Abstract
A 76-year-old man with hypogammaglobulinemia on monthly intravenous immunoglobulin infusions presented to the hospital with fever, cough, and shortness of breath and was diagnosed with COVID-19 pneumonia requiring intensive care unit admission but not intubation. He was treated with convalescent plasma, remdesivir and corticosteroids. Sixteen days into his hospitalisation he began to report weakness without sensory symptoms and was found on biopsy to have a necrotising myopathy.
Keywords: COVID-19, muscle disease, neuromuscular disease
Background
COVID-19 caused by the novel virus SARS-CoV-2 has resulted in approximately 150 million cases worldwide with >3 million deaths. Patients infected with COVID-19 typically present with fever, dyspnoea, dry cough, myalgias, diarrhoea, anosmia and dysgeusia. We report a case of necrotising myopathy and thyroiditis in a patient with SARS-CoV-2 infection who was treated with convalescent plasma.
Case presentation
A 76-year-old man presented to the hospital with 1 week of shortness of breath, cough, fatigue and myalgias. His medical history was significant for hyperlipidemia, hypogonadism on testosterone replacement and bronchiolitis associated with hypogammaglobulinemia for which he received monthly intravenous immunoglobulin (IVIG) infusions, with last infusion 12 weeks prior to admission. He was a former athlete who participated in 45 min of cardiovascular workouts daily before this admission.
His oxygen saturation on hospital presentation was 89% on room air and he required 4 L/min of supplemental oxygen via a nasal cannula which increased saturations to 93%. His heart rate was 54 beats/min. He was afebrile and otherwise stable. The only significant physical examination abnormality was scattered coarse inspiratory breath sounds. Neurological examination including strength testing was normal.
Investigations
His symptoms were highly suspicious for a COVID-19 infection and PCR testing for SARS-CoV-2 testing was found to be positive. Initial chest X-ray demonstrated patchy bilateral mid-to-lower lung infiltrates suspicious for pneumonia. Given hypoxia and chest infiltrates with positive PCR, he was diagnosed with severe COVID-19 pneumonia. C reactive protein was elevated on admission at 5.93 mg/dL (normal:<1.00 mg/dL) and peaked at 9.57 mg/dL on hospital day 6, then declined thereafter. Ferritin was high on admission and peaked at day 3 at 2767 µg/mL (normal: 24–336 µg/mL) and declined thereafter. D-dimer was elevated on admission and peaked on day 9 at 3.83 µg/mL (normal: <0.5 µg/mL). Interleukin-6 levels were not checked.
He initially received empiric treatment for bacterial pneumonia with one-time doses of ceftriaxone and doxycycline, which were discontinued after normal procalcitonin and negative blood cultures. In addition, he was treated for COVID-19 pneumonia with a 5-day course of remdesivir and 6 mg intravenous dexamethasone daily for the first 5 days. His home atorvastatin 80 mg was continued; however, his home testosterone replacement was held. On hospital day 5, his hypoxia and oxygen requirements acutely worsened, necessitating transfer to the intensive care unit (ICU). In the ICU, given his severe COVID-19 infection, he was administered convalescent plasma and high-flow oxygen, and his steroids were increased to methylprednisolone 60 mg every 6 hours. His condition improved in the ICU without the need for intubation, antibiotics or paralytics, and he was discharged to a medical ward on hospital day 14.
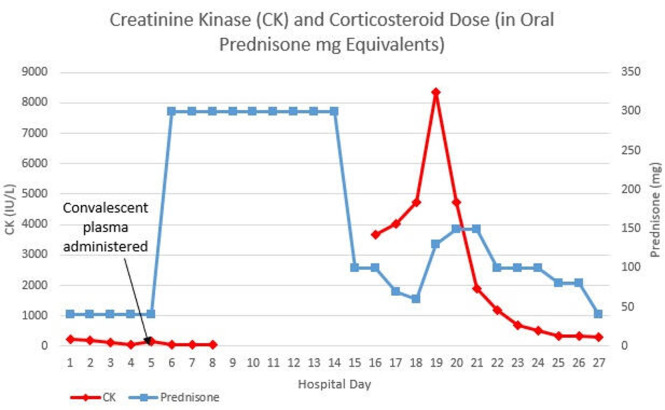
Initial creatine kinase (CK) was 236 IU/L (normal: 30–223 IU/L), which trended down to 39 IU/L in the following week (figure 1). On hospital day 16, the patient reported new-onset muscle weakness amid an attempt to taper his steroid treatment. Physical examination was significant for symmetrical 4/5 proximal upper extremity strength and 3/5 proximal lower extremity strength. Upper and lower distal extremity strengths were 5/5 bilaterally. Repeat CK was found to be elevated to 3665 IU/L. Transaminases were elevated with an aspartate aminotransferase (AST) of 123 (normal: 30–39) IU/L and an alanine aminotransferase (ALT) of 137 (normal: 7–52) IU/L. Owing to concern that his hypolipidemic agents were contributing to his clinical decline, his atorvastatin and fenofibrate were discontinued. The patient’s IVIG treatment was continued, and he received an infusion on hospital day 18.
Figure 1.
Graphical representation showing the relation between steroid dose and CK levels.
Despite discontinuing his hypolipidemic agents and tapering steroids, his serum CK continued to rise. A thyroid function panel was ordered to investigate potential thyroid myopathy. The panel revealed thyroid stimulating hormone (TSH) of 0.026 µIU/mL (normal: 0.45–5.3 µIU/mL), free thyroxine (T4) of 2.66 ng/dL (normal: 0.58–1.64 ng/dL) and free triiodothyronine (T3) of 2.30 pg/mL (normal: 2.2–4.1 pg/mL). He had no tremor, tachycardia, eye symptoms or prior thyroid disease. Thyroid examination revealed symmetrical, normal-sized and non-tender thyroid without nodules. A thyroid uptake and scan following capsule of I-123 revealed diffusely decreased thyroid uptake (3.3%, normal: 5%–30%), consistent with thyroiditis. Anti-thyroid peroxidase was 1.7 IU/mL (normal:<9 IU/mL). TSH and free T4 levels were followed 10 days later still demonstrating a hyperthyroid pattern. Two weeks after, the TSH and free T4 levels normalised without antithyroid treatment.
Differential diagnosis
A broad differential diagnosis was considered as the cause for this patient’s myopathy including hyperthyroidism, corticosteroids, dermatomyositis and polymyositis, statin-induced and critical illness myopathy. Hyperthyroidism was our initial consideration as the cause for his myopathy in light of his newly suppressed TSH and elevated T4 levels. However, his hyperthyroidism was mild, otherwise asymptomatic (no tremor, tachycardia, diarrhoea and weight loss) and resolved without antithyroid therapy. Furthermore, his myopathy persisted for months after the full recovery of his thyroid hormone levels. Most importantly, although hyperthyroid patients do lose muscle through the catabolic effects of increased metabolism, the necrosis we witnessed on biopsy was entirely inconsistent with this diagnosis. The patient was receiving a significant dose of corticosteroids; however, it is not believed to be steroid myopathy as his CK rose with tapering steroids then subsequently decreased with increasing his dose. Necrosis on biopsy would also not be consistent with steroid myopathy. Additionally, dermatomyositis and polymyositis were considered but an extensive myositis panel including Jo-1 antibody and Mi-2 antibody were all negative. Statin-associated muscle symptoms, including statin myonecrosis and necrotising autoimmune myopathy, were considered given the patient’s exposure to atorvastatin both before and during admission. Although muscle complaints are typical in statin users, 1 per 10 000 person-years will develop myopathy (defined as CK >10 times upper limit of normal) that resolves with discontinuation of the statin.1 A small subset of these, however, will develop an immune-mediated myopathy that progresses despite statin discontinuation. 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase receptor (HMGCR) antibodies are highly sensitive (94.4%) and specific (99.3%) in these cases.2 Given his myopathy progressed despite discontinuation of statins and antibodies were negative, we believed this was less likely. Finally, critical illness myopathy is not believed to be the cause because the patient improved with steroids and he did not have commonly associated risk factors including neuromuscular blocking agents, antibiotics or invasive ventilation. Although the patient regularly received testosterone replacement, his acutely developing weakness with muscle necrosis is inconsistent with purely testosterone deficiency. Finally, an immune-mediated myopathy was considered, especially since myopathy occurred 10 days after administration of convalescent plasma, and the patient was also found to have a concurrent acute autoimmune disorder (autoimmune thyroiditis), and this was ultimately our final diagnosis.
Treatment
CK level peaked at 8335 IU/L on day 19 of hospitalisation. The decision was made to increase his steroid dose from prednisone 30 mg twice daily to methylprednisolone 40 mg every 8 hours. Subsequently, his CK then continued to decrease (figure 1).
The anti-HMGCR antibody was <3 (normal: 1–19 units). An extensive myositis panel was negative (table 1).
Table 1.
Myositis extended panel
| Myositis extended panel | |
| Marker | Reference range and units |
| SAE1 (SUMO activating enzyme) | Negative |
| NXP-2 (nuclear matrix proten-2) | Negative |
| MDA5 (CADM-140) antibody (Ab) | Negative |
| TIF-1 gamma (155 kDA) Ab | Negative |
| Mi-2 (nuclear helicase protein) Ab | Negative |
| P155/140 Ab | Negative |
| PL-12 (alanyl-tRNA synthetase) Ab | Negative |
| PL-7 (threonyl-tRNA synthetase) Ab | Negative |
| OJ (isoleucyl-tRNA synthetase) Ab | Negative |
| EJ (glycyl-tRNA synthetase) Ab | Negative |
| SRP (Signal Recognition Particle) Ab | Negative |
| Jo-1 (histidyl-tRNA synthetase) Ab IgG | 2 AU/mL (<30 AU/mL=negative) |
| Ku Ab | Negative |
| Smith/RNP Ab | 5 AU/mL (<30 AU/mL=negative) |
| PM/SCL-100 Ab IgG | Negative |
| SSA-52 (Ro) Ab IgG | 13 AU/mL (<30 AU/mL=negative) |
| SSA-60 (Ro) Ab IgG | 14 AU/mL (<30 AU/mL=negative) |
| Fibrillarin (U3 RNP) Ab IgG | Negative |
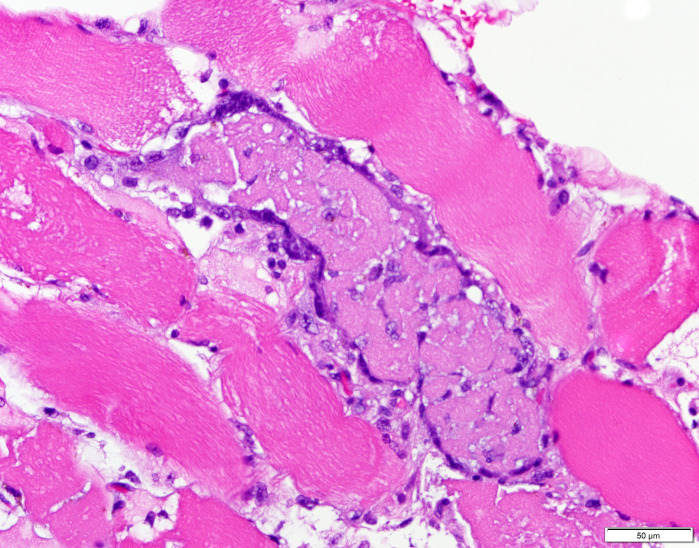
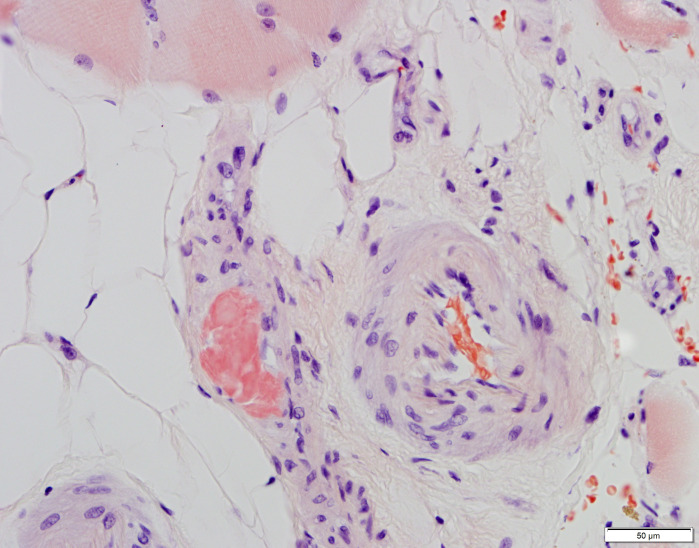
In addition to his laboratory studies, a MRI of his right thigh was obtained. The MRI revealed mild oedema of the musculature, most prominently involving the vastus lateralis. Given the patient’s elevated CK and muscle weakness, along with a nonspecific diagnostic laboratory workup, the decision was made to obtain a muscle biopsy on day 21. The biopsy revealed a necrotising myopathy with focal perivascular amyloid (figures 2 and 3). Serum light chains showed elevated kappa quantitative-free light chains of 32.36 mg/L (normal: 3.30–19.40 mg/L) but kappa-lambda-free light chain ratio and lambda quantitative-free light chains were normal. Electromyography (EMG) performed 6 weeks after illness onset showed diffuse small polyphasic units with early recruitment but also evidence of small fibrillation potentials that persisted proximally and distally compatible with residual abnormalities from the acute myopathic process. Nerve conduction studies revealed decreased amplitudes in lower extremities greater than upper extremities. The findings were specified as being most compatible with inflammatory/immune myositis potentially associated with COVID-19 infection.
Figure 2.
H&E, 400×. Necrotic myofibre, centre of field.
Figure 3.
Congo red, 400×. Blood vessels in epimysial connective tissue. See muscle fibres at the top of thefield and vascular mural deposit of bright-red Congo red-positive amyloid in the middle left of this field.
In general, patients with evidence of myopathy who do not have classic manifestations of dermatomyositis and who do not exhibit myositis-specific antibodies should be considered for muscle biopsy, possibly guided by MRI imaging to increase likelihood of adequate sampling of involved muscle.
Outcome and follow-up
The patient was discharged from the hospital on 4 L/min of supplemental oxygen via nasal cannula and significant muscle weakness to an acute rehabilitation facility after 27 days in the hospital. At the rehabilitation facility, he was able to be weaned to on room air. He is still on a slow prednisone taper at 8 weeks post infection. He regained strength slowly, and weekly CK testing remains normal. He was discharged from his rehabilitation facility to home after 90 days, able to walk with the assistance of a cane.
Discussion
Since first reported in Wuhan, China in December 2019, the deadly SARS-CoV-2 has claimed >2 million lives worldwide and the numbers continue to rise to date. Most patients present with symptoms like cough, sputum production, fever, myalgias, diarrhoea, vomiting and non-specific abdominal pain.3 However, neurological manifestations are being increasingly reported with both peripheral and central nervous system symptoms.4 Although myopathy as a part of critical illness myopathy is not uncommon among intubated patients with SARS-CoV-2 infection, there have been very few case reports of myopathy in patients with SARS-CoV-2 infection who were not intubated.5 6 One case report described a patient with SARS-CoV-2 virus-related myositis who presented with severe bulbar and proximal weakness. Biopsies revealed human leucocyte antigen class ABC expression on non-necrotic fibres. EMG of the affected muscle fibres revealed sparse fibrillation potentials. Interestingly, CK level was only mildly elevated to 700 U/L.7 Moreover, there has been mention of elevated CK levels 29 800 U/L in a case report that also demonstrated major histocompatibility complex (MHC) class I antigen and myxovirus resistance protein A on muscle biopsy and immunohistochemical stain analysis. However, no features of necrosis were noted on biopsy despite the high levels of CK.8 Although the SARS-CoV-2 virus has been known to cause some cases of subacute thyroiditis, there have been no case reports showing concurrent thyroiditis and myopathy.9 10
Our case is unique as it demonstrates two distinct disease processes occurring concomitantly with COVID-19 infection including thyroiditis and necrotising myopathy. Our patient does have amyloid deposits seen in his muscle biopsy of unclear significance, without other clinical signs or biochemical evidence of systemic disease and in the face of underproduction of his own antibodies. Whether the amyloid deposits here are incidental, a risk for this condition, or truly pathologic, is unclear. Ultimately, we believe that either SARS-CoV-2 itself or antibodies (from either convalescent plasma, IVIG, or less likely his native antibodies) resulted in necrotising myopathy and concurrent thyroiditis. Given his hypogammaglobulinemia, we suspect his own immune system the least. Given that it occurred soon after we gave him convalescent plasma, we believe temporally it is more likely that convalescent plasma is more likely than IVIG to be the cause. This side effect has not been reported in a case series to date.11
Learning points.
It is important to regularly assess for muscle weakness in patients with SARS-CoV-2 virus infection even if they are not mechanically ventilated to identify an inflammatory myopathy.
The diagnosis of myopathy in acutely ill patients can be challenging especially when a patient is also on corticosteroids and/or immunoglobulin therapy. Medication effects, thyroid disease and inflammatory conditions should be closely considered.
In a large percentage of cases of necrotizing myopathy, no cause is found despite comprehensive workup including a muscle biopsy.
Footnotes
Contributors: AAD, CR and AP: responsible for planning, conduct, reporting, conception and design, acquisition of data and analysis and interpretation of data. CSS : contributed the pictures, assisted in writing and reviewed the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gallo A, Perregaux J, Bruckert E. Advances in the management of statin myopathy. Curr Opin Endocrinol Diabetes Obes 2021;28:142–51. 10.1097/MED.0000000000000595 [DOI] [PubMed] [Google Scholar]
- 2.Shovman O, Gilburd B, Chayat C, et al. Anti-HMGCR antibodies demonstrate high diagnostic value in the diagnosis of immune-mediated necrotizing myopathy following statin exposure. Immunol Res 2017;65:276–81. 10.1007/s12026-016-8867-x [DOI] [PubMed] [Google Scholar]
- 3.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985. 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagnato S, Boccagni C, Marino G, et al. Critical illness myopathy after COVID-19. Int J Infect Dis 2020;99:276–8. 10.1016/j.ijid.2020.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper JE, Uner M, Priemer DS. Muscle biopsy findings in a case of sars-cov-2-associated muscle injury. J Neuropathol Exp Neurol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Charmchi Z, Seidman RJ, et al. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020;62:E57–60. 10.1002/mus.27003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manzano GS, Woods JK, Amato AA. Covid-19-associated myopathy caused by type I interferonopathy. N Engl J Med 2020;383:2389–90. 10.1056/NEJMc2031085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty U, Ghosh S, Chandra A, et al. Subacute thyroiditis as a presenting manifestation of COVID-19: a report of an exceedingly rare clinical entity. BMJ Case Rep 2020;13. 10.1136/bcr-2020-239953. [Epub ahead of print: 18 Dec 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M, et al. Subacute thyroiditis associated with COVID-19. Case Rep Endocrinol 2020;2020:1–4. 10.1155/2020/8891539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest 2020;130:4791–7. 10.1172/JCI140200 [DOI] [PMC free article] [PubMed] [Google Scholar]