Abstract
Background
We previously showed that levels of prebeta‐1 high‐density lipoprotein (HDL), the principal acceptor of cholesterol effluxed from cells, including artery wall macrophages, are positively associated with coronary heart disease (CHD) and myocardial infarction (MI) risk.
Methods and Results
In a multiethnic follow‐up cohort of 1249 individuals from University of California–San Francisco clinics, we determined the degree to which prebeta‐1 HDL levels, both absolute and percentage of apolipoprotein AI, are associated with CHD and history of MI. Independent, strong, positive associations were found. Meta‐analysis revealed for the absolute prebeta‐1 HDL for the top tertile versus the lowest, unadjusted odds ratios of 1.90 (95% CI, 1.40–2.58) for CHD and 1.79 (95% CI, 1.35–2.36) for MI. For CHD, adjusting for established risk factors, the top versus bottom tertiles, quintiles, and deciles yielded sizable odds ratios of 2.37 (95% CI, 1.74–3.25, P<0.001), 3.20 (95% CI, 2.07–4.94, P<0.001), and 4.00 (95% CI, 2.11–7.58, P<0.001), respectively. Men and women were analyzed separately in a combined data set of 2507 individuals. The odds ratios for CHD and MI risk were similar. Higher levels of prebeta‐1 HDL were associated with all 5 metabolic syndrome features. Addition of prebeta‐1 HDL to these 5 features resulted in significant improvements in risk‐prediction models.
Conclusions
Analysis of 2507 subjects showed conclusively that levels of prebeta‐1 HDL are strongly associated with a history of CHD or MI, independently of traditional risk factors. Addition of prebeta‐1 HDL can significantly improve clinical assessment of risk of CHD and MI.
Keywords: apolipoprotein A‐1, coronary heart disease; myocardial infarction, prebeta‐1 HDL, reverse cholesterol transport
Subject Categories: Clinical Studies, Myocardial Infarction, Risk Factors, Coronary Artery Disease, Metabolic Syndrome
Nonstandard Abbreviations and Acronyms
- ABCA1
ATP binding cassette subfamily A member 1
- ABCG1
ATP binding cassette subfamily G member 1
- CETP
cholesteryl ester transfer protein
- LCAT
lecithin‐cholesterol acyltransferase
- PLTP
phospholipid transfer protein
- SR‐BI
scavenger receptor class B type I
- VLDL
very‐low‐density lipoprotein
Clinical Perspective
What Is New?
A strong positive association exists between levels of prebeta‐1 high‐density lipoprotein (HDL), the principal acceptor of cholesterol effluxed from cells, and risk of both coronary heart disease and myocardial infarction; the extent of this association is similar in both men and women.
The top versus bottom tertiles, quintiles, and deciles of prebeta‐1 HDL yielded odds ratios for coronary heart disease of 2.37 (95% CI, 1.74–3.25, P<0.001), 3.20 (95% CI, 2.07–4.94, P<0.001), and 4.00 (95% CI, 2.11–7.58, P<0.001), respectively.
Higher levels of prebeta‐1 HDL were associated with all 5 metabolic syndrome features.
What Are the Clinical Implications?
The associations of prebeta‐1 HDL with coronary heart disease and myocardial infarction strongly suggests that impaired efflux of cholesterol from the artery wall by this lipoprotein species is involved in coronary atherogenesis.
Inclusion of prebeta‐1 HDL into traditional risk models alongside the 5 metabolic syndrome risk factors leads to improvement in patient risk stratification for coronary heart disease and myocardial infarction.
Therefore, prebeta‐1 HDL should be included alongside standard clinical measurements for prediction of risk.
Atherosclerosis is the leading cause of death in the United States and in Europe resulting from myocardial infarction (MI), stroke, and peripheral vascular disease. A central process in the development of atherosclerosis is the infiltration of lipid‐rich lipoproteins containing B apolipoproteins into the artery wall. High‐density lipoproteins (HDLs), on the other hand, are antiatherogenic because they mediate the removal of cholesterol from the artery wall via reverse cholesterol transport. Several prospective epidemiological studies have found that low serum HDL‐cholesterol (HDL‐C) concentrations constitute an independent risk factor for coronary heart disease (CHD). 1 , 2 , 3 , 4 , 5 A low plasma level of HDL‐C is a characteristic of the metabolic syndrome, a constellation of clinical features linking insulin resistance, hypertriglyceridemia, HDL deficiency, diabetes mellitus, obesity, and hypertension with CHD. 6
It is now recognized that prebeta‐1 HDL is the quantum species of HDL in the reverse cholesterol transport process. 7 We, and others, have reported that levels of prebeta‐1 in plasma are independently and positively associated with the risk of CHD, 8 , 9 , 10 , 11 MI, 9 and carotid intima media thickness. 12 , 13
Prebeta‐1 HDL is the primary acceptor of cholesterol effluxed from macrophages by the ATP‐binding cassette transporters ABCA1 (ATP binding cassette subfamily A member 1) and ABCG1 (ATP binding cassette subfamily G member 1). 7 , 14 , 15 It also serves as the substrate for LCAT (lecithin‐cholesterol acyltransferase), which esterifies the cholesterol. As the prebeta‐1 HDL particles accrue cholesteryl esters, they are transformed into larger‐diameter α‐migrating HDL. 7 Prebeta‐1 HDL is regenerated from these particles during transfer of cholesteryl esters to acceptor particles by a process mediated by CETP (cholesteryl ester transfer protein). They can also be formed from α‐HDL by the action of PLTP (phospholipid transfer protein), hepatic lipase, and endothelial lipase. 16 , 17 , 18 , 19 , 20 , 21 This process is dependent on the presence of apo M (apolipoprotein M). 20 Prebeta‐1 HDL is also generated from chylomicrons and very‐low‐density lipoproteins during lipolysis by lipoprotein lipase, and probably because of the selective uptake of cholesteryl esters from mature HDL particles by hepatocytes via the SR‐BI (scavenger receptor class B type I) receptor. 20 , 21 Our previous study reported positive associations between percentage levels of prebeta‐1 HDL and CHD, and showed for the first time that it was a novel predictor of MI, independent of traditional risk factors. 9 We found that the inclusion of prebeta‐1 HDL into traditional risk models led to an improvement in patient risk stratification. Here, we report on a replication cohort of similar size to that in our initial study. Both cohorts were recruited at the University of California–San Francisco Lipid and Endocrinology Clinics. Our aims were to provide evidence to confirm our previous findings of significant associations of percent levels of prebeta‐1 HDL with CHD and MI, and to test the associations with absolute levels of prebeta‐1. We undertook a meta‐analysis of the 2 studies that included a total of 2507 participants. The additional cohort, with the added statistical power, allowed us to examine men and women separately. We also determined the degree to which prebeta‐1 HDL associates with each of the 5 characteristic elements of the metabolic syndrome.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Participants in this study were recruited in the University of California–San Francisco Lipid and Endocrinology Clinics. They were referred for evaluation of cardiovascular risk factors. We aimed to include all new patients who were willing to be recruited into our Genomic Resource in Arteriosclerosis and Metabolic Disease. The University of California–San Francisco committee on human research approved the study, which adhered to the Declaration of Helsinki, and informed written consent was obtained from all participants. Patients were considered to have CHD as in our previous study, 9 that is, if they matched these criteria: MI, coronary disease on angiogram, stent placement, angioplasty, or coronary revascularization. As before, 9 diagnosis of MI was determined based on one of these criteria: troponin levels, electrocardiographic evidence, or echocardiographic studies.
Measurement of Lipids and Lipoproteins
The method to measure the level of prebeta‐1 HDL in plasma was as described in our previous studies. 9 , 22 , 23 The original technique article detailed several experiments demonstrating this ultrafiltration technique was free of artifacts. 22 Briefly, tritium‐labeled purified prebeta‐1 HDL tracer was added initially to the aliquots of plasma, which were always stored at 4°C. We showed the behavior of the tracer to be identical to the analyte. 22 Prebeta‐1 HDL in the samples was then separated from α‐HDL particles by ultrafiltration. This method allows the purification of the 67‐kDa prebeta‐1 HDL particle from α‐migrating HDL species, and importantly, from the larger prebeta components, prebeta‐2 and prebeta‐3 HDL. 22 In addition, we observed little, if any, monomeric apo AI (apolipoprotein AI) on gradient gels or fast protein liquid chromatography. 22 The particle isolated by ultrafiltration is identical to that seen by 2‐dimensional gel electrophoresis. The concentration of apo AI in the filtrate (prebeta‐1 HDL) and in plasma were determined using an ELISA. 24 This isotope dilution technique allows the measurement of prebeta‐1 HDL over a wide range of concentrations. The level in plasma of prebeta‐1 HDL–associated apo AI is reported as a percentage of the total concentration of apo AI in plasma and in absolute terms (milligrams per deciliter).
Lipid and other lipoprotein measurements were performed as previously described. 9
Statistical Analysis
For the present cohort, we analyzed the data as described previously, 9 but in addition to analyzing the prebeta‐1 HDL percentage values, we also evaluated the absolute values and undertook a meta‐analysis of data from this and our previous study using a random‐effects model. For logistic regression analysis, the Hosmer‐Lemeshow test for goodness of model fit was used. For consistency, the same covariates were chosen as in our previous study. 9 Meta‐analysis was performed, and forest plots were obtained using the open‐source software OpenMetaAnalyst developed at Brown University (School of Public Health, Providence, RI). Descriptive statistics were used to summarize clinical, demographic, and biomarker characteristics. For continuous variables, differences were tested by ANOVA or t test as appropriate, and for categorical variables by χ2 test. All skewed continuous variables were log transformed prior to analysis. Statistical testing was done using SPSS (IBM, Armonk, NY) or Stata (StataCorp, College Station, TX). To evaluate prediction performance of adding prebeta‐1 HDL as an additional biomarker, we used the “incrisk” module for Stata developed at the Fred Hutchinson Cancer Research Center (University of Washington, Seattle, WA). All demographic and clinical variables associated (P<0.10) with CHD or MI were evaluated as potential covariates in logistic regression analysis. Age and sex were forced into the analysis. Covariates were retained in the final model if their significance was P<0.05.
Results
Patient Characteristics
Table 1 shows the characteristics of the new cohort of 1249 patients. Shown is the entire cohort and as divided into 3 tertiles of percent prebeta‐1 HDL. The means or percentages, as appropriate, for each variable are similar to the values for the first cohort. 9 There were fewer patients with CHD (27.9% versus 36.2%) or with a history of MI (12.1% versus 17.0%). In this new cohort, we found significant positive and independent associations between the bottom and top tertiles of prebeta‐1 HDL and both CHD and MI. In our previous study, we presented the prebeta‐1 HDL data only in terms of a percentage of total apo AI levels in plasma. 9 For comparison, we have presented the characteristics of the original cohort in Table S1. For the new cohort, we have presented the data as both percentages and as absolute values (Table 2). For percent values, the odds ratios (ORs), after adjusting for other covariables, including traditional risk factors, were 1.81 (95% CI, 1.20–2.73; P=0.005) for CHD and 2.12 (95% CI, 1.24–3.63; P<0.001) for MI. These ORs are similar in magnitude to what we reported previously. 9 The ORs for tertile 2 compared with tertile 1 were in all 4 cases no longer significant after adjusting for the traditional risk factors. We also conducted a meta‐analysis of the results from the present and previous studies (2507 total participants) (Figure). This shows, for percentage values, highly significant ORs (unadjusted) of 1.82 (95% CI, 1.40–2.36) for CHD and 1.93 (95% CI, 1.46–2.57) for MI for the highest tertile of prebeta‐1 HDL compared with the lowest tertile. For absolute values of prebeta‐1 HDL, the corresponding ORs were 1.90 (95% CI, 1.40–2.58) for CHD and 1.79 (95% CI, 1.35–2.36) for MI.
Table 1.
Characteristics of All Participants in Cohort 2 in Total and Based on Tertiles of Percent Prebeta‐1 HDL
| All Participants, n=1249 | Prebeta‐1 HDL Tertiles | P Value | |||
|---|---|---|---|---|---|
| <4.06%, n=416 | 4.06% to 9.18%, n=416 | >9.18%, n=417 | |||
| Age, y | 51.3±14.4 | 51.7±14.7 | 51.9±14.6 | 50.4±13.7 | 0.244 |
| Sex, % men | 42.4 | 32.5 | 43.3 | 51.3 | <0.001 |
| BMI, kg/m2 | 26.6±5.3 | 25.2±4.5 | 26.4±5.1 | 28.0±5.8 | <0.001 |
| Race/ethnicity % | |||||
| White | 78.5 | 76.4 | 77.9 | 81.3 | 0.215 |
| East Asian | 7.7 | 9.9 | 7.2 | 6.0 | 0.102 |
| Hispanic | 6.0 | 4.1 | 7.7 | 6.2 | 0.088 |
| Black | 4.0 | 4.6 | 4.1 | 3.4 | 0.669 |
| Mixed, other* | 3.7 | 5.0 | 2.9 | 3.1 | 0.191 |
| CHD, % | 27.9 | 20.0 | 29.3 | 34.3 | <0.001 |
| MI, % | 12.1 | 7.7 | 12.7 | 15.8 | 0.001 |
| Diabetes mellitus, % | 12.6 | 7.9 | 12.0 | 17.7 | <0.001 |
| Hypertension, % | 30.8 | 26.6 | 30.8 | 35.0 | 0.031 |
| Smoker (current), % | 7.8 | 6.8 | 8.9 | 7.7 | 0.516 |
| Triglycerides, mg/dL | 135 (56) | 105 (36) | 130 (41) | 197 (80) | <0.001 |
| LDL cholesterol, mg/dL | 154±62 | 147±55 | 153±54 | 161±73 | 0.021 |
| HDL cholesterol, mg/dL | 51.8±19.8 | 60.3±21.3 | 52.0±17.4 | 43.2±16.8 | <0.001 |
| Apolipoprotein AI, mg/dL | 126±37 | 127±38 | 128±39 | 123±35 | 0.137 |
| Lipid‐lowering medication, % | 18.2 | 12.0 | 19.5 | 23.0 | <0.001 |
For continuous variables, differences between the tertile groups were tested by ANOVA and for categorical variables by χ2 test. Prior to testing, skewed variables (triglycerides, LDL cholesterol, HDL cholesterol, and BMI) were log transformed. Data are presented as number of individual participants (percentage), mean±SD, or median (median absolute deviation). BMI indicates body mass index; CHD, coronary heart disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; and MI, myocardial infarction.
Other indicates Native American, North Asian, and South Asian.
Table 2.
Associations of Prebeta‐1 HDL Tertiles With CHD and MI in Cohort 2
| ORs | CHD | MI | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Unadjusted | ||||
| Percent prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 2 | 1.67 (1.21–2.29) | 0.002 | 1.75 (1.10–2.78) | 0.017 |
| Tertile 3 | 2.09 (1.53–2.87) | <0.001 | 2.26 (1.44–3.53) | <0.001 |
| Adjusted | ||||
| Percent prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 2 | 1.42 (0.97–2.09) | 0.076 | 1.38 (0.82–2.31) | 0.226 |
| Tertile 3 | 1.81 (1.20–2.73) | 0.005 | 2.12 (1.24–3.63) | 0.006 |
| Unadjusted | ||||
| Absolute prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 2 | 1.62 (1.18–2.24) | 0.003 | 1.74 (1.11–2.71) | 0.015 |
| Tertile 3 | 2.24 (1.64–3.07) | <0.001 | 1.79 (1.15–2.79) | 0.010 |
| Adjusted | ||||
| Absolute prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 2 | 1.28 (0.86–1.90) | 0.220 | 1.53 (0.92–2.57) | 0.105 |
| Tertile 3 | 2.12 (1.41–3.20) | <0.001 | 1.90 (1.09–3.30) | 0.024 |
Unadjusted and adjusted ORs and 95% CIs for tertiles of both percent prebeta‐1 HDL (percent of total plasma apolipoprotein A‐I) and absolute prebeta‐1 HDL. ORs were determined by logistic regression and adjusted for age, sex, body mass index, ethnicity, hypertension, current smoking status, type 2 diabetes mellitus, HDL cholesterol, LDL cholesterol, triglycerides, apolipoprotein AI, and use of lipid medication. CHD indicates coronary heart disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; and OR, odds ratio.
Figure 1. Forest plots showing odds ratios and 95% confidence intervals from our previous report, Study 1 9 ; this report, Study 2; and a meta‐analysis using a random‐effects model.
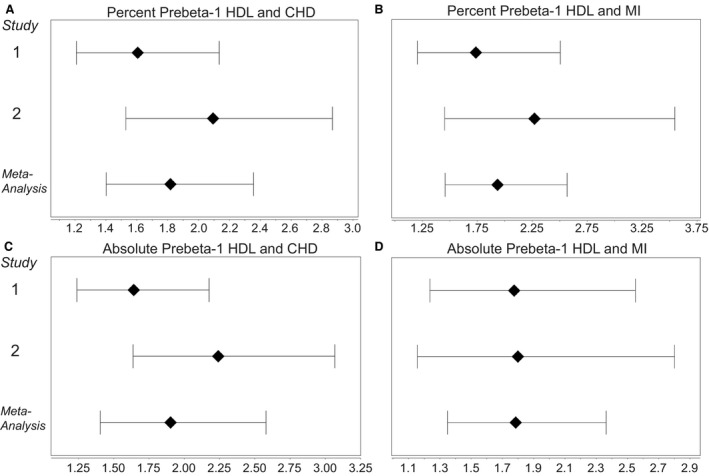
A, Association between percent prebeta‐1 high‐density lipoprotein (HDL) and presence of coronary heart disease (CHD). B, Association between percent prebeta‐1 HDL and history of myocardial infarction (MI ). C, Association between absolute level of prebeta‐1 HDL and presence of CHD. D, Association between absolute level of prebeta‐1 HDL and history of MI.
We and others have shown that statins lower the level of prebeta‐1 HDL. 25 , 26 , 27 In our new cohort, 18.2% were taking lipid‐altering medications (Table 1) as were 15.4% in our previous cohort, 9 and of these, 75% were taking a statin. We compared those with and without a history of CHD or MI (Table 3). To arrive at more accurate assessments of the characteristics, we analyzed the combined data sets from the 2 cohorts, but only included those participants who were not taking lipid medications. Again, we have presented these data as both percentages and absolute values. There were more women and patients with diabetes mellitus among the patients with CHD and MI, and they were older and had higher body mass indexes. There was a higher proportion who were White, and considerably more had hypertension. Current smoking was almost twice as prevalent among the affected groups. Among the lipid and lipoprotein parameters, triglycerides were higher and HDL‐C lower. Percent prebeta‐1 HDL was 21.3% higher in the CHD group and 25.1% higher in the MI group. The corresponding increases for absolute prebeta‐1 HDL were 31.9% and 33.1%, respectively. Mean levels of low‐density lipoprotein cholesterol were high in all 3 groups (non‐CHD, CHD, and MI). This is not surprising, because most were recruited in a tertiary lipid clinic and were therefore mainly selected for hypercholesterolemia.
Table 3.
Characteristics of All Participants Not Taking Lipid Medication (Cohorts 1and 2): Comparison of Those With and Without CHD or a History of MI (n=2083)
| Non‐CHD | CHD | P Value* | MI | P Value † | |
|---|---|---|---|---|---|
| (n=1507) | (n=576) | (n=249) | |||
| Age, y | 46.5±15.8 | 60.7±11.9 | <0.001 | 60.7±12.8 | <0.001 |
| Sex, % women | 57.9 | 71.9 | <0.001 | 69.9 | <0.001 |
| BMI, kg/m2 | 26.0±5.1 | 27.5±5.7 | <0.001 | 27.0±5.8 | 0.006 |
| Race/ethnicity % | |||||
| White | 74.3 | 82.5 | <0.001 | 84.3 | 0.001 |
| East Asian | 11.6 | 7.5 | 0.006 | 8.0 | 0.099 |
| Hispanic | 6.2 | 3.6 | 0.021 | 2.0 | 0.007 |
| Black | 3.1 | 3.5 | 0.625 | 2.4 | 0.576 |
| Mixed, other ‡ | 4.8 | 3.0 | 0.0.57 | 3.2 | 0.253 |
| Diabetes mellitus, % | 7.56 | 9.20 | 0.219 | 12.4 | 0.009 |
| Hypertension, % | 19.2 | 55.3 | <0.001 | 59.8 | <0.001 |
| Smoker (current), % | 6.0 | 11.2 | <0.001 | 11.0 | 0.004 |
| Triglycerides, mg/dL | 128 (51) | 168 (58) | <0.001 | 171 (55) | <0.001 |
| LDL cholesterol, mg/dL | 155±60 | 162±61 | 0.001 | 159±62 | 0.183 |
| HDL cholesterol, mg/dL | 53.8±19.9 | 48.3±16.3 | <0.001 | 46.8±16.2 | <0.001 |
| Apolipoprotein AI, mg/dL | 119±34 | 131±40 | <0.001 | 129±39 | <0.001 |
| Percent Prebeta‐1 HDL | 7.45±6.51 | 9.04±6.91 | <0.001 | 9.32±6.95 | <0.001 |
| Absolute prebeta‐1 HDL, mg/dL | 8.71±8.29 | 11.49±8.83 | <0.001 | 11.59±8.42 | 0.001 |
For continuous variables, differences between the CHD and the normal group were tested by t test and for categorical variables by the χ2 test. Prior to testing, skewed variables were log transformed. For continuous variables, data are presented as mean±SD or median (median absolute deviation). Absolute prebeta‐1 HDL is given as apolipoprotein AI content in milligrams per deciliter. BMI indicates body mass index; CHD, coronary heart disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; and MI, myocardial infarction.
Non‐CHD vs CHD.
Non‐CHD vs MI.
Other includes Native American, North Asian, and South Asian.
To arrive at more accurate assessments of the ORs, we analyzed the combined data sets (Table 4), and again only included those participants who were not taking lipid medications. Table 4 shows the adjusted ORs for the top and bottom tertiles of prebeta‐1 HDL with CHD and MI. For the total participants, the ORs are highly significant, with those for absolute values being higher than for percent values. In our previous report, with respect to associations with CHD and MI, we stated that there was no significant interaction between percent prebeta‐1 HDL and sex. When examining the combined data from the 2 studies (cohorts 1 and 2) we found small, but significant, similar interactions with both percent and absolute prebeta‐1 HDL. To assess whether there were any profound differences in ORs between the sexes, we undertook a separate analysis of men and women in this combined data set (Table 4). For women, the ORs for absolute values were higher than for percentages. This was not the case for men, where the absolute and percent ORs were similar. The ORs for absolute levels of prebeta‐1 HDL were higher in women compared with men, though this was not significant (P=0.1442), whereas for percent values, ORs were similar. The ORs for men and MI did not reach statistical significance, presumably because of the lower proportion of men and resulting reduced statistical power.
Table 4.
Associations of Prebeta‐1 HDL With CHD and MI Using the Combined Data From Cohorts 1 and 2
| ORs | CHD | MI | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Total participants | ||||
| Absolute prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 3 | 2.37 (1.74–3.25) | <0.001 | 2.08 (1.42–3.04) | <0.001 |
| Total participants | ||||
| Percent prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 3 | 1.85 (1.33–2.58) | <0.001 | 1.69 (1.15–2.48) | 0.008 |
| Women | ||||
| Absolute prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 3 | 2.65 (1.80–3.88) | <0.001 | 2.50 (1.54–4.06) | <0.001 |
| Men | ||||
| Absolute prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 3 | 2.04 (1.19–3.51) | 0.010 | 1.96 (0.97–3.99) | 0.062 |
| Women | ||||
| Percent prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 3 | 2.00 (1.32–3.02) | 0.001 | 1.84 (1.15–2.96) | 0.011 |
| Men | ||||
| Percent prebeta‐1 HDL tertile 1 | 1.00 (reference) | — | 1.00 (reference) | — |
| Tertile 3 | 2.01 (1.14–3.54) | 0.016 | 1.90 (0.90–4.02) | 0.093 |
Data from both cohorts for those not taking lipid medication. Adjusted ORs and 95% CIs for tertiles of both percent prebeta‐1 HDL (percent of total plasma apolipoprotein AI) and absolute prebeta‐1 HDL for the 2 combined cohorts. For total participants: tertile 1, n=836; tertile 3, n=837. For women: tertile 1, n=474; tertile 3, n=474. For men: tertile 1, n=362; tertile 3, n=365. ORs were determined by logistic regression and adjusted for age, sex, body mass index, ethnicity, hypertension, current smoking status, type 2 diabetes mellitus, HDL cholesterol, LDL cholesterol, triglycerides, and apolipoprotein AI. CHD indicates coronary heart disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; and OR, odds ratio.
We separately calculated the adjusted OR for CHD for the bottom and top quintiles using the combined data sets from both studies for those not taking lipid medication (Table 5). (This was not possible for MI because of the small number of individuals with a history of MI and thus much diminished statistical power.) The OR for absolute prebeta‐1 HDL was 3.20 (95% CI, 2.07–4.94, P<0.001) and for percent prebeta‐1 HDL was 2.70 (95% CI, 1.72–4.24, P≤0.001). We were able to calculate the adjusted ORs for the bottom and top 10th percentiles (Table 5). Here again, the absolute levels of prebeta‐1 HDL were a better predictor of CHD risk. For absolute values the OR was 4.00 (95% CI, 2.11–7.58, P<0.001) and for percent values was 2.79 (95% CI, 1.44–5.42, P=0.002).
Table 5.
Associations of Prebeta‐1 HDL Upper and Lower Quintiles and Deciles With CHD
| ORs | CHD | |
|---|---|---|
| OR (95% CI) | P Value | |
| Absolute prebeta‐1 HDL quintile 1 | 1.00 (reference) | — |
| Quintile 5 | 3.20 (2.07–4.94) | <0.001 |
| Percent prebeta‐1 HDL quintile 1 | 1.00 (reference) | — |
| Quintile 5 | 2.70 (1.72–4.24) | <0.001 |
| Absolute prebeta‐1 HDL decile 1 | 1.00 (reference) | — |
| Decile 10 | 4.00 (2.11–7.58) | <0.001 |
| Percent prebeta‐1 HDL decile 1 | 1.00 (reference) | — |
| Decile 10 | 2.79 (1.44–5.42) | 0.002 |
Data from both cohorts for those not taking lipid medication. Adjusted ORs and 95% CIs for upper and lower quintiles and deciles of both absolute prebeta‐1 HDL and percent prebeta‐1 HDL (percent of total plasma apolipoprotein AI). ORs were determined by logistic regression and adjusted for age, sex, body mass index, ethnicity, hypertension, current smoking status, type 2 diabetes mellitus, HDL cholesterol, LDL cholesterol, triglycerides, and apolipoprotein AI. CHD indicates coronary heart disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein, and OR, odds ratio.
The ORs for all the other covariates, including traditional risk factors, entered in multivariable risk models are shown in Table 6. This was limited to the participants who were not taking lipid‐lowering medication. Except for men, all associations for established risk factors were in the same direction as described in studies of general populations. Not surprisingly, for both CHD and MI, age was a positive risk factor, and the OR for men reflected the greater proportion of women with CHD in these cohorts. Being of East Asian, Hispanic, or mixed/other ethnicity was associated with lower risk of CHD. Unlike prebeta‐1 HDL and HDL‐C, total plasma apo AI was not significantly associated with either CHD or MI. Four of the characteristic features of the metabolic syndrome were associated with significant increased risk of CHD, the exception being diabetes mellitus. We compared the mean levels of prebeta‐1 HDL for those with and without each of these 5 metabolic syndrome features (Table 7). Significantly higher levels were observed, both percent and absolute values, for all 5 characteristics. Notably, prebeta‐1 HDL was 57.8% higher among those with elevated triglyceride levels, 24.2% higher among those who were obese, and 22.9% higher among those with low HDL‐C. Percent values for men were significantly higher than for women, whereas absolute levels were the same. Table 8 shows that the inclusion of prebeta‐1 HDL in the risk model for CHD resulted in a highly significant change in the area under the receiver operating characteristic curve and improvements in classification as indicated by the net reclassification index, and in the integrated discrimination improvement index. There were similar changes with respect to MI.
Table 6.
Other Predictors of CHD and MI Entered in Multivariable Risk Models in Combined Cohorts in Participants Not Using Lipid Medication
| CHD | P Value | MI | P Value | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age, 5 y* | 1.42 (1.36–1.48) | <0.001 | 1.29 (1.23–1.36) | <0.001 |
| Sex, men | 0.54 (0.44–0.66) | <0.001 | 0.66 (0.50–0.88) | 0.005 |
| BMI † | 1.34 (1.22–1.48) | <0.001 | 1.12 (0.98–1.28) | 0.086 |
| Race/ethnicity | ||||
| White | 1.00 (reference) | — | 1.00 (reference) | — |
| East Asian | 0.58 (0.41–0.82) | 0.002 | 0.67 (0.41–1.08) | 0.101 |
| Black | 1.02 (0.60–1.75) | 0.930 | 0.66 (0.28–1.54) | 0.336 |
| Hispanic | 0.53 (0.32–0.86) | 0.009 | 0.30 (0.12–0.74) | 0.009 |
| Mixed, other ‡ | 0.55 (0.32–0.94) | 0.029 | 0.64 (0.31–1.35) | 0.241 |
| Diabetes mellitus | 1.24 (0.88–1.74) | 0.219 | 1.79 (1.18–2.71) | 0.006 |
| Hypertension | 5.22 (4.23–6.42) | <0.001 | 4.48 (3.40–5.89) | <0.001 |
| Smoker, current | 1.97 (1.41–2.76) | <0.001 | 1.64 (1.06–2054) | 0.027 |
| Triglycerides, >200 mg/dL | 1.62 (1.32–1.99) | <0.001 | 1.55 (1.17–2.04) | 0.002 |
| HDL cholesterol † | 0.78 (0.71–0.86) | <0.001 | 0.75 (0.66–0.85) | <0.001 |
| LDL cholesterol † | 1.19 (1.08–1.32) | 0.001 | 1.07 (0.93–1.23) | 0.324 |
| Apolipoprotein AI, >0.9 mg/dL | 1.28 (0.98–1.67) | 0.063 | 1.18 (0.82–1.69) | 0.382 |
Data from both cohorts for those not taking lipid medication. Odds ratios were determined by logistic regression. BMI indicates body mass index; CHD, coronary heart disease; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; and OR, odds ratio.
OR represents risk per 5‐year increment.
Log transformed and standardized. OR represents increased risk of CHD or MI prevalence per 1 SD increment.
Other includes Native American, North Asian, and South Asian.
Table 7.
Comparison of Prebeta‐1 HDL Levels According to Sex and Presence of Metabolic Syndrome Characteristics
| Trait (n) | Percent Prebeta‐1 HDL (SD) | P Value | Absolute Prebeta‐1 HDL, mg/dL (SD) | Percent Increase | P Value |
|---|---|---|---|---|---|
| Men (1088) | 8.96 (7.46) | <0.001 | 9.50 (8.36) | — | 0.862 |
| Women (1419) | 7.36 (6.34) | 9.66 (8.76) | |||
| Diabetic (263) | 9.82 (7.65) | <0.001 | 11.07 (8.69) | 17.5 | 0.005 |
| Nondiabetic (2244) | 7.85 (6.77) | 9.42 (8.56) | |||
| Low HDL* (996) | 10.18 (7.55) | <0.001 | 10.80 (8.80) | 22.9 | <0.001 |
| High HDL (1489) | 6.64 (6.05) | 8.79 (8.37) | |||
| High triglycerides † (1147) | 10.20 (7.55) | <0.001 | 11.96 (9.68) | 57.8 | <0.001 |
| Low triglycerides (1348) | 6.24 (5.70) | 7.58 (6.93) | |||
| Obese ‡ (512) | 10.10 (8.14) | <0.001 | 11.43 (10.38) | 24.2 | <0.001 |
| Nonobese (1942) | 7.60 (6.48) | 9.20 (8.04) | |||
| Hypertensive (810) | 8.52 (6.58) | 0.005 | 10.44 (8.45) | 13.6 | <0.001 |
| Normotensive (1695) | 7.84 (7.03) | 9.19 (8.63) |
Data are presented for the 2 combined cohorts with the number of individual participants in each instance and mean±SD. Percent prebeta‐1 HDL is defined as the percent of total plasma apolipoprotein AI present in prebeta‐1 HDL. The absolute level is the amount of apolipoprotein AI present in prebeta‐1 HDL expressed as milligrams per deciliter. HDL indicates high‐density lipoprotein.
Low HDL is defined as <40 mg/dL for men and <50 mg/dL for women.
High triglycerides are defined as ≥150 mg/dL.
Obesity is defined as a body mass index >30 kg/m2.
Table 8.
Effect of Adding Prebeta‐1 HDL to Metabolic Syndrome‐Related Risk Factors on Risk‐Prediction Models
| AUC | NRI | IDI | |||
|---|---|---|---|---|---|
| Change (95% CI) | P Value | Estimate (95% CI) | Estimate (95% CI) | ||
| Absolute | CHD | 0.047 (0.021–0.073) | <0.001 | 0.341 (0.219–0.450) | 0.019 (0.006–0.038) |
| Prebeta‐1 HDL | MI | 0.052 (0.012–0.092) | 0.011 | 0.402 (0.224–0.576) | 0.012 (0.001–0.033) |
Incremental change for 3 calibration statistics because of inclusion in risk models of top and bottom quintiles of absolute prebeta‐1 HDL compared to metabolic syndrome risk factors alone. The metabolic syndrome–related risk factors used in the models: low HDL <40 mg/dL for men and <50 mg/dL for women, high triglycerides defined as ≥150 mg/dL, obesity defined as a body mass index >30 kg/m2, diabetes mellitus, hypertension. AUC in risk models that exclude prebeta‐1 HDL are 0.622 for CHD and 0.589 for MI. AUC indicates area under receiver operating characteristic curve; CHD, coronary heart disease; HDL, high‐density lipoprotein; IDI, integrated discrimination improvement index; MI, myocardial infarction; and NRI, net reclassification improvement index.
Discussion
This study reinforces the evidence that we and others have presented that levels in plasma of prebeta‐1 HDL associate positively and independently with the presence of CHD, 8 , 9 , 10 , 11 MI, 9 and carotid intima media thickness. 12 , 13 More recently, it was shown that levels of prebeta‐1 were positively associated with patients with coronary artery disease. 28 Thus, the measurement of prebeta‐1 HDL adds an important dimension to the assessment of risk of coronary and cerebral vascular disease. Here we have shown clearly that the association with CHD and MI holds true for both men and women. In the case of CHD, for those not taking lipid medication, the top versus the bottom tertiles, quintiles, and deciles of absolute prebeta‐1 HDL show significant and sizable ORs of 2.37, 3.20, and 4.00, respectively. We have also shown here that the levels of prebeta‐1 HDL were significantly higher for all 5 characteristics of the metabolic syndrome. More importantly, we have shown here using risk reclassification analysis a significant improvement with the addition of prebeta‐1 HDL to these metabolic syndrome‐related risk factors in risk‐prediction models. Thus, prebeta‐1 HDL should be considered a significant additional major risk factor for CHD and MI.
A positive association might at first be considered counterintuitive because high levels of prebeta‐1 HDL could be considered antiatherogenic for the reason that this subspecies of HDL is the principal acceptor of cholesterol from the artery wall and plays a critical role in reverse cholesterol transport. However, the measured amount of prebeta‐1 HDL in plasma reflects the steady‐state level and tells us nothing directly about its rate of formation and removal. The removal of prebeta‐1 HDL begins after the transfer of free cholesterol effluxed from cells by ABCA1, ABCG1, and SR‐BI. 7 , 14 , 15 The cholesterol is esterified by LCAT, for which prebeta‐1 HDL acts as a substrate. 29 The resultant cholesteryl esters move rapidly through larger prebeta‐2 and prebeta‐3 species and then into α‐HDL particles and low‐density lipoprotein. 30 Prebeta‐1 HDL can be removed by the kidney. 21 Decreased cholesterol efflux because of diminished capacity of SR‐BI or the ABCA1 and ABCG1 transporters, low LCAT activity, or for other reasons, could result in an accumulation of prebeta‐1 HDL and a rise in its steady‐state level.
There are several processes involved in the formation of prebeta‐1 HDL. 21 It can enter the circulation directly as nascent apo AI from hepatocytes and enterocytes. 7 Because cholesteryl esters in α‐HDL particles are transferred via CETP to chylomicrons, very‐low‐density lipoproteins, and low‐density lipoprotein, a process that also involves PLTP, 31 hepatic lipase, 32 endothelial lipase, 33 and apo M, 20 , 34 the alpha HDL particles are remodeled, and prebeta‐1 HDL is generated. 16 , 17 , 18 , 19 , 20 It can be formed from chylomicrons and very‐low‐density lipoproteins as a result of lipolysis by lipoprotein lipase. 7 , 35 It is likely that prebeta‐1 HDL may also be formed as part of a selective process involving SR‐BI, whereby cholesteryl esters are transferred directly to hepatocytes and other tissues from α‐HDL. Interestingly, it has been shown that both levels of CETP and prebeta‐1 HDL were higher in patients with coronary heart disease, and that CETP was a determinant of prebeta‐1 HDL levels. 28
Overall, these processes of formation and removal constitute the prebeta‐1 HDL cycle. 7 , 36 It has been suggested recently 37 that a novel apo AI metabolic pathway exists. Nevertheless, no matter what the intricacies of the pathway of de novo synthesized apo AI to more mature HDL species, our study was focused on determining the levels in plasma of prebeta‐1 HDL under steady‐state conditions in individuals with and without CHD. Prebeta‐1 HDL, as we have quantified it, is an integral of all the different pathways.
A defect in the overall process of cholesterol efflux from cells not only would account for an accumulation of prebeta‐1 HDL, but would also contribute to the increased atherosclerosis that underlies CHD and MI. As an independent risk factor, prebeta‐1 HDL levels are indicators of the relative efficiency of the efflux of cholesterol from the artery wall, a crucial step in protecting against atherosclerosis. 7 It has been shown by others that the acceptor properties of serum 38 or plasma 39 for cholesterol vary among individuals and is another determinant of the rate of cholesterol efflux. In case–control studies, HDL‐mediated cholesterol efflux capacity measured in J774 cells 40 and in THP‐1 monocytes 41 was associated with lower risk of CHD and deemed a highly important atheroprotective process. 41 Using a different, novel, nuclear magnetic resonance technique, a similar lower risk was observed by another group. 42 In addition to the increased risk of CHD associated with higher levels of prebeta‐1 HDL, others have shown that patients with CHD have circulating prebeta‐1 HDL with reduced functionality. 43
Interestingly, a new additional mechanism for the removal of surplus cholesterol from macrophages has recently been suggested, whereby it is transferred to adjacent smooth muscle cells. This process has been termed transcellular cholesterol movement. 44 It has been suggested that because ABCA1 is highly expressed in medial smooth muscle cells, this might represent an important antiatherogenic process. 44
The strength of this study lies in the much higher number of patients we could investigate relative to comparable reports and thus look deeper while retaining sufficient statistical power. In addition, we could rule out the effects of lipid medications because of the high percentage of patients not taking these drugs. However, this was a cross‐sectional observational study that lacked longitudinal information and was not a prospective cohort study. Also, our results may not be fully comparable to that of all other populations, which may have different underlying clinical conditions or ethnic composition. Another limitation is that we cannot completely rule out the existence of CHD in controls, because they did not undergo the testing required to diagnose CHD or MI.
We believe that this and our previous study 9 provide evidence that strongly suggests that impaired efflux by prebeta‐1 HDL of cholesterol from the artery wall is involved in coronary atherogenesis. Because prebeta‐1 HDL assesses the mechanisms of atherosclerosis independent of classic lipid disorders and reveals significant risk associations, this lipoprotein species should be included in clinical measurements for prediction of risk.
Sources of Funding
This work was supported in part by grants HL3120, HL50782, Hl50779, and AA11205 from the National Institutes of Health (Bethesda, MA) and by the Joseph Drown Foundation, the Campini Foundation, the Dhanem Foundation, the Foundation Leducq, and by gifts from Peter Read, Harold Dittmer, Susan Boeing, Donald Yellon, and the Mildred V. Strouss Charitable Trust.
Disclosures
None.
Supporting information
Table S1
Acknowledgments
We wish to thank the patients for their cooperation and willingness to participate in this study.
Author contributions: Dr Kane, Dr Malloy, and Dr Frost recruited the study participants. Dr Pullinger wrote the article. Dr Kane and Dr Malloy assisted in editing and revising the article. Dr O’Connor, Dr Kunitake, and Dr Kane, with the assistance of Dr Naya‐Vigne, developed the prebeta‐1 HDL assay. Dr Naya‐Vigne ran the assays and produced the data. I. Movsesyan was responsible for sample processing and curating the demographic data. Dr Pullinger analyzed the data and performed the statistical analysis. All authors have approved the final article.
(J Am Heart Assoc. 2021;10:e018381. DOI: 10.1161/JAHA.120.018381.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018381.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med. 1977;62:707–714. DOI: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2. Gotto AM, Brinton EA. Assessing low levels of high‐density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J Am Coll Cardiol. 2004;43:717–724. DOI: 10.1016/j.jacc.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 3. Maron DJ. The epidemiology of low levels of high‐density lipoprotein cholesterol in patients with and without coronary artery disease. Am J Cardiol. 2000;86:11L–14L. DOI: 10.1016/S0002-9149(00)01462-4. [DOI] [PubMed] [Google Scholar]
- 4. Miller NE, Thelle DS, Forde OH, Mjos OD. The tromso heart‐study. High‐density lipoprotein and coronary heart‐disease: a prospective case‐control study. Lancet (London, England). 1977;1:965–968. [DOI] [PubMed] [Google Scholar]
- 5. Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W; ARICS Study Group . Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A‐I and B, and HDL density subfractions: the atherosclerosis risk in communities (ARIC) study. Circulation. 2001;104:1108–1113. DOI: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 6. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 7. Kane JP, Malloy MJ. Prebeta‐1 HDL and coronary heart disease. Curr Opin Lipidol. 2012;23:367–371. DOI: 10.1097/MOL.0b013e328353eef1. [DOI] [PubMed] [Google Scholar]
- 8. Asztalos BF, Collins D, Cupples LA, Demissie S, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Value of high‐density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the veterans affairs HDL intervention trial. Arterioscler Thromb Vasc Biol. 2005;25:2185–2191. DOI: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 9. Guey LT, Pullinger CR, Ishida BY, O'Connor PM, Zellner C, Francone OL, Laramie JM, Naya‐Vigne JM, Siradze KA, Deedwania P, et al. Relation of increased prebeta‐1 high‐density lipoprotein levels to risk of coronary heart disease. Am J Cardiol. 2011;108:360–366. DOI: 10.1016/j.amjcard.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 10. Miida T, Nakamura Y, Inano K, Matsuto T, Yamaguchi T, Tsuda T, Okada M. Pre beta 1‐high‐density lipoprotein increases in coronary artery disease. Clin Chem. 1996;42:1992–1995. DOI: 10.1093/clinchem/42.12.1992. [DOI] [PubMed] [Google Scholar]
- 11. Sethi AA, Sampson M, Warnick R, Muniz N, Vaisman B, Nordestgaard BG, Tybjaerg‐Hansen A, Remaley AT. High pre‐beta1 HDL concentrations and low lecithin: cholesterol acyltransferase activities are strong positive risk markers for ischemic heart disease and independent of HDL‐cholesterol. Clin Chem. 2010;56:1128–1137. DOI: 10.1373/clinchem.2009.139931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Vries R, Perton FG, van Tol A, Dullaart RPF. Carotid intima media thickness is related positively to plasma pre ß‐high density lipoproteins in non‐diabetic subjects. Clin Chim Acta. 2012;413:473–477. DOI: 10.1016/j.cca.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13. Hirayama S, Miida T, Miyazaki O, Aizawa Y. Pre beta1‐HDL concentration is a predictor of carotid atherosclerosis in type 2 diabetic patients. Diabetes Care. 2007;30:1289–1291. [DOI] [PubMed] [Google Scholar]
- 14. Baldán A, Tarr P, Lee R, Edwards PA. ATP‐binding cassette transporter G1 and lipid homeostasis. Curr Opin Lipidol. 2006;17:227–232. DOI: 10.1097/01.mol.0000226113.89812.bb. [DOI] [PubMed] [Google Scholar]
- 15. Favari E, Calabresi L, Adorni MP, Jessup W, Simonelli S, Franceschini G, Bernini F. Small discoidal pre‐beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 2009;48:11067–11074. DOI: 10.1021/bi901564g. [DOI] [PubMed] [Google Scholar]
- 16. Hennessy LK, Kunitake ST, Kane JP. Apolipoprotein A‐I‐containing lipoproteins, with or without apolipoprotein A‐II, as progenitors of pre‐beta high‐density lipoprotein particles. Biochemistry. 1993;32:5759–5765. DOI: 10.1021/bi00073a006. [DOI] [PubMed] [Google Scholar]
- 17. Kunitake ST, Mendel CM, Hennessy LK. Interconversion between apolipoprotein A‐I‐containing lipoproteins of pre‐beta and alpha electrophoretic mobilities. J Lipid Res. 1992;33:1807–1816. DOI: 10.1016/S0022-2275(20)41338-0. [DOI] [PubMed] [Google Scholar]
- 18. Rye K‐A, Barter PJ. Formation and metabolism of prebeta‐migrating, lipid‐poor apolipoprotein A‐I. Arterioscler Thromb Vasc Biol. 2004;24:421–428. DOI: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 19. Tall A. Plasma lipid transfer proteins. Annu. Rev Biochem. 1995;64:235–257. [DOI] [PubMed] [Google Scholar]
- 20. Zannis VI, Chroni A, Krieger M. Role of apoA‐I, ABCA1, LCAT, and SR‐BI in the biogenesis of HDL. J Biol Med (Berl). 2006;84:276–294. DOI: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 21. Zannis VI, Fotakis P, Koukos G, Kardassis D, Ehnholm C, Jauhiainen M, Chroni A. HDL biogenesis, remodeling, and catabolism. Handb Exp Pharmacol. 2015;224:53–111. DOI: 10.1007/978-3-319-09665-0_2. [DOI] [PubMed] [Google Scholar]
- 22. O'Connor PM, Naya‐Vigne JM, Duchateau PN, Ishida BY, Mazur M, Schoenhaus SA, Zysow BR, Malloy MJ, Kunitake ST, Kane JP. Measurement of prebeta‐1 HDL in human plasma by an ultrafiltration‐isotope dilution technique. Anal Biochem. 1997;251:234–240. DOI: 10.1006/abio.1997.2258. [DOI] [PubMed] [Google Scholar]
- 23. O'Connor PM, Zysow BR, Schoenhaus SA, Ishida BY, Kunitake ST, Naya‐Vigne JM, Duchateau PN, Redberg RF, Spencer SJ, Mark S, et al. Prebeta‐1 HDL in plasma of normolipidemic individuals: influences of plasma lipoproteins, age, and gender. J Lipid Res. 1998;39:670–678. DOI: 10.1016/S0022-2275(20)33304-6. [DOI] [PubMed] [Google Scholar]
- 24. Kunitake ST, O'Connor P, Naya‐Vigne J. Heterogeneity of high‐density lipoproteins and apolipoprotein A‐I as related to quantification of apolipoprotein A‐I. Method Enzymol. 1996;263:260–267. DOI: 10.1016/s0076-6879(96)63018-3. [DOI] [PubMed] [Google Scholar]
- 25. Asztalos BF, Le Maulf F, Dallal GE, Stein E, Jones PH, Horvath KV, McTaggart F, Schaefer EJ. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high‐density lipoproteins. Am J Cardiol. 2007;99:681–685. DOI: 10.1016/j.amjcard.2006.09.117. [DOI] [PubMed] [Google Scholar]
- 26. Kawano M, Nagasaka S, Yagyu H, Ishibashi S. Pitavastatin decreases plasma prebeta1‐HDL concentration and might promote its disappearance rate in hypercholesterolemic patients. J Atheroscler Thromb. 2008;15:41–46. DOI: 10.5551/jat.E532. [DOI] [PubMed] [Google Scholar]
- 27. Quinn AG, Schwemberger R, Stock EO, Movsesyan I, Axtell A, Chang S, Ishida BY, Malloy MJ, Kane JP, Pullinger CR. Moderate statin treatment reduces prebeta‐1 high‐density lipoprotein levels in dyslipidemic patients. J Clin Lipidol. 2017;11:908–914. DOI: 10.1016/j.jacl.2017.04.118. [DOI] [PubMed] [Google Scholar]
- 28. Bu X‐M, Niu D‐M, Wu J, Yuan Y‐L, Song J‐X, Wang J‐J. Elevated levels of preβ1‐high‐density lipoprotein are associated with cholesterol ester transfer protein, the presence and severity of coronary artery disease. Lipids Health and Dis. 2017;16:4. DOI: 10.1186/s12944-016-0394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. DOI: 10.1016/S0022-2275(20)39898-9. [DOI] [PubMed] [Google Scholar]
- 30. Castro GR, Fielding CJ. Early incorporation of cell‐derived cholesterol into pre‐beta‐migrating high‐density lipoprotein. Biochemistry. 1988;27:25–29. DOI: 10.1021/bi00401a005. [DOI] [PubMed] [Google Scholar]
- 31. Huuskonen J, Olkkonen VM, Jauhiainen M, Ehnholm C. The impact of phospholipid transfer protein (PLTP) on HDL metabolism. Atherosclerosis. 2001;155:269–281. DOI: 10.1016/S0021-9150(01)00447-6. [DOI] [PubMed] [Google Scholar]
- 32. Barrans A, Collet X, Barbaras R, Jaspard B, Manent J, Vieu C, Chap H, Perret B. Hepatic lipase induces the formation of pre‐ß1 high density lipoprotein (HDL) from triacylglycerol‐rich HDL2. A study comparing liver perfusion to in vitro incubation with lipases. J Biol Chem. 1994;269:11572–11577. [PubMed] [Google Scholar]
- 33. Maugeais C, Tietge UJF, Broedl UC, Marchadier D, Cain W, McCoy MG, Lund‐Katz S, Glick JM, Rader DJ. Dose‐dependent acceleration of high‐density lipoprotein catabolism by endothelial lipase. Circulation. 2003;108:2121–2126. DOI: 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- 34. Christoffersen C, Jauhiainen M, Moser M, Porse B, Ehnholm C, Boesl M, Dahlbäck B, Nielsen LB. Effect of apolipoprotein m on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock‐out mice. J Biol Chem. 2008;283:1839–1847. DOI: 10.1074/jbc.M704576200. [DOI] [PubMed] [Google Scholar]
- 35. Daerr WH, Minzlaff U, Greten H. Quantitative determination of apolipoprotein A‐I in high‐density lipoproteins and 'free' apolipoprotein A‐I by two‐dimensional agarose gel lipoprotein‐'rocket' immunoelectrophoresis of human serum. Biochim Biophys Acta. 1986;879:134–139. DOI: 10.1016/0005-2760(86)90095-0. [DOI] [PubMed] [Google Scholar]
- 36. Stock EO, Ferrara CT, O'Connor PM, Naya‐Vigne JM, Frost PH, Malloy MJ, Kane JP, Pullinger CR. Levels of prebeta‐1 high‐density lipoprotein are elevated in 3 phenotypes of dyslipidemia. J Clin Lipidol. 2018;12:99–109. DOI: 10.1016/j.jacl.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 37. Mendivil CO, Furtado J, Morton AM, Wang L, Sacks FM. Novel pathways of apolipoprotein A‐I metabolism in high‐density lipoprotein of different sizes in humans. Arterioscler Thromb Vasc Biol. 2016;36:156–165. DOI: 10.1161/ATVBAHA.115.306138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de la Llera‐Moya M, Drazul‐Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high‐density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. DOI: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, et al. HDL cholesterol efflux capacity and incident cardiovascular events. New Engl J Med. 2014;371:2383–2393. DOI: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case‐control study. Lancet Diabetes Endocrinol. 2015;3:507–513. DOI: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shea S, Stein JH, Jorgensen NW, McClelland RL, Tascau L, Shrager S, Heinecke JW, Yvan‐Charvet L, Tall AR. Cholesterol mass efflux capacity, incident cardiovascular disease, and progression of carotid plaque. Arterioscler Thromb Vasc Biol. 2019;39:89–96. DOI: 10.1161/ATVBAHA.118.311366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuusisto S, Holmes MV, Ohukainen P, Kangas AJ, Karsikas M, Tiainen M, Perola M, Salomaa V, Kettunen J, Ala‐Korpela M. Direct estimation of HDL‐mediated cholesterol efflux capacity from serum. Clin Chem. 2019;65:1042–1050. DOI: 10.1373/clinchem.2018.299222. [DOI] [PubMed] [Google Scholar]
- 43. Asztalos BF, Horvath KV, Schaefer EJ. High‐density lipoprotein particles, cell‐cholesterol efflux, and coronary heart disease risk. Arterioscler Thromb Vasc Biol. 2018;38:2007–2015. DOI: 10.1161/ATVBAHA.118.311117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Westerterp M, Tall AR. A new pathway of macrophage cholesterol efflux. Proc Natl Acad Sci USA. 2020;117:11853–11855. DOI: 10.1073/pnas.2007836117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


