 2018. DTCT indicates door‐to‐computed tomography time.
In a secondary analysis examining DTCT time in patients treated by IV tPA within 4.5 hours of stroke (25%; 15 877 of 63 265), 81% of patients received DTCT ≤25 when compared with 56% in the overall population. Similar temporal trends in DTCT ≤25 akin to the total cohort were observed across all sex and race‐ethnic groups, as well as by region (Figure 2). Within this subset of patients, women were less likely to achieve DTCT ≤25, (OR, 0.85; 95% CI, 0.77–0.94) when compared with men (Table 3). Stratification by ON/OFF hours, showed similar results with women being less likely to achieve DTCT ≤25 in nonworking hours (OR, 0.85; 95% CI, 0.73–0.97), and trending toward similar results in working hours (OR, 0.86; 95% CI, 0.75–1.00) hours. There were no significant differences in the likelihood of DTCT ≤25 between race‐ethnic groups in this secondary analysis, even when stratified by ON/OFF hours.
Figure 2. % DTCT ≤25 minutes in subgroup treated with tPA over time across sex, race‐ethnicity, and region.
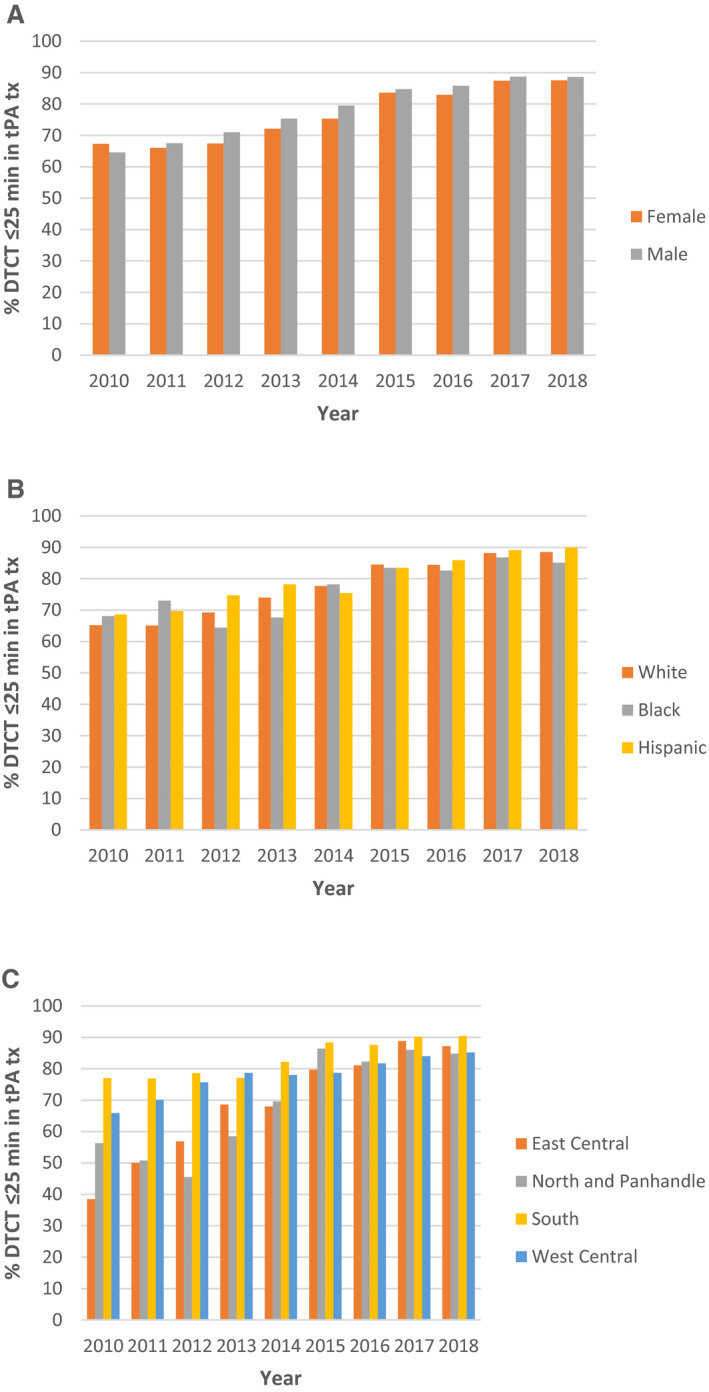
Among patients with acute ischemic stroke who received intravenous tissue plasminogen activator treatment, the percentage achieving DTCT ≤25 minutes increased over time in all (A) sex, (B) race‐ethnic, and (C) regional subgroups from 2010 to 2018. DTCT indicates door‐to‐computed tomography time; tPA, tissue plasminogen activator; and tx, treatment.
Table 3.
Door‐to‐CT Time ≤25 Minutes in Patients Treated With IV‐tPA by Sex and Race/Ethnicity
| White | Black | Hispanic | Male | Female | |
|---|---|---|---|---|---|
| No. eligible patients | 10 768 | 2619 | 2490 | 8061 | 7816 |
| % DTCT ≤25 min | 81.0 | 80.1 | 83.2 | 82.1 | 80.3 |
| Model 1, OR and 95% CI | [REF] | 0.99 (0.88–1.13) | 0.99 (0.81–1.20) | [REF] | 0.84 (0.78–0.90) |
| Model 2, OR and 95% CI | [REF] | 1.01 (0.89–1.15) | 1.03 (0.84–1.25) | [REF] | 0.84 (0.78–0.90) |
| Model 3, OR and 95% CI | [REF] | 1.03 (0.88–1.19) | 1.06 (0.84–1.33) | [REF] | 0.85 (0.77–0.94) |
| ON hours*, OR and 95% CI | [REF] | 1.09 (0.87–1.38) | 0.91 (0.70–1.19) | [REF] | 0.86 (0.75–1.00) |
| OFF hours*, OR and 95% CI | [REF] | 0.99 (0.80–1.23) | 1.26 (0.94–1.67) | [REF] | 0.85 (0.73–0.97) |
All secondary analysis models were run restricting arrival time from onset of stroke to 4.5 hours. Model 1: adjusted for age plus either race/ethnicity or sex. Model 2: includes Model 1 with additional adjustments for health insurance status, mode of arrival, and hospital‐level factors including number of beds, years in Get With The Guidelines‐Stroke, and academic status. Model 3: includes Model 2 with additional adjustments for vascular risk factors (current smoker, hypertension, diabetes mellitus, dyslipidemia), and past medical history (atrial fibrillation, coronary artery disease/prior myocardial infarction, and previous stroke/transient ischemic attack). DTCT indicates door‐to‐computed tomography time; and IV‐tPA, intravenous tissue plasminogen activator.
Adjusted for Model 3.
Discussion
Few studies have examined race‐ethnic differences in secondary stroke care metrics, such as DTCT time, despite well‐established disparities in acute stroke care. 5 , 15 , 25 Our current study examining disparities in DTCT in the FSR reveals significant disparities in receiving DTCT ≤25 that affect female and Black patients who had strokes, despite notable improvements in this acute stroke care metric from 2010 to 2018. These disparities in DTCT ≤25 persist during both working and nonworking hours. Sex disparities persist regardless of arrival time whereas racial disparities existed only in patients who arrived late, 6 to 24 hours after stroke onset. Interestingly, Hispanic stroke patients showed increased odds of achieving DTCT ≤25 compared with White patients, a finding not seen in our DTN analysis 10 and door‐to‐imaging times in the national GWTG‐S. 5
In our prior work, we reported that Floridian and Puerto Rican Hispanic patients had the lowest median DTN times but no statistically significant difference in odds of achieving DTN ≤60 minutes compared with White patients. 10 Our study shows no race‐ethnic difference in DTCT in the subgroup of patients who received IV tPA within 4.5 hours from stroke onset. This suggests the lack of DTN disparities by race/ethnicity may be secondary to equitable achievement of DTCT in the subgroup treated with IV tPA. However, in the overall AIS population, which includes patients who do not receive IV tPA, there is a statistically significant increase in DTCT ≤25 among Hispanic patients and a decrease among Black patients. The improved DTCT among Hispanic patients has been noted in other studies and may be the result of socioeconomic factors, social support, interaction and connectivity, and patient and family knowledge about stroke, access to care, and baseline prevalence rates, among other factors that were not collected in the GWTG‐S protocol and not included in this analysis. 17 , 29 , 30 Conversely, such factors may have contributed to the decreased achievement of DTCT ≤25 among Black patients in addition to racism, discrimination, and implicit bias.
Lower likelihood of DTCT ≤25 for non‐White patients has been previously reported in a study of GWTG patients from 2003 to 2009. 5 Specifically, Black patients are less likely to receive DTCT ≤25 when compared with White patients, even if the quality of hospital care is relatively similar. 25 Black patients have high rates of diabetes mellitus and the lowest rates of arrival by EMS, both of which are known to contribute to increased DTCT time. 4 , 5 , 23 , 27 , 29 Interestingly, those race‐ethnic disparities do not appear in the subgroup of patients treated with IV tPA. However, in the general cohort with AIS, the persistence of race‐ethnic disparities despite adjustment for demographic, clinical, and hospital characteristics suggests the presence of either unaccounted clinical and hospital characteristics or the influence of prehospital factors, social determinants of health, and other sociocultural and patient‐level factors that have been linked to DTN time 10 , 26 , 31 but not yet clearly for DTCT time. Black patients also tend to be younger while still experiencing the highest rate of previous stroke or TIA, making symptom identification more difficult. Similarly, lower knowledge of stroke symptoms and treatment in Black communities may contribute to delays in calling 911, arrival to the hospital, and arrival outside the tPA treatment window. 32 However, although health literacy may contribute to delays in stroke detection outside the hospital, their influence on DTCT is poorly understood and needs to be considered amid a larger picture of physician–patient communication, language barriers, reliance on patient or family history for stroke identification, implicit biases, and other factors.
Our study shows that significant disparities in DTCT time persist for women within the subgroup treated with IV tPA, regardless of working or nonworking hours. These disparities parallel those identified in DTN time in the same patient population. 10 It has been recognized that early stroke symptoms in women patients who are eligible for IV tPA may not be as quickly recognized or may differ when compared with early stroke symptoms in men, leading to a sex difference in DTCT, potentially explaining some of the sex difference in DTCT ≤25 seen in the subgroup treated with IV‐tPA . 33 , 34 Given that early identification of symptoms and treatment with IV tPA is associated with favorable outcomes, this sex disparity in DTCT ≤25 may manifest in subsequent sex disparities in multiple poststroke outcomes. 2 , 3 Such disparities lead to decreased defect‐free care for women. 9 , 35 This "sex gap" in the treatment of acute stroke has been previously established and resulted in multiple meta‐analyses confirming such sex differences in quality of care and stroke outcomes. 22 , 35 Despite this well‐established disparity, women continue to receive a lower standard of care when compared with men. Our study reveals that the absolute difference in proportion of men versus women who achieve DTCT ≤25 has changed from 0.8 in 2010 to 1.5 in 2018, with significant yearly variation (minimum of 0.8, maximum of 2.4) and with men achieving the higher proportion since 2011.
Overall, DTCT times and the percentage of eligible patients meeting the DTCT ≤25 target showed steady annual improvements from 2010 to 2018. A comprehensive review examining reasons for delay times in acute stroke care showed that across 23 population groups there was ≈6 min/year in improvements in DTCT. 21 In our study, we observed a decline in DTCT from a median of 35 minutes to 13 minutes, or 2.75 minutes/year, and analogous improvement in DTCT ≤25 from 36% to 72%. Although outcome is not yet optimal, it is comparable to previously reported findings and underscores the considerable progress made in this arena. 4 , 5 , 8 , 29 Furthermore, the improvement in DTCT from 2010 to 2018 is likely secondary to a combination of factors: increased education and awareness among patients, families, and staff; increased availability of CT imaging; increased numbers of and experience within primary stroke center hospitals; and national incentives for quality improvement in conjunction with the AHA GWTG‐S initiative and stroke quality improvement recognition awards. 16 , 17 , 18 , 19 It is important to note that these annual improvements in the DTCT ≤25 occur both before and following the implementation of new guidelines for door‐to‐imaging time in acute reperfusion strategies in 2015. 2 Determination of the impact of these guidelines on the annualized percent change in the improving DTCT ≤25 metric will require test of trend analysis in future work.
AHA/ASA recommends the time of arrival to treatment with recombinant tPA to be <60 minutes. 2 , 20 In prior work conducted on our cohort for the years 2010 to 2015, few race‐ethnic differences in DTN times were observed, whereas significant sex disparities in DTN times were identified. 10 Sex disparities in received care were evident in both DTCT and DTN times, indicating that disparities in DTN times resulted at least in part from disparities in DTCT.
The prior analysis of DTN time in this cohort also revealed significant geographic variation, with South Florida having the greatest proportion of patients (50%) achieving DTN ≤60 and West Central Florida having the lowest (28%). Our work also identified regional variations in DTCT time, though, interestingly, the geographic variation in DTCT did not correlate well with that seen in DTN times. All regions showed similar annual improvements in the proportion of patients achieving DTCT ≤25 from 2010 to 2018.
Analysis of a national cohort showed that longer delay in DTCT was associated with lower likelihood of receiving IV tPA among eligible patients. 36 Moreover, many of the patient and hospital characteristics contributing to decreased likelihood of DTCT ≤25 in our study, such as female sex, non‐White race, diabetes mellitus, prior stroke, and not arriving by EMS, were also associated with decreased likelihood of treatment with IV tPA, even among eligible patients, and have been noted in national studies of disparities in tPA use. 24 , 37 In addition, improvements in DTCT are not necessarily indicative of improved DTN times. Although it is currently suggested to initiate IV tPA in the CT suite, variations in imaging‐to‐needle times also contribute to increased DTN times. 15
Our FSR contains 164 173 cases of suspected strokes across 93 hospitals throughout Florida, out of 124 Florida hospitals that treat stroke. The accuracy and reliability of the GWTG‐S data has been validated by a national audit. 38 The generalizability of the data has been studied and shown to be representative of the AIS Medicare‐aged population—who constitute most, but not all, of our study cohort—despite substantial differences between GWTG‐S and non‐GWTG‐S hospitals. 39 The GWTG‐S hospitals tend to be large, urban teaching hospitals in proportions greater than the nationally proportionate value. 39 Though our study includes a diverse patient population, that same diversity may limit generalizability of study findings to regional locations with similar race‐ethnic breakdown. Furthermore, given the noted differences within and between Hispanic subgroups (eg, Cuban, Puerto Rican, Dominicans, Mexican, South/Central American), these findings in our predominantly Cuban and Puerto Rican population should be investigated in other Hispanic subgroups, given intra‐ and intergroup differences in immigration status, socioeconomic status, cultural differences, perceptions and biases, and given geographic variation in systems of care and social determinants of health. 30 , 40 , 41 Discrepancies in DTCT reporting, where the definition varies between "initiation of imaging," "completion of imaging," or "interpretation of imaging," can also contribute to variations in DTCT times and limitations of generalizability across studies. However, we are among the few studies to examine the predictors of DTCT and improvements in DTCT over time in a culturally and racially‐ethnically diverse cohort. Implementation of best practice interventions advocated by the AHA Target Stroke Initiative have been shown to result in substantial decreases in DTCT and DTN times and should be considered when attempting to ameliorate identified disparities and improve DTCT times in general. 42 , 43 Future studies should be performed for identification of factors that precede brain imaging and lead to race‐ethnic and sex DTCT time disparities, to address the effect of these observed disparities in DTCT time on stroke care, and to evaluate the causal effect that DTCT disparities alone have in contributing to stroke morbidity and mortality.
Sources of Funding
This study is supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant U54‐NS081763 and the Florida Department of Health Funding #:COHAN‐A1.
Disclosures
Dr Romano receives salary support to the Department of Neurology at the University of Miami from the Florida Department of Health for work on the Florida Stroke Registry. Dr Romano is a principal investigator of the Transition of Care Stroke Disparity Study (National Institutes of Health/National Institute of Mental Health 1R01MD012467), the Mechanisms of Early Recurrence in Intracranial Atherosclerotic Disease study (1R01NS084288), the Florida Regional Coordinating Center for StrokeNet (1U24NS107267), and receives salary support from Genentech for his role as principal investigator of the MaRISS (Mild and Rapidly Improving Stroke Study). Dr Sacco is a recipient and principal investigator of the Stroke Prevention and Intervention Research Program cooperative grant (National Institutes of Health/National Institute of Neurological Disorders and Stroke U54NS081763‐01S1). Dr Rundek is funded by the Florida Department of Health for work on the Florida Stroke Registry and by the grants from National Institutes of Health (R01 MD012467, R01 NS029993, R01 NS040807, 1U24 NS107267), and the National Center for Advancing Translational Sciences (UL1 TR002736 and KL2 TR002737). The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
We acknowledge our collaborators: the Florida Stroke Registry participating hospitals, investigators, coordinators, and others involved in collecting GWTG‐S data.
(J Am Heart Assoc. 2021;10:e017543. DOI: 10.1161/JAHA.120.017543.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017543
For Sources of Funding and Disclosures, see page 9.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update a report from the American Heart Association. Circulation. 2019;139:E56–E528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e99. DOI: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 3. American Stroke Association . Get With the Guidelines–Stroke. American Heart Association web site. 2011.
- 4. Abdullah AR, Smith EE, Biddinger PD, Kalenderian D, Schwamm LH. Advance hospital notification by EMS in acute stroke is associated with shorter door‐to‐computed tomography time andincreased likelihood of administration of tissue‐plasminogen activator. Prehosp Emerg Care. 2008;12:426–431. DOI: 10.1080/10903120802290828. [DOI] [PubMed] [Google Scholar]
- 5. Kelly AG, Hellkamp AS, Olson D, Smith EE, Schwamm LH. Predictors of rapid brain imaging in acute stroke: analysis of the Get With the Guidelines–Stroke program. Stroke. 2012;43:1279–1284. DOI: 10.1161/STROKEAHA.111.626374. [DOI] [PubMed] [Google Scholar]
- 6. Rodríguez‐Rivera IV, Santiago F, Estape ES, Sepúlveda LG, Brau R. Impact of day of the week and time of arrival on ischemic stroke management. P R Health Sci J. 2015;34:164–169. [PMC free article] [PubMed] [Google Scholar]
- 7. Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, Bhatt DL, Grau‐Sepulveda MV, Peterson ED, Fonarow GC. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines–Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6:543–549. DOI: 10.1161/CIRCOUTCOMES.111.000303. [DOI] [PubMed] [Google Scholar]
- 8. Smith E, Liang L, Hernandez A, Reeves M, Cannon C, Fonarow G, Schwamm L. Influence of stroke subtype on quality of care in the Get With the Guidelines–Stroke program. Neurology. 2009;73:709–716. DOI: 10.1212/WNL.0b013e3181b59a6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asdaghi N, Romano JG, Wang K, Ciliberti‐Vargas MA, Koch S, Gardener H, Dong C, Rose DZ, Waddy SP, Robichaux M, et al. Sex disparities in ischemic stroke care: Fl‐PR CReSD study (Florida–Puerto Rico Collaboration to Reduce Stroke Disparities). Stroke. 2016;47:2618–2626. DOI: 10.1161/STROKEAHA.116.013059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oluwole SA, Wang K, Dong C, Ciliberti‐Vargas MA, Gutierrez CM, Yi L, Romano JG, Perez E, Tyson BA, Ayodele M, et al. Disparities and trends in door‐to‐needle time: the FL‐PR CReSD study (Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities). Stroke. 2017;48:2192–2197. DOI: 10.1161/STROKEAHA.116.016183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sacco RL, Gardener H, Wang K, Dong C, Ciliberti‐Vargas MA, Gutierrez CM, Asdaghi N, Burgin WS, Carrasquillo O, Garcia‐Rivera EJ, et al. Racial‐ethnic disparities in acute stroke care in the Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities Study. J Am Heart Assoc. 2017;6:e004073. DOI: 10.1161/JAHA.116.004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Heart Association . Target: stroke phase II. 2018.
- 13. Fonarow GC, Zhao X, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Xian Y, Hernandez AF, Peterson ED, Schwamm LH. Door‐to‐needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311:1632–1640. DOI: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 14. Kamal N, Sheng S, Xian Y, Matsouaka R, Hill MD, Bhatt DL, Saver JL, Reeves MJ, Fonarow GC, Schwamm LH, et al. Delays in door‐to‐needle times and their impact on treatment time and outcomes in Get With the Guidelines‐Stroke. Stroke. 2017;48:946–954. DOI: 10.1161/STROKEAHA.116.015712. [DOI] [PubMed] [Google Scholar]
- 15. Sauser K, Levine DA, Nickles AV, Reeves MJ. Hospital variation in thrombolysis times among patients with acute ischemic stroke: the contributions of door‐to‐imaging time and imaging‐to‐needle time. JAMA Neurol. 2014;71:1155–1161. DOI: 10.1001/jamaneurol.2014.1528. [DOI] [PubMed] [Google Scholar]
- 16. Prabhakaran S, McNulty M, O'Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke. 2012;43:875–877. DOI: 10.1161/STROKEAHA.111.640060. [DOI] [PubMed] [Google Scholar]
- 17. Sauser K, Bravata DM, Hayward RA, Levine DA. A national evaluation of door‐to‐imaging times among acute ischemic stroke patients within the Veterans Health Administration. J Stroke Cerebrovasc Dis. 2015;24:1329–1332. DOI: 10.1016/j.jstrokecerebrovasdis.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 18. Sauser K, Burke JF, Levine DA, Scott PA, Meurer WJ. Time to brain imaging in acute stroke is improving: secondary analysis of the INSTINCT trial. Stroke. 2014;45:287–289. DOI: 10.1161/STROKEAHA.113.003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, Ellrodt G, Cannon CP, Liang L, Peterson E, et al. Get With the Guidelines‐Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107–115. DOI: 10.1161/CIRCULATIONAHA.108.783688. [DOI] [PubMed] [Google Scholar]
- 20. Alberts MJ, Hademenos G, Latchaw RE, Jagoda A, Marler JR, Mayberg MR, Starke RD, Todd HW, Viste KM, Girgus M, et al. Recommendations for the establishment of primary stroke centers. JAMA. 2000;283:3102–3109. DOI: 10.1001/jama.283.23.3102. [DOI] [PubMed] [Google Scholar]
- 21. Evenson KR, Foraker R, Morris DL, Rosamond WD. A comprehensive review of prehospital and in‐hospital delay times in acute stroke care. Int J Stroke. 2009;4:187–199. DOI: 10.1111/j.1747-4949.2009.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giralt D, Domingues‐Montanari S, Mendioroz M, Ortega L, Maisterra O, Perea‐Gainza M, Delgado P, Rosell A, Montaner J. The gender gap in stroke: a meta‐analysis. Acta Neurol Scand. 2012;125:83–90. DOI: 10.1111/j.1600-0404.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 23. Haršány M, Kadlecová P, Švigelj V, Kõrv J, Kes VB, Vilionskis A, Krespi Y, Mikulík R; Investigators S‐E . Factors influencing door‐to‐imaging time: analysis of the safe implementation of treatments in Stroke–EAST registry. J Stroke Cerebrovasc Dis. 2014;23:2122–2129. DOI: 10.1016/j.jstrokecerebrovasdis.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 24. Hsia AW, Edwards DF, Morgenstern LB, Wing JJ, Brown NC, Coles R, Loftin S, Wein A, Koslosky SS, Fatima S, et al. Racial disparities in tissue plasminogen activator treatment rate for stroke: a population‐based study. Stroke. 2011;42:2217–2221. DOI: 10.1161/STROKEAHA.111.613828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacobs B, Birbeck G, Mullard A, Hickenbottom S, Kothari R, Roberts S, Reeves M. Quality of hospital care in African American and white patients with ischemic stroke and TIA. Neurology. 2006;66:809–814. DOI: 10.1212/01.wnl.0000203335.45804.72. [DOI] [PubMed] [Google Scholar]
- 26. Lachkhem Y, Rican S, Minvielle E. Understanding delays in acute stroke care: a systematic review of reviews. Eur J Public Health. 2018;28:426–433. DOI: 10.1093/eurpub/cky066. [DOI] [PubMed] [Google Scholar]
- 27. Morris DL, Rosamond W, Madden K, Schultz C, Hamilton S. Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey. Stroke. 2000;31:2585–2590. DOI: 10.1161/01.STR.31.11.2585. [DOI] [PubMed] [Google Scholar]
- 28. American Heart Association . Get With the Guidelines—stroke patient management tool. Available at: https://www.heart.org/en/professional/quality‐improvement/get‐with‐the‐guidelines/get‐with‐the‐guidelines‐stroke/get‐with‐the‐guidelines‐stroke‐patient‐management‐tool Accessed October 8, 2020.
- 29. Oostema JA, Nasiri M, Chassee T, Reeves MJ. The quality of prehospital ischemic stroke care: compliance with guidelines and impact on in‐hospital stroke response. J Stroke Cerebrovasc Dis. 2014;23:2773–2779. DOI: 10.1016/j.jstrokecerebrovasdis.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B, et al. Status of cardiovascular disease and stroke in hispanics/latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. DOI: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pandian JD, Padma V, Vijaya P, Sylaja P, Murthy JM. Stroke and thrombolysis in developing countries. Int J Stroke. 2007;2:17–26. DOI: 10.1111/j.1747-4949.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 32. Sharrief AZ, Johnson B, Abada S, Urrutia VC. Stroke knowledge in African Americans: a narrative review. Ethn Dis. 2016;26:255–262. DOI: 10.18865/ed.26.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau‐Sepulveda MV, Pan W, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. DOI: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 34. Strbian D, Ahmed N, Wahlgren N, Lees KR, Toni D, Roffe C, Surakka IL, Tatlisumak T. Trends in door‐to‐thrombolysis time in the safe implementation of stroke thrombolysis registry: effect of center volume and duration of registry membership. Stroke. 2015;46:1275–1280. DOI: 10.1161/STROKEAHA.114.007170. [DOI] [PubMed] [Google Scholar]
- 35. Reeves MJ, Fonarow GC, Zhao X, Smith EE, Schwamm LH. Quality of care in women with ischemic stroke in the GWTG program. Stroke. 2009;40:1127–1133. DOI: 10.1161/STROKEAHA.108.543157. [DOI] [PubMed] [Google Scholar]
- 36. Messé SR, Khatri P, Reeves MJ, Smith EE, Saver JL, Bhatt DL, Grau‐Sepulveda MV, Cox M, Peterson ED, Fonarow GC, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87:1565–1574. DOI: 10.1212/WNL.0000000000003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nasr DM, Brinjikji W, Cloft HJ, Rabinstein AA. Racial and ethnic disparities in the use of intravenous recombinant tissue plasminogen activator and outcomes for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:154–160. DOI: 10.1016/j.jstrokecerebrovasdis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 38. Xian Y, Fonarow GC, Reeves MJ, Webb LE, Blevins J, Demyanenko VS, Zhao X, Olson DM, Hernandez AF, Peterson ED, et al. Data quality in the American Heart Association Get With the Guidelines‐Stroke (GWTG‐Stroke): results from a national data validation audit. Am Heart J. 2012;163:392–398. DOI: 10.1016/j.ahj.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 39. Reeves MJ, Fonarow GC, Smith EE, Pan W, Olson D, Hernandez AF, Peterson ED, Schwamm LH. Representativeness of the Get With the Guidelines–Stroke Registry: comparison of patient and hospital characteristics among Medicare beneficiaries hospitalized with ischemic stroke. Stroke. 2012;43:44–49. DOI: 10.1161/STROKEAHA.111.626978. [DOI] [PubMed] [Google Scholar]
- 40. Daviglus ML, Talavera GA, Avilés‐Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among hispanic/latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. DOI: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rich DQ, Gaziano JM, Kurth T. Geographic patterns in overall and specific cardiovascular disease incidence in apparently healthy men in the United States. Stroke. 2007;38:2221–2227. DOI: 10.1161/STROKEAHA.107.483719. [DOI] [PubMed] [Google Scholar]
- 42. Kalnins A, Mickelsen LJ, Marsh D, Zorich C, Casal S, Tai WA, Vora N, Olalia G, Wintermark M, Larson DB. Decreasing stroke code to CT time in patients presenting with stroke symptoms. Radiographics. 2017;37:1559–1568. DOI: 10.1148/rg.2017160190. [DOI] [PubMed] [Google Scholar]
- 43. Ruff IM, Ali SF, Goldstein JN, Lev M, Copen WA, McIntyre J, Rost NS, Schwamm LH. Improving door‐to‐needle times: a single center validation of the target stroke hypothesis. Stroke. 2014;45:504–508. DOI: 10.1161/STROKEAHA.113.004073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


