Abstract
We investigated the effects of dietary fiber (DF) supplementation in normal or low crude protein (CP) diets on reproductive performance and nitrogen (N) utilization in primiparous gilts. In total, 77 Landrace × Yorkshire pregnant gilts were randomly allocated to four dietary treatments in a 2 × 2 factorial design. The groups comprised 1) equal intake of normal CP (12.82% and 0.61% total lysine), 2) low CP (LP) (10.53% and 0.61% total lysine), and 3) with or 4) without DF supplementation (cellulose, inulin, and pectin in a 34:10:1 ratio). A low-protein diet during gestation significantly reduced daily weight gain from days 91 to 110 of pregnancy (−162.5 g/d, P = 0.004). From N balance trials conducted at days 35 to 38, 65 to 68, and 95 to 98 of pregnancy, DF addition increased fecal N excretion at days 65 to 68 (+24.1%) and 95 to 98 (+13.8%) of pregnancy (P < 0.05) but reduced urinary N excretion (P < 0.05), resulting in greater N retention at each gestational stage. DF increased fecal microbial protein levels and excretion during gestation. An LP diet also reduced urinary N excretion at different gestational stages. An in vitro fermentation trial on culture media with nonprotein N urea and ammonium bicarbonate (NH4HCO3) as the only N sources revealed that microbiota derived from feces of gestating gilts fed the high DF diet exhibited a greater capacity to convert nonprotein N to microbial protein. Microbial fecal diversity, as measured by 16S rRNA sequencing, revealed significant changes from DF but not CP diets. Gilts fed an LP diet had a higher number of stillbirths (+0.83 per litter, P = 0.046) and a lower piglet birth weight (1.52 vs. 1.37 kg, P = 0.006), regardless of DF levels. Collectively, DF supplementation to gestation diets shifted N excretion from urine to feces in the form of microbial protein, suggesting that the microbiota had a putative role in controlling N utilization from DF. Additionally, a low-protein diet during gestation negatively affected the litter performance of gilts.
Keywords: dietary fiber, gestating gilt, low protein diet, microbiota, nitrogen utilization
Introduction
The excretion of nitrogen (N) in feces and urine during swine production has received considerable attention due to its polluting effects on the environment (Millet et al., 2018). Nitrogen excretion is largely determined by dietary protein quality, which depends on digestion and absorption processes in the gastrointestinal tract, and the utilization of amino acids (AA) for tissue deposition or accretion. It is common practice to limit N excretion by reducing dietary protein levels for growing pigs (Wang et al., 2018). However, it is unclear whether low-protein diets are applicable to gestating sows without affecting reproductive performance.
The efficacy of low-protein diets for gestating gilts may be affected by the microbiota. Firstly, swine pregnancy is associated with dramatic shifts in microbiota (Zhuo et al., 2020), suggesting that increased microbial proliferation and activity increasingly convert dietary protein to microbial protein. Secondly, gestating gilts are usually fed high dietary fiber (DF) levels to promote animal welfare, satiety, and gastrointestinal tract health (Jarrett and Ashworth, 2018). Importantly, DF is a carbon fuel source that drives microbial protein synthesis, which is a valuable AA source for the host. Additionally, DF has long been considered an anti-nutritional factor in terms of protein digestibility. Indeed, DF was previously shown to shift N excretion away from urine to feces in growing pigs (Patrás et al., 2012), but whether this was due to an altered microbiota capacity to use N remains unclear.
The gut intestinal mucosa is considered the largest semipermeable biological tissue allowing N-containing metabolite exchange between the blood circulatory system and the gastrointestinal tract (Zheng et al., 2020). Also, blood-borne small-molecule nonprotein waste, for example, urea, can be transported to the gut lumen and further converted to microbial proteins by the microbiota to reduce nitrogenous waste in the blood (Zheng et al., 2020). Therefore, the microbial metabolism of nitrogenous components may have crucial roles in N balance homeostasis. Currently, most of the research has focused on fiber effects by investigating high levels of fiber-rich ingredients, for example, sugar beet pulp and soybean hull. However, other nutrients (e.g., vitamins and minerals) complicate the direct effects of DF. Therefore, our objective was to examine the effects of purified fiber in normal- or low-protein diets during gestation on the reproductive outcomes and N utilization in primiparous gilts.
Materials and Methods
Ethical considerations
All experimental procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University. Additionally, this study was performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Animals, diets, and study design
Ninety-two (92) Landrace × Yorkshire gilts (average body weight [BW] = 90 kg) were obtained from Tianzow Breeding Co., Ltd. and reared in the Sichuan Agricultural University research center. At approximately 143 kg (140 to 148 kg) BW and 240 d (235 to 245 d) old, we induced and synchronized estrous in gilts by orally administering altrenogest (20 mg, Ningbo Sansheng, Co. Ltd., Hangzhou, China) for 18 consecutive days. This was followed by the intramuscular injection of pregnant mare serum gonadotropin (PMSG, 1,000 IU, Ningbo Sansheng) and gonadorelin (100 μg, Ningbo Sansheng) on the 20th and 23rd d post first oral altrenogest administration. Gilts were artificially inseminated twice with pooled semen from three Duroc boars at 24 and 40 h post-gonadorelin administration. The day of the last insemination (40 h post-gonadorelin administration) was considered day 1 of pregnancy. Finally, 77 gilts became pregnant and were randomly allocated to four dietary treatments in a 2 × 2 factorial design, comprising two crude protein (CP) intake levels, with or without additional DF supplementation. The four dietary treatment groups were as follows: 1) a normal CP (NP) diet without DF supplementation (n = 19), 2) a NP diet with DF supplementation (n = 19), 3) a low CP (LP) diet without DF supplementation (n = 20), and 4) an LP diet with DF supplementation (n = 19). The NP diet contained 12.9% CP, and the LP diet contained 10.6% CP, and both were formulated by reducing the soybean meal content while adding crystalline AA to ensure a similar level of dietary lysine and other AA (Tables 1 and 2). Pregnant gilts were fed a 2.15-kg NP or LP diet from day 1 to 90 of gestation and 2.68 kg/d NP or LP diet from day 91 of gestation to delivery. Moreover, additional DF was added to NP and LP rations at 193.5 and 241.2 g/d from days 1 to 90 of pregnancy, and from day 91 of pregnancy to delivery, respectively, to maintain a constant ratio of DF to daily diet levels. All gilts consumed similar DE, lysine, and other nutrients per day with the exception of DF and CP during gestation (Table 3). The concentration of DF in the diet, including soluble and insoluble DF, was measured using an enzymatic-gravimetric method (Association of Official Analytical Chemists [AOAC]; method 991.43) as previously described, but with minor modifications (Zhuo et al., 2020, 2021), which is different from the definitions of crude fiber by a modified Weende procedure or neutral detergent fiber/acid detergent fiber by a modified Van Soest procedure.
Table 1.
Ingredients of gestation diets (as-fed basis)
| Ingredients | Diets1 | |
|---|---|---|
| NP | LP | |
| Corn | 772.6 | 853.4 |
| Wheat bran | 50 | 35 |
| Soybean meal (44% CP) | 120 | 50 |
| Fish meal (65% CP) | 12 | 12 |
| Soy oil | 15 | 14 |
| Limestone | 8.3 | 8.8 |
| Dicalcium phosphate | 12.3 | 12.5 |
| Sodium chloride (feed grade, >99.0%) | 4 | 4 |
| l-Lysine HCl (98.5%) | 0.3 | 2.6 |
| dl-Methionine (99%) | 0 | 0.7 |
| l-Threonine (98.5%) | 0 | 1.1 |
| l-Tryptophan (98.5%) | 0 | 0.4 |
| Choline chloride (50%) | 1.5 | 1.5 |
| Vitamins and minerals2 | 4 | 4 |
| Total | 1,000 | 1,000 |
1NP, normal CP; LP, low CP.
2Provided the following quantities of vitamins and trace minerals per kg of diet: Fe, 100 mg as ferrous sulfate; Cu, 6.6 mg as copper sulfate; Mn, 30 mg as manganese sulfate; Se, 0.15 mg as sodium selenite; Zn, 100 mg as zinc sulfate; I, 0.6 mg as potassium iodide; 4,000 IU of vitamin A; 800 IU of vitamin D3; 441 IU of vitamin E; 0.5 mg of vitamin K; 1.0 mg of thiamine; 3.75 mg of riboflavin; 10 mg of niacin; 15 mg of pantothenic acid; 15 µg of vitamin B12; 0.8 mg of pyridoxine; 1.0 mg of folic acid; and 0.20 mg of biotin.
Table 2.
Nutrient compositions of gestation diets (as-fed basis)
| Diets1 | ||
|---|---|---|
| NP | LP | |
| Digestible energy, Mcal/kg | 3.3 | 3.3 |
| CP2, % | 12.82 (12.86) | 10.53 (10.63) |
| Soluble fiber, % | 0.50 (0.52) | 0.50 (0.52) |
| Insoluble fiber, % | 10.50 (10.58) | 10.50 (10.84) |
| Total DF3, % | 11.0 (11.11) | 11.0 (11.36) |
| Calcium, % | 0.8 | 0.8 |
| Total phosphorus, % | 0.64 | 0.61 |
| Available phosphorus, % | 0.37 | 0.37 |
| Total indispensable AA, % | ||
| Arginine | 0.79(0.78) | 0.56(0.55) |
| Isoleucine | 0.47(0.48) | 0.37(0.36) |
| Histidine | 0.35(0.33) | 0.28(0.27) |
| Leucine | 1.17(1.19) | 1.04(1.05) |
| Lysine | 0.61(0.61) | 0.61(0.62) |
| Met + Cys | 0.44(0.42) | 0.44(0.41) |
| Phenylalanine | 0.62(0.60) | 0.49(0.47) |
| Threonine | 0.46(0.48) | 0.46(0.47) |
| Tryptophan | 0.13(0.14) | 0.13(0.13) |
| Valine | 0.59(0.55) | 0.46(0.47) |
| Expected standard ileal digestible indispensable AA, % | ||
| Arginine | 0.70 | 0.50 |
| Isoleucine | 0.41 | 0.29 |
| Histidine | 0.28 | 0.22 |
| Leucine | 1.05 | 0.91 |
| Lysine | 0.51 | 0.53 |
| Met + Cys | 0.36 | 0.37 |
| Phenylalanine | 0.53 | 0.41 |
| Threonine | 0.38 | 0.40 |
| Tryptophan | 0.11 | 0.12 |
| Valine | 0.49 | 0.38 |
1NP, normal CP; LP, low CP.
2Nutrient content was calculated based on Tables of Feed Composition and Nutritive Values in China listed by Institute of Animal Science of CAAS and China State Key Laboratory of Animal Nutrition (2020), and values in the brackets are analyzed value.
3Total DF (%) = soluble fiber (%) + insoluble fiber (%).
Table 3.
Nutrient intake of different dietary treatment groups1
| NP | LP | |||
|---|---|---|---|---|
| −DF | +DF | −DF | +DF | |
| Days 0 to 90 of pregnancy | ||||
| Intake of basal NP diet, kg/d | 2.15 | 2.15 | 0 | 0 |
| Intake of basal LP diet, kg/d | 0 | 0 | 2.15 | 2.15 |
| Intake of DF mixture, g/d | 0 | 193.5 | 0 | 193.5 |
| Total feed intake, kg/d | 2.15 | 2.34 | 2.15 | 2.34 |
| DE intake, Mcal/d | 7.1 | 7.1 | 7.1 | 7.1 |
| Lysine intake, g/d | 13.1 | 13.1 | 13.1 | 13.1 |
| CP intake, g/d | 275.6 | 275.6 | 228.5 | 228.5 |
| Nitrogen intake, g/d | 44.10 | 44.10 | 36.56 | 36.56 |
| Total DF intake, g/d | 238.8 | 393.4 | 238.8 | 393.4 |
| Soluble fiber, g/d | 11.2 | 54.9 | 11.2 | 54.9 |
| Insoluble fiber, g/d | 227.6 | 338.5 | 227.6 | 338.5 |
| Days 91 to parturition | ||||
| Intake of basal NP diet, kg/d | 2.68 | 2.68 | 0 | 0 |
| Intake of basal LP diet, kg/d | 0 | 0 | 2.68 | 2.68 |
| DF mixture intake, g/d | 0 | 241.2 | 0 | 241.2 |
| Total feed intake, kg/d | 2.68 | 2.92 | 2.68 | 2.92 |
| DE intake, Mcal/d | 8.85 | 8.85 | 8.85 | 8.85 |
| Lysine intake, g/d | 16.35 | 16.35 | 16.62 | 16.62 |
| CP intake, g/d | 344.6 | 344.6 | 284.9 | 284.9 |
| Nitrogen intake, g/d | 55.14 | 55.14 | 45.58 | 45.58 |
| Total DF intake, g/d | 297.7 | 538.9 | 304.4 | 545.6 |
| Soluble fiber, g/d | 13.9 | 68.4 | 13.9 | 68.4 |
| Insoluble fiber, g/d | 283.8 | 470.5 | 290.5 | 477.2 |
1Nutrient intake was calculated based on estimated nutrient content or actual analyzed nutrient content. NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
The DF mixture comprised pectin, inulin, and cellulose (Guangxi Shangda Tech Co., Ltd, Nanning, China). Pectin and inulin are water soluble and easily fermented by microbiota in the gut. Cellulose is insoluble in water and poorly fermented by gut microbiota. Pectin, inulin, and cellulose were added to diets at a 1:10:34 ratio. In our work, digestible energy and AA intakes were designed to meet or exceed the nutrient requirements of gestating gilts, according to NRC (2012). Gestating gilts were housed in individual gestation stalls (2.20 × 0.65 m) from days 1 to 110 of gestation. On day 111, gilts were moved to an individual farrowing pen, where they continued to consume their gestation diets. Animals were fed twice daily at 0800 hours and 1600 hours and had free access to water. The average ambient temperature was 20 to 24 °C.
Measurements of reproductive outcomes
Backfat (BF) thickness at 65 mm on the left side of the dorsal midline of the last rib (P2) on days 1 (initial), 30, 60, 90, and 110 of gestation was measured using a digital BF indicator (Renco Lean-Meater, Renco Corporation, Minneapolis, MN, USA). We assessed BW on days 1, 30, 60, 90, and 110 of gestation and farrowing, and BW and BF metrics for each gilt during gestation were calculated during each 30-d period.
Farrowing was attended, and farrowing duration (the time from first to last piglet birth), the total numbers of piglets born, piglets born alive, and stillborn or mummified were recorded. Piglet birth weight was recorded immediately after birth, prior to suckling. Intrauterine growth retarded was defined as birth weight < 2 standard deviations of average birth weight. Newborn uniformity was determined using the intra-litter coefficient of variation (CV).
Nitrogen balance trial
Three N balance trials were conducted over a 9-d period, including a 5-d adaptation and a 4-d sample collection period, at days 35 to 38, 65 to 68, and 95 to 98 of pregnancy. Gilts were transferred to individual stainless-steel metabolism crates equipped with water supply and feeders at day 1 of the N balance trial (which occurred at days 30, 60, and 90 of pregnancy) for a 5-d adaptation. At each stage, 8 to 9 gestating gilts were randomly selected for the N balance trial; therefore, each gilt experienced at least one N balance trial. Gilts were provided with half of their daily diet allowance at 0800 hours and 1600 hours and had free access to water. Mechanistically, a metal gauze strip between the metabolism cage and the urine-collecting bottle allowed urine to pass through while retaining the feces and protects the direct contamination of feces by the urine. A 24-h defecation schedule was maintained to collect urine and feces. Gilts were excluded from the N balance trail if urine was contaminated with feces. Approximately, 5 g of chromic oxide was added to 100 g of feed at 0800 hours on the 5th day of each trial to indicate the start of feces collection and label feces and on the 10th day to indicate the end of feces collection and label feces. Several methylbenzene drops were added to feces immediately after collection to prevent microbial fermentation. Feces collected every 24 h were pooled, weighed, and placed on ice. Then, 1 mL of 10% HCl was added to every 10 g to prevent N loss. Gilt urine was collected in a plastic container and measured every 24 h, from 0800 hours of day 5 to 0800 hours of day 10 during each trial. Sulfuric acid was added to urine to ensure a pH < 3.0, and representative urine subsamples (5%) were stored at 4 °C until analysis. Collected feces and urine samples were pooled at the end of each N balance trial.
Chemical analysis
NP or LP diet samples from the three batches were pooled, homogenized, and stored at 4 °C until analysis. The AA composition was analyzed on an automatic AA analyzer (LA8080; Hitachi Co., Tokyo, Japan). Dry matter feed and feces levels were measured using forced-air oven drying methods (method 930.15; AOAC Int., 2007). A 150 g of fecal subsample collected at each N balance trial was freeze-dried and homogenized. These freeze-dried fecal samples, diet samples, and liquid urine subsamples were used for N content analysis by the Kjeldahl method. Nitrogen balance concentrations were calculated as follows:
Microbial protein measurement
Fecal microbial protein levels were determined using a previously described method, with minor modifications (Espinosa et al., 2019). Briefly, ~0.2 g of thawed fecal matter from each animal in N balance trials was diluted in 5 mL of ice-cold phosphate-buffered saline (PBS, pH = 7.2). The mixture, after homogenization with a handheld mixer for 30 s, was centrifuged at 250 × g for 15 min at 4 °C to pellet undigested feed particles. The supernatant was then centrifuged at 14,500 × g for 30 min at 4 °C to form a pellet containing microbial cells. The pellet was washed in ice-cold PBS, re-centrifuged, and lysed in lysis buffer (Beyotime Biotechnology, Shanghai, China) containing 100 mM Tris (hydroxymethyl) aminomethane (pH 7.2), 0.5% sodium deoxycholate, 0.5% sodium dodecyl sulfate, and a protease inhibitor cocktail (Roche, USA). Lysis was accompanied by ultrasonic treatment for 30 s twice on ice. Then, protein levels were measured using a bicinchoninic acid protein assay kit (Thermo Scientific, IL, USA) in a plate reader. The percentage of microbial protein levels was presented based on the dry matter in feces, and the daily excretions of microbial protein were calculated based on the dry matter excretion in feces derived from the N balance trial.
In vitro fermentation trial
Microbiota in feces from each gilt at days 30 and 90 of pregnancy were collected and used for an in vitro fermentation trial. This trial was conducted as previously described but with minor modifications (Mou et al., 2020). Briefly, feces were rectally collected from pregnant gilts, immediately placed in plastic bags containing carbon dioxide, placed in a foam box filled with ice, and transferred to the laboratory within 2 h. Samples were weighed, and a volume of ice-cold sterile PBS (pH 7.0) was added at 1:5 (g/mL). Mixtures were homogenized by a hand-mixer for 30 s and filtered through four sterile cheesecloth layers to collect the filtrate. After this, a 0.2-mL filtrate aliquot was used to measure microbial protein levels as previously described. Filtrates were then adjusted to similar protein levels (as described) and transferred to a glass syringe containing pre-warmed fermentation medium at 39 °C. Per liter, the media contained the following: urea 0.07 g, NH4HCO3 0.4 g, NaHCO3 35 g, Na2HPO4·12H2O 9.45 g, K2HPO4·6.2 g, MgSO4·7H2O 0.6 g, CaCl2·2H2O 13.2 mg, MnCl2·4H2O 10 mg, CoCl2·6H2O 1 mg, FeCl3·6H2O 8 mg, l-cysteine HCl 1 g, and pectin 120 mg. Several resazurin drops were added to indicate anaerobic fermentation. Syringes were sealed with glycerinum, and an anaerobic environment was achieved by flushing the system with N2 gas during the fermentation period. Any gas generated by the fermentation process was recorded at 24 h using a glass syringe reading. Fermentation was stopped at 24 h by immersing the syringe in iced water. Fermentation media were collected to assay for total N, microbial protein levels, and short-chain fatty acids (SCFA) (described below).
SCFA analysis
SCFA analysis, including acetate, propionate, butyrate, isovaleric acid, and valeric acid in fermentation media, feces, and bloods, was performed using Varian CP-3800 gas chromatography as previously described (Zhuo et al., 2020). Briefly, 0.8 g of fecal matter was suspended in 1.5 mL of deionized water in a 15-mL centrifuge tube, vortexed for 10 s, and placed on ice for 30 min. Then, 1 mL of supernatant was collected after centrifugation at 13,500 × g at 4 °C for 15 min. To this volume, 0.2 mL of 25% metaphosphoric acid and 23.3 μL of crotonic acid (210 mmol/L, internal standard) were added, mixed by vortexing, incubated at 4 °C for 30 min, and centrifuged at 13,500 × g for 10 min at 4 °C. From this, 0.3 mL of supernatant was mixed with 0.9 mL methanol and filtered through a 0.22-μm filter (Millipore Co., Bedford, MA, USA). A 1-μL filtrate aliquot was analyzed by gas chromatography (Varian Inc., Lake Forest, CA, USA).
For SCFA detection in serum, 400 μL of the sample was mixed with 50 μL of metaphosphoric acid (25%, w/v) and 4 μL of crotonic acid (21 mmol/L, internal standard). The solution was incubated at 4 °C for 30 min, followed by centrifugation at 14,500 × g at 4 °C for 10 min. Then, 100 μL of supernatant was added to 100 μL of methanol and further centrifuged at 13,500 × g for 15 min. The supernatant was collected and filtered through a 0.22-μm filter (Millipore). A 1 μL aliquot was then analyzed using gas chromatography (Varian). Total acetate, propionate, and butyrate (APB) levels were calculated; total isovaleric and valeric acid levels were calculated as branched-chain fatty acids; and total acetate, propionate, butyrate, isovaleric acid, and valeric acid were calculated as total SCFA.
Microbial analysis
Fecal sample microbial diversity from gilts (n = 10 to 12/group) at days 30, 60, and 90 of pregnancy was determined using high-throughput microbial 16S rRNA sequencing. Briefly, fecal samples were directly collected from the rectum, rapidly frozen in liquid nitrogen, and stored at −80 °C. Microbial DNA was extracted from 0.25 g thawed feces using a QIAamp DNA stool mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Extracted genomic DNA integrity was determined by 1% (w/v) agarose gel electrophoresis, and an absorption ratio of 1.8 to 2.0 at 260/280 nm on a NanoPhotometer spectrophotometer (IMPLEN, CA, USA). DNA samples were sent to Novogene, Beijing, China, for amplicon pyrosequencing on an Illumina MiSeq platform. Microbial 16S rRNA sequencing analysis was conducted as previously described (Cao et al., 2019).
Statistical analysis
A completely randomized design using a 2 × 2 factorial treatment arrangement was used. The data were checked for variance, and normal data distribution was verified using Kolmogorov–Smirnov tests. Data were analyzed by a mixed procedure approach in SAS 9.4 (SAS Institute Inc., Cary, NC, USA) using the following model:
where Yijk is the response variable; μ is the overall mean; α i denotes the CP levels (i = NP and LP levels); β j denotes the DF intake levels (low [LF] and high DF [HF] levels); αβ ij denotes the interaction among fixed effects, and eij is the residual error.
For microbiota analysis at phylum and genus levels, microbiota data were transformed to a log basis before analysis. Microbiota relative abundance data were analyzed using the MIXED procedure in SAS 9.4:
where Yijkl denotes the response variable; µ is the overall mean; αi, βj, and γk denote the fixed effects of dietary CP levels, DF levels, and gestational stages (days 30, 60, and 90). (αβγ)ijk is the interaction among fixed effects, tƖ denotes the random effects of gilts to account for repeated measurements within gilt, and ε ijk is the residual error.
Data were represented as the mean and maximum SEM. The Tukey test was used to compare group means. Differences were considered statistically significant at P < 0.05, and a trend toward significance was considered at 0.05 ≤ P ≤ 0.10.
Results
Culling data
Of the 77 gestating gilts, 6 were removed from the study—2 due to illness, 2 that became lame, and 2 because of very low litter sizes (<6). Their reproductive performance data were not included in the analysis.
Gilt growth traits during gestation
The growth characteristics (BW, BF, and average daily gain [ADG]) of gilts on different diets during gestation are presented in Table 4. The BW and BF thickness at days 1, 30, 60, 90, and 110 of gestation were not significantly affected by CP, DF, or interactions between DF and CP levels (P > 0.05). The ADG during days 1 to 30, 31 to 60, and 61 to 90 of gestation were not significantly influenced by CP, DF, or interactions between CP and DF levels (P > 0.05). Feeding animals an LP diet resulted in a 162.5 g/d and 36.2 g/d lower ADG during days 91 to 110 (P = 0.004) and 1 to 110 (P = 0.024) of gestation, respectively. The ADG was not neither affected by DF levels nor the interaction between CP and DF levels (P > 0.05).
Table 4.
Growth traits of gilts fed different diets during gestation1,2
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Ingredients | −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF |
| BW, kg | ||||||||
| Initial (day 1) | 164.87 | 164.76 | 164.85 | 164.57 | 2.22 | 0.961 | 0.924 | 0.968 |
| Day 30 | 181.46 | 181.32 | 180.62 | 180.75 | 2.65 | 0.757 | 0.999 | 0.954 |
| Day 60 | 200.53 | 199.61 | 201.00 | 199.72 | 2.90 | 0.901 | 0.638 | 0.938 |
| Day 90 | 216.32 | 215.64 | 215.37 | 214.92 | 2.97 | 0.717 | 0.807 | 0.962 |
| Day 110 | 237.86 | 236.31 | 233.92 | 232.08 | 3.14 | 0.100 | 0.492 | 0.953 |
| BF thickness, mm | ||||||||
| Initial (day 1) | 17.65 | 17.71 | 17.32 | 17.00 | 0.82 | 0.409 | 0.832 | 0.755 |
| Day 30 | 18.36 | 18.50 | 18.29 | 17.90 | 0.81 | 0.588 | 0.838 | 0.663 |
| Day 60 | 18.93 | 19.02 | 18.69 | 18.69 | 0.84 | 0.645 | 0.938 | 0.943 |
| Day 90 | 18.95 | 19.44 | 18.94 | 18.70 | 0.88 | 0.561 | 0.847 | 0.566 |
| Day 110 | 19.96 | 19.63 | 19.03 | 19.14 | 0.78 | 0.301 | 0.872 | 0.741 |
| Daily gain, g/d | ||||||||
| Day 1 to 30 | 552.94 | 552.22 | 525.44 | 541.95 | 29.32 | 0.496 | 0.776 | 0.756 |
| Day 31 to 60 | 635.69 | 609.63 | 679.44 | 632.49 | 40.96 | 0.333 | 0.289 | 0.761 |
| Day 61 to 90 | 526.27 | 534.44 | 479.11 | 506.62 | 38.84 | 0.182 | 0.523 | 0.729 |
| Day 91 to 110 | 1,077.06 | 1,033.33 | 927.33 | 858.00 | 74.00 | 0.004 | 0.304 | 0.815 |
| Day 1 to 110 | 663.53 | 650.51 | 627.88 | 613.70 | 19.59 | 0.024 | 0.388 | 0.971 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2 N = 17 to 18 per group.
Feces and urine excretion
Feces and urine excretion quantities and dry matter quantities in feces are presented in Table 5. Daily fecal excretion was significantly increased by DF supplementation on days 35 to 38, 65 to 68, and 95 to 98 of gestation (P < 0.01) and was decreased in the LP diet on days 95 to 98 of gestation (P = 0.002). Dry matter in feces was increased in the LP diet on days 35 to 38 (P = 0.030) of gestation and displayed an elevated tendency in the LP diet on days 65 to 68 (P = 0.089) and 95 to 98 of gestation (P = 0.100), but levels were unaffected by DF or interactions between CP and DF levels (P > 0.05). Urinary excretion on days 35 to 38, 65 to 68, and 95 to 98 of gestation were unaffected by CP, DF, or interactions between CP and DF levels (P > 0.05).
Table 5.
Excretions of dry matter, feces, and urine of gilts fed different diets during gestation1,2
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF | |
| Days 35 to 38 | ||||||||
| Fecal excretions, g/d | 460.1 | 786.6 | 504.2 | 690.4 | 95.9 | 0.662 | <0.001 | 0.245 |
| Dry matter in feces, % | 40.80 | 41.64 | 44.74 | 44.04 | 1.99 | 0.030 | 0.961 | 0.580 |
| Dry matter excretions in feces, g/d | 185.9 | 343.9 | 226.9 | 297.9 | 35.47 | 0.785 | <0.001 | 0.154 |
| Urinary excretions, mL/d | 3,470.7 | 4,688.6 | 4,835.8 | 4,346.3 | 1,077.9 | 0.581 | 0.684 | 0.377 |
| Days 65 to 68 | ||||||||
| Fecal excretions, g/d | 471.6 | 864.1 | 425.8 | 735.3 | 60.1 | 0.186 | <0.001 | 0.227 |
| Dry matter in feces, % | 38.43 | 38.27 | 41.16 | 39.51 | 1.37 | 0.089 | 0.515 | 0.616 |
| Dry matter excretions in feces, g/d | 180.5 | 330.5 | 186.9 | 289.6 | 20.15 | 0.342 | <0.001 | 0.195 |
| Urinary excretions, mL/d | 2,861.3 | 3,597.5 | 2,261.7 | 1,520.0 | 963.5 | 0.635 | 0.453 | 0.412 |
| Days 95 to 98 | ||||||||
| Fecal excretions, g/d | 759.96 | 1,053.85 | 584.77 | 707.85 | 82.3 | 0.002 | 0.001 | 0.438 |
| Dry matter in feces, % | 36.00 | 36.31 | 38.60 | 37.20 | 1.31 | 0.100 | 0.612 | 0.425 |
| Dry matter excretions in feces, g/d | 271.4 | 374.5 | 230.1 | 287.4 | 27.9 | 0.003 | <0.001 | 0.289 |
| Urinary excretions, mL/d | 4,424.17 | 4,113.44 | 4,835.83 | 2,910.00 | 1,490.3 | 0.508 | 0.518 | 0.704 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2 N = 6 to 9 gilts per group at different gestation stages.
Nitrogen utilization
The N utilization levels of gilts fed different diets during gestation are presented in Table 6. At days 35 to 38 of gestation, N levels in feces and N digestibility were unaffected by DF or interactions between CP and DF levels (P > 0.05). Feeding animals an LP diet resulted in significantly lower N urinary levels (P < 0.001), greater N retention (P = 0.029), and higher N utilization (P = 0.011). DF addition resulted in lower urinary N levels (P = 0.015), greater N retention (P = 0.044), higher N utilization (P = 0.049), and increased N net utilization (P = 0.028).
Table 6.
Nitrogen utilization of gilts fed different diets during gestation1,2
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF | |
| Days 35 to 38 | ||||||||
| N intake, g/d | 44.10 | 44.10 | 36.56 | 36.56 | — | — | — | — |
| N in feces, g/d | 5.93 | 6.83 | 6.27 | 5.68 | 0.84 | 0.523 | 0.810 | 0.246 |
| N in urine, g/d | 19.68 | 18.15 | 15.26 | 12.57 | 0.98 | <0.001 | 0.015 | 0.314 |
| N retention, g/d | 18.49 | 19.11 | 15.03 | 18.31 | 1.24 | 0.029 | 0.044 | 0.161 |
| N digestibility, % | 86.54 | 84.51 | 82.85 | 84.47 | 2.29 | 0.254 | 0.899 | 0.266 |
| N utilization (% of absorbed) | 48.29 | 51.27 | 49.78 | 59.20 | 2.66 | 0.011 | 0.049 | 0.115 |
| N net utilization (% of intake) | 41.93 | 43.35 | 41.11 | 50.07 | 2.82 | 0.135 | 0.028 | 0.101 |
| Days 65 to 68 | ||||||||
| N intake, g/d | 44.10 | 44.10 | 36.56 | 36.56 | — | — | — | — |
| N in feces, g/d | 4.90 | 6.60 | 4.90 | 5.56 | 0.63 | 0.273 | 0.019 | 0.273 |
| N in urine, g/d | 21.86 | 19.12 | 17.08 | 12.23 | 1.94 | 0.001 | 0.026 | 0.512 |
| N retention, g/d | 17.34 | 18.38 | 14.58 | 18.77 | 2.24 | 0.477 | 0.126 | 0.349 |
| N digestibility, % | 88.89 | 85.03 | 86.60 | 84.79 | 1.43 | 0.281 | 0.022 | 0.382 |
| N utilization (% of absorbed) | 44.25 | 49.08 | 46.01 | 60.23 | 6.56 | 0.172 | 0.049 | 0.315 |
| N net utilization (% of intake) | 39.32 | 41.68 | 39.88 | 51.33 | 6.13 | 0.233 | 0.099 | 0.286 |
| Days 95 to 98 | ||||||||
| N intake, g/d | 54.96 | 54.96 | 45.58 | 45.14 | — | — | — | — |
| N in feces, g/d | 7.88 | 8.61 | 5.65 | 6.79 | 0.77 | 0.001 | 0.094 | 0.705 |
| N in urine, g/d | 18.37 | 16.60 | 15.06 | 10.89 | 1.94 | 0.002 | 0.029 | 0.356 |
| N retention, g/d | 28.71 | 29.75 | 24.87 | 27.46 | 1.70 | 0.018 | 0.147 | 0.528 |
| N digestibility, % | 85.66 | 84.33 | 87.60 | 84.96 | 1.40 | 0.242 | 0.077 | 0.548 |
| N utilization (% of absorbed) | 61.09 | 64.17 | 62.22 | 71.95 | 4.91 | 0.149 | 0.041 | 0.280 |
| N net utilization (% of intake) | 52.23 | 54.12 | 54.57 | 60.83 | 3.77 | 0.076 | 0.087 | 0.323 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2 N = 6 to 9 per group.
At days 65 to 68 of gestation, the LP diet decreased urinary N levels (P = 0.001), but N in feces, N retention, N digestibility, N utilization, and N net utilization were unaffected (P > 0.05). DF supplementation increased N levels in feces (P = 0.019), decreased urinary N levels (P = 0.026), lowered N digestibility (P = 0.026), increased N utilization (P = 0.049), and displayed an increasing tendency toward N net utilization (P = 0.099).
At days 95 to 98 of gestation, the LP diet resulted in higher N levels in feces (P = 0.001), lower urinary N (P = 0.002), higher N retention (P = 0.018), and an increasing tendency toward N net utilization (P = 0.076). A HF diet resulted in greater N levels in feces (P = 0.094), lower urinary N levels (P = 0.029), increased N utilization (P = 0.041), a decreasing tendency toward N digestibility (P = 0.077), and increased N net utilization (P = 0.076).
No N balance parameters at different gestational stages were affected by interactions between CP and DF levels (P > 0.05).
Microbial protein levels in feces
Microbial protein levels in feces at different gestation stages are presented in Table 7. Microbial protein percentages in feces and daily microbial protein excretion levels at days 35 to 38 and 65 to 68 of gestation were increased by DF supplementation (P < 0.01 or P < 0.05), respectively, but unaffected by CP levels (P > 0.05). At days 95 to 98 of gestation, microbial protein percentages in feces and daily microbial protein excretion levels were increased by DF (P < 0.01), and daily microbial protein excretion levels were decreased by an LP diet (P < 0.001). Neither microbial protein levels in feces nor daily microbial protein excretion levels at different gestational stages were affected by interactions between CP and DF levels (P > 0.05).
Table 7.
Effects of dietary treatment on the microbial protein excretions in feces of gilts during gestation1,2
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF | |
| Days 35 to 38 | ||||||||
| Microbial protein (% of dry matter) | 66.91 | 82.88 | 64.52 | 83.27 | 4.30 | 0.831 | <0.001 | 0.772 |
| Microbial protein excretion, g/d | 3.86 | 5.56 | 4.02 | 4.69 | 0.48 | 0.468 | 0.022 | 0.298 |
| Days 65 to 68 | ||||||||
| Microbial protein (% of dry matter) | 67.63 | 76.45 | 65.36 | 83.34 | 4.34 | 0.86 | 0.002 | 0.891 |
| Microbial protein excretion, g/d | 3.32 | 5.04 | 3.21 | 4.63 | 0.44 | 0.628 | 0.035 | 0.312 |
| Days 95 to 98 | ||||||||
| Microbial protein (% of dry matter) | 67.42 | 77.30 | 65.97 | 80.00 | 3.42 | 0.861 | 0.003 | 0.564 |
| Microbial protein excretion, g/d | 5.17 | 6.58 | 3.57 | 5.43 | 0.28 | <0.001 | <0.001 | 0.420 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2 N = 6 to 9 gilts per group at different gestation stages.
Litter performance
The litter performance of gilts fed different diets during gestation is presented in Table 8. The numbers of total piglets born alive and total piglets born effective were unaffected by CP, DF, and interactions between CP and DF levels (P > 0.05). Total piglets born were elevated by an LP diet (P = 0.022), and the numbers of stillborns were also increased by an LP diet (P = 0.046). The birth weight of total piglets born (P = 0.006) and birth weights of piglets born alive (P = 0.019) were decreased by an LP diet, but not by DF, nor were there interactions between CP and DF levels (P > 0.05). Litter weight, intra-litter CV, placenta weight, and placenta efficiency were unaffected by CP, DF, and interactions between CP and DF levels (P > 0.05).
Table 8.
Reproductive performance of gilts fed different diets during gestation1
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF | |
| No. of gilts | 18 | 18 | 18 | 17 | — | — | — | |
| No. of total born2 | 12.76 | 12.69 | 13.73 | 14.93 | 0.81 | 0.022 | 0.417 | 0.356 |
| Total born alive | 11.82 | 11.63 | 11.87 | 13.14 | 0.64 | 0.208 | 0.383 | 0.234 |
| Total born effective3 | 11.53 | 11.31 | 11.53 | 12.50 | 0.75 | 0.348 | 0.554 | 0.351 |
| Stillborn | 0.94 | 1.06 | 1.87 | 1.79 | 0.54 | 0.046 | 0.960 | 0.803 |
| Number of IUGR per litter4 | 0.29 | 0.31 | 0.33 | 0.64 | 0.50 | 0.469 | 0.521 | 0.568 |
| Litter weight of total born without mummies, kg | 19.24 | 18.80 | 18.74 | 19.98 | 1.07 | 0.717 | 0.672 | 0.374 |
| Birthweight of total born without mummies, kg | 1.52 | 1.51 | 1.37 | 1.36 | 0.07 | 0.006 | 0.894 | 0.990 |
| Litter weight alive, kg | 17.69 | 17.39 | 16.61 | 17.81 | 0.97 | 0.721 | 0.630 | 0.416 |
| Birthweight of alive born, kg | 1.51 | 1.51 | 1.41 | 1.37 | 0.07 | 0.019 | 0.771 | 0.666 |
| Intra-litter CV | 17.10 | 17.95 | 18.07 | 19.16 | 1.89 | 0.494 | 0.544 | 0.940 |
| Placenta weight, kg | 4.24 | 4.05 | 3.76 | 4.43 | 0.38 | 0.862 | 0.429 | 0.165 |
| Placenta efficiency | 4.54 | 4.62 | 4.97 | 4.53 | 0.52 | 0.427 | 0.522 | 0.297 |
| Parturition length, min | 290.3 | 216.3 | 282.1 | 218.9 | 49.92 | 0.939 | 0.070 | 0.885 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2Including stillborn piglets and without mummies.
3Born effective = born alive – IUGR.
4IUGR, intrauterine growth-retarded piglets, birth weight below 2 SD of average birth weight.
SCFA levels in feces
The effects of DF supplementation on normal or LP diets during gestation on SCFA levels in gilt feces are presented in Table 9. Levels of acetate, propionate, butyrate, isobutyric acid, isovaleric acid, valeric acid, and total SCFAs were unaffected by CP, DF, or interactions between CP and DF levels during gestation (P > 0.05). At day 90 of gestation, acetate, propionate, butyrate (P = 0.057), isobutyric acid, and total SCFA levels (P = 0.053) were affected by interactions between CP and DF levels (P < 0.05). Levels of isovaleric acid were decreased by DF (P = 0.036) but not by CP or interactions between CP and DF levels (P > 0.05).
Table 9.
SCFA in feces of gestating gilts fed different diets1,2,3
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF | |
| Days 30 of gestation | ||||||||
| Acetate, μmol/g | 54.39 | 49.41 | 44.01 | 41.44 | 5.29 | 0.090 | 0.480 | 0.820 |
| Propionate, μmol/g | 17.81 | 17.83 | 16.49 | 14.73 | 1.82 | 0.232 | 0.636 | 0.628 |
| Butyrate, μmol/g | 7.16 | 8.50 | 8.39 | 7.49 | 0.85 | 0.896 | 0.798 | 0.191 |
| Isobutyric acid, μmol/g | 1.52 | 1.74 | 1.47 | 1.58 | 0.18 | 0.550 | 0.351 | 0.768 |
| Isovaleric acid, μmol/g | 3.19 | 2.64 | 3.21 | 2.05 | 0.61 | 0.639 | 0.167 | 0.615 |
| Valeric acid, μmol/g | 1.99 | 1.41 | 1.35 | 1.85 | 0.47 | 0.835 | 0.929 | 0.259 |
| Sum of SCFAs, μmol/g | 88.06 | 85.51 | 75.92 | 72.14 | 8.13 | 0.124 | 0.699 | 0.939 |
| Days 90 of gestation | ||||||||
| Acetate, μmol/g | 54.02b | 44.72ab | 42.16a | 48.28ab | 3.46 | 0.236 | 0.647 | 0.031 |
| Propionate, μmol/g | 19.97b | 16.23ab | 15.05a | 18.04ab | 1.56 | 0.324 | 0.813 | 0.037 |
| Butyrate, μmol/g | 10.89b | 8.38a | 8.19a | 8.94ab | 0.84 | 0.207 | 0.300 | 0.057 |
| Isobutyric acid, μmol/g | 2.39b | 1.73a | 1.61a | 1.97ab | 0.18 | 0.139 | 0.403 | 0.006 |
| Isovaleric acid, μmol/g | 3.18 | 2.35 | 2.75 | 2.52 | 0.25 | 0.610 | 0.036 | 0.229 |
| Valeric acid, μmol/g | 1.44 | 1.26 | 1.22 | 1.30 | 0.11 | 0.446 | 0.689 | 0.266 |
| Sum of SCFAs, μmol/g | 93.88b | 78.50ab | 71.99a | 84.05ab | 5.98 | 0.178 | 0.783 | 0.026 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2 N = 12 per group.
3SCFA, the sum of acetate, propionate, butyrate, isovaleric acid, and valeric acid contents.
a,bMeans with different superscript letters denote P < 0.05.
SCFA serum levels
The effects of DF in normal or LP diets on serum SCFA levels in gilt serum are presented in Table 10. At day 30 of gestation, serum butyrate levels were increased by an LP diet. Valeric acid levels displayed an increasing tendency by DF (P = 0.088). Serum acetate, propionate, isovaleric acids, and total SCFA levels at day 30 of gestation were unaffected by CP, DF, and interactions between CP and DF levels (P > 0.05).
Table 10.
SCFA in serum of gestating gilts fed different diets1,2,3
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF | |
| Day 30 of gestation | ||||||||
| Acetate, μmol/L | 275.26 | 235.60 | 225.03 | 251.40 | 37.15 | 0.414 | 0.752 | 0.121 |
| Propionate, μmol/L | 38.33 | 37.81 | 32.71 | 33.63 | 6.79 | 0.339 | 0.968 | 0.888 |
| Butyrate, μmol/L | 2.45 | 2.79 | 4.49 | 4.19 | 1.19 | 0.007 | 0.977 | 0.593 |
| Isovaleric acid, μmol/L | 18.63 | 18.71 | 17.43 | 20.31 | 2.74 | 0.920 | 0.465 | 0.490 |
| Valeric acid, μmol/L | 21.71 | 18.49 | 21.39 | 18.39 | 2.40 | 0.906 | 0.088 | 0.950 |
| Total SCFAs, μmol/g | 356.39 | 314.46 | 301.05 | 327.91 | 39.50 | 0.378 | 0.750 | 0.151 |
| Day 90 of gestation | ||||||||
| Acetate, μmol/L | 256.01 | 258.88 | 259.91 | 261.05 | 18.98 | 0.812 | 0.876 | 0.947 |
| Propionate, μmol/L | 23.42 | 23.62 | 27.01 | 35.45 | 2.85 | 0.002 | 0.066 | 0.079 |
| Butyrate, μmol/L | 3.57 | 4.65 | 3.63 | 3.13 | 0.78 | 0.182 | 0.593 | 0.149 |
| Isovaleric acid, μmol/L | 22.30ab | 18.38ab | 17.36a | 23.09b | 3.03 | 0.955 | 0.655 | 0.021 |
| Valeric acid, μmol/L | 29.58 | 21.69 | 20.15 | 18.15 | 8.77 | 0.115 | 0.226 | 0.469 |
| Total SCFAs, μmol/g | 334.92 | 327.21 | 328.07 | 340.87 | 22.59 | 0.830 | 0.873 | 0.519 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2 N = 12 per group.
3SCFA, the sum of acetate, propionate, butyrate, isovaleric acid, and valeric acid contents.
a,bMeans with different superscript letters denote P < 0.05.
On day 90, serum propionate levels were increased by the LP diet (P = 0.002) and displayed an increasing tendency from DF supplementation (P = 0.066). Serum propionate levels displayed a tendency to be affected by interactions between CP and DF levels (P = 0.079). Serum isovaleric acid levels were affected by interactions between CP and DF levels (P = 0.021), and serum isovaleric acid levels were increased by DF in gilts fed the LP diet (P < 0.05) but were unaffected by DF in gilts fed the NP diet (P > 0.05). Serum acetate, butyrate, valeric acid, and total SCFA levels at day 90 were unaffected by CP, DF, and interactions between CP and DF levels (P > 0.05).
Microbial metabolism during the in vitro fermentation trial
Microbial protein levels in culture media during the in vitro fermentation trial are presented in Table 11. For samples taken at days 30 and 90 of gestation, initial microbial protein levels (baseline) were approximately 1.0 mg/mL (P > 0.05). Microbial protein levels at 24 h and percentage changes in microbial protein levels at 24 h on days 30 and 90 of gestation were greater in the fecal microbiota of gilts fed HF during gestation (P < 0.05 or P < 0.01) but were unaffected by CP levels or interactions between CP and DF levels during gestation (P > 0.05). Gas production and percentage changes in gas production at 24 h on days 30 and 90 of gestation were unaffected by dietary treatments during gestation.
Table 11.
Microbial protein content in culture media during in vitro fermentation trial1,2,3
| NP | LP | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| −DF | +DF | −DF | +DF | SEM | CP | DF | CP × DF | |
| Days 30 of gestation | ||||||||
| Microbial protein at baseline, mg/mL | 0.99 | 1.01 | 0.97 | 1.00 | 0.021 | 0.397 | 0.351 | 0.711 |
| Microbial protein at 24 h, mg/mL | 1.33 | 1.45 | 1.31 | 1.63 | 0.072 | 0.146 | <0.001 | 0.075 |
| Percentage changes of microbial protein at 24 h, % | 33.90 | 44.55 | 34.66 | 63.53 | 6.84 | 0.078 | <0.001 | 0.103 |
| Gas production at 24 h, mL | 24.0 | 26.8 | 24.0 | 27.1 | 1.84 | 0.062 | 0.916 | 0.836 |
| Percentage changes of Gas production at 24 h, % | 41.60 | 47.07 | 41.62 | 46.18 | 3.41 | 0.078 | 0.875 | 0.871 |
| Days 90 of gestation | ||||||||
| Microbial protein at baseline, mg/mL | 1.01 | 1.02 | 0.96 | 0.97 | 0.041 | 0.069 | 0.771 | 0.963 |
| Microbial protein at 24 h, mg/mL | 1.30 | 1.46 | 1.30 | 1.56 | 0.10 | 0.470 | 0.010 | 0.492 |
| Percentage changes of microbial protein at 24 h, % | 29.60 | 42.59 | 35.14 | 62.56 | 8.36 | 0.064 | 0.004 | 0.288 |
| Gas production at 24 h, mL | 25.08 | 26.92 | 25.33 | 27.33 | 2.09 | 0.928 | 0.347 | 0.964 |
| Percentage changes of Gas production at 24 h, % | 42.94 | 47.30 | 44.08 | 46.87 | 3.90 | 0.913 | 0.207 | 0.810 |
1NP, normal CP; LP, low CP; DF, dietary fiber. DF mixture consisted of pectin, inulin, and cellulose at the ratio of 1:10:34.
2Data are means and maximum SEM.
3 N = 12 at each stage of gestation.
The effects of dietary treatments during gestation on gilt fecal microbial diversity
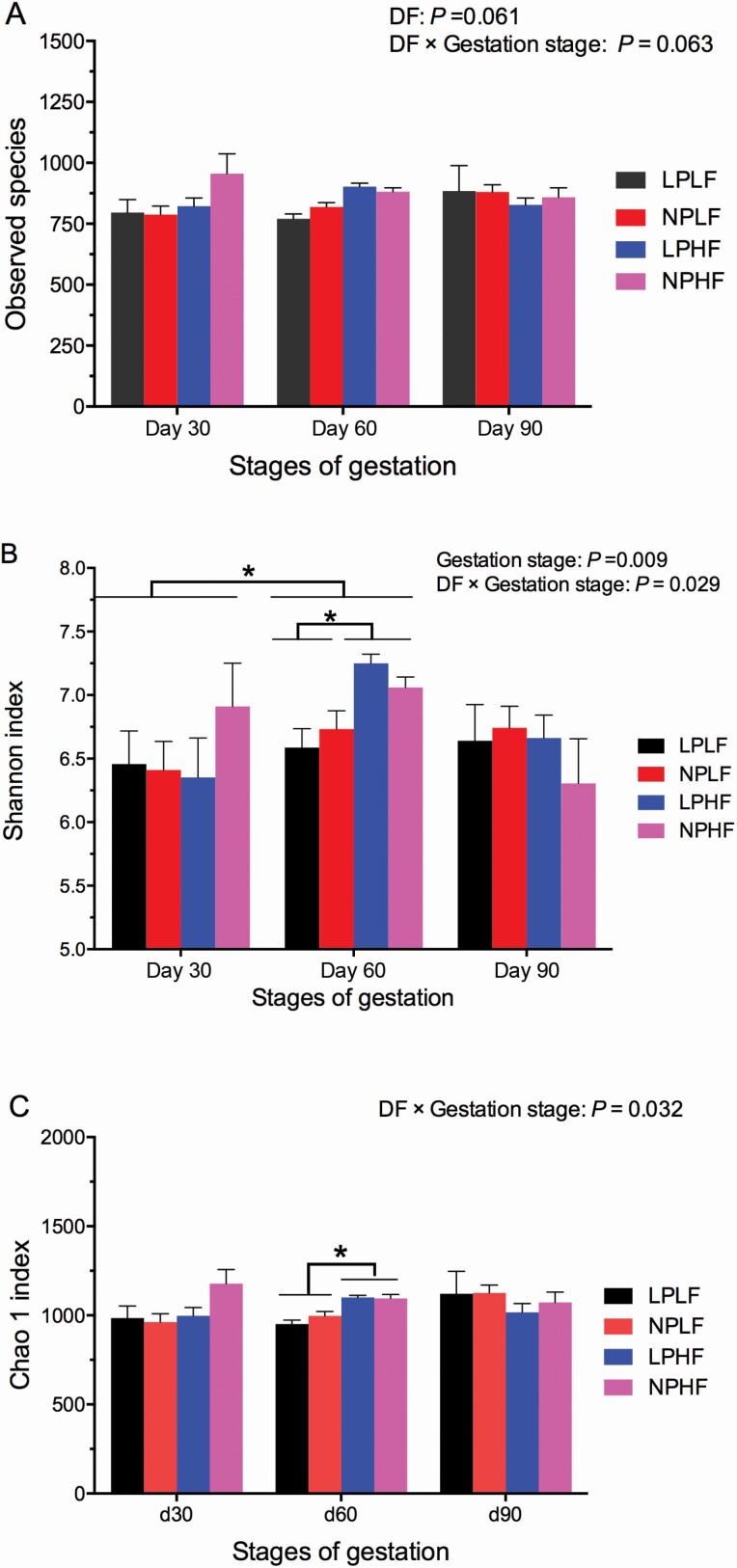
To assess changes in fecal microbial alpha-diversity, observed species, Shannon, and Chao 1 indices were measured (Figure 1A–C). The observed species displayed an increasing tendency from DF supplementation (P = 0.061, Figure 1A) or were affected by interactions between CP and DF levels (P = 0.063, Figure 1A). The Shannon index was significantly increased by gestation stage (P = 0.009, Figure 1B) and interactions between DF and gestation stage (P = 0.029, Figure 1B). The Chao 1 index was affected by interactions between DF and gestation stage (P = 0.032, Figure 1C).
Figure 1.
Microbiota alpha-diversity in feces of sows fed different DF levels at different gestation stages. Sow was regarded as the experimental units (n = 10 to 12). (A) Observed species; (B) Shannon index, and (C) Chao 1 index. NPLF, NP level without DF supplement; NPHF, NP level with DF supplement; LPLF, LP level without DF supplement; and LPHF, LP level with DF supplement. *denote P < 0.05.
The relative phylum abundance in gilt feces during gestation is presented in Table 12. We observed two dominant phyla: Firmicutes and Bacteroidetes, which accounted for >90% of total fecal microbiota. Bacteroidetes levels were affected by the gestation stage (P = 0.018), which were greater at day 60 than day 30. Proteobacteria levels displayed an increasing tendency from DF supplementation (P = 0.087). Tenericutes levels were increased by gestation stage (P = 0.030) and interactions between DF levels and gestation stage (P = 0.028). Actinobacteria levels displayed a tendency to be affected by the interaction of DF, CP, and gestational stage (P = 0.058). Spirochaetes (P = 0.011) and unidentified_Bacteria (P = 0.052) were increased at gestational stage. Euryarchaeota were affected by gestational stage (P < 0.001) and interactions between CP and gestational stage (P < 0.044). The Firmicutes to Bacteroidetes ratio displayed a decreasing tendency from DF supplementation (P = 0.061) and interactions between DF levels and gestational stage (P = 0.076).
Table 12.
The relative abundances of nine phyla (%, >1% in at least one sample) and Firmicutes/Bacteroidetes ratio during gestation1,2,3
| DF levels | CP level | Gestation stage | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LF | HF | LP | NP | Day 30 | Day 60 | Day 90 | SEM | DF | CP | S | DF × CP | DF × S | CP × S | |
| Firmicutes | 79.00 | 75.92 | 78.15 | 76.81 | 77.59 | 76.63 | 78.18 | 2.15 | 0.138 | 0.425 | 0.682 | 0.973 | 0.073 | 0.368 |
| Bacteroidetes | 13.35 | 15.99 | 14.63 | 14.71 | 13.76b | 16.64a | 13.66ab | 1.18 | 0.127 | 0.753 | 0.018 | 0.124 | 0.085 | 0.853 |
| Proteobacteria | 1.10 | 1.79 | 1.16 | 1.72 | 1.81 | 1.01 | 1.49 | 0.87 | 0.087 | 0.953 | 0.062 | 0.101 | 0.084 | 0.639 |
| Tenericutes | 2.06 | 1.96 | 2.06 | 1.97 | 2.10ab | 1.28b | 2.69a | 0.53 | 0.873 | 0.906 | 0.030 | 0.131 | 0.028 | 0.089 |
| Acidobacteria | 0.16 | 0.61 | 0.14 | 0.71 | 0.74 | 0.02 | 0.15 | 0.60 | 0.204 | 0.470 | 0.765 | 0.764 | 0.349 | 0.791 |
| Actinobacteria | 0.36 | 0.52 | 0.35 | 0.53 | 0.68 | 0.27 | 0.34 | 0.32 | 0.696 | 0.712 | 0.639 | 0.362 | 0.829 | 0.134 |
| Spirochaetes | 3.40 | 2.46 | 2.88 | 2.97 | 2.69b | 3.49a | 2.61b | 0.49 | 0.196 | 0.816 | 0.011 | 0.450 | 0.878 | 0.270 |
| unidentified_Bacteria | 0.20 | 0.27 | 0.25 | 0.22 | 0.19ab | 0.16b | 0.36a | 0.11 | 0.297 | 0.188 | 0.052 | 0.808 | 0.205 | 0.932 |
| Euryarchaeota | 0.25 | 0.21 | 0.19 | 0.27 | 0.15b | 0.29a | 0.25b | 0.06 | 0.676 | 0.346 | <0.001 | 0.401 | 0.122 | 0.044 |
| Firmicutes/Bacteroidetes | 9.20 | 6.65 | 8.70 | 7.20 | 10.04 | 5.46 | 8.06 | 2.16 | 0.061 | 0.729 | 0.470 | 0.546 | 0.076 | 0.537 |
1Data are means and maximum SEM. N = 10 to 12 at each stage of gestation.
2S, stage of gestation; LF, low DF; HF, high DF.
3There was no three-way interaction of DF × CP × S.
a,bMeans with different superscript letters denote P < 0.05 between groups.
The relative abundance of microbiota at the genus level (>1%) is presented in Table 13. The relative abundances of Bacteroides, unidentified_Ruminococcaceae, and unidentified_Christensenellaceae were decreased by DF supplementation (P < 0.05 or P < 0.01), and the relative abundance of Faecalibacterium, Phascolarctobacterium, Oscillibacter, and Alloprevotella was increased by DF supplementation (P < 0.05 or P < 0.01). The relative abundance of Faecalibacterium was increased by CP levels during gestation (P = 0.020). The relative abundance of 17 of the 27 genera, including unidentified_Clostridiales, Streptococcus, Lactobacillus, Terrisporobacter, Turicibacter, Succinivibrio, unidentified_Spirochaetaceae, unidentified_Lachnospiraceae, Romboutsia, unidentified_Christensenellaceae, Enterococcus, Methanobrevibacter, unidentified_Prevotellaceae, Phascolarctobacterium, Anaerovibrio, Oscillibacter, and Desulfovibrio, was all increased by gestational stages (P < 0.05 or P < 0.01). Microbiota relative abundance was unaffected by interactions between DF and CP levels (P > 0.05). The relative abundance of Terrisporobacter, Succinivibrio, Romboutsia, unidentified_Christensenellaceae, Enterococcus, Methanobrevibacter, unidentified_Prevotellaceae, Phascolarctobacterium, unidentified_Bacteroidales, Alloprevotella, and Desulfovibrio was affected by interactions between DF levels and gestational stages (P < 0.05 or P < 0.01). The relative abundance of unidentified_Clostridiales, Terrisporobacter, Turicibacter, Enterococcus, Methanobrevibacter, Oscillospira, and Phascolarctobacterium was affected by interactions between CP levels and gestational stages (P < 0.05 or P < 0.01). The relative abundance of unidentified_Bacteroidales was affected by interactions between DF levels, CP levels, and gestational stages (P = 0.028).
Table 13.
The relative abundances of 27 genera (%, >1% in at least one sample) during gestation1,2,3
| Fiber level | Protein level | Gestation stage | P-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LF | HF | LP | NP | Day 30 | Day 60 | Day 90 | SEM | DF | CP | S | DF × CP | DF × S | CP × S | |
| unidentified_Clostridiales | 13.55 | 13.00 | 14.65 | 11.99 | 11.86b | 15.89a | 12.16b | 2.19 | 0.413 | 0.230 | <0.001 | 0.380 | 0.389 | 0.012 |
| Bacteroides | 0.52a | 0.40b | 0.49 | 0.43 | 0.46 | 0.45 | 0.47 | 0.09 | 0.022 | 0.108 | 0.495 | 0.289 | 0.067 | 0.622 |
| Streptococcus | 10.76 | 6.94 | 7.89 | 9.74 | 6.56b | 13.69a | 6.41b | 1.20 | 0.112 | 0.947 | <0.001 | 0.319 | 0.649 | 0.101 |
| Lactobacillus | 1.93 | 2.46 | 2.42 | 1.99 | 2.09b | 2.72a | 1.77b | 0.59 | 0.588 | 0.657 | 0.001 | 0.079 | 0.546 | 0.211 |
| Terrisporobacter | 2.05 | 2.24 | 2.07 | 2.21 | 1.81b | 2.88a | 1.76b | 0.36 | 0.427 | 0.802 | <0.001 | 0.326 | 0.026 | 0.041 |
| Turicibacter | 2.12 | 2.29 | 2.27 | 2.14 | 1.90b | 2.80a | 1.92b | 0.40 | 0.357 | 0.456 | <0.001 | 0.535 | 0.169 | 0.009 |
| Succinivibrio | 0.28 | 0.18 | 0.22 | 0.25 | 0.12b | 0.23a | 0.36ab | 0.14 | 0.605 | 0.820 | 0.045 | 0.257 | 0.043 | 0.416 |
| unidentified_Ruminococcaceae | 3.86 | 2.97 | 3.39 | 3.44 | 3.31 | 2.99 | 3.99 | 0.39 | 0.053 | 0.957 | 0.234 | 0.098 | 0.529 | 0.160 |
| unidentified_Spirochaetaceae | 2.38 | 1.78 | 2.03 | 2.12 | 1.75b | 2.60a | 1.90ab | 0.44 | 0.372 | 0.811 | 0.023 | 0.343 | 0.908 | 0.246 |
| unidentified_Lachnospiraceae | 0.51 | 0.64 | 0.51 | 0.64 | 0.57b | 0.63a | 0.52ab | 0.18 | 0.935 | 0.816 | 0.003 | 0.300 | 0.180 | 0.255 |
| Sarcina | 0.05 | 0.08 | 0.08 | 0.04 | 0.06 | 0.04 | 0.08 | 0.04 | 0.347 | 0.339 | 0.385 | 0.946 | 0.263 | 0.634 |
| Campylobacter | 0.12 | 0.21 | 0.19 | 0.14 | 0.11 | 0.12 | 0.27 | 0.11 | 0.171 | 0.911 | 0.276 | 0.459 | 0.418 | 0.583 |
| Romboutsia | 1.20 | 1.26 | 1.18 | 1.27 | 1.08b | 1.52a | 1.10b | 0.20 | 0.405 | 0.887 | <0.001 | 0.304 | 0.046 | 0.093 |
| unidentified_Christensenellaceae | 0.86a | 0.50b | 0.62 | 0.73 | 0.58b | 0.85a | 0.63b | 0.15 | 0.035 | 0.293 | 0.018 | 0.763 | 0.167 | 0.516 |
| Enterococcus | 0.03 | 0.03 | 0.04 | 0.02 | 0.03a | 0.02b | 0.04a | 0.01 | 0.357 | 0.456 | 0.000 | 0.535 | 0.169 | 0.009 |
| Methanobrevibacter | 0.25 | 0.20 | 0.18 | 0.26 | 0.15b | 0.29a | 0.25b | 0.06 | 0.542 | 0.366 | <0.001 | 0.450 | 0.127 | 0.037 |
| unidentified_Prevotellaceae | 1.42 | 1.58 | 1.43 | 1.57 | 1.52 | 1.73 | 1.24 | 0.17 | 0.594 | 0.344 | 0.041 | 0.055 | 0.050 | 0.235 |
| Oscillospira | 0.55 | 0.65 | 0.54 | 0.65 | 0.65 | 0.59 | 0.54 | 0.11 | 0.289 | 0.205 | 0.718 | 0.237 | 0.567 | 0.028 |
| Faecalibacterium | 0.11b | 0.13a | 0.06b | 0.17a | 0.07 | 0.10 | 0.21 | 0.12 | <0.001 | 0.020 | 0.200 | 0.678 | 0.405 | 0.415 |
| Parabacteroides | 0.88 | 0.97 | 1.01 | 0.85 | 0.93 | 0.95 | 0.90 | 0.12 | 0.960 | 0.312 | 0.077 | 0.102 | 0.230 | 0.324 |
| Phascolarctobacterium | 0.45b | 0.67a | 0.51 | 0.60 | 0.52b | 0.66a | 0.50ab | 0.07 | 0.033 | 0.455 | 0.023 | 0.168 | 0.144 | 0.017 |
| Bifidobacterium | 0.15 | 0.08 | 0.13 | 0.10 | 0.14 | 0.12 | 0.09 | 0.04 | 0.960 | 0.312 | 0.077 | 0.102 | 0.230 | 0.324 |
| Anaerovibrio | 0.08 | 0.17 | 0.12 | 0.13 | 0.08b | 0.14a | 0.15ab | 0.04 | 0.155 | 0.902 | 0.031 | 0.657 | 0.330 | 0.768 |
| unidentified_Bacteroidales | 0.13 | 0.16 | 0.14 | 0.15 | 0.20 | 0.09 | 0.15 | 0.05 | 0.487 | 0.355 | 0.997 | 0.413 | 0.003 | 0.267 |
| Oscillibacter | 0.25b | 0.37a | 0.33 | 0.29 | 0.23b | 0.43a | 0.27b | 0.04 | 0.011 | 0.137 | <0.001 | 0.700 | 0.325 | 0.766 |
| Alloprevotella | 0.13b | 0.30a | 0.20 | 0.24 | 0.16 | 0.28 | 0.21 | 0.05 | <0.001 | 0.520 | 0.052 | 0.385 | 0.011 | 0.805 |
| Desulfovibrio | 0.22 | 0.32 | 0.31 | 0.23 | 0.18b | 0.30a | 0.34a | 0.05 | 0.243 | 0.092 | <0.001 | 0.132 | 0.046 | 0.719 |
1Data are means and maximum SEM. N = 10 to 12 at each stage of gestation.
2S, stage of gestation; LF, low DF; HF, high DF.
3There was no three-way interaction of DF × CP × S.
a,bMeans with different superscript letters denote P < 0.05 between groups.
Discussion
The effects of DF supplementation in NP or LP diets on N utilization in gilts
Most of the research on the effects of low-protein diets in pigs has focused on young piglets or growing-finisher pigs (Wang et al., 2018). However, the efficacy of such diets in gilts remains unclear. One reason for this may be that gilts have a mature body composition, and their AA requirements are usually lower than growing and finisher pigs. In this study, CP dietary levels were reduced from 12.86% to 10.63% by replacing soybean meal with crystalline AA to balance essential AA. Thus, reduced CP was potentially attributable to decreased nonessential AA. Similar to the effects of LP diets for growing pigs (Wang et al., 2018), the ADG of gilts during days 1 to 30, 31 to 60, and 61 to 90 of gestation was unaffected by an LP diet, suggesting such diets can be used for gestating gilts at early or middle gestation stages without affecting growth performance, if essential AA are balanced. However, the ADG of gestating gilts was reduced by the LP diet when compared with the NP diet at days 91 to 110 of gestation, suggesting that N limitation by decreased nonessential AA from an LP diet was a limiting factor affecting whole-body N metabolism at late gestational stages. This observation may be attributed to the greater demands of certain nonessential AA requirements for fetal growth or mammary gland development at late gestation. However, the identification of limiting AA requires further investigation.
To examine N utilization in gestating gilts, an N balance trial was conducted over three gestational stages. Nitrogen excretion in feces was not influenced by an LP diet at days 35 to 38 and 65 to 68 of gestation. However, fecal N excretion at days 95 to 98 was significantly decreased by the LP diet. When associated with decreased ADG at days 91 to 110 of gestation, this N decrease in feces may be an adaptive response to increased N demands for fetal growth at late gestation. Our findings also revealed that urinary N levels at days 35 to 38, 65 to 68, and 95 to 98 of gestation were decreased by the LP diet, which agreed with a previous study in growing pigs (Wang et al., 2018) and lactating sows (Pedersen et al., 2019). However, N retention (retained N/absorbed N) was differentially affected by gestational CP levels at different gestational stages, with significant increases in N retention ratios at days 35 to 38 of gestation but unchanged at days 65 to 68 and 95 to 98. The reasons for these gestation stage-dependent differences in N retention are unclear but may be related to increased AA requirements for pregnancy-associated growth, that is, increased AA levels were mobilized for mammary gland growth at late gestation, which potentially decreased N retention (Li et al., 2009; Chen et al., 2018)
In this study, extra DF was supplemented to gestational diets because HF levels are considered essential for gestating sows (Jarrett and Ashworth, 2018). DF inclusion in previous studies was achieved by formulating high levels of fiber-rich ingredients, for example, sugar beet pulp and soybean hull (Jarrett and Ashworth, 2018). However, many of the nutrients are contained in these ingredients. Therefore, it is difficult to assess whether beneficial effects were exclusively attributed to DF. Thus, the extracted DF components (pectin, inulin, and cellulose) were used to evaluate the direct effects of DF (Zhuo et al., 2020, 2021). In this study, two soluble DF sources, pectin and inulin, and one insoluble source, cellulose, were used, because a mixture of dietary fermentable fibers was previously shown to increase microbial diversity and metabolic change (Mohamed et al., 2019; Uerlings et al., 2019). In our study, DF supplementation did not affect growth rates and BF traits, which was inconsistent with our previous work showing that DF altered BF thickness in growing replacement gilts and gestating sows when supplemented with high-energy-density diets (Wang et al., 2016; Zhuo et al., 2020). However, this current study data agreed with other research from our laboratory (Li et al., 2019; Zhuo et al., 2020) and other work (Jarrett and Ashworth, 2018).
One key finding from the N balance trial was that DF supplementation decreased urinary N excretion and increased fecal N excretion, which agreed with previous study showing that dietary beet pulp in growing pigs increased fecal N and reduced urinary N (Patrás et al., 2012). From our work, DF decreased urinary N excretion by 2.1, 3.8, and 3.0 g/d but increased fecal N excretion by 0.16, 1.2, and 0.94 g/d at days 35 to 38, 65 to 68, and 95 to 98 of gestation, respectively. The net N retention in gestating gilts fed DF was elevated by 1.9, 2.6, and 2.0 g/d at days 35 to 38, 65 to 68, and 95 to 98 of gestation, respectively. DF has long been eschewed in animal nutrition due to its anti-nutritional effects on nutrient digestion and absorption. However, our findings revealed the beneficial effect of DF on N utilization. Therefore, in combination with previous data in growing piglets (Patrás et al., 2012), our studies provide new insights into DF effects on nutrient utilization. In the present study, the N utilization appeared to be affected by the stage of gestation, which was greater at days 95 to 98 of gestation (on average, 64.9%) than at day 35 to 38 of gestation (on average, 52.1%) or at day 65 to 68 of gestation (on average, 49.9%). However, feed intake during days 95 to 98 of gestation was greater than in early or middle gestation, we could not exclude the effects of feed intake on the N utilization, since feeding level affected the N utilization (Miller et al., 2016).
Fecal N can be generally divided into two components: microbial protein N and non-microbial protein N. To measure the N form in feces, fecal microbial protein levels were determined. We observed that microbial protein N was the main source (60% to 80%) of total N in feces, suggesting gut microbiota may have an important role in regulating N excretion. Since DF was considered the primary factor driving the proliferation and the diversity of microbiota in the porcine gut (Aluthge et al., 2019; Tiwari et al., 2019), and the growth of microbiota required both carbon and N as substrates (Oliphant and Allen-Vercoe, 2019), the microbial protein content was measured and found that DF addition significantly increased the microbial protein content and excretions in feces. Our findings also suggested another DF benefit that was related to environmental pollution. Urea from excreted urine enters the environment and may be rapidly converted to ammonia by ureases to generate unpleasant air pollution, whereas microbial proteins are more stable than urea which slow hydrolysis and decrease unpleasant air such as NH3 (Ndegwa et al., 2008).
Gut microbiota may have important roles in N balance regulation. Intestinal permeability characteristics have revealed that the gastrointestinal tract mucosa is considered the largest semipermeable tissue enabling small nutrient metabolite exchange between the bloodstream and gut lumen (Balimane and Chong, 2005; Zheng et al., 2020). Previously, it was shown that several microbiota species efficiently exploit nonprotein N sources to synthesize AA, peptides, and proteins for their own growth (Torrallardona et al., 2003; Mansilla et al., 2015; Zheng et al., 2020), suggesting that gut microbiota effectively clear nitrogenous metabolic waste from the body. Thus, we conducted an in vitro fermentation trial to test this hypothesis and asked whether DF-directed changes in microbial diversity could alter N metabolism with a nonprotein N source. Gas production, which is an indicator of microbial metabolism (Mou et al., 2020), increased as in vitro fermentation progressed, suggesting that microbiota survived and executed normal functions when urea and ammonium bicarbonate were the only N sources. Microbial protein synthesis, as represented by fold change in microbial protein levels at 24 h fermentation, was significantly increased in feces from gestating gilts fed HF levels, suggesting that their gut microbiota had an increased capacity to use nonprotein nitrogenous components. Indeed, Mansilla et al. (2017a, 2017b, 2018) conducted elegant studies showing that the fecal infusion of nonprotein N to pigs fed low-protein diets deficient in nonessential AA improved whole-body metabolism. One explanation could be that microbiota converted nonprotein N to AA and peptides that benefited whole-body metabolism. In our study, several microbiota species such as Bacteroides, unidentified_Ruminococcaceae, unidentified_Christensenellaceae, Faecalibacterium, Phascolarctobacterium, Oscillibacter, and Alloprevotella were altered by DF treatments during gestation. Therefore, it will be interesting to ascertain which specific species were responsible for these N-usage effects. However, we cannot exclude the possibility that other physiological mechanisms were involved in N control by DF. For example, the SCFA, acetate, propionate, and butyrate could be used as primary nutrients for gut epithelial cells and hepatocytes, which may, in turn, reduce the oxidation of AA as energy sources, since this process largely occurs in gut epithelial cells and hepatocytes (Chen et al., 2009; Ji et al., 2019). However, this hypothesis requires further investigation. Additionally, N utilization is largely determined by protein turnover. However, it remains unclear whether the microbial metabolism of DF regulates N metabolism by this mechanism.
The effects of DF supplementation in NP or LP diets during gestation on the reproductive performance of the first parity sows
In this study, the reproductive performance of the first parity sows was significantly affected by dietary treatments. The number of newborn piglets or birth weight of piglets was unaffected by DF levels, which was inconsistent with our recent research on purified DF as a DF source (Zhuo et al., 2020) or the inclusion of fiber-rich ingredients, such as wheat bran, wheat straw, or sugar beet pulp as DF sources (van der Peet-Schwering et al., 2004; Veum et al., 2009). Inconsistencies may have been due to the fact that this latter research was conducted in multiparous sows, whereas our early research revealed that the effects of DF on litter performance may have been dependent on parity (Che et al., 2011). In this study, total newborn piglet numbers were increased by an LP diet without affecting total litter weight, but the average piglet birth weight was significantly decreased by this diet during gestation, suggesting increased litter size was achieved at the expense of birth weight. Despite the increase of total born without mummies, stillborn levels were also elevated by an LP diet, resulting in similar numbers of effective newborn piglets among groups. Therefore, an LP diet appeared unsuitable for gestating gilts, and the effectiveness of an LP diet for multiparous gestating sows requires comprehensive investigation.
Conclusions
Collectively, DF supplementation variably affected N levels in feces and urine excretion in gilts. Microbiota appeared to exert important roles in controlling N utilization from DF. Additionally, feeding primiparous gilts an LP diet during gestation appeared to impair reproductive performance.
Acknowledgments
This study was supported by SAU-Adisseo Center of Research on Nutrition and Health (20190010). We also wish to thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Glossary
Abbreviations
- AA
amino acids
- ADG
average daily gain
- AOAC
Association of Official Analytical Chemists
- BF
backfat
- BW
body weight
- CP
crude protein
- DF
dietary fiber
- PBS
phosphate-buffered saline
- SCFA
short-chain fatty acids
Conflict of interest statement
The authors declare no conflict of interest.
Literature Cited
- Aluthge, N. D., Sambeek D. M. V., Hinkle E. E. C., Li Y. S., and Burkey T. E.. . 2019. Board Invited Review: The pig microbiota and the potential for harnessing the power of the microbiome to improve growth and health. J. Anim. Sci. 97:3741–3757. doi: 10.1093/jas/skz208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC Int. 2007. Official methods of analysis of AOAC international. 18th ed. Gaithersburg (MD): AOAC International.
- Balimane, P. V., and Chong S.. . 2005. Cell culture-based models for intestinal permeability: a critique. Drug Discov. Today 10: 335–343. doi: 10.1016/S1359-6446(04)03354-9 [DOI] [PubMed] [Google Scholar]
- Cao, M., Li Y., Wu Q. J., Zhang P., Li W. T., Mao Z. Y., Wu D. M., Jiang X. M., Zhuo Y., Fang Z. F., . et al. 2019. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota. J. Anim. Sci. 97:3426–3439. doi: 10.1093/jas/skz186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, L., Feng D., Wu D., Fang Z., Y.Lin, and Yan T.. . 2011. Effect of dietary fiber on reproductive performance of sows during the first two parities. Reprod. Domest. Anim. 46:1061–1066. doi: 10.2527/1994.7271754x [DOI] [PubMed] [Google Scholar]
- Chen, L., Li P., Wang J., Li X., Gao H., Yin Y., Hou Y., and Wu G.. . 2009. Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 37:143–152. doi: 10.1007/s00726-009-0268-1 [DOI] [PubMed] [Google Scholar]
- Chen, F., Zhang S., Deng Z., Zhou Q., Cheng L., Kim S. W., Chen J., and Guan W.. . 2018. Regulation of amino acid transporters in the mammary gland from late pregnancy to peak lactation in the sow. J. Anim. Sci. Biotechnol. 9:35. doi: 10.1186/s40104-018-0250-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa, C. D., Fry R. S., Kocher M. E., and Stein H. H.. . 2019. Effects of copper hydroxychloride and distillers dried grains with solubles on intestinal microbial concentration and apparent ileal and total tract digestibility of energy and nutrients by growing pigs. J. Anim. Sci. 97:4904–4911. doi: 10.1093/jas/skz340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Animal Science of CAAS and China State Key Laboratory of Animal Nutrition. 2020. Tables of Feed Composition and Nutritive Values in China. 31th rev. ed. Beijing: Chinese Feed-database Information Network Centre.
- Jarrett, S., and Ashworth C. J.. . 2018. The role of dietary fiber in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 9:59. doi: 10.1186/s40104-018-0270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, F. J., Wang L. X., Yang H. S., Hu A., and Yin Y. L.. . 2019. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal 13:2727–2735. doi: 10.1017/S1751731119001800 [DOI] [PubMed] [Google Scholar]
- Li, P., Knabe D. A., Kim S. W., Lynch C. J., Hutson S. M., and Wu G.. . 2009. Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J. Nutr. 139:1502–1509. doi: 10.3945/jn.109.105957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Zhang L., Liu H., Yang Y., He J., Cao M., Yang M., Zhong W., Lin Y., Zhuo Y., . et al. 2019. Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals (Basel). 9:422. doi: 10.3390/ani9070422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla, W. D., Columbus D. A., Htoo J. K., and de Lange C. F.. . 2015. Nitrogen absorbed from the large intestine increases whole-body nitrogen retention in pigs fed a diet deficient in dispensable amino acid nitrogen. J. Nutr. 145:1163–1169. doi: 10.3945/jn.115.212316 [DOI] [PubMed] [Google Scholar]
- Mansilla, W. D., Htoo J. K., and de Lange C. F. M.. . 2017a. Nitrogen from ammonia is as efficient as that from free amino acids or protein for improving growth performance of pigs fed diets deficient in nonessential amino acid nitrogen. J. Anim. Sci. 95:3093–3102. doi: 10.2527/jas.2016.0959 [DOI] [PubMed] [Google Scholar]
- Mansilla, W. D., Silva K. E., Zhu C. L., Nyachoti C. M., Htoo J. K., Cant J. P., and de Lange C. F.. . 2017b. Ammonia nitrogen added to diets deficient in dispensable amino acid nitrogen is poorly utilized for urea production in growing pigs. J. Nutr. 147: 2228–2234. doi: 10.3945/jn.117.251314 [DOI] [PubMed] [Google Scholar]
- Mansilla, W. D., Silva K. E., Zhu C., Nyachoti C. M., Htoo J. K., Cant J. P., and de Lange C. F. M.. . 2018. Ammonia-nitrogen added to low-crude-protein diets deficient in dispensable amino acid-nitrogen increases the net release of alanine, citrulline, and glutamate post-splanchnic organ metabolism in growing pigs. J. Nutr. 148:1081–1087. doi: 10.1093/jn/nxy076 [DOI] [PubMed] [Google Scholar]
- Miller, E. G., Levesque C., Trottier N., and de Lange C. F. M.. . 2016. Dynamics of nitrogen retention in gestating gilts at two feeding levels. J. Anim. Sci. 94, 3353–3361. doi: 10.2527/jas.2016-0539 [DOI] [PubMed] [Google Scholar]
- Millet, S., Aluwé M., Van den Broeke A., Leen F., De Boever J., and De Campeneere S.. . 2018. Review: Pork production with maximal nitrogen efficiency. Animal 12:1060–1067. doi: 10.1017/S1751731117002610 [DOI] [PubMed] [Google Scholar]
- Mohamed, A. B., Rémond D., Chambon C., Sayd T., Hébraud M., Capel F., Cohade B., Hafnaoui N., Béchet D., Coudy-Gandilhon C., . et al. 2019. A mix of dietary fermentable fibers improves lipids handling by the liver of overfed minipigs. J. Nutr. Biochem. 65:72–82. doi: 10.1016/j.jnutbio.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Mou, D., Li S., Yan C., Zhang Q., Li J., Wu Q., Qiu P., He Y., Li Y., Liu H., . et al. 2020. Dietary fiber sources for gestation sows: Evaluations based on combined in vitro and in vivo methodology. Anim. Feed. Sci. Tech. 269:114636. doi: 10.1016/j.anifeedsci.2020.114636 [DOI] [Google Scholar]
- Ndegwa, P. M., Hristovb A. N., Arogoc J., and Sheffieldd R. E.. . 2008. A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst. Eng. 100:453–469. doi: 10.1016/j.biosystemseng.2008.05.010 [DOI] [Google Scholar]
- NRC . 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): The National Academy Press. [Google Scholar]
- Oliphant, K., and Allen-Vercoe E.. . 2019. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7:91. doi: 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrás, P., Nitrayová S., Brestenský M., and Heger J.. . 2012. Effect of dietary fiber and crude protein content in feed on nitrogen retention in pigs. J. Anim. Sci. 90:158–160. doi: 10.2527/jas.53837 [DOI] [PubMed] [Google Scholar]
- Pedersen, T. F., Bruun T. S., Trottier N. L., and Theil P. K.. . 2019. Nitrogen utilization of lactating sows fed increasing dietary protein. J. Anim. Sci. 97:3472–3486. doi: 10.1093/jas/skz213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Peet-Schwering, C. M., Kemp B., Plagge J. G., Vereijken P. F., den Hartog L. A., Spoolder H. A., and Verstegen M. W.. . 2004. Performance and individual feed intake characteristics of group-housed sows fed a nonstarch polysaccharides diet ad libitum during gestation over three parities. J. Anim. Sci. 82:1246–1257. doi: 10.2527/2004.8241246x [DOI] [PubMed] [Google Scholar]
- Tiwari, U. P., Singh A. K., and Jha R.. . 2019. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: a review. Anim. Nutr. 5:217–226. doi: 10.1016/j.aninu.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrallardona, D., Harris C. I., and Fuller M. F.. . 2003. Pigs’ gastrointestinal microflora provide them with essential amino acids. J. Nutr. 133:1127–1131. doi: 10.1093/jn/133.4.1127 [DOI] [PubMed] [Google Scholar]
- Uerlings, J., Bindelle J., Schroyen M., Richel A., Bruggeman G., Willems E., and Everaert N.. . 2019. Fermentation capacities of fructan- and pectin-rich by-products and purified fractions via an in vitro piglet faecal model. J. Sci. Food Agric. 99:5720–5733. doi: 10.1002/jsfa.9837 [DOI] [PubMed] [Google Scholar]
- Veum, T. L., Crenshaw J. D., Crenshaw T. D., Cromwell G. L., Easter R. A., Ewan R. C., Nelssen J. L., Miller E. R., Pettigrew J. E., and Ellersieck M. R.; North Central Region-42 Committee On Swine Nutrition . 2009. The addition of ground wheat straw as a fiber source in the gestation diet of sows and the effect on sow and litter performance for three successive parities. J. Anim. Sci. 87:1003–1012. doi: 10.2527/jas.2008-1119 [DOI] [PubMed] [Google Scholar]
- Wang, Y. S., Zhou P., Liu H., Li S., Zhao Y., Deng K., Cao D. D., Che L. Q., Fang Z. F., Xu S. Y., . et al. 2016. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod. Domest. Anim. 51:492–500. doi: 10.1111/rda.12707 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Zhou J., Wang G., Cai S., Zeng X., and Qiao S.. . 2018. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 9:60. doi: 10.1186/s40104-018-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, D. W., Pan P., Chen K. W., Fan J. X., Li C. X., Cheng H., and Zhang X. Z.. . 2020. An orally delivered microbial cocktail for the removal of nitrogenous metabolic waste in animal models of kidney failure. Nat. Biomed. Eng. 4:853–862. doi: 10.1038/s41551-020-0582-1 [DOI] [PubMed] [Google Scholar]
- Zhuo, Y., Cao M., Gong Y., Tang L., Jiang X., Li Y., Yang M., Xu S., Li J., Che L., . et al. 2021. Gut microbial metabolism of dietary fiber protects against high energy feeding induced ovarian follicular atresia in a pig model. Br. J. Nutr. 125:38–49. doi: 10.1017/S0007114520002378 [DOI] [PubMed] [Google Scholar]
- Zhuo, Y., Feng B., Xuan Y., Che L., Fang Z., Lin Y., Xu S., Li J., Feng B., and Wu D.. . 2020. Inclusion of purified dietary fiber during gestation improved the reproductive performance of sows. J. Anim. Sci. Biotechnol. 11:47. doi: 10.1186/s40104-020-00450-5 [DOI] [PMC free article] [PubMed] [Google Scholar]