Abstract
Background
We investigated the clinical significance of derivatives of reactive oxygen metabolites (DROMs), a new marker of reactive oxygen species, in patients with nonischemic heart failure (HF) and compared them among new categories of HF.
Methods and Results
We recruited 201 consecutively hospitalized patients with HF and measured DROM under stable conditions. Then, we divided them according to new categories of HF (HF with reduced ejection fraction [EF], HF with midrangeEF, and HF with preserved EF) without coronary artery disease. In subgroup analysis, we followed EF changes in patients with HF with reduced EF and classified them into HF with recovered EF or nonrecovered EF according to whether EF had improved to >40%. DROMs are significantly and independently associated with HF‐related events in patients with NIHF. There were no significant differences in DROM and the probability of HF‐related events among HF categories in Kaplan–Meier analysis. However, patients with HF with reduced EF and HF with preserved EF but not HF with midrange EF with HF‐related events had higher DROM than those without HF‐related events. In subgroup analysis, Kaplan–Meier analysis demonstrated that the probabilities of HF‐related events in HF with recovered EF were dramatically decreased. DROM were significantly higher in patients with HF with nonrecovered EF than in HF with recovered EF. In receiver operating characteristic analysis, the cutoff level of DROM for predicting improvements in HF with recovered EF was 347 Carratelli units. Furthermore, the C‐statistic value for predicting EF improvement for the DROM levels was 0.703. In multivariable logistic regression analysis, DROM was independently and significantly associated with the prediction of HF with recovered EF.
Conclusions
DROM measurements can provide important prognostic information for risk stratification in any category of NIHF.
Registration
URL: https://www.umin.ac.jp/ctr/; Unique identifier: UMIN000035827.
Keywords: follow‐up studies, nonischemic heart failure, prognosis, reactive oxygen species
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- CHART‐2
Chronic Heart Failure Analysis and Registry in the Tohoku District‐2
- DROMs
derivatives of reactive oxidative metabolites
- HFmrEF
heart failure with midrange ejection fraction
- HFnonrecEF
heart failure with nonrecovered ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrecEF
heart failure with recovered ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- NIHF
nonischemic heart failure
- ROS
reactive oxygen species
- U.CARR
Carratelli unit
Clinical Perspective
What Is New?
Derivatives of reactive oxidative metabolites, a new marker of reactive oxygen species, are significantly associated with heart failure (HF)‐related events in patients with nonischemic HF.
Derivatives of reactive oxidative metabolites significantly correlated with ejection fraction improvement in patients with nonischemic HF with reduced ejection fraction.
What Are the Clinical Implications?
Derivatives of reactive oxidative metabolites could provide important prognostic information for predicting HF‐related events in all phenotypes of nonischemic HF and therapeutic responders, especially in patients with HFrEF.
Until the 1990s, left ventricular (LV) systolic dysfunction (heart failure [HF] with reduced LV ejection fraction [EF; HFrEF]) was regarded as the main target of HF treatment, but since the 2000s, as the number of elderly patients with HF has increased, HF with preserved LVEF (HFpEF) has received increasing attention. Furthermore, in the 2010s, patients with an LVEF in the range of 40% to 49% represented a “gray area” and were defined as having HF with midrange EF (HFmrEF), and HFmrEF was considered separately from HFrEF and HFpEF because of the clinical differences. 1 Although LVEF is initially reduced when HF symptoms develop, cases in which the LVEF improves with treatment and time (HF with recovered EF [HFrecEF]) have also been noted. 2 Thus, the definition of HF according to LVEF has changed over time. Understanding the pathology of each type of HF and the development of appropriate interventions are considered important issues in clinical settings.
Previous clinical reports have shown increased levels of reactive oxygen species (ROS) in the failing heart and the potential importance of oxidative stress. 3 , 4 , 5 Moreover, several biomarkers of ROS provide important information on the pathogenesis, risk stratification, and diagnosis of HF and on the efficacy of treatment. 4 , 6 Recently, the serum levels of reactive oxidative metabolites were recently shown to be a reliable ROS biomarker in various diseases. 7 , 8 Measurements of the derivatives of reactive oxidative metabolites (DROMs) provide a direct and simple assay of total oxidant capacity, mainly hydroperoxide. 9 We previously reported that DROMs might provide clinical benefits for risk stratification of HFpEF, HFrEF, and coronary artery disease. 10 , 11 , 12 Also, the efficacy of the DROM test as a predictor of initial HF hospitalization in elderly patients with congestive HF had already reported. 13 However, the comparison of oxidative stress among the above‐mentioned new HF category remains still unclear. In the present study, hence, we tested the hypothesis that the oxidative stress level, indicated by the serum DROM concentration, is associated with the presence and severity of various types of HF based on LVEF. Because the differentiation of patients with HF based on LVEF is considered important because of differences in underlying etiologies, demographics, comorbidities, and responses to therapies, 1 we also investigated whether DROM is a useful prognostic biomarker for predicting HF‐related events and EF improvements in patients with HF without coronary artery disease.
Methods
The authors declare that all supporting data are available within the article.
Study Subjects and Protocol
In this study, we defined patients with nonischemic HF (NIHF) as patients with symptoms suspicious of HF and with no significant coronary artery stenosis (>75%), which was confirmed by coronary angiography or coronary computed tomography angiography, and we enrolled only patients with NIHF. We retrospectively investigated 1107 consecutive patients with NIHF who were hospitalized in the Kumamoto University Hospital between January 2007 and December 2017 and recorded each patient's medical history and relevant clinical characteristics. We excluded 906 patients for the following reasons: severe valvular disease (n=353), end‐stage renal disease (estimated glomerular filtration ratio <15 mL/min per 1.73 m2) (n=82), a history of malignancy (n=49), active infective disease (n=14), and failure to meet the diagnostic criteria of various HF categories described below (n=406). We enrolled 201 patients with NIHF and followed them from the date of DROM measurement at admission, for 40 months, until June 2018, or until the occurrence of HF‐related events. The oxidative status of the patients was determined by measuring the serum DROM concentration in patients with NIHF under stable conditions. We further divided them into 3 groups (HFrEF, n=79; HFmrEF, n=35; and HFpEF, n=87) according to the new categories of HF and compared the clinical characteristics and prognosis.
Furthermore, we focused on patients with HFrEF and followed LVEF changes. We excluded 8 patients because of a lack of echocardiography data (n=6) and death before echocardiography follow‐up (n=2), and we evaluated the LVEF value at least 6 months after optimal medical therapy. Then, we classified patients with HFrEF into HFrecEF (n=46) and HF with nonrecovered EF (HFnonrecEF) (n=26), as described below, according to LVEF improvements and compared the clinical characteristics and prognosis (Figure 1).
Figure 1. Flowchart showing the enrollment protocol.
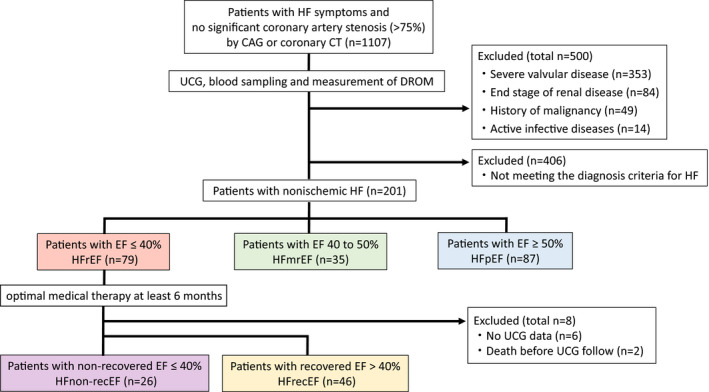
CAG indicates coronary angiography; CT, computed tomography; DROM, derivatives of reactive oxidative metabolites; EF, ejection fraction; HF, heart failure; HFmrEF, HF with midrange EF; HFnonrecEF, HF with nonrecovered EF; HFpEF, HF with preserved EF; HFrecEF, HF with recovered EF; HFrEF, HF with reduced EF; and UCG, ultrasonic echocardiography.
All procedures were conducted in accordance with the Declaration of Helsinki and its amendments. The study protocol was approved by the institutional review board of Kumamoto University (approval number: Senshin 2225). This study is registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000035827). The requirement for informed consent was waived because of the low‐risk nature of this retrospective study and the inability to directly obtain consent from all subjects. Instead, the study protocol was extensively promoted at Kumamoto University Hospital and on the website (https://kumadai‐junnai.com/wp‐content/uploads/houkatsu.pdf); notably, patients were provided the opportunity to withdraw from the study.
Clinical Parameters
The baseline demographic data, cardiovascular risk factors, and medications upon admission were documented. Hypertension was defined as a recorded blood pressure >140/90 mm Hg or the use of any antihypertensive medications. Diabetes mellitus was defined as the presence of symptoms of diabetes mellitus and a random plasma glucose concentration ≥200 mg/dL, a fasting plasma glucose concentration ≥126 mg/dL, and a 2‐hour plasma glucose concentration ≥200 mg/dL according to a 75‐g oral glucose tolerance test or the use of any medications for diabetes mellitus. Dyslipidemia was defined as a low‐density lipoprotein level ≥140 mg/dL (≥3.63 mmol/L), a high‐density lipoprotein level <40 mg/dL (1.04 mmol/L), or a triglyceride level ≥150 mg/dL (≥1.7 mmol/L) or the use of any medications for dyslipidemia. Chronic kidney disease was defined as was an estimated glomerular filtration rate <60 mL/min per 1.73 m2. 14 A history of smoking was determined via interview.
Echocardiography and Blood Sampling
Echocardiography was performed under stable conditions upon admission by experienced cardiac sonographers who had no knowledge of the study data. LVEF was measured using a modified Simpson's method. The ratio of early transmitral flow velocity to early diastolic mitral annular velocity (E/e′) was assessed by tissue Doppler imaging. Echocardiography was performed using the Vivid 7 or Vivid E9 (GE Vingmed, Horten, Norway), Aplio XG (Toshiba, Tokyo, Japan), and Epiq 7 (Philips, Bothell, WA) systems equipped with a 2.5‐MHz phased‐array transducer, as previously reported. 15
We performed a blood test early in the morning with patients in the fasting state before taking any medications. Blood tests were performed to measure levels of plasma BNP (B‐type natriuretic peptide), high‐sensitivity troponin T, serum hs‐CRP (high‐sensitivity C‐reactive protein), and other biochemical markers. The blood samples were kept frozen at −80°C until analysis.
Assessment of Serum DROM
The DROM test has been described previously. 16 , 17 Serum DROM levels, reflecting total oxidant capacity, were measured in all patients under stable conditions using an automated method (DROM test; Diacron srl, Grosseto, Italy) and a free‐radical elective evaluator (F.R.E.E.; Diacron srl). The total oxidant capacity is composed of hydroperoxide, ferroxidase, and myeloperoxidase activities. Accordingly, the DROM test detects ROS produced by metabolic and inflammatory activities. Specifically, oxidation of the chromogenic substrate N,N‐diethyl‐para‐phenylenediamine by radicals generated mainly from hydroperoxide is detected spectrophotometrically. Measurements are expressed as an arbitrary unit, the Carratelli unit (U.CARR). The DROM test was measured using the assay kit's manufacturer (Diacron srl) with intra‐ and interassay coefficient of variations of 2.07% and 1.79%. Normal reference levels of DROM as stated by the assay kit's manufacturer are 250 to 300 U.CARR. 16 , 17
Definition of Various Categories of HF and HF‐Related Events
We defined various categories of HF clinically according to the criteria of the European Working Group as follows. 1 HFrEF: (1) symptoms of HF and (2) reduced LV systolic function (LVEF <40%); HFmrEF: (1) symptoms of HF, (2) mildly reduced LV systolic function (40%–50% of LVEF), (3) elevated levels of natriuretic peptides (BNP >35 pg/mL), and (4) evidence of abnormal LV diastolic dysfunction (E/e′ ≥13); HFpEF: (1) symptoms of HF, (2) normal or mildly reduced LV systolic function (LVEF ≥50%), (3) BNP >35 pg/mL, and (4) E/e′ ≥13. We excluded patients with HFmrEF and HFpEF who had a confirmed reduction in EF in the past. Furthermore, among patients with HFrEF who had undergone optimal medical therapy for at least 6 months according to the Japanese Circulation Society guidelines for the treatment of HF, we defined HF with EF improvement of >40% as HFrecEF, and HF with EF <40% as HFnonrecEF. We defined HF‐related events as hospitalization for HF decompensation or as ventricular assist device placement.
Definition and Etiology of HF
HF‐related events were ascertained from a review of medical records and were confirmed by direct contact with patients, their families, or their physicians or via an annual telephone interview conducted with each patient. Hospitalization for HF decompensation was defined as admission of a patient with symptoms typical of HF who had objective signs of worsening HF. During their hospitalization, all patients with HF were under optimal medical therapy for HF according to the European Society of Cardiology guideline 18 and the Japanese Circulation Society guidelines, including stable doses of an angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker, a β‐blocker, and an aldosterone blocker, if not contraindicated. Physicians confirmed that patients had HF by determining the New York Heart Association functional class, 19 and the quality of life was assessed under stable conditions after optimal therapy by the standard questionnaire.
The etiology of HF was diagnosed on the basis of the Japanese Circulation Society guidelines. The etiology was as follows: dilated cardiomyopathy, hypertrophic cardiomyopathy, hypertensive heart disease, cardiac amyloidosis, cardiac sarcoidosis, valvular heart disease, arrhythmogenic cardiomyopathy, and others (mitochondrial cardiomyopathy, alcoholic cardiomyopathy, diabetic cardiomyopathy, LV noncompaction, puerperal cardiomyopathy, and collagen disease–related cardiomyopathy).
Statistical Analysis
Statistical Package for Social Science version 22.0 (IBM Japan, Tokyo, Japan) software was used for the statistical analyses. Nonnormally distributed data are expressed as the median (interquartile range), and P<0.05 was considered statistically significant. Data from multiple groups were compared by the chi‐squared test, 1‐way ANOVA, or the Kruskal‐Wallis test. Differences between the 2 groups were assessed by the chi‐squared test for categorical variables, and Student unpaired t test or the Mann‐Whitney U‐test (as appropriate) was used to analyze differences between groups for continuous variables. Kaplan–Meier curves were used to determine the cumulative incidence of HF‐related events, and log‐rank tests were used to compare the incidence of HF‐related events between groups. The Cox proportional hazard model was used to estimate the HF‐related event hazard ratio and its 95% CI in patients with NIHF by univariate and multivariable analysis with forward stepwise method. Significant clinical parameters associated with HF‐related events in univariate Cox hazard analysis were entered into multivariable Cox hazard analysis. We calculated the Akaike's information criterion to evaluate the suitability of the multivariate model. Receiver operating characteristic curves were constructed to analyze the value of DROM for the prediction of HFrecEF. The area under the curve, sensitivity, and specificity were calculated for the predictive value of DROM with regard to EF recovery in patients with HFrEF. We defined the cutoff value of DROM for the prediction of HFrecEF by maximizing the sum of the sensitivity and specificity by using Youden's J statistic. 20 Logistic regression analysis was performed to identify significant parameters related to EF recovery in patients with HFrEF. Significant clinical parameters associated with EF recovery in univariate logistic regression analysis were entered into multivariable logistic regression analysis. Multivariable logistic regression analysis was then performed using the forward stepwise method. The Hosmer–Lemeshow test was applied to assess model calibration.
Results
Baseline Characteristics and Follow‐Up of Patients With NIHF
The study population included 201 patients with NIHF. The mean time from the first day of hospitalization to the day of the study evaluation was median 8.0 days (interquartile range, 3.0–14.0 days). We calculated the median follow‐up period for each patient. The median follow‐up period was 31.5 months (interquartile range, 9.8–40 months), and 37 HF‐related events were recorded. Baseline characteristics of patients with NIHF are shown in Table 1. Patients with NIHF were classified into the low‐DROM (≤354 U.CARR; n=101) and high‐DROM (>354 U.CARR, n=100) groups using the median value of DROM. Patients with NIHF in the high‐DROM group had higher BNP and hs‐CRP levels than those in the low‐DROM group (P=0.005 and P<0.001, respectively) Kaplan–Meier analysis showed that the high‐DROM group had a higher probability of HF‐related events than the low‐DROM group (P=0.001 by log‐rank test; Figure 2A). DROM levels were significantly higher in patients with NIHF with HF‐related events than in those without HF‐related events (411 [350–506] U.CARR versus 348 [300–397] U.CARR; P<0.001; Figure 2B). Futhermore, DROM levels were significantly higher in patients with NIHF with New York Heart Association class III/IV than in those with class II (344 [294–384] versus 399 [344–467] U.CARR, respectively; P<0.001) (data not shown).
Table 1.
Baseline Characteristics of Patients With NIHF
| All Patients With NIHF (n=201) | Low DROM (n=101) | High DROM (n=100) | P Value | |
|---|---|---|---|---|
| Age, y | 65.0±13.3 | 66.6±12.7 | 63.3±13.8 | 0.080 |
| Male sex (%) | 118 (58.7) | 65 (64.4) | 53 (53.0) | 0.102 |
| Body mass index, kg/m2 | 24.4±4.9 | 23.4±3.9 | 25.5±5.5 | 0.002 |
| Hypertension (%) | 125 (62.2) | 65 (64.4) | 60 (60.0) | 0.524 |
| Diabetes mellitus (%) | 57 (28.4) | 20 (19.8) | 37 (37.0) | 0.007 |
| Dyslipidemia (%) | 100 (49.8) | 47 (46.5) | 53 (53.0) | 0.359 |
| History of smoking (%) | 99 (49.3) | 55 (54.5) | 44 (44.0) | 0.138 |
| NYHA III or IV (%) | 68 (33.8) | 21 (20.8) | 47 (47.0) | <0.001 |
| DROM, U.CARR | 354 [307–441] | 308 [277–339] | 411 [379–455] | <0.001 |
| BNP, pg/mL | 168 [74–403] | 154 [64–249] | 206 [92–466] | 0.005 |
| eGFR, mL/min per 1.73 m2 | 62.1±16.2 | 62.9±15.0 | 61.2±17.3 | 0.465 |
| Hemoglobin, g/dL | 13.7±2.1 | 13.9±2.0 | 13.5±2.1 | 0.167 |
| Hs‐CRP, mg/dL | 0.08 [0.03–0.24] | 0.05 [0.03–0.09] | 0.13 [0.06–0.38] | <0.001 |
| Hs‐troponinT, ng/L | 18.7 [9.7–34.6] | 15.9 [9.1–28.3] | 19.2 [10.2–38.4] | 0.319 |
| Serum sodium, mEq/L | 140.0±2.9 | 140.3±2.8 | 139.7±3.0 | 0.162 |
| LVEF, % | 46.5±16.3 | 48.1±16.1 | 44.9±16.5 | 0.166 |
| LVDd, mm | 50.6±11.1 | 48.5±9.6 | 52.7±12.2 | 0.008 |
| LAD, mm | 41.8±8.0 | 40.0±8.2 | 43.6±7.3 | 0.001 |
| E/e′ | 17.8±7.5 | 16.8±5.4 | 18.9±9.0 | 0.043 |
| CCB (%) | 60 (29.9) | 30 (29.7) | 30 (30.0) | 0.963 |
| ACEI or ARB (%) | 133 (66.2) | 65 (64.4) | 68 (68.0) | 0.585 |
| β‐blockers (%) | 126 (62.7) | 61 (60.4) | 65 (65.0) | 0.500 |
| Diuretics (%) | 109 (54.2) | 48 (47.5) | 61 (61.0) | 0.055 |
| Statins (%) | 58 (28.9) | 25 (24.8) | 33 (33.0) | 0.197 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CCB, calcium channel blocker; DROM, derivatives of reactive oxidative metabolites; E/e′, ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity; HF, heart failure; hs‐CRP, high‐sensitivity C‐reactive protein; LAD, left atrium diameter; LVDd, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NIHF, nonischemic heart failure; OR, odds ratio; and U.CARR, Carratelli unit.
Figure 2. Follow‐up analysis in 201 patients with NIHF.
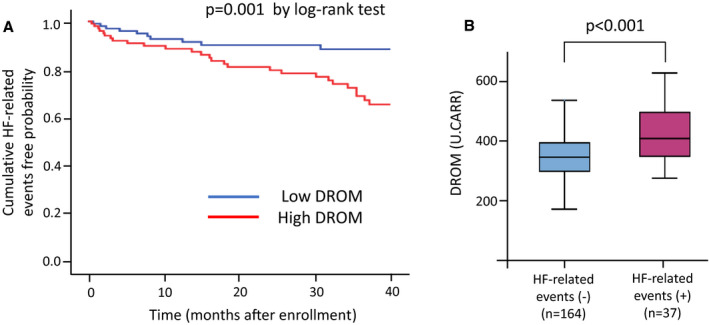
A, Kaplan–Meier analysis of probability of HF‐related events in patients with NIHF with low or high DROM value. B, Serum DROM levels without or with HF‐related events. A, Using the median value of DROM (354 U.CARR), patients with HFrEF were divided into 2 groups. Kaplan–Meier analysis showed a significantly higher probability of HF‐related events in patients with NIHF in the high‐DROM group than in those in the low‐DROM group (P=0.001 by log‐rank test). B, DROM levels were significantly higher in patients with NIHF with HF‐related events than in those without HF‐related events (P<0.001 by Mann–Whitney U test). DROM indicates derivatives of reactive oxidative metabolites; HF, heart failure; and U.CARR, Carratelli unit.
Cox Proportional Hazard Analysis of HF‐Related Events
The results of univariate and multivariable regression Cox proportional hazard analysis for HF‐related events are shown in Table 2. A univariate Cox hazard analysis identified 9 variables as significant predictors (body mass index, DROM, log‐transformed BNP, log‐transformed hs‐CRP, hemoglobin, log‐transformed high‐sensitivity troponinT, serum sodium, left atrial diameter, and E/e′). In consideration of the internal correlations of DROM with log‐transformed BNP (r=0.238; P=0.001), with hs‐CRP (r=0.465; P<0.001), we excluded BNP and hs‐CRP from multivariable Cox hazard analysis. In a multivariable Cox hazard analysis including significant predictors in univariate Cox hazard analysis by forward stepwise methods, DROM was independently and significantly associated with HF‐related events (hazard ratio, 1.004; 95% CI, 1.000–0.008; P=0.030). The Akaike's information criterion value of DROM was 370.8 in univariate model and 345.0 in multivariate model.
Table 2.
Cox Hazard Analysis of HF‐Related Events in Patients With NIHF
| Variables | Cording | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Age | Per 1 y | 0.995 | 0.971–1.018 | 0.649 | … | ||
| Sex | Male (vs female) | 0.661 | 0.347–1.261 | 0.209 | … | ||
| Body mass index | Per 1 kg/m2 | 1.089 | 1.023–1.159 | 0.008 | Not selected | ||
| Hypertension | Yes (vs no) | 0.714 | 0.373–1.369 | 0.311 | … | ||
| Diabetes mellitus | Yes (vs no) | 1.013 | 0.490–2.092 | 0.973 | … | ||
| Dyslipidemia | Yes (vs no) | 0.877 | 0.460–1.671 | 0.689 | … | ||
| History of smoking | Yes (vs no) | 0.824 | 0.430–1.579 | 0.559 | … | ||
| Chronic kidney disease | Yes (vs no) | 1.059 | 0.549–2.042 | 0.864 | … | ||
| DROM | Per 1 U.CARR | 1.008 | 1.005–1.012 | <0.001 | 1.004 | 1.000–1.008 | 0.030 |
| BNP | Per 1 ln‐BNP | 2.452 | 1.703–3.531 | <0.001 | … | ||
| Hemoglobin | Per 1 g/dL | 0.799 | 0.684–0.933 | 0.005 | Not selected | ||
| Hs‐CRP | Per 1 ln‐hs‐CRP | 1.694 | 1.342–2.140 | <0.001 | … | ||
| Hs‐troponinT | Per 1 ln‐hs‐troponinT | 2.097 | 1.609–2.733 | <0.001 | 2.336 | 1.646–3.313 | <0.001 |
| Serum sodium | Per 1 mEq/L | 0.871 | 0.784–0.967 | 0.010 | Not selected | ||
| LVEF | Per 1 % | 0.985 | 0.964–1.005 | 0.143 | … | ||
| LVDd | Per 1 mm | 1.027 | 0.996–1.059 | 0.092 | … | ||
| LAD | Per 1 mm | 1.062 | 1.014–1.112 | 0.011 | 1.058 | 1.009–1.109 | 0.019 |
| E/e′ | Per 1 | 1.035 | 1.007–1.062 | 0.012 | Not selected | ||
BNP indicates B‐type natriuretic peptide; DROM, derivatives of reactive oxidative metabolites; E/e′, the ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity; HF, heart failure; Hs‐CRP, high‐sensitivity C‐reactive protein; Hs‐troponinT, high‐sensitivity troponin T; LAD, left atrial diameter; LVDd, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NIHF, nonischemic heart failure; and OR, odds ratio.
Follow‐Up and Comparison of Baseline Characteristics Among HF Categories
There were no differences in the frequency of HF‐related events among the HF categories (HFrEF; n=16, 20.3% versus HFmrEF; n=7, 20.0% versus HFpEF; n=14, 16.1%; P=0.760). Kaplan–Meier analysis revealed that there were no significant differences in the probability of HF‐related events among HF categories (P=0.796 by log‐rank test; Figure 3).
Figure 3. Kaplan–Meier analysis of the probability of HF‐related events in patients with HFrEF, HFmrEF and HFpEF.

Kaplan–Meier analysis revealed that there were no significant differences in the probability of HF‐related events among HF categories (P=0.796 by log‐rank test). EF indicates ejection fraction; HF, heart failure; HFmrEF, HF with midrange EF; HFpEF, HF with preserved EF; and HFrEF, HF with reduced EF.
A comparison among baseline characteristics of each HF category is shown in Table 3. Patients with HFrEF were significantly younger and more frequently had a history of smoking and New York Heart Association classifications of III and IV than patients with both HFmrEF and HFpEF. Patients with HFrEF also constituted a significantly higher percentage of men, a lower prevalence of hypertension, and higher levels of hemoglobin than patients with HFpEF. Patients with HFpEF had a higher percentage of calcium channel blocker usage as well as a lower percentage of diuretic, renin‐angiotensin system inhibitor, and β‐blocker usage than patients with HFrEF and HFmrEF. Regarding etiology, patients with HFrEF were mostly patients with dilated cardiomyopathy (62.0%), while patients with HFpEF were mostly patients with hypertrophic cardiomyopathy (31.0%) and hypertensive heart disease (14.9%). There was no significant difference in DROM levels among HF categories. However, we compared the DROM levels between those with or without HF events in each HF category and found that the DROM levels in patients with HFrEF and HFpEF with HF‐related events were significantly higher than those in patients without HF‐related events (P=0.003 and P<0.001, respectively; Figure 4).
Table 3.
Baseline Characteristics of HFrEF, HFmrEF and HFpEF Patients
| HFrEF (n=79) | HFmrEF (n=35) | HFpEF (n=87) | P Value | |
|---|---|---|---|---|
| Age, y | 60.3±14.4* , * | 67.7±13.1 | 68.0±11.1 | 0.001 |
| Male sex (%) | 61 (77.2) † | 21 (60.0) | 36 (41.4) | <0.001 |
| Body mass index, kg/m2 | 23.9±5.1 | 25.1±5.0 | 24.7±4.6 | 0.437 |
| Hypertension (%) | 36 (45.6) † | 21 (60.0) | 68 (78.2) | <0.001 |
| Diabetes mellitus (%) | 26 (32.9) | 9 (25.7) | 22 (25.3) | 0.518 |
| Dyslipidemia (%) | 30 (38.0) | 18 (51.4) | 52 (59.8) | 0.019 |
| History of smoking (%) | 57 (72.2)* , * | 16 (45.7) | 26 (29.9) | <0.001 |
| NYHA III or IV (%) | 41 (51.9)* , * | 8 (22.9) | 19 (21.8) | <0.001 |
| DROM, U.CARR | 362 [309–432] | 358 [330–417] | 348 [284–401] | 0.116 |
| BNP, pg/mL | 182 [75–486] | 178 [99–413] | 149 [68–292] | 0.254 |
| eGFR, mL/min per 1.73 m2 | 62.6±16.2 | 58.4±13.5 | 63.1±15.5 | 0.333 |
| Hemoglobin, g/dL | 14.2±2.1 † | 13.8±2.0 | 13.2±2.0 | 0.004 |
| Hs‐CRP, mg/dL | 0.12 [0.04–0.36] | 0.06 [0.03–0.21] | 0.08 [0.03–0.13] | 0.190 |
| Hs‐troponinT, ng/L | 19.4 [10.0–38.2] | 18.6 [9.9–32.3] | 18.0 [8.7–31.9] | 0.234 |
| Serum sodium, mEq/L | 139.3±3.3 | 140.5±2.6 | 140.4±2.4 | 0.023 |
| LVEF, % | 29.3±7.1* , * | 44.9±2.9 † | 62.8±5.0 | <0.001 |
| LVDd, mm | 58.7±10.5* , * | 52.3±7.7 † | 42.6±6.1 | <0.001 |
| LAD, mm | 42.0±9.1 | 41.2±7.1 | 41.8±7.2 | 0.901 |
| E/e′ | 15.6±6.3* , * | 18.3±5.1 | 19.7±8.7 | 0.002 |
| CCB (%) | 15 (19.0) † | 5 (14.3) † | 40 (46.0) | <0.001 |
| ACEI or ARB (%) | 63 (79.7) † | 28 (80.0) † | 42 (48.3) | <0.001 |
| β‐blockers (%) | 59 (74.7) † | 28 (80.0) † | 39 (44.8) | <0.001 |
| Diuretics (%) | 60 (75.9) † | 20 (57.1) | 29 (33.3) | <0.001 |
| Statins (%) | 16 (20.3) | 11 (31.4) | 31 (35.6) | 0.079 |
| Etiology of heart failure | ||||
| Dilated cardiomyopathy | 49 (62.0)* , * | 11 (31.4) † | 2 (2.3) | <0.001 |
| Hypertrophic cardiomyopathy | 1 (1.3) † | 4 (11.4) † | 27 (31.0) | <0.001 |
| Hypertensive heart disease | 9 (11.4) | 2 (5.7) | 13 (14.9) | 0.251 |
| Cardiac amyloidosis | 2 (2.5) | 6 (17.1) | 7 (8.0) | 0.045 |
| Cardiac sarcoidosis | 1 (1.3) | 2 (5.7) | 2 (2.3) | 0.546 |
| Valvular heart diseases | 4 (5.1) | 2 (5.7) | 7 (8.0) | 0.726 |
| Arrhythmogenic cardiomyopathy | 4 (5.1) | 2 (5.7) | 7 (8.0) | 0.726 |
| Others | 9 (11.4) | 6 (17.1) | 22 (25.3) | 0.069 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CCB, calcium channel blocker; DROM, derivatives of reactive oxidative metabolites; E/e′, ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity; EF, ejection fraction; eGFR, estimated glomerular filtration ratio; HF, heart failure; HFmrEF, HF with midrange EF; HFpEF, HF with preserved EF; HFrEF, HF with reduced EF; Hs‐CRP, high‐sensitivity C‐reactive protein; Hs‐troponinT, high‐sensitivity troponin T; LAD, left atrial diameter; LVDd, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; and U.CARR, Carratelli unit.
P<0.05 compared with HFmrEF group.
P<0.05 compared with HFpEF group.
Figure 4. Serum DROM levels between patients with and without HF‐related events in each HF category.
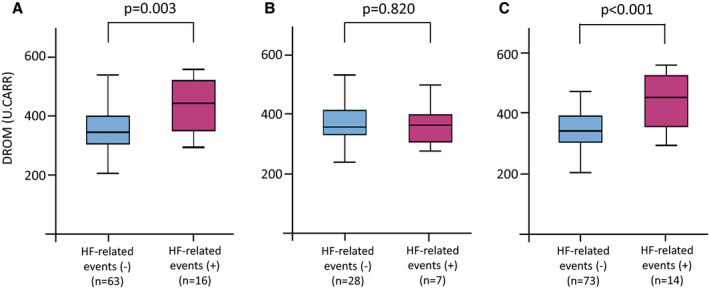
A, HFrEF, (B) HFmrEF, (C) HFpEF. DROM levels in patients with HFrEF and HFpEF but not patients with HFmrEF with HF‐related events were significantly higher than those in patients without HF‐related events (P<0.003, P=0.820, and P<0.001, respectively, by Mann–Whitney U test). EF indicates ejection fraction; HF, heart failure; HFmrEF, HF with midrange EF; HFpEF, HF with preserved EF; and HFrEF, HF with reduced EF.
Follow‐Up and Baseline Characteristics HFnonrecEF and HFrecEF
The frequency of HF‐related events was significantly higher in the patients with HFnonrecEF than in the patients with HFrecEF (n=12, 46.2% versus n=3, 6.5%; P<0.001). Kaplan–Meier analysis revealed that patients with HFrecEF had a significantly and dramatically lower probability of HF events than did patients with HFnon‐recEF (P<0.001 by log‐rank test; Figure 5).
Figure 5. Kaplan–Meier analysis for the probability of HF‐related events in patients with HFrecEF and HFnonrecEF.
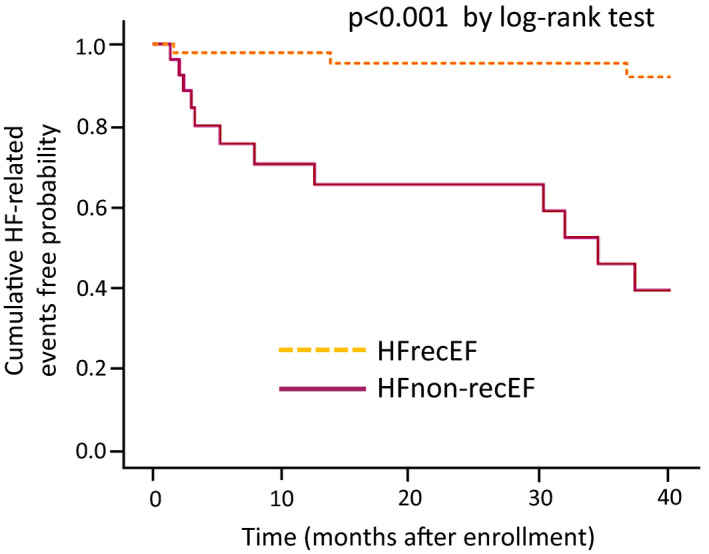
Kaplan–Meier analysis revealed that patients with HFrecEF had a significantly lower probability of HF events than did patients with HFnonrecEF (P<0.001 by log‐rank test). HF indicates heart failure; HFnonrecEF, HF with nonrecovered EF; and HFrecEF, HF with recovered EF.
Table 4 shows the comparison among baseline characteristics of patients with HFnonrecEF (n=26) and HFrecEF (n=46). The DROM levels were significantly higher in patients with HFnonrecEF than in patients with HFrecEF (395 [351–478] U.CARR versus 335 [304–392] U.CARR; P=0.004). The BNP levels were significantly lower, and the serum sodium and estimated glomerular filtration rate levels were higher in patients with HFrecEF than in patients with HFnonrecEF. Patients with HFrecEF showed a significantly higher LVEF before treatment and a lower LV end‐diastolic dimension than patients with HFnonrecEF. No significant difference was observed in the rate of drug usage at the moment of DROM measurement.
Table 4.
Baseline Characteristics of Patients With HFnonrecEF and HFrecEF
| HFnonrecEF (n=26) | HFrecEF (n=46) | P Value | |
|---|---|---|---|
| Age, y | 60.8±13.7 | 60.6±14.6 | 0.968 |
| Male sex (%) | 20 (76.9) | 35 (76.1) | 0.936 |
| BMI, kg/m2 | 23.3±4.6 | 24.0±5.5 | 0.548 |
| Hypertension (%) | 7 (26.9) | 26 (56.5) | 0.015 |
| Diabetes mellitus (%) | 7 (26.9) | 13 (28.3) | 0.903 |
| Dyslipidemia (%) | 8 (30.8) | 20 (43.5) | 0.288 |
| History of smoking (%) | 20 (76.9) | 33 (71.7) | 0.632 |
| NYHA III or IV (%) | 19 (73.1) | 18 (39.1) | 0.006 |
| DROM, U.CARR | 395 [351–478] | 335 [304–392] | 0.004 |
| BNP, pg/mL | 395 [163–680] | 147 [52–287] | 0.001 |
| eGFR, mL/min per 1.73 m2 | 54.8±19.2 | 66.4±16.1 | 0.008 |
| Hemoglobin, g/dL | 14.4±2.1 | 14.5±1.8 | 0.930 |
| Hs‐CRP, mg/dL | 0.26 [0.11–0.36] | 0.05 [0.03–0.21] | 0.002 |
| Hs‐troponinT, ng/L | 26.8 [20.7–40.4] | 13.5 [9.1–30.3] | 0.013 |
| Serum sodium, mEq/L | 137.8±3.1 | 140.4±3.1 | 0.001 |
| LVEF, % | 26.2±8.0 | 30.9±6.0 | 0.011 |
| LVDd, mm | 64.7±9.1 | 55.5±9.8 | <0.001 |
| LAD, mm | 44.8±9.3 | 39.9±8.9 | 0.031 |
| E/e′ | 17.0±7.8 | 14.6±5.4 | 0.135 |
| CCB (%) | 2 (7.7) | 11 (23.9) | 0.077 |
| ACEI or ARB (%) | 20 (76.9) | 39 (84.8) | 0.299 |
| β‐blockers (%) | 20 (76.9) | 37 (80.4) | 0.725 |
| Diuretics (%) | 21 (80.8) | 33 (71.7) | 0.395 |
| Statins (%) | 5 (19.2) | 11 (23.9) | 0.646 |
| Etiology of heart failure | |||
| Dilated cardiomyopathy | 21 (80.8) | 25 (54.3) | 0.025 |
| Hypertrophic cardiomyopathy | 0 (0) | 0 (0) | … |
| Hypertensive heart disease | 0 (0) | 8 (17.4) | 0.022 |
| Cardiac amyloidosis | 1 (3.8) | 0 (0) | 0.361 |
| Cardiac sarcoidosis | 1 (3.8) | 0 (0) | 0.361 |
| Valvular heart diseases | 1 (3.8) | 3 (6.5) | 0.542 |
| Arrhythmogenic cardiomyopathy | 0 (0) | 4 (8.7) | 0.159 |
| Others | 2 (7.7) | 6 (13.0) | 0.392 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CCB, calcium channel blocker; DROM, derivatives of reactive oxidative metabolites; E/e′, the ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity; EF, ejection fraction; eGFR, estimated glomerular filtration ratio; HF, heart failure; HFnonrecEF, HF with nonrecovered EF; HFrecEF, HF with recovered EF; Hs‐CRP, high‐sensitivity C‐reactive protein; Hs‐troponinT, high‐sensitivity troponin T; LAD, left atrial diameter; LVDd, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; and U.CARR, Carratelli unit.
Receiver Operating Characteristic Curve Analysis and Logistic‐Regression Analysis for the Prediction of HFrecEF
To predict whether patients with HFrEF changed to HFrecEF or HFnonrecEF, we constructed receiver operating characteristic curve analysis. DROM values significantly correlated with LVEF improvement, and the cutoff value for the prediction of HFrecEF was 347 U.CARR (sensitivity, 0.808; specificity, 0.609; Figure 6). Furthermore, the C‐statistic value to predict LVEF improvement in terms of the DROM levels was 0.703 (95% CI, 0.575–0.831; P=0.004). These results indicated that the DROM value could predict future EF improvements in patients with HFrEF.
Figure 6. Receiver operating characteristic (ROC) analysis of DROM for the prediction of HFrecEF.
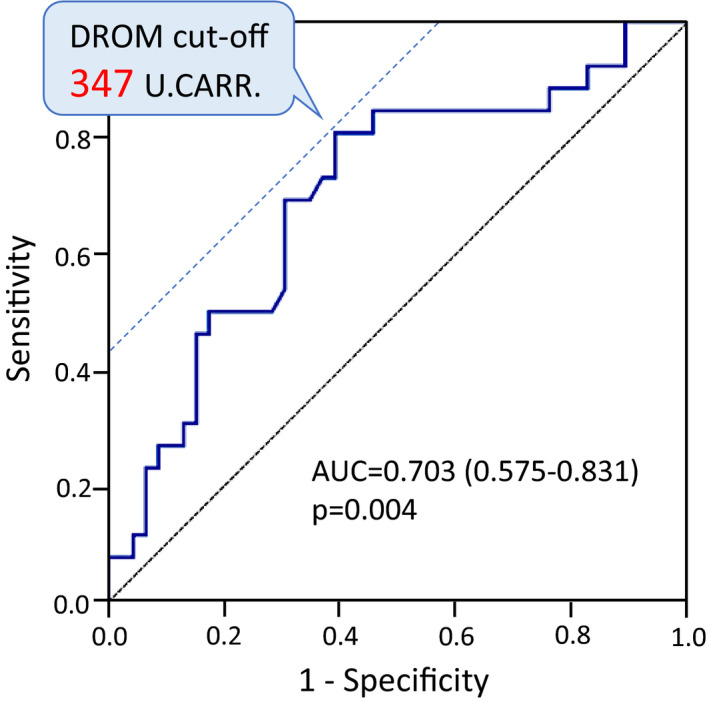
ROC analysis showed that DROM values were significantly correlated with EF recovery, and the cutoff value for the prediction of HFrecEF was 347 U.CARR. The C‐statistic value for the DROM levels identified in patients with HFrEF was 0.703 (95% CI, 0.575–0.831; P=0.004). AUC indicates area under curve; HFrecEF, heart failure with recovered ejection fraction; and U.CARR, Carratelli unit.
In a univariate logistic regression analysis, 9 variables were identified as significant parameters related to the prediction of HFrecEF. In a multivariable logistic regression analysis, concomitant with hypertension and chronic kidney disease, LV end‐diastolic dimension and DROM <347 U.CARR were independently and significantly associated with the prediction of HFrecEF (DROM <347 U.CARR [odds ratio, 4.681; 95% CI, 1.228–17.844; P=0.024]; Table 5). The Akaike's information criterion value of DROM <347 U.CARR was 85.9 in the univariate model and 70.6 in the multivariate model.
Table 5.
Logistic Regression Analysis for the Prediction of HFrecEF
| Variables | Cording | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | ||
| Age, y | Per 1 y | 0.999 | 0.966–1.034 | 0.968 | … | ||
| Sex (male) | Male (vs female) | 0.955 | 0.306–2.974 | 0.936 | … | ||
| Body mass index, kg/m2 | Per 1 kg/m2 | 1.030 | 0.936–1.135 | 0.543 | … | ||
| Hypertension (yes) | Yes (vs no) | 3.529 | 1.242–10.027 | 0.018 | 4.813 | 1.252–18.497 | 0.022 |
| Diabetes mellitus (yes) | Yes (vs no) | 1.069 | 0.364–3.143 | 0.903 | … | ||
| Dyslipidemia (yes) | Yes (vs no) | 1.731 | 0.626–4.783 | 0.290 | … | ||
| History of smoking (yes) | Yes (vs no) | 0.762 | 0.250–2.323 | 0.632 | … | ||
| Chronic kidney disease (yes) | Yes (vs no) | 0.289 | 0.105–0.792 | 0.016 | 0.183 | 0.048–0.702 | 0.013 |
| DROM, U.CARR | Per 1 U.CARR | 0.991 | 0.984–0.997 | 0.005 | … | ||
| DROM <347 U.CARR (yes) | Yes (vs no) | 6.533 | 2.087–20.488 | 0.001 | 4.681 | 1.228–17.844 | 0.024 |
| BNP, pg/mL | Per 1 ln‐BNP | 0.997 | 0.995–0.999 | 0.003 | Not selected | ||
| Hemoglobin, g/dL | Per 1 g/dL | 1.012 | 0.782–1.308 | 0.929 | … | ||
| Serum sodium, mEq/L | Per 1 mEq/L | 1.392 | 1.113–1.739 | 0.004 | Not selected | ||
| LVEF, % | Per 1 % | 1.103 | 1.026–1.187 | 0.008 | Not selected | ||
| LVDd, mm | Per 1 mm | 0.885 | 0.822–0.954 | 0.001 | 0.900 | 0.832–0.975 | 0.010 |
| LAD, mm | Per 1 mm | 0.935 | 0.878–0.996 | 0.037 | Not selected | ||
| E/e′ | Per 1 | 0.944 | 0.873–1.020 | 0.143 | … | ||
| CCB (yes) | Yes (vs no) | 3.771 | 0.766–18.562 | 0.103 | … | ||
| ACEI or ARB (yes) | Yes (vs no) | 1.671 | 0.495–5.641 | 0.408 | … | ||
| β‐blockers (yes) | Yes (vs no) | 1.233 | 0.384–3.964 | 0.725 | … | ||
| Diuretics (yes) | Yes (vs no) | 0.604 | 0.188–1.943 | 0.398 | … | ||
| Statins (yes) | Yes (vs no) | 1.320 | 0.403–4.328 | 0.647 | … | ||
| Dilated cardiomyopathy (yes) | Yes (vs no) | 0.283 | 0.091–0.882 | 0.029 | Not selected | ||
| Hosmer–Lemeshow χ2 | 8.589 | ||||||
| P value | 0.378 | ||||||
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, B‐type natriuretic peptide; CCB, calcium channel blocker; DROM, derivatives of reactive oxidative metabolites; E/e′, ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity; EF, ejection fraction; HF, heart failure; HFrecEF, HF with recovered EF; LAD, left atrial diameter; LVDd, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; and U.CARR, Carratelli unit.
Discussion
We have been investigating the association of various biomarkers with the prognosis of HF since before, 21 , 22 and previously reported that DROM might be a useful biomarker of ROS for the risk stratification and the prediction of future cardiovascular events in patients with both HFpEF and HFrEF. 10 , 11 Although patients with HF in the present study overlapped with 10% to 20% of study subjects in these previous studies 11 , 22 because of the difference of study period, we newly found that the DROMs are significantly and independently associated with HF‐related events in patients with NIHF in this study. To examine whether DROM is not a marker of HF disease etiology, moreover, we further compared the enrolled patients with NIHF in this study with patients with HF coexisting with coronary artery disease. As a result, patients with high DROM were closely associated with significantly increased risk of HF‐related events than patients with low DROM regardless of whether NIHF was ischemic or nonischemic (data not shown). Thus, we speculate that DROM could not be a marker of HF disease etiology but rather disease progression and instability. Among the HF categories, moreover, patients with HFrEF and HFpEF but not HFmrEF with HF‐related events had higher DROM levels than those without HF‐related events in this study. Although the DROM test has been reported to predict initial HF hospitalization in elderly patients with HF, 13 the present work is the first report of a significant association of serum DROM levels with future HF‐related events in nonischemic HFrEF, and our findings are similar to those in a previous report of other ROS biomarkers in patients with HF with dilated cardiomyopathy. 23 However, our approach differed in that the method used in that study to measure oxidative stress reflected the overall oxidative status and excluded non–hydroperoxide‐related ROS‐mediated biomarkers. Furthermore, one of the limitations of this study is that we have not examined antioxidative status in patients with HF. The measurement of biological antioxidative potential, an index of antioxidative activity, in addition to DROM is needed to provide insightful information about mechanisms of ROS in HF by further clinical study.
Interestingly, only patients with HFmrEF with HF‐related events had no significant difference in DROM levels compared with those without HF‐related events, differing from patients with HFrEF and HFpEF. In 2016, the European Society of Cardiology introduced the category HFmrEF to acknowledge the “gray area” between HFrEF and HFpEF, 1 and this range of LVEF is less well studied compared with HFpEF and HFrEF. The recent largest prospective observational cohort in Japan, the CHART‐2 (Chronic Heart Failure Analysis and Registry in the Tohoku District‐2) study clearly demonstrated that HFmrEF dynamically transitions to HFpEF or HFrEF, especially within 1 year, suggesting that HFmrEF represents a transitional status or an overlap zone between HFpEF and HFrEF, rather than an independent entity of HF. 24 As described above, DROM values in patients with HFrecEF were significantly lower than those in patients with HFnonrecEF, indicating that DROM values in patients with HFmrEF transitioning from HFrEF to HFrecEF could be lower than those in patients with stable chronic HFmrEF. Hence, we speculate that DROM values in patients with HFmrEF can be counterbalanced by the presence of various phenotypes of patients with HFmrEF because HFmrEF represents a transitional status between HFpEF and HFrEF. In the present study, unfortunately, the changes in LVEF in over half of the enrolled patients with NIHF were not examined, while HF phenotypes in the present study were categorized by LVEF values assessed only at study registration. Hence, further studies with follow‐up data are needed to examine the effects of oxidative status by changes in LVEF in patients with HFmrEF.
In the present study of patients with NIHF, the prevalence of HFmrEF was 17.4%, while that of HFpEF and HFrEF was 43.2% and 39.3%, respectively. Although the present study was a single‐center design with a relatively small patient population that enrolled only patients with NIHF, these prevalences of HFmrEF, HFrEF and HFpEF were comparable with previous reports in Western countries. 25 , 26 , 27 In terms of the prognosis of patients with NIHF, a previous report by Cheng et al 25 demonstrated that patients with HFmrEF had higher readmission rates than patients with HFpEF and that mortality rates were comparable to those of HFrEF and HFpEF. By contrast, CHART‐2 demonstrated that HFmrEF had an intermediate incidence of all‐cause death, cardiovascular death, and HF admission between HFpEF and HFrEF, while incidences of noncardiovascular death, acute myocardial infarction, and stroke were comparable among the 3 groups. 24 In the present study, there were no significant differences in the probability of HF‐related events among HF categories according to the Kaplan–Meier analysis. This discrepancy between these previous studies and the present study could be explained by the difference in the inclusion criteria of the patients (hospitalized HF versus stable HF) and the difference in the etiology of HF (NIHF versus ischemic HF), rather than geographic and racial differences. Further clinical studies are needed to determine the underlying pathophysiology of HFmrEF.
Few reports have discussed the association of biomarkers of ROS with adverse clinical outcomes and LVEF recovery in patients with NIHF, but the identification of predictors of future HF‐related events and therapeutic responders (recovered LVEF) in nonischemic HFrEF is of great clinical importance. A recent study by Breathett et al 28 reported that LVEF changes predicted both survival and hospitalization for HF in patients with HFrEF. Indeed, Kaplan–Meier analysis in the present study revealed that patients with HFrecEF had a significantly and dramatically lower probability of HF events than did patients with HFnonrecEF. Moreover, DROM values in patients with HFrecEF were significantly lower than those in patients with HFnon‐recEF. To examine the cutoff value of DROM for the prediction of HFrecEF, we further performed receiver operating characteristic analysis and found that DROM was a significant predictor of HFrecEF (cutoff value of DROM, 347 U.CARR). Therefore, the results of this study suggest not only that the DROM response to optimal therapy is predictive of HF‐related events in NIHF but also that ROS provides a novel therapeutic target, especially of nonischemic HFrEF. However, in several of the clinical studies investigating the role of ROS in cardiovascular diseases as a therapeutic target, the initial trials were of limited success 29 , 30 ; additionally, as far as we know, there have been no positive clinical data on antioxidant therapy for HFrEF, including for patients with NIHF. Our study was observational rather than interventional and did not test antioxidative drugs. Large‐scale interventional studies in nonischemic patients with HFrEF are still necessary.
Previous reports including CHART‐2 24 , 31 , 32 showed that ischemic heart disease etiology was negatively associated with LVEF recovery in patients with HF. To examine whether DROM is not a marker of HF disease etiology, we further examined patients with HFrecEF with coexisting coronary artery disease (ischemic HFrecEF). As a result, patients with high DROM levels were closely associated with a significantly increased risk of HF‐related events compared with patients with low DROM levels regardless of whether HFrecEF was ischemic or nonischemic (data not shown). Thus, we speculate that DROM may not be a marker of HF disease etiology but rather of disease progression and instability. However, our present results, which first demonstrate the usefulness of DROM for predicting HFrecEF, may provide clinically important information because the role of biomarkers in predicting HFrecEF and HF‐related events in patients with NIHF remains an active area of investigation. 33
In conclusion, serum DROM level could provide important prognostic information for risk stratification in any category of NIHF and was a useful and novel biomarker of ROS for predicting HFrecEF and future HF‐related events in patients with HFrEF.
Sources of Funding
None.
Disclosures
None.
Acknowledgments
The authors thank all paramedical staff and clinical secretaries for their kind support during this work.
(J Am Heart Assoc. 2021;10:e016765. DOI: 10.1161/JAHA.120.016765.)
For Sources of Funding and Disclosures, see page 13.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the Esc. Eur Heart J. 2016;37:2129–2200.DOI: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2. Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17:527–532.DOI: 10.1016/j.cardfail.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto E, Kataoka K, Shintaku H, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ichijo H, Ogawa H, Kim‐Mitsuyama S. Novel mechanism and role of angiotensin II induced vascular endothelial injury in hypertensive diastolic heart failure. Arterioscler Thromb Vasc Biol. 2007;27:2569–2575.DOI: 10.1161/ATVBAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim‐Mitsuyama S. Role of xanthine oxidoreductase in the reversal of diastolic heart failure by candesartan in the salt‐sensitive hypertensive rat. Hypertension. 2007;50:657–662.DOI: 10.1161/HYPERTENSIONAHA.107.095315. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto E, Yamashita T, Tanaka T, Kataoka K, Tokutomi Y, Lai ZF, Dong YF, Matsuba S, Ogawa H, Kim‐Mitsuyama S. Pravastatin enhances beneficial effects of olmesartan on vascular injury of salt‐sensitive hypertensive rats, via pleiotropic effects. Arterioscler Thromb Vasc Biol. 2007;27:556–563.DOI: 10.1161/01.ATV.0000254855.24394.f9. [DOI] [PubMed] [Google Scholar]
- 6. Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2:608–615.DOI: 10.1161/CIRCHEARTFAILURE.109.868513. [DOI] [PubMed] [Google Scholar]
- 7. Tang WH, Wu Y, Mann S, Pepoy M, Shrestha K, Borowski AG, Hazen SL. Diminished antioxidant activity of high‐density lipoprotein‐associated proteins in systolic heart failure. Circ Heart Fail. 2011;4:59–64.DOI: 10.1161/CIRCHEARTFAILURE.110.958348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kishi T, Hirooka Y, Sunagawa K. Telmisartan reduces mortality and left ventricular hypertrophy with sympathoinhibition in rats with hypertension and heart failure. Am J Hypertens. 2014;27:260–267.DOI: 10.1093/ajh/hpt188. [DOI] [PubMed] [Google Scholar]
- 9. Shimano M, Shibata R, Inden Y, Yoshida N, Uchikawa T, Tsuji Y, Murohara T. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm. 2009;6:935–940.DOI: 10.1016/j.hrthm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 10. Hirata Y, Yamamoto E, Tokitsu T, Kusaka H, Fujisue K, Kurokawa H, Sugamura K, Maeda H, Tsujita K, Yamamuro M, et al. Reactive oxidative metabolites are associated with the severity of heart failure and predict future cardiovascular events in heart failure with preserved left ventricular ejection fraction. Int J Cardiol. 2015;179:305–308.DOI: 10.1016/j.ijcard.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 11. Nishihara T, Tokitsu T, Sueta D, Oike F, Takae M, Fujisue K, Usuku H, Ito M, Kanazawa H, Araki S, et al. Clinical significance of reactive oxidative metabolites in patients with heart failure with reduced left ventricular ejection fraction. J Card Fail. 2021;27:57–66. [DOI] [PubMed] [Google Scholar]
- 12. Hirata Y, Yamamoto E, Tokitsu T, Kusaka H, Fujisue K, Kurokawa H, Sugamura K, Maeda H, Tsujita K, Kaikita K, et al. Reactive oxygen metabolites are closely associated with the diagnosis and prognosis of coronary artery disease. J Am Heart Assoc. 2015;4:e001451. DOI: 10.1161/JAHA.114.001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hitsumoto T. Efficacy of the reactive oxygen metabolite test as a predictor of initial heart failure hospitalization in elderly patients with chronic heart failure. Cardiol Res. 2018;9:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National KF. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 15. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 16. Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R, Nicolaides A. A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- 17. Iamele L, Fiocchi R, Vernocchi A. Evaluation of an automated spectrophotometric assay for reactive oxygen metabolites in serum. Clin Chem Lab Med. 2002;40:673–676. [DOI] [PubMed] [Google Scholar]
- 18. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, et al.; Guidelines ESCCfP . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 19. The Criteria Committee of the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, Mass: Little, Brown & Co; 1994: 253–256. [Google Scholar]
- 20. Zou KH, O'Malley AJ, Mauri L. Receiver‐operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. [DOI] [PubMed] [Google Scholar]
- 21. Nishihara T, Tokitsu T, Sueta D, Takae M, Oike F, Fujisue K, Usuku H, Takashio S, Hanatani S, Kanazawa H, et al. Serum potassium and cardiovascular events in heart failure with preserved left ventricular ejection fraction patients. Am J Hypertens. 2018;31:1098–1105.DOI: 10.1093/ajh/hpy101. [DOI] [PubMed] [Google Scholar]
- 22. Komorita T, Yamamoto E, Sueta D, Tokitsu T, Fujisue K, Usuku H, Nishihara T, Oike F, Takae M, Egashira K, et al. The controlling nutritional status score predicts outcomes of cardiovascular events in patients with heart failure with preserved ejection fraction. Int J Cardiol Heart Vasc. 2020;29:100563. DOI: 10.1016/j.ijcha.2020.100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsutamoto T, Wada A, Matsumoto T, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, et al. Relationship between tumor necrosis factor‐alpha production and oxidative stress in the failing hearts of patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:2086–2092.DOI: 10.1016/S0735-1097(01)01299-2. [DOI] [PubMed] [Google Scholar]
- 24. Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, Abe R, Oikawa T, Kasahara S, Sato M, et al.; CHART‐2 Investigators . Characterization of heart failure patients with mid‐range left ventricular ejection fraction‐a report from the CHART‐2 study. Eur J Heart Fail. 2017;19:1258–1269.DOI: 10.1002/ejhf.807. [DOI] [PubMed] [Google Scholar]
- 25. Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the medicare population. Am Heart J. 2014;168:721–730.DOI: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 26. Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail. 2016;4:464–472. [DOI] [PubMed] [Google Scholar]
- 27. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, et al.; Candesartan in Heart Failure Reduction in Mortality I . Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744.DOI: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 28. Breathett K, Allen LA, Udelson J, Davis G, Bristow M. Changes in left ventricular ejection fraction predict survival and hospitalization in heart failure with reduced ejection fraction. Circ Heart Fail. 2016;9:e002962. DOI: 10.1161/CIRCHEARTFAILURE.115.002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salonen RM, Nyyssönen K, Kaikkonen J, Porkkala‐Sarataho E, Voutilainen S, Rissanen TH, Tuomainen T‐P, Valkonen V‐P, Ristonmaa U, Lakka H‐M, et al.; Antioxidant Supplementation in Atherosclerosis Prevention Study . Six‐year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study. Circulation. 2003;107:947–953.DOI: 10.1161/01.CIR.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 30. Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin e after myocardial infarction: results of the GISSI‐Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 31. Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726.DOI: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clarke CL, Grunwald GK, Allen LA, Baron AE, Peterson PN, Brand DW, Magid DJ, Masoudi FA. Natural history of left ventricular ejection fraction in patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:680–686.DOI: 10.1161/CIRCOUTCOMES.111.000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL, Kiernan MS, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135:e1054–e1091.DOI: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]


