Abstract
The interest and demand for healthy and less processed foods for human consumption have been mirrored in the pet industry, with an explosion of alternative diets available. Several nontraditional feeding methodologies including raw meat-based diets (RMBDs) are believed by many dog owners to be superior to traditional extruded commercial dog foods. Despite the strong opinions, limited data are available comparing objective health measures among healthy dogs fed using different methods of diet preparation. Therefore, we compared health markers in client-owned dogs fed an RMBD to markers in dogs fed a high-quality extruded kibble. We hypothesized that healthy adult dogs fed RMBD would show differences in biochemical and hematological parameters and improved clinical health scores (e.g., dental, external ear canal, and integument scores) compared with dogs fed a kibble diet. A cross-sectional observational study was performed comparing hematology, serum biochemistry, urinalysis management history, and clinical health scores in healthy client-owned dogs reported as fed RMBD (n = 28) or kibble (n = 27) for >1 yr. Dental, external ear canal, and integument health scores were assigned by a single veterinary evaluator blinded to feed group, using a scale where 0 was normal and 3 was most severely affected. Spearman correlation coefficient (rs) was calculated to assess the strength and direction of the relationship of biochemical outcomes with age and body condition score (BCS), while analysis of variance was used to determine if biochemical analytes differed by breed or gender. Biochemical data were analyzed using multiple linear regression models, adjusting for the covariates gender, breed, age, and BCS. A composite clinical health score, (CCS) = 9 − (dental score + otitis score + integument score), was compared between feeding groups using Mann–Whitney test. Serum alkaline phosphatase activity (P < 0.001) and globulin concentration (P < 0.001) were lower, while lymphocyte count (P < 0.05) was higher in dogs fed RMBD. No differences were found in urinalysis between diet groups. Dogs fed RMBD showed a slight improvement in CCS compared with kibble-fed dogs (CCS: P = 0.03). Owner management significantly differed with a greater likelihood of management interventions including dietary supplements and sporting activities in the RMBD group. Further work is needed to specifically determine the impact of diet processing and nutrient content on canine health.
Keywords: canine nutrition, hematology, integument score, otitis score, periodontal score, serum biochemistry
Introduction
The impact of diet on health is irrefutable, for humans as well as our companion animal species. The interest and demand for healthy, locally sourced, fresh, or less processed foods for humans consumption have been mirrored in the pet industry, with an explosion of available diets labeled as natural, fresh, or grain free. Several nontraditional feeding methodologies including raw, home-cooked, and naturally sourced ingredients are believed by many dog owners and practitioners to be superior to traditional extruded commercial dog foods. A current trend among pet owners advocating for more natural diets is the use of raw fed or minimally processed diets (Schlesinger and Joffe, 2011). Anecdotal reports of health benefits of raw meat-based diets (RMBD) include cleaner teeth, decrease in fecal output, improvement of skin and coat, improved immunity, and decrease in inflammatory-related diseases (Freeman et al., 2013). However, little to no evidence is present in the scientific literature to support assertions of improved clinical outcomes in RMBD-fed dogs, and what has been reported only followed dogs for a short-term feeding trial, was limited by small sample sizes, or failed to report clinical outcomes (Algya et al., 2018; Frisk, 2018; Anturaniemi et al., 2020).
Feeding raw meat is not without risk. Multiple studies have reported the presence of pathogenic bacteria in RMBD as well in the feces, and the Centers for Disease Control and Prevention and Food and Drug Administration recommend against feeding raw diets to pets due to the risk of exposing owners to disease-causing enteric bacteria or parasites in the raw food products (LeJeune and Hancock, 2001; Joffe and Schlesinger, 2002; Weese et al., 2005; Finley et al., 2006; Strohmeyer et al., 2006; Lefebvre et al., 2008; Freeman et al., 2013; Nemser et al., 2014; van Bree et al., 2018). Owners may also unwittingly create nutritional deficiencies or excesses in their pets due to poorly formulated diets (Köhler et al., 2012; Pedrinelli et al., 2019). Because of the potential risks to human and dog health, it is important to identify if there are benefits to warrant these feeding practices.
The type, duration, and temperature of processing of feedstuffs can all affect nutrient composition and digestibility, with raw diets being generally associated with higher digestibility than dry kibble (Beloshapka et al., 2012; Kerr et al., 2012; Bermingham et al., 2017). Rendering meat lowers the protein quality and amino acid availability compared with raw meat meals (Cramer et al., 2007). Processing may also result in chemical transformations such as the production of advanced glycation end products (AGEs). Several reports have shown that multiple methods and conditions used in the production of commercial dog food result in the creation of AGEs (Tran et al., 2008; Van Rooijen et al., 2014a, 2014b). AGEs, through interaction with the receptor for AGEs, activate signaling pathways that increase the production of reactive oxygen species and sustained the activation of pro-inflammatory signals, such as nuclear factor kappa-light-chain-enhancer of activated B cells (Cepas et al., 2020). There is mounting evidence that AGEs contribute to the development of several important chronic and age-associated diseases in humans and dogs, such as diabetes, heart disease, cancer, and neurodegeneration (Langhendries et al., 1992; Hamelin et al., 2003; Comazzi et al., 2008; Poulsen et al., 2013; Ribeiro et al., 2019). Taken together, increased bioavailability of nutrients and reduction of low-grade inflammation could explain the anecdotal results of improved health and reduction in disease states reported by owners.
Additionally, previous research has shown that the nutrient composition of a diet is a strong driver of differences in the intestinal tract microbial populations (i.e., the gut microbiota), which has a substantial impact on the overall health (Gilbert et al., 2016; Lynch and Pedersen, 2016; Mondo et al., 2019; Burr et al., 2020). RMBD diets differ substantially in the overall macronutrient composition, with a typical RMBD consisting of primarily protein and fat, while the addition of plant products in commercial kibble results in 30% to 40% simple carbohydrates and 3% to 5% crude fiber. Therefore, naturally sourced ingredients or raw foods theoretically could result in different health outcomes in dogs due to changes in absorbed nutrients or by modifying the gut microbiota which, in turn, would alter clinical health markers. We hypothesized that healthy dogs fed RMBD would show improved general health markers, including a modest clinical benefit (such as improved dental, ear, and skin health scores) compared with dogs fed a kibble diet. Furthermore, we hypothesized that animal factors, such as age, body condition score (BCS), breed, and gender, would also contribute to differences in general health markers in dogs fed RMBD or kibble diets. To examine the interaction of diet and health in dogs, we compared hematology, serum biochemical, and clinical health outcomes in client-owned dogs fed RMBD or commercial extruded kibble diets for a period of at least 1 yr.
Methods and Materials
The study was performed under the approval and oversight of the Oklahoma State University Institutional Animal Care and Use Committee, in accordance with applicable provisions of the Animal Welfare Act, Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, the USDA Animal Welfare Regulations, and University policies. All procedures were performed only after receiving written, informed client consent.
Inclusion criteria
Healthy, adult (1.5 to 13 yr) dogs fed either an RMBD or extruded commercial kibble diet for longer than 1 yr were recruited for enrollment. Health was confirmed by physical examination by a single veterinary clinician blinded to the dog’s feeding method. Dogs that received treatment with antimicrobial, immune modifying (e.g., Apoquel or Cytopoint), or steroid drugs within 4 mo; vaccinated with 2 wk; or pregnant were excluded. Recruitment of dogs was through outreach to area raw food cooperatives, dog events/competitions, social media targeted to local dog show and performance groups, veterinary teaching hospital clients, employees, and students. To facilitate ease of sample collection, only dogs >9 kg were included.
Historical data
Extensive general and nutritional historical information was obtained to determine the dog’s overall health and fitness. Historical information collected included vaccination status, parasite prevention, dental care (including date of last profession dental cleaning, use of dental chews, or frequency of teeth brushing), ear cleaning, bath and grooming frequency, lifestyle, travel, ongoing medical issues, recent surgery, current medications (including nutraceuticals and over the counter), health habits, and activity level. A modified World Small Animal Veterinary Association (WSAVA) Global Nutrition Assessment checklist was completed by the pet owner in order to obtain a complete dietary history, including information regarding current food type, amount, feeding frequency, length on diet, palatability, food storage, and access to foods not offered by the owner (Baldwin et al., 2010; Freeman et al., 2011).
Physical evaluation
Participant dogs received a thorough physical examination completed by a blinded clinician. Basic parameters included rectal body temperature, heart rate, respiratory rate, and body weight, as well as evaluation of total body health. Utilizing the WSAVA’s standardized scale, BCS was assessed (Laflamme, 1997; Mawby et al., 2004). The oral cavity of the dog was examined for the amount and distribution of plaque and calculus, health of the gingiva (gums), and loose or missing teeth and graded on a scale of 0 to 3, using an adaptation of the American Veterinary Dental College Stages of Periodontal Disease (Bauer et al., 2018). Due to the infrequency of animals with advanced dental disease in the study population and to equally weight the three clinical scores (dental, otitis, and integument score), dental grades 3 and 4 were combined. The condition of the dog’s integument was evaluated for dryness, scaling, ease of epilation, and presence of lesions (erythema, lichenification, excoriations, and alopecia) and graded on a scale of 0 to 3 (Olivry et al., 2014). In addition, ears were visually evaluated for erythema, edema, erosion/ulceration, and exudate and graded on a scale of 0 to 3 (Nuttall and Bensignor, 2014). For each of the clinical grading scales, 0 was normal and 3 was the most severally affected. A composite clinical score (CCS: 0 to 9) was calculated from the three individual clinical health scores using the formula CCS = 9 − (dental score + otitis score + integument score).
Sample collection
Free catch urine samples were collected from the dogs on the day of examination for urinalysis. Specific gravity and pH were determined on fresh urine samples by refractometry and urine test strip (Siemens Multistix 10 SG, Siemens Healthcare Diagnostics Inc, Tarrytown, NY), respectively. Hematology and serum chemistry analyses were performed by an on-site commercial laboratory (Antech) on fresh (submitted within 30 min) blood samples collected via jugular venipuncture into tubes containing appropriate anticoagulants (ethylenediaminetetraacetic acid and lithium heparin, respectively). Dogs were not fasted prior to blood collection; all samples were collected 60 to 360 min after their typical meal. To further characterize the specific isotype contributing to the total alkaline phosphatase (ALP) activity, bone ALP concentration was determined using a canine-specific enzyme-linked immunosorbent assay kit (MyBioSource, San Diego, CA). All samples were measured on a single plate with an intraassay coefficient of variation of 2.78% at a concentration of 6 ng/mL (n = 4). Blood samples for serum thyroxine (T4), free T4, and thyroid stimulating hormone (TSH) were allowed to clot, then separated within 3 h and stored at −80 °C until assayed using a canine-validated chemiluminescence assay (Immulite 1000, Siemens). All thyroid hormone assays were performed within 3 mo of sample collection.
Statistics
Sample size calculation indicated that 25 animals per group were needed to detect a 1.2-grade difference in the CCS with an 80% power and an alpha of 0.05%. To perform this calculation, we assumed a mean CCS of 7/9 (SD 1.5) in the kibble-fed dogs based on the summation of the published mean score (±SD) for each individual clinical scoring system (integument, otitis, and periodontal; Nuttall and Bensignor, 2014; Olivry et al., 2014; Stella et al., 2018).
Animal factors were compared between feeding groups by Mann–Whitney test (age and BCS) or chi-square test (gender and breed). Spearman correlation coefficient (rs) was calculated to assess the strength and direction of the relationship of biochemical outcomes with age and BCS. Analysis of variance was used to determine if biochemical analytes differed by breed or gender. Biochemical data were analyzed using multiple linear regression models to understand the difference in the biochemical parameters across the two feeding groups while adjusting for other covariates determined in the above analyses to be potential cofounders and/or not balanced across the feeding groups, namely gender, breed, age, and BCS. For the purposes of statistics, breed was coded as border collies (n = 13), other purebred dogs (n = 27), or mixed breed dogs (n = 14). Border collies were considered as their own group due to the substantial number enrolled, whereas all other breeds had no more than four participants (Table 1). We performed regression diagnostics with the residuals to check the necessary assumptions of the regression models and used appropriate logarithmic transformation whenever necessary to resolve violation of the normality assumption.
Table 1.
Characteristics of dogs enrolled in the study1
| Kibble | Raw | P-value | |
|---|---|---|---|
| Number enrolled | n = 27 | n = 28 | |
| Gender | M = 5; MC = 11; F = 0; FS = 11 | M = 4; MC =7 ; F = 6; FS = 11 | 0.06 |
| Age (mean ± SD) | 4.5 ± 2.1 yr | 6.9 ± 2.6 yr | <0.001 |
| Weight (mean ± SD) | 27.81 ± 13.6 kg | 24.14 ± 11.1 kg | 0.28 |
| BCS (mean ± SD) | 5.1 ± 1.4 | 3.8 ± 1.2 | 0.001 |
| Breed (multiples) | BC = 3; Lab = 3; GD = 3; Aussie = 2; Mixed = 9 | BC = 11; Rott = 4; ESS = 3; Lab = 2; Mixed = 5 | 0.08 |
| Breed (singles) | Corgi, Golden, Husky, Heeler, Greyhound, Staffy, and Beagle | GSD, GSP, Mal, and BM |
1Age, body weight, and BCS were compared between the two feeding groups by Mann–Whitney test, while the frequency of breed and gender in the two groups was compared using a chi-square test. M, male; MC, male castrate; F, female; FS, female spayed; BC, Border Collie; Lab, Labrador Retriever; GD, Great Dane; Aussie, Australian Shepherd; Golden, Golden Retriever; Staffy, American Staffordshire Terrier; Rott, Rottweiler; ESS, English Springer Spaniel; GSD, German Shepherd Dog; GSP, German Shorthair Pointer; Mal, Malinois; BM, Bullmastiff.
Bone ALP concentration was compared with total ALP activity by Spearman rank test. The bone ALP concentration in RMBD-fed dogs was compared with kibble-fed dogs by Mann–Whitney test.
To test the overall hypothesis that feeding RMBD improves the overall clinical outcomes, a CCS was compared between feeding groups using Mann–Whitney test, with P < 0.05 considered significant (one-sided). For clinical data for which limited dogs had abnormal findings (otitis and integument score), data were scored as normal vs. abnormal and the feeding groups compared using Fisher’s exact test. Fecal consistency and dental score were compared between feeding groups using a chi-square test.
Owner responses regarding animal care and history were summarized. Fisher’s exact test was used to compare the frequency of historical skin disease and frequency of owner interventions between feeding groups for all interventions except bathing practice, which used a Mann–Whitney test. To assess if management practices influenced clinical outcomes, a chi-square test was performed comparing dental scores in those dogs that did or did not have a history of professional dentistry, teeth brushing, or provision of dental chews (tested individually and combined). Otitis score (normal vs. abnormal) was compared in dogs that did or did not have a history of ear cleaning by Fisher’s exact test. Logistic regression was performed to test the association between the integument score (normal: grade 0; abnormal: grade ≥ 1) and frequency of baths (baths/yr). Statistical analysis and graph creation were performed using statistical software R and Graph Pad Prism 5.01.
Results
Sixty-one dogs were recruited for the study. Six dogs were deemed unsuitable due to abnormalities on physical examination (five kibble-fed dogs and 1 RMBD dog); 27 kibble-fed dogs and 28 RMBD dogs were enrolled. All dogs had been on their current method of feeding (raw vs. kibble) for >2 yr, except for one dog that had switched from kibble to raw the previous year. Table 1 summarizes the signalment of the dogs included. In the kibble group, diets included Purina ProPlan (n = 12), Hills (n = 9), Iams (n = 2), Taste of the Wild, Royal Canin, Diamond, and Pedigree (1 each). In the RMBD group, all dogs were fed RMBD distributed by two companies (Titan blends, Ross Wells Distributor: n = 22 and meat blends from Texas Tripe distributor, n = 6). RMBD dogs often received a combination of prepared blends and locally sourced (groceries, self-prepared, and hunters) feedstuffs. Dogs in the RMBD group were significantly older and had a lower BCS than the kibble-fed group (Table 1, P < 0.001 for both).
Table 2 summarizes the correlation of age and BCS with the biochemical and hematological parameters, while Table 3 presents the group mean (or median) differences in analyte concentration based on gender or breed. Age was weakly correlated with several biochemical and hematological parameters, whereas BCS showed a moderate correlation to many outcomes. There were numerous differences in hematological parameters among the breed and gender groups. Therefore, all four of these animal factors were considered potential confounders and included in the subsequent regression models.
Table 2.
Correlation (Spearman rank test, rs) between age or BCS and biochemical parameters
| Analyte1 | Age | P-value | BCS | P-value |
|---|---|---|---|---|
| r s | r s | |||
| Total protein | −0.00756 | 0.9563 | 0.2514 | 0.0641 |
| Albumin | −0.1159 | 0.3995 | 0.1936 | 0.1566 |
| Globulin | 0.02548 | 0.8535 | 0.1535 | 0.2631 |
| A/G ratio | −0.03453 | 0.8024 | −0.04118 | 0.7653 |
| ALT activity | 0.1498 | 0.275 | −0.1067 | 0.438 |
| ALP activity | −0.3006 | 0.0257 | 0.3777 | 0.0045 |
| BUN | 0.271 | 0.0454 | −0.4431 | 0.0007 |
| Creatinine | −0.01523 | 0.9121 | 0.255 | 0.0602 |
| BUN/creatinine ratio | 0.2518 | 0.0637 | −0.5392 | <0.0001 |
| Glucose | −0.3311 | 0.0135 | 0.1453 | 0.2898 |
| WBC count | −0.228 | 0.0941 | −0.1463 | 0.2865 |
| RBC count | −0.1076 | 0.4343 | 0.4189 | 0.0015 |
| HGB | −0.1706 | 0.2129 | 0.4242 | 0.0012 |
| HCT | −0.1528 | 0.2654 | 0.4229 | 0.0013 |
| MCV | −0.1179 | 0.3912 | 0.09045 | 0.5113 |
| MCH | −0.1657 | 0.2267 | 0.1631 | 0.234 |
| MCHC | −0.05452 | 0.6926 | 0.05176 | 0.7074 |
| Platelet count | 0.3118 | 0.0205 | −0.3027 | 0.0247 |
| Neutrophils | −0.1698 | 0.2153 | −0.05825 | 0.6727 |
| Lymphocytes | −0.2891 | 0.0323 | −0.1682 | 0.2196 |
| Monocytes | −0.124 | 0.367 | 0.009606 | 0.9445 |
| Eosinophils | 0.08021 | 0.5605 | −0.2354 | 0.0836 |
| Urine SG | −0.1349 | 0.3354 | −0.2413 | 0.0817 |
| Urine pH | −0.06589 | 0.6359 | 0.06329 | 0.6493 |
| T4 | 0.1317 | 0.338 | −0.01294 | 0.9253 |
| fT4 | 0.128 | 0.3518 | −0.1702 | 0.2141 |
| TSH | 0.2321 | 0.0882 | 0.2269 | 0.0957 |
1A/G, Albumin/Globulin; ALT, alanine transferase; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin, MCHC, mean corpuscular hemoglobin concentration; SG, specific gravity; fT4, free thyroxine; TSH, thyroid-stimulating hormone.
Table 3.
Differences in measured analytes by gender or breed (analysis of variance [ANOVA], P < 0.05 considered significant)
| Analyte1 | Gender | Breed |
|---|---|---|
| P-value | P-value | |
| Total protein | 0.5443 | 0.0938 |
| Albumin | 0.3249 | 0.1654 |
| Globulin | 0.7496 | 0.5126 |
| A/G ratio | 0.6263 | 0.8707 |
| ALT activity | 0.6918 | 0.1016 |
| ALP activity | 0.8029 | 0.0733 |
| BUN | 0.3585 | 0.5776 |
| Creatinine | 0.6055 | 0.1358 |
| BUN/creatinine ratio | 0.3108 | 0.166 |
| Glucose | 0.409 | 0.3132 |
| WBC count | 0.0092 | 0.757 |
| RBC count | 0.021 | 0.0006 |
| HGB | 0.0127 | 0.0003 |
| HCT | 0.0153 | 0.0015 |
| MCV | 0.8006 | 0.656 |
| MCH | 0.5586 | 0.5306 |
| MCHC | 0.4236 | 0.0541 |
| Platelet count | 0.0095 | 0.0009 |
| Neutrophils2 | 0.0785 | 0.2468 |
| Lymphocytes2 | 0.4073 | 0.968 |
| Monocytes | 0.1774 | 0.6819 |
| Eosinophils2 | 0.0366 | 0.1436 |
| Urine SG | 0.9052 | 0.0799 |
| Urine pH | 0.5776 | 0.6567 |
| T4 | 0.2489 | 0.5928 |
| fT42 | 0.294 | 0.3071 |
| TSH2 | 0.2325 | 0.3698 |
1A/G, Albumin/Globulin; ALT, alanine transferase; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin, MCHC, mean corpuscular hemoglobin concentration; SG, specific gravity; fT4, free thyroxine; TSH, thyroid-stimulating hormone.
2Data was log transformed prior to analysis.
The hematology, serum chemistry, and urinalysis results (mean or median) for each feeding group are summarized in Table 4 along with the results of the multiple linear regression models. Laboratory-specific reference intervals are presented in Supplementary Table S1. ALP activity was nearly 50% less in dogs fed raw compared with kibble-fed dogs. Despite the large difference in ALP activity, all dogs were within the normal laboratory reference interval. Multiple linear regression revealed that feed group, but not BCS, age, gender, or breed, contributed to the observed difference in ALP activity (P < 0.0001). To further characterize the source of the difference in ALP activity, bone ALP concentration was determined (n = 41). Total ALP activity was not correlated with bone ALP concentration (rs = −0.14, P = 0.4), and there was no difference between bone ALP concentration in RMBD- or kibble-fed dogs (Mann–Whitney, P = 0.8).
Table 4.
Hematology, serum chemistry, and urinalysis results with multiple linear regression analysis
| Descriptive statistics | Multiple linear regression analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Kibble group, n = 27 | Raw group, n = 28 | Feed | Age | Gender | BCS | Breed | |||
| Variable1 | Units | Mean ± SD or median (IQR) | Mean ± SD or median (IQR) | Beta coefficient (RMBD) |
P-value | P-value | P-value | P-value | P-value |
| Total protein | g/dL | 6.43 ± 0.32 | 6.24 ± 0.42 | −0.3498 | 0.0101 | 0.0125 | 0.0326 | 0.1329 | 0.0566 |
| Albumin | g/dL | 3.58 ± 0.23 | 3.54 ± 0.25 | 0.1372 | 0.135 | 0.0203 | 0.2037 | 0.7007 | 0.3499 |
| Globulin | g/dL | 2.85 ± 0.33 | 2.7 ± 0.44 | −0.4871 | 5.0E-04 | 1.00E-04 | 0.002 | 0.2096 | 0.3196 |
| A:G ratio | 1.27 ± 0.17 | 1.35 ± 0.25 | 0.269 | 9.0E-04 | 5.0E-04 | 0.0051 | 0.4268 | 0.8479 | |
| ALT activity | IU/L | 38.4 ± 15.04 | 39.4 ± 16.14 | −4.4262 | 0.4746 | 0.2933 | 0.7335 | 0.5559 | 0.3918 |
| ALP activity | IU/L | 41.89 ± 19.5 | 20.64 ± 10.18 | −24.6262 | 1.0E-04 | 0.2268 | 0.0611 | 0.1766 | 0.2366 |
| BUN | mg/dL | 17.37 ± 4.51 | 21.79 ± 6.24 | 1.4478 | 0.4885 | 0.2177 | 0.5069 | 0.018 | 0.898 |
| Creatinine (creat) | mg/dL | 1.13 ± 0.17 | 1.04 ± 0.22 | −0.0665 | 0.3979 | 0.651 | 0.8242 | 0.4138 | 0.2441 |
| BUN:creat ratio | 15.44 ± 3.77 | 21.96 ± 8.12 | 2.8099 | 0.2423 | 0.3389 | 0.5026 | 0.01 | 0.5356 | |
| Glucose | mg/dL | 106.6 ± 8.64 | 98.82 ± 15.06 | −4.3343 | 0.3793 | 0.6335 | 0.4893 | 0.9423 | 0.2702 |
| WBC count | × 103/µL | 7.53 ± 1.9 | 8.46 ± 2.25 | 1.1185 | 0.1445 | 0.2357 | 0.1493 | 0.7267 | 0.2978 |
| RBC count | × 106/µL | 7.29 ± 0.56 | 6.85 ± 0.67 | −0.0406 | 0.8555 | 0.5202 | 0.638 | 0.1999 | 0.0687 |
| HGB | g/dL | 17.89 ± 1.63 | 16.65 ± 1.67 | 0.2234 | 0.696 | 0.0822 | 0.25 | 0.0821 | 0.1448 |
| HCT | % | 51.52 ± 4.15 | 47.96 ± 4.78 | 0.178 | 0.9119 | 0.146 | 0.3552 | 0.085 | 0.2687 |
| MCV | fL | 70.7 ± 2.73 | 70.29 ± 2.34 | 0.9979 | 0.301 | 0.0446 | 0.455 | 0.1019 | 0.083 |
| MCH | pg | 24.54 ± 1.02 | 24.31 ± 0.8 | 0.429 | 0.2232 | 0.016 | 0.2867 | 0.2233 | 0.7314 |
| MCHC | g/dL | 34.81 ± 0.88 | 34.79 ± 0.74 | 0.3943 | 0.2089 | 0.1368 | 0.4102 | 0.883 | 0.2743 |
| Platelet | × 103/µL | 219.4 ± 70.69 | 316.5 ± 79.17 | 44.4249 | 0.1014 | 0.0348 | 0.0482 | 0.5858 | 0.0526 |
| Neutrophils2 | /µL | 4,846 ± 1,356 | 5,263 ± 1,675 | 430.0646 | 0.4334 | 0.6787 | 0.051 | 0.8109 | 0.0352 |
| Lymphocytes2 | /µL | 1,850 (1,426 to 2,272) | 1,890 (1,606 to 2,455) | 0.2782 | 0.0466 | 0.012 | 0.5453 | 0.9299 | 0.9505 |
| Monocytes | /µL | 328 (213 to 412) | 256 (212 to 481) | −0.3029 | 0.1162 | 0.2273 | 0.0352 | 0.8305 | 0.4156 |
| Eosinophils2 | /µL | 268 (100 to 568) | 459 (312 to 916) | 0.4315 | 0.1395 | 0.7597 | 0.5925 | 0.7121 | 0.6019 |
| Urine SG | 1.041 ± 0.0113 | 1.040 ± 0.013 | −0.003 | 0.5107 | 0.5386 | 0.771 | 0.1528 | 0.0916 | |
| Urine pH | 7.0 ± 0.863 | 6.75 ± 0.55 | −0.277 | 0.3433 | 0.7549 | 0.4759 | 0.7631 | 0.6454 | |
| T4 | µg/dL | 1.12 ± 0.36 | 1.25 ± 0.4 | 0.2226 | 0.1356 | 0.5412 | 0.1357 | 0.5869 | 0.4239 |
| fT42 | ng/dL | 1.32 (0.99 to 1.75) | 1.38 (1.13 to 1.87) | 0.1336 | 0.4876 | 0.4192 | 0.1558 | 0.3947 | 0.3249 |
| TSH2 | ng/mL | 0.153 (0.11 to 0.24) | 0.142 (0.09 to 0.2) | −0.263 | 0.3377 | 0.1784 | 0.2879 | 0.5281 | 0.3292 |
1ALT, alanine transferase; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin, MCHC, mean corpuscular hemoglobin concentration; SG, specific gravity; fT4, free thyroxine; TSH, thyroid-stimulating hormone; IQR, interquartile range.
2The response variables for neutrophils, lymphocytes, fT4, and TSH are log-transformed using y = log(y). For eosinophils, the transformation is y = log(100 + y).
3 N = 26.
Dogs fed RMBD had a lower serum globulin concentration resulting in a lower total protein concentration and greater albumin:globulin ratio. Both age and gender also contributed to the model, with older animals and females having higher globulin concentrations. Although blood urea nitrogen (BUN) was lower in the kibble-fed group, this difference was statistically associated with BCS rather than diet.
Feeding RMBD was significantly associated with a greater lymphocyte count, while increasing age was associated with lower lymphocyte counts. Although several other hematological parameters differed between the raw and kibble groups including platelet count and red blood cell indices, multiple linear regression models revealed these differences to be primarily an effect of animal factor differences rather than diet.
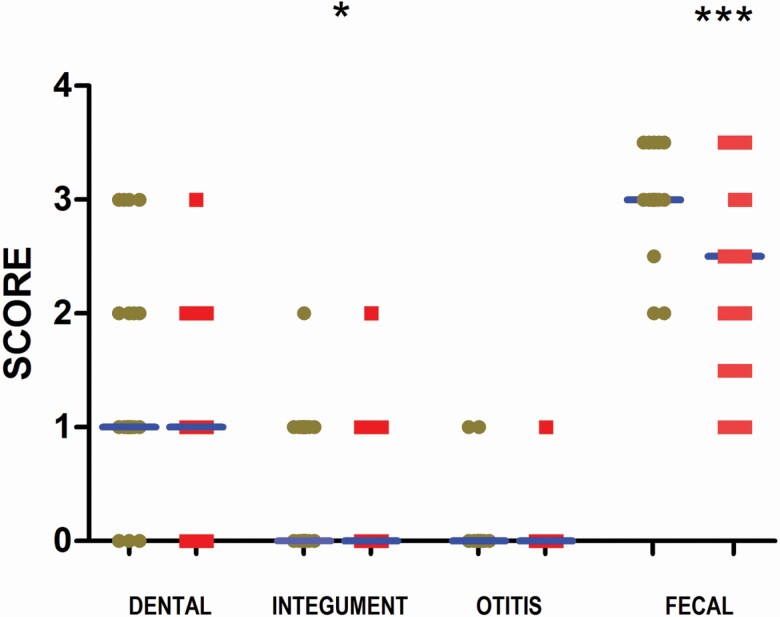
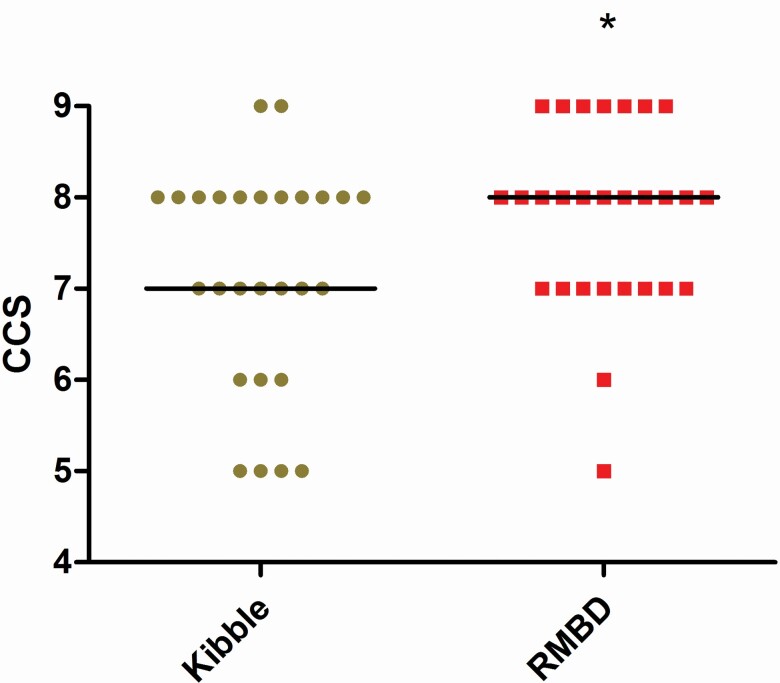
Several clinical differences were observed between the RMBD- and kibble-fed dogs. Figures 1 and 2 summarize the clinical examination results. Integument health was improved in raw-fed dogs (P = 0.04); however, there was no difference in dental and otitis clinical scores between the groups (Figure 1) and no difference in the prevalence of previous skin disease as reported by the owner between groups (raw: 7/28; kibble: 5/27; P = 0.7). Fecal consistency was firmer in RMBD dogs compared with kibble-fed dogs (Figure 1, P < 0.0001). CCSs were calculated with the maximum score of nine assigned if no abnormalities were present in dental, integument and otitis examinations. The CCS was higher in RMBD dogs compared with the kibble-fed dogs (Figure 2; P = 0.02) suggesting a modest improvement in the overall health.
Figure 1.
Dental, integument, otitis, and fecal scores for dogs fed kibble (brown circles) and dogs fed RMBD (red boxes). Median values shown as horizontal bars (overlap with x-axis for integument and otitis scores). *P < 0.05, ***P < 0.001.
Figure 2.
CCS was calculated as CCS = 9 – (integument score + otitis score + dental score). CCS was lower (more abnormal) in dogs fed extruded kibble (brown circles) compared with those fed RMBD (red boxes). Median values shown as horizontal bars. *P = 0.03.
The client histories collected revealed distinct differences in behavioral practices between owners who fed kibble diets and those that fed RMBD. When management of the two groups was considered, there was no statistically significant difference in the frequency of tooth brushing (8% of RMBD and 12% kibble-fed dog owners brushed their dogs’ teeth, P = 0.66) or professional cleaning (38% of RMBD compared with 21% of kibble-fed dogs, P = 0.36). Nearly half of all dog owners reported using a dental intervention; however, 50% of RMBD dogs received raw meaty bones as dental cleaning aids, while 48% of kibble-fed dogs received dental chews or wipes. Raw-fed dogs did have a higher frequency of bathing with 81% of owners reporting bathing at least monthly intervals for an average of 5 baths per 6 mo, while 25% of kibble-fed dogs were bathed at least once a month, and 49% bathed only every 3 mo or less for an average of 2.96 baths per 6 mo (Mann–Whitney, P < 0.01). RMBD owners also provided a greater number of dietary supplements, as 85% of raw dogs received some sort of supplement, while 24% of kibble dogs received additional nutritional aids (P < 0.001). As there was minimal consensus in type of supplements provided, it was not possible to statistically account for this potential confounder using the current study design.
To determine if owner management contributed to the clinical findings, frequency of individual dog husbandry interventions was compared to its associated clinical scores. There was no significant association in dental, otitis, or integument score with provision of dental interventions (veterinary cleaning, brushing, chews, or any one of the three), ear cleaning, or baths, respectively.
Discussion
Advocates strongly believe in the perceived health benefits of alternative diets, such as raw or home cooked, compared with conventional commercial kibble diets. In the current study, 86% of owners reported feeding RMBD for the perceived health benefits. Anecdotal reports of health benefits of RMBD include cleaner teeth, decrease in fecal output, improvement of skin and coat, improved immunity, and decrease in inflammatory-related diseases (Schlesinger and Joffe, 2011; Freeman et al., 2013; Algya et al., 2018). To more critically assess whether feeding an RMBD had an observable effect on canine health, clinical health markers, hematologic and biochemical parameters, urinalysis, and thyroid hormones were compared in client-owned dogs that were reported to have been fed either kibble or RMBD for over a year.
To our knowledge, this is the first study to report that veterinarian-assessed clinical outcomes associated with feeding RMBD compared with kibble diet. Using a single-blinded veterinary observer and previously validated scoring systems, the clinical consequence of the feeding method was evaluated. Assessment was focused on those clinical outcomes anecdotally suggested to be improved by RBMD. Integument, dental, and external ear canal scores were combined to make a CCS. Selection criteria for the study, which included the exclusion of dogs receiving medications typically prescribed for common pruritic or dermatological problems, may have contributed to the majority of enrolled dogs having minimal to no active skin or ear abnormalities. Despite this limitation, dogs fed RBMD had modest improvement in integument and CCS compared with kibble-fed dogs. Although improvement of skin allergies is a reason provided by owners for feeding raw, there was no difference in reported history of skin disease between the two feeding groups. As suggested in other publications, the consistency of feces in dogs fed RBMD was firmer than those fed kibble (Beloshapka et al., 2012; Kerr et al., 2012; Freeman et al., 2013; Algya et al., 2018).
To address the concern that owner management might differ between the two groups, owner responses from a questionnaire were used to determine the frequency of dental interventions, baths and grooming, and ear cleaning. While there were substantial differences in management between the two groups, the frequency of these interventions was not correlated with individual clinical health scores, suggesting that differences in owner management alone were not the reason for the observed difference in health markers between dogs fed RMBD compared with kibble. However, we cannot eliminate the possibility that differences in some owner practices, such as dog activity level and dietary supplementation, both of which were more common in the RMBD group, may have confounded the ability to identify the effects due to diet alone.
In addition to clinical assessment, biochemical parameters were compared as a rough indicator of physiological function. For all measured analytes, results from both feeding groups were within the normal reference interval, although several differences between groups were observed. Similar to previous studies, kibble-fed dogs had a 50% greater total serum ALP activity compared with raw-fed dogs (Algya et al., 2018; Frisk, 2018; Anturaniemi et al., 2020). Frisk (2018) found a 50% greater ALP in dogs fed kibble with a similar number of client-owned dogs assessed as in the current study (n = 26 kibble-fed and n = 28 raw-fed dogs). However, in the Frisk (2018) study, 80% of the enrolled dogs were a single breed (Staffordshire terriers) and the specific diet fed was not reported. Algya et al. (2018) also reported a 50% greater ALP in eight beagles when fed kibble compared with when the same dogs were fed a commercially available raw diet. Recently, Anturaniemi et al. (2020) reported blood chemistry and hematology results from 33 Staffordshire bull terriers, fed either a commercial kibble or raw diet in a long-term prospective feeding trial. Serum ALP activity decreased 50% from baseline in dogs receiving the raw diet intervention (Anturaniemi et al., 2020). Total serum ALP in dogs is a combination of liver, bone, and corticosteroid ALP. Dogs in the study population had no history of corticosteroid administration or evidence (historical or physical) of hyperadrenocorticism, making it unlikely that the differences observed were due to change in the corticosteroid-induced ALP. Further analysis also found no difference in bone-specific ALP between groups, leading us to conclude that the source of the increased ALP in kibble-fed dogs was most likely due to the release of liver isotype. Induction of hepatocyte activity with associated increase in ALP activity could occur in response to nutrient modifications secondary to processing or due to differences in ingredient sourcing. AGEs, a known consequence of dog food processing, have been implicated in liver injury (Hyogo and Yamagishi, 2008; Bijnen et al., 2019). In human nutrition, it is well accepted that processed food consumption is associated with both low-grade chronic inflammation and oxidative stress, both of which are known to induce hepatotoxicity and ALP activity (Srour et al., 2019; Alonso-Pedrero et al., 2020; Pagliai et al., 2021). Although the median ALP in the kibble-fed dogs was still within the reference interval, the consistency of the observed difference among multiple studies suggests that further investigation into the mechanism of this change is warranted.
Additional differences observed in serum biochemistry analytes in the current study were not consistent with other reported studies (Algya et al., 2018; Frisk, 2018; Anturaniemi et al., 2020). In the current study, globulin and total protein concentration was lower, while albumin:globulin ratio was higher in raw-fed dogs, suggesting a decrease in inflammation. However, this finding was in contrast to data from other studies, which reported no difference in globulin concentration between dogs fed kibble and those on RMBD (Algya et al., 2018; Frisk, 2018; Anturaniemi et al., 2020). These differences may reflect variation in study design, inclusion criteria, duration on diet, specific study diets, and animal management including exercise or dog-specific factors rather than a function of feed processing alone. In our data analysis, both age and gender were confounding animal factors that contributed to the globulin concentration in addition to diet.
Only two other studies reported the effect of raw diet in dogs on hematologic parameters, although a white blood cell differential was not included in either study (Frisk, 2018; Anturaniemi et al., 2020). Results from client-owned purebred terriers showed greater platelet counts in dogs fed a raw diet (Frisk, 2018; Anturaniemi et al., 2020). In our study, platelet count was also higher in RMBD-fed dogs; however, regression analysis suggested that this effect was primarily due to age and gender of the animals rather than diet. A modestly higher lymphocyte count was found in our population of RMBD-fed dogs. This is similar to what has been reported in cats fed raw food (Freeman et al., 2013). However, the cats in that study were also found to be shedding Salmonella in the feces, so it is not clear if this finding was due to the diet or infection. Lymphocytes have many functions in immune response, as both inflammatory and anti-inflammatory agents. Without further characterization of the specific population of lymphocytes that differed, and the role enteric pathogens might have played, it is not possible to attribute any potential consequence to this observation.
The current study differs from previous studies in the diversity of breeds, gender, and age of dogs included (Algya et al., 2018; Frisk, 2018; Anturaniemi et al., 2020). Although restricting the study to a single-breed or laboratory-maintained dogs would decrease variability in the data, this approach would limit the broad applicability of the results and fail to adequately address this controversial topic for most of the dog owners. Furthermore, data from the present study were used in the design of subsequent, more controlled studies. Therefore, an understanding of which animal factors contributed to observed differences in health markers is considered useful. Multiple linear regression analysis was used to compare the feeding groups while controlling for animal factors. Results from the multivariate linear regression model suggested that age, gender, BCS, and breed contributed to several biochemical outcomes. As has been previously reported, age was associated with an increase in globulin concentration as well as contributed modestly to hematological parameters, such as red blood cell indices, platelet, and lymphocyte counts (Lee et al., 2020; Scarpa et al., 2020). Gender also had an effect on globulin concentration as well as a slight influence on monocyte count. BCS was only negatively associated with BUN concentration. Although mean BCS differed between the two feed groups, dogs were screened to be healthy prior to enrollment. Thus, extremely thin or morbidly obese animals were not included. This may have limited the impact of BCS on the measured health variables. Breed also had a minimal effect on outcomes, only modestly contributing to neutrophil count. However, characterization of breed as purebred, mixed breed, or border collie may have failed to account for individual breed differences. The most common breed in the current study was border collies; most of these received regular exercise, likely due to targeted recruitment from dog sport participants. It is possible that activity level or other owner management factors contributed to the differences in health outcomes observed between the two feeding groups. This is now being investigated in an ongoing subsequent study by our group. Additional studies with more subjects also would help to confirm our findings and potentially explain differences between the current and previously reported studies.
Another unique feature of the present study is the duration for which the dogs had been being fed using the same type of diet. Dogs in this study were recruited based on having been fed either raw or kibble for more than a year, and, in fact, all but one dog had been on the current feeding method for more than 2 yr. While the specific diet and amount fed differed among dogs, most of the dogs were fed a high-quality kibble or an RBMD from a single cooperative. RMBD diets differ substantially from kibble in the overall macronutrient composition, with a typical RMBD consisting of primarily protein and fat, while the addition of plant products in commercial kibble results in 30% to 40% simple carbohydrates and 3% to 5% crude fiber. Extruded kibble undergoes heat and pressure processing, while the two RMBD fed in this trial are ground to a form a homogenized blend and frozen until fed. Thus, the differences in diet between the groups were more pronounced than within the groups. Still, it is not possible to conclude that any observed differences between the two feeding groups were due to the diet processing, differences in nutrient composition (protein, fat, or carbohydrate content and quality), or added supplements. Regardless, the current data do support the differences in physiological and general health markers between dogs based on diet. Ultimately, the accuracy of the investigated health markers at predicting future disease risk and the role of diet type in the development of chronic canine disease are important questions that need to be investigated.
Supplementary Data
Supplementary data are available at Journal of Animal Science online.
Supplementary Table S1: Adult reference intervals
Acknowledgment
The funding for this study was provided by the American Holistic Veterinary Medical Foundation.
Glossary
Abbreviations
- AGEs
advanced glycation end products
- BCS
body condition score
- BUN
blood urea nitrogen
- CCS
clinical composite score
- HCT
hematocrit
- HGB
hemoglobin
- RBC
red blood cell
- WBC
white blood cell
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Algya, K. M., Cross T. W. L., Leuck K. N., Kastner M. E., Baba T.. Lye L., de Godoy M. R., and Swanson K. S.. . 2018. Apparent total-tract macronutrient digestibility, serum chemistry, urinalysis, and fecal characteristics, metabolites and microbiota of adult dogs fed extruded, mildly cooked and raw diets. J. Anim. Sci. 96:3670–3683. doi: 10.1093/jas/sky235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Pedrero, L., Ojeda-Rodríguez A., Martínez-González M. A., Zalba G., Bes-Rastrollo M., and Marti A.. . 2020. Ultra-processed food consumption and the risk of short telomeres in an elderly population of the Seguimiento Universidad de Navarra (SUN) Project. Am. J. Clin. Nutr. 111(6):1259–1266. doi: 10.1093/ajcn/nqaa075 [DOI] [PubMed] [Google Scholar]
- American Veterinary Dental College (AVDC). n.d. Nomenclature, Stages of Periodontal Disease. https://avdc.org/avdc-nomenclature/ [accessed May 18, 2021].
- Anturaniemi, J., Zaldivar-Lopez S., Moore R., Kosola M., Sankari S., Barrouin-Melo S. M., and Hielm-Bjorkman A.. . 2020. The effect of a raw vs dry diet on serum biochemical, hematologic, blood iron, B12 and folate levels in Staffordshire Bull Terriers. Vet. Clin. Pathol. 49:258–269. doi: 10.1111/vcp.12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin, K., Bartges J., Buffington T., Freeman L. M., Grabow M., Legred J., and Ostwald D. Jr. 2010. AAHA nutritional assessment guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 46:285–296. doi: 10.5326/0460285 [DOI] [PubMed] [Google Scholar]
- Bauer, A. E., Stella J., Lemmons M., and Croney C. C.. . 2018. Evaluating the validity and reliability of a visual dental scale for detection of periodontal disease (PD) in non-anesthetized dogs (Canis familiaris). PLoS One. 13:e0203930. doi: 10.1371/journal.pone.0203930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloshapka, A. N., Duclos L. M., Vester Boler B. M., and Swanson K. S.. . 2012. Effects of inulin or yeast cell-wall extract on nutrient digestibility, fecal fermentative end-product concentrations, and blood metabolite concentrations in adult dogs fed raw meat-based diets. Am. J. Vet. Res. 73:1016–1023. doi: 10.2460/ajvr.73.7.1016 [DOI] [PubMed] [Google Scholar]
- Bermingham, E. N., Maclean P., Thomas D. G., Cave N. J., and Young W.. . 2017. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ. 5:e3019. doi: 10.7717/peerj.3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnen, M., van Greevenbroek M. M. J., van der Kallen C. J. H., Scheijen J. L., van de Waarenburg M. P. H., Stehouwer C. D. A., Wouters K., and Schalkwijk C. G.. . 2019. Hepatic fat content and liver enzymes are associated with circulating free and protein-bound advanced glycation end products, which are associated with low-grade inflammation: the CODAM study. J. Diabetes Res. 2019:6289831. doi: 10.1155/2019/6289831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bree, F. P. J., Bokken G. C. A. M., Mineur R., Franssen F., Opsteegh M., van der Giessen J. W. B., Lipman L. J. A., and Overgaauw P. A. M.. . 2018. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet. Rec. 182:50. doi: 10.1136/vr.104535 [DOI] [PubMed] [Google Scholar]
- Burr, A. H. P., Bhattacharjee A., and Hand T. W.. . 2020. Nutritional modulation of the microbiome and immune response. J. Immunol. 205:1479–1487. doi: 10.4049/jimmunol.2000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepas V., Collino M., Mayo J. C. and Sainz R. M.. . 2020. Redox signaling and advanced glycation endproducts (AGEs) in diet-related disease. Antioxidants. 9:142. doi: 10.3390/antiox9020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comazzi, S., Bertazzolo W., Bonfanti U., Spagnolo V., and Sartorelli P.. . 2008. Advanced glycation end products and sorbitol in blood from differently compensated diabetic dogs. Res. Vet. Sci. 84:341–346. doi: 10.1016/j.rvsc.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Cramer, K. R., Greenwood M. W., Moritz J. S., Beyer R. S., and Parsons C. M.. . 2007. Protein quality of various raw and rendered by-product meals commonly incorporated into companion animal diets. J. Anim. Sci. 85:3285–3293. doi: 10.2527/jas.2006-225 [DOI] [PubMed] [Google Scholar]
- Finley, R., Reid-Smith R., and Weese J. S.. . 2006. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin. Infect. Dis. 42:686–691. doi: 10.1086/500211 [DOI] [PubMed] [Google Scholar]
- Freeman, L., Becvarova I., Cave N., MacKay C., Nguyen P., Rama B., Takashima G., Tiffin R., Tsjimoto H., and van Beukelen P.. . 2011. WSAVA nutritional assessment guidelines. J. Small Anim. Pract. 52:385–396. doi: 10.1111/j.1748-5827.2011.01079.x [DOI] [PubMed] [Google Scholar]
- Freeman, L. M., Chandler M. L., Hamper B. A., and Weeth L. P.. . 2013. Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. J. Am. Vet. Med. Assoc. 243:1549–1558. doi: 10.2460/javma.243.11.1549 [DOI] [PubMed] [Google Scholar]
- Frisk, C. 2018. The effect of different diets on haematology and serum biochemistry in dogs [licentiate thesis]. Finland: University of Helskinki. Available from https://helda.helsinki.fi/bitstream/handle/10138/301238/Frisk%20Camilla%20Licentiatavhandling_CamillaFrisk.pdf?sequence=2&isAllowed=y [accessed May 19, 2021]. [Google Scholar]
- Gilbert, J. A., Quinn R. A., Debelius J., Xu Z. Z., Morton J., Garg N., Jansson J. K., Dorrestein P. C., and Knight R.. . 2016. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 535:94–103. doi: 10.1038/nature18850 [DOI] [PubMed] [Google Scholar]
- Hamelin, M., Borot-Laloi C., Friguet B., and Bakala H.. . 2003. Increased level of glycoxidation product N(epsilon)-(carboxymethyl)lysine in rat serum and urine proteins with aging: link with glycoxidative damage accumulation in kidney. Arch. Biochem. Biophys. 411:215–222. doi: 10.1016/s0003-9861(02)00735-x [DOI] [PubMed] [Google Scholar]
- Hyogo, H., and Yamagishi S.. . 2008. Advanced glycation end products (AGEs) and their involvement in liver disease. Curr. Pharm. Des. 14:969–972. doi: 10.2174/138161208784139701 [DOI] [PubMed] [Google Scholar]
- Joffe, D. J., and Schlesinger D. P.. . 2002. Preliminary assessment of the risk of Salmonella infection in dogs fed raw chicken diets. Can. Vet. J. 43:441–442. [PMC free article] [PubMed] [Google Scholar]
- Kerr, K. R., Vester Boler B. M., Morris C. L., Liu K. J., and Swanson K. S.. . 2012. Apparent total tract energy and macronutrient digestibility and fecal fermentative end-product concentrations of domestic cats fed extruded, raw beef-based, and cooked beef-based diets. J. Anim. Sci. 90:515–522. doi: 10.2527/jas.2010-3266 [DOI] [PubMed] [Google Scholar]
- Köhler, B., Stengel C., and Neiger R.. . 2012. Dietary hyperthyroidism in dogs. J. Small Anim. Pract. 53:182–184. doi: 10.1111/j.1748-5827.2011.01189.x [DOI] [PubMed] [Google Scholar]
- Laflamme, D. P. 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22:10–15. [Google Scholar]
- Langhendries, J. P., Hurrell R. F., Furniss D. E., Hischenhuber C., Finot P. A., Bernard A., Battisti O., Bertrand J. M., and Senterre J.. . 1992. Maillard reaction products and lysinoalanine: urinary excretion and the effects on kidney function of preterm infants fed heat-processed milk formula. J. Pediatr. Gastroenterol. Nutr. 14:62–70. [PubMed] [Google Scholar]
- Lee, S. H., Kim J. W., Lee B. C., and Oh H. J.. . 2020. Age-specific variations in hematological and biochemical parameters in middle- and large-sized of dogs. J. Vet. Sci. 21(1):e7. doi: 10.4142/jvs.2020.21.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre, S. L., Reid-Smith R., Boerlin P., and Weese J. S.. . 2008. Evaluation of the risks of shedding Salmonellae and other potential pathogens by therapy dogs fed raw diets in Ontario and Alberta. Zoonoses Public Health 55:470–480. doi: 10.1111/j.1863-2378.2008.01145.x [DOI] [PubMed] [Google Scholar]
- LeJeune, J. T., and Hancock D. D.. . 2001. Public health concerns associated with feeding raw meat diets to dogs. J. Am. Vet. Med. Assoc. 219:1222–1225. doi: 10.2460/javma.2001.219.1222 [DOI] [PubMed] [Google Scholar]
- Lynch, S. V., and Pedersen O.. . 2016. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375:2369–2379. doi: 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- Mawby, D. I., Bartges J. W., d′Avignon A., Laflamme D. P., Moyers T. D., and Cottrell T.. . 2004. Comparison of various methods for estimating body fat in dogs. J. Am. Anim. Hosp. Assoc. 40:109–114. doi: 10.5326/0400109 [DOI] [PubMed] [Google Scholar]
- Mondo, E., Marliani G., Accoris P. A., Cocchi M., and Di Leone A.. . 2019. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 9:253–258. doi: 10.4314/ovj.v9i3.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemser, S. M., Doran T., Grabenstein M., McConnell T., McGrath T., Pamboukian R., Smith A. C., Achen M., Danzeisen G., Kim S., . et al. 2014. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog. Dis. 11:706–709. doi: 10.1089/fpd.2014.1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall, T., and Bensignor E.. . 2014. A pilot study to develop an objective clinical score for canine otitis externa. Vet. Dermatol. 25:530–537, e91. doi: 10.1111/vde.12163 [DOI] [PubMed] [Google Scholar]
- Olivry, T., Saridomichelakis M., Nuttall T., Bensignor E., Griffin C. E., and Hill P. B.. . 2014. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)‐4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet. Derm. 25: 77–85. doi: 10.1111/vde.12107 [DOI] [PubMed] [Google Scholar]
- Pagliai, G., Dinu M., Madarena M. P., Bonaccio M., Iacoviello L., and Sofi F.. . 2021. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br. J. Nutr. 125:308–318. doi: 10.1017/S0007114520002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrinelli, V., Zafalon R. V. A., Rodrigues R. B. A., Perini M. P., Conti R. M. C., Vendramini T. H. A., de Carvalho Balieiro J. C., and Brunetto M. A.. . 2019. Concentrations of macronutrients, minerals and heavy metals in home-prepared diets for adult dogs and cats. Sci. Rep. 9:13058. doi: 10.1038/s41598-019-49087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen, M. W., Hedegaard R. V., Andersen J. M., de Courten B., Bügel S., Nielsen J., Skibsted L. H., and Dragsted L. O.. . 2013. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 60:10–37. doi: 10.1016/j.fct.2013.06.052 [DOI] [PubMed] [Google Scholar]
- Q & A Salmonella Outbreak. n.d. National Center for Zoonotic, Vector-Borne, and Enteric Diseases (ZVED). https://www.cdc.gov/salmonella/schwarzengrund_faq.html [accessed May 19, 2021].
- Ribeiro, P. V. M., Tavares J. F., Costa M. A. C., Mattar J. B., and Alfenas R. C. G.. . 2019. Effect of reducing dietary advanced glycation end products on obesity-associated complications: a systematic review. Nutr. Rev. 77:725–734. doi: 10.1093/nutrit/nuz034 [DOI] [PubMed] [Google Scholar]
- Scarpa, P., Ruggerone B., Gironi S., Vitiello T., and Paltrinieri S.. . 2020. Haematological and biochemical reference intervals in healthy racing and retired Italian Greyhounds. Acta Vet. Hung. 68:71–78. doi: 10.1556/004.2020.00006 [DOI] [PubMed] [Google Scholar]
- Schlesinger, D. P., and Joffe D. J.. . 2011. Raw food diets in companion animals: a critical review. Can. Vet. J. 52:50–54. [PMC free article] [PubMed] [Google Scholar]
- Srour, B., Fezeu L. K., Kesse-Guyot E., Allès B., Méjean C., Andrianasolo R. M., Chazelas E., Deschasaux M., Hercberg S., Galan P., . et al. 2019. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ 365:l1451. doi: 10.1136/bmj.l1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella, J. L., Bauer A. E., and Croney C. C.. . 2018. A cross-sectional study to estimate prevalence of periodontal disease in a population of dogs (Canis familiaris) in commercial breeding facilities in Indiana and Illinois. PLoS One. 13:e0191395. doi: 10.1371/journal.pone.0191395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmeyer, R. A., Morley P. S., Hyatt D. R., Dargatz D. A., Scorza A. V., and Lappin M. R.. . 2006. Evaluation of bacterial and protozoal contamination of commercially available raw meat diets for dogs. J. Am. Vet. Med. Assoc. 228:537–542. doi: 10.2460/javma.228.4.537 [DOI] [PubMed] [Google Scholar]
- Tran, Q. D., Hendriks W. H., and van der Poel A. F. B.. . 2008. Effects of extrusion processing on nutrients in dry pet food. J. Sci. Food Agric. 88:1487–1493. doi: 10.1002/jsfa.3247 [DOI] [Google Scholar]
- Van Rooijen, C., Bosch G., van der Poel A. F., Wierenga P. A., Alexander L., and Hendriks W. H.. . 2014a. Quantitation of Maillard reaction products in commercially available pet foods. J. Agric. Food Chem. 62:8883–8891. doi: 10.1021/jf502064h [DOI] [PubMed] [Google Scholar]
- Van Rooijen, C., Bosch G., van der Poel A. F. B., Wierenga P. A., Alexander L., and Hendriks W. H.. . 2014b. Reactive lysine content in commercially available petfoods. J. Nutr. Sci. 3:e35. doi: 10.1017/jns.2014.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese, J. S., Rousseau J., and Arroyo L.. . 2005. Bacteriological evaluation of commercial canine and feline raw diets. Can. Vet. J. 46:513–516. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.