Abstract
In our laboratory, the negative rapid group A streptococcal (GAS) antigen assays are backed up by the Solana® GAS Assay by Quidel instead of a Group A streptococcal throat culture. Another FDA cleared RT-PCR assay is the Xpert® Xpress Strep A, which detects Streptococcus pyogenes DNA, and is performed on the Cepheid GeneXpert instrument. Three hundred seventy-five positive and negative specimens were randomly selected from 5489 throat specimens that had been tested by the Solana® GAS Assay during January 2018 and were tested with the Xpress Strep A assay. A throat culture was also set up (sheep blood agar at 35 °C in 5% CO2). All beta-hemolytic streptococci were purified and identified by MALDI-TOF mass spectrometry. Of the 375 samples, 185 were positive by Solana® GAS Assay, and 187 were positive by the Xpress Strep A. The total agreement between the Solana® GAS Assay and the Xpert® Xpress Strep A was 99.5%. The agreement of the Xpert® Xpress Strep A assay with culture was 90.1%. The sensitivity and specificity for Xpress Strep A versus culture were 100% and 83.5%, respectively. The Xpert® Xpress Strep A assay’s performance was equivalent to the Solana® GAS Assay, and was highly sensitive. The lower specificity was likely due to the Xpress Strep A assay having higher sensitivity as compared to throat culture.
Introduction
Streptococcal pharyngitis, most commonly called “strep throat,” is caused by Group A Streptococcus (GAS), a beta-hemolytic Gram-positive coccus. GAS is responsible for 15–30% of the bacterial pharyngitis cases in children and 5–15% of bacterial pharyngitis cases in adults [1].
GAS can cause various diseases that can range from minor illnesses like impetigo to very deadly infections like streptococcal toxic shock syndrome.
In general, streptococcal pharyngitis is a mild infection that can easily be treated with an antibiotic. However, patients are at risk of secondary complications, such as rheumatic fever and glomerulonephritis, if they are not treated for their streptococcal pharyngitis [2].
The symptoms of pharyngitis are nonspecific to the causative agents. Thus, it is challenging for clinicians to differentiate between viral and bacterial causes of pharyngitis. Therefore, the guidelines in the United States recommend testing for GAS pharyngitis by rapid antigen detection test (RADT) and performing back-up on negative RADT tests with a throat culture [3]. Throat cultures for detecting GAS can require up to 48 h before providing an answer, delaying appropriate antimicrobial treatment.
Nucleic acid testing offers an alternative way to improve speed and accuracy in GAS pharyngitis diagnosis and has been shown to have superior sensitivity and specificity compared to conventional throat cultures and clinical diagnosis [4, 5].
In our laboratory, we use the Quidel Solana® GAS Assay (Quidel Corporation, 2005 East State Street, Athens, OH 45,701 USA), which is a nucleic acid amplification assay (NAAT) as a substitute for back-up culture on throat specimens with a negative rapid group A streptococcal antigen assay (McKesson Consult® Diagnostics Strep A Test, Richmond, VA).
When Cepheid received U.S. Food and Drug Administration (FDA) 510(k) clearance and waiver under the Clinical Laboratory Improvement Amendments (CLIA) for the Xpert® Xpress Strep A test (Cepheid, 904 Caribbean Drive, Sunnyvale, CA 94,089), our laboratory evaluated the performance of the automated real-time polymerase chain reaction (PCR) Xpert® Xpress Strep A test on the Cepheid GeneXpert instrument at detecting Streptococcus pyogenes DNA.
Methods
During the month of January 2018, 5489 ESwab™ throat specimens negative by the rapid McKesson Consult® Diagnostics Strep A Test were tested by the NAAT Solana® GAS Assay.
From the 5489 ESwab™ throat specimens tested on the Solana® GAS Assay, 375 specimens (185 positive and 190 negative) were randomly selected to be tested with the Xpert® Xpress Strep A assay and conventional throat culture [Remel™ Blood Agar (TSA w/5% Sheep Blood) at 35 °C in 5% CO2]. Culture processing was performed, as previously described [6].
Cultures were reviewed at 24 and 48 h; all beta-hemolytic streptococci (consistent morphology, catalase negative) were purified to subculture plates. Isolate colonies were selected, and following the manufacturer’s instructions, set up for identification on the MALDI-TOF mass spectrometry VITEK® MS (BioMérieux, Marcy-l’Etoile, France). Each isolate was identified using the Vitek MS V3.0 knowledge base. The VITEK MS identification was accepted if the confidence value was 99.9. Isolates with a confidence value < 99.9 were retested on the VITEK MS. Two authors (P.F. and S.A.) performed all the culture reading, subculturing, and processing.
The Xpert® Xpress Strep A assay was performed on the GeneXpert® XVI instrument following the manufacturer’s instructions.
The statistical analysis was performed using the Fisher exact test (2 × 2 contingency table). The Fisher exact test was chosen for the statistical analysis instead of Pearson’s chi-square test because of the relatively small sample size.
Results
Of the 375 tested specimens, 187 were positive by Xpert® Xpress Strep A, and 185 were positive by the Solana® GAS Assay with an overall total agreement of 99.5% between the two amplified GAS assays. There were 12 culture-negative specimens with discordant results between the two amplified GAS assays: 6 specimens that were Xpert® Xpress Strep A positive and Solana® GAS Assay negative, and six specimens Xpert® Xpress Strep A negative and Solana® GAS Assay positive. After retesting of the twelve specimens by both systems, only two remained discordant (#184 and #331); both were positive by the Xpert® Xpress Strep A, but negative by the Solana® GAS Assay (see Table 1).
Table 1.
Composite results of the 12 discordant results between the Solana® group A streptococcus assay and the Xpert® Xpress strep A assay
| Specimen # | Original testing result | Culture | Repeat testing result | ||
|---|---|---|---|---|---|
| Xpert® Xpress | Solana® | Xpert® Xpress | Solana® | ||
| 53 | Detected (31.4)* | Negative | Negative | Detected (30.9)* | Positive |
| 59 | Detected (38.9)* | Negative | Negative | Not Detected | Negative |
| 69 | Detected (41.3)* | Negative | Negative | Not Detected | Negative |
| 104 | Detected (37.2)* | Negative | Negative | Not Detected | Negative |
| 184 | Detected (32.3)* | Negative | Negative | Detected (30.5)* | Negative |
| 236 | Detected (33.1)* | Negative | Negative | Detected (33.5)* | Positive |
| 96 | Not Detected | Positive | Negative | Not Detected | Negative |
| 141 | Not Detected | Positive | Negative | Not Detected | Negative |
| 202 | Not Detected | Positive | Negative | Not Detected | Negative |
| 282 | Not Detected | Positive | Negative | Not Detected | Negative |
| 305 | Not Detected | Positive | Negative | Not Detected | Negative |
| 331 | Not Detected | Positive | Negative | Detected (38.8)* | Negative |
The Solana assay uses isothermal helicase-dependent amplification and is unable to yield a “Ct” value
*Ct value
The Fisher exact test analysis of the data showed that there was no statistical difference between the performance of the Xpert® Xpress Strep A and the Solana® GAS Assay at detecting GAS from throat samples. The Fisher exact test statistical value was 0.9418 (P < 0.05).
The overall total agreement between culture (the gold standard) and the Xpert® Xpress Strep A assay was 90.1%. Thirty-seven of the 187 positive Xpert® Xpress Strep A were culture negative. The sensitivity and specificity for the Xpert® Xpress Strep A were 100% and 83.5%, respectively. The sensitivity and specificity of the Solana® GAS Assay as compared to culture were 100% and 84.4%, respectively (35 of the 185 positive Solana® GAS Assay were culture negative). The statistical analysis of the data with the Fisher exact test showed that there was a statistical difference in the performance of the Xpert® Xpress Strep A compared to culture at detecting GAS from throat samples. The Fisher exact test statistical value was 0.0082 (P < 0.05).
Analyses of the 165 beta-hemolytic streptococcal isolates recovered by culture revealed: 150 isolates of Streptococcus pyogenes, 4 Streptococcus agalactiae, 7 groups C/G Streptococcus (identified to species level by MALDI-TOF mass spectrometry as Streptococcus dysgalactiae ssp dysgalactiae, Streptococcus dysgalactiae ssp equisimilis); and 4 miscellaneous beta-hemolytic streptococcal species (2 Streptococcus constellatus and 2 Streptococcus anginosus) (Table 2).
Table 2.
Distribution of non-group A beta-hemolytic Streptococcus isolated from primary culture
| Specimen # | Solana result | Cepheid result | Culture result |
|---|---|---|---|
| 114 | NEGATIVE | NOT DETECTED | Strep. agalactiae |
| 117 | NEGATIVE | NOT DETECTED | Strep. agalactiae |
| 168 | NEGATIVE | NOT DETECTED | Strep. agalactiae |
| 360 | NEGATIVE | NOT DETECTED | Strep. agalactiae |
| 20 | NEGATIVE | NOT DETECTED | Strep. anginosus/Group C |
| 152 | NEGATIVE | NOT DETECTED | Strep. constellatus |
| 32 | NEGATIVE | NOT DETECTED | Strep. constellatus/Group C |
| 127* | POSITIVE | DETECTED | Strep. dysgalactiae |
| 293* | POSITIVE | DETECTED | Strep. dysgalactiae |
| 322* | POSITIVE | DETECTED | Strep. dysgalactiae |
| 107 | NEGATIVE | NOT DETECTED | Strep. dysgalactiae |
| 170 | NEGATIVE | NOT DETECTED | Strep. dysgalactiae |
| 242 | NEGATIVE | NOT DETECTED | Strep. dysgalactiae |
| 342 | NEGATIVE | NOT DETECTED | Strep. dysgalactiae |
| 358 | NEGATIVE | NOT DETECTED | Strep. anginosus |
Large-colony forming beta-hemolytic S. dysgalactiae isolates have been shown to cause streptococcal pharyngitis; however, if untreated do not increase the risk of post-streptococcal sequelae [5]
*For samples #127, #293, and #322, both Streptococcus pyogenes and Streptococcus dysgalactiae were present in the samples
In our experience, it is not uncommon to detect these other non-group A beta-hemolytic streptococcal species in the pharynx.
The age groups for which the rapid antigen detection test had the highest failure rate in detecting GAS were the 5–9 yrs (81), 10–14 yrs (39), and 30–39 yrs (26) (Fig. 1).
Fig. 1.
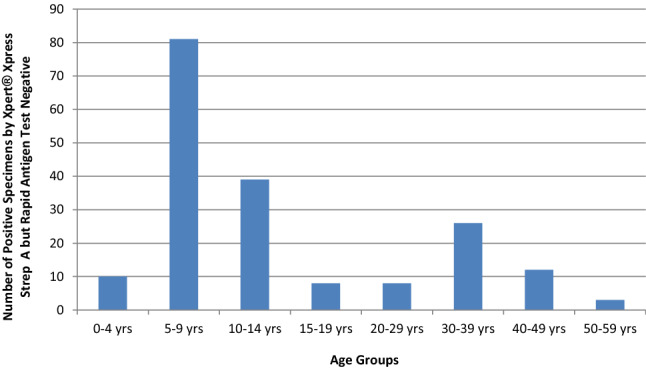
Summary of Final GAS Positivity by Xpert® Xpress Strep A Assay with Negative Rapid Streptococcal Antigen Test by Age Groups
This distribution of Xpert® Xpress Strep A positive specimens, but rapid antigen test negative, showed a peak for the 5–9 yrs and 10–14 yrs groups, which traditionally have the highest culture positive results. The relatively high positivity rate in the 30–39 yrs group may reflect child to parent transmission of GAS.
The time needed to set up one Xpert® Xpress Strep A test on the Cepheid GeneXpert instrument (hands-on time) was 1 min per specimen. The time to results was 18–24 min, depending on whether the specimen was positive or negative for GAS. For the Solana® GAS Assay, hands-on time per specimen was 7 min and the time to result was 37 min [4] (Table 3). The risk of cross-contamination between specimens is minimal as the Xpert® instrument requires that each cartridge be processed and installed separately.
Table 3.
Comparison of the Two GAS molecular platforms
| Solana® group A Streptococcus assay | Xpert® Xpress strep A assay* | |
|---|---|---|
| Complexity and “hands-on” time | Several pipetting steps “7 min” | Fully automated “1 min” |
| Time to result | 37 min | 18–24 min** |
| Molecular target amplified | DNase B (sdaB) gene | speB gene |
*Product information brochure
**Positive results are usually available after 18 min of initiation in the GeneXpert® instrument
Discussion
The detection of GAS by the Xpert® Xpress Strep A assay was comparable to the Solana® GAS Assay with a 99.5% agreement. It was more sensitive than rapid antigen detection assay and culture for GAS with a sensitivity as compared to the routine culture of 100% and specificity of 83.5%. In the 510(k) document (#K173398) submitted to the Food and Drug Administration (FDA), Cepheid reported a sensitivity of 99.4% and a specificity of 94.1% when compared with culture.
The original discordant results of six throat specimens tested on the Solana® GAS Assay, with positive results that, on repeat testing, became negative, were likely due to cross-contamination during the manual pipetting process. Of the six specimens that were originally positive by the Xpert® Xpress assay, only three remained positive on repeat testing. For the specimens that did not repeat positive, it is possible that there was degradation of nucleic acid in the freeze/thaw handling, and thus accounting for the lack of detection on repeat testing. Of the three specimens that remained positive by the Xpert® Xpress assay on repeat testing (#53,#184,#236), two of them (#53,#236) became positive by the Solana® GAS Assay. The sampling effect could be a reason for the discordant results on repeat testing of the Solana® GAS Assay. Sample #184 stayed positive by the Xpert® Xpress assay and negative by the Solana® GAS Assay on repeat testing. It is possible that a mutation was present in the primers or probe regions of the sdaB gene that affected the Solana® GAS Assay’s ability to detect GAS.
Although rapid GAS antigen tests are heavily used in primary care settings due to their ease of performance and rapid time to result, there are drawbacks. The specificity of the rapid antigen tests is high, but the sensitivity of various rapid antigen detection assays for GAS ranges from 55 to 100% [1, 7, 8]. NAAT testing also permitted a faster turnaround time, and thus, timely treatment of GAS as demonstrated by other NAATs described in the literature.
In contrast, the capability of NAAT at detecting very small quantities of GAS can be seen as a disadvantage by some physicians, as it may represent colonization and not a true infection, leading to overtreatment of GAS and thus defeating antimicrobial stewardship efforts. A counter argument to this is that studies have shown specific GAS antibody development in patients with only 1 + − 2 + semiquantitative GAS cultures [9]. Thus, there may be benefits to detect lower quantities of GAS with sensitive molecular technologies in symptomatic patients.
Furthermore, a recent study evaluated GAS antibody responses to 21 GAS antigens from sera collected over a consecutive 24-month period from 41 pediatric subjects who experienced a new pharyngeal GAS acquisition [10]. Observations that 65% of new GAS acquisition caused no symptoms, but were immunologically significant, based on the rises in GAS antibodies, suggested that the majority of infections were not diagnosed. These were missed opportunities for the prevention of rheumatic fever and rheumatic heart disease by the use of appropriate antibiotic therapy [10]. In addition, in a study in high GAS burden settings, molecular testing for GAS nearly tripled the detection of GAS in throat samples in 25 patients with rheumatic fever or post-streptococcal glomerulonephritis [11]. The complexity of GAS acquisitions and the immune response is illustrated by these findings and supports further investigation.
The molecular target that was amplified in the Solana® group A streptococcus assay is the highly conserved sdaB gene, which encodes for DNase B, an extracellular antigen of GAS, and the basis for the anti-DNase B antibody test used to substantiate a true GAS infection [10]. The target for the Xpert® Xpress GAS assay is speB, also highly conserved, and it encodes for streptococcal pyrogenic exotoxin B or SPEB, a cysteine protease, major virulence factor, and superantigen whose expression mediates toxic shock seen with various syndromes of severe acute pyogenic infections due to GAS [12]. Both of these genes are specific for GAS, and both are single gene copies in the Streptococcus pyogenes chromosome [13]. Thus, the congruence of the two assays’ performance was related to the high conservation, single gene copy, and specificity of these two different gene targets of GAS [12, 13].
Conclusions
The Xpert® Xpress Strep A PCR assay is fully automated and; thus, requires minimal pipetting and decreases the opportunity of cross-contamination during set up.
In summary, the performance of the Xpert® Xpress Strep A assay was equivalent to the Solana® GAS Assay and was highly sensitive. The lower specificity was likely due to the Xpress Strep A assay having higher sensitivity as compared to throat culture.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patricia Ferrieri and Sophie Arbefeville equally contributed to the data acquisition and the manuscript.
References
- 1.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, Martin JM, Van Beneden C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the infectious diseases society of America. Clin Infect Dis. 2012;55:e86–e102. doi: 10.1093/cid/cis629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karthikeyan G, Guilherme L. Acute rheumatic fever. Lancet. 2018;14:161–174. doi: 10.1016/S0140-6736(18)30999-1. [DOI] [PubMed] [Google Scholar]
- 3.Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, Taubert KA. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, endocarditis, and kawasaki disease committee of the council on cardiovascular disease in the young, the interdisciplinary council on functional genomics and translational biology, and the interdisciplinary council on quality of care and outcomes research: endorsed by the American Academy of Pediatrics. Circulation. 2009;24:1541–1551. doi: 10.1161/CIRCULATIONAHA.109.191959. [DOI] [PubMed] [Google Scholar]
- 4.Uphoff TS, Buchan BW, Ledeboer NA, Granato PA, Daly JA, Marti TN. Multicenter evaluation of the Solana group A streptococcus assay: comparison with culture. J Clin Microbiol. 2016;54:2388–2390. doi: 10.1128/JCM.01268-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyanton BL, Jr, Darnell EM, Prada AE, Hansz DM, Robinson-Dunn B. Evaluation of the lyra direct strep assay to detect group A streptococcus and group C and G beta-hemolytic streptococcus from pharyngeal specimens. J Clin Microbiol. 2016;54:175–177. doi: 10.1128/JCM.02405-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbefeville S, Nelson K, Thonen-Kerr E, Ferrieri P. Prospective post implementation study of solana group A streptococcal nucleic acid amplification test vs conventional throat culture. Am J Clin Pathol. 2018;150(4):333–337. doi: 10.1093/ajcp/aqy051. [DOI] [PubMed] [Google Scholar]
- 7.Stewart EH, Davis B, Clemans-Taylor BL, Littenberg B, Estrada CA, Centor RM. Rapid antigen group A streptococcus test to diagnose pharyngitis: a systematic review and meta-analysis. PLoS ONE. 2014;9:e111727. doi: 10.1371/journal.pone.0111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17:571–580. doi: 10.1128/CMR.17.3.571-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber MA, Randolph MF, Chanatry J, Wright LL, DeMeo KK, Anderson LR. Antigen detection test for streptococcal pharyngitis: evaluation of sensitivity with respect to true infections. J Pediatr. 1986;108:654–658. doi: 10.1016/S0022-3476(86)81036-8. [DOI] [PubMed] [Google Scholar]
- 10.Hysmith ND, Kaplan EL, Cleary PP, Johnson DR, Penfound TA, Dale JB. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of group A streptococci. J Pediatric Infect Dis Soc. 2017;6(2):187–196. doi: 10.1093/jpids/piw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralph AP, Holt DC, Islam S, Osowicki J, Carroll DE, Tong SYC, Bowen AC. Potential for molecular testing for group A streptococcus to improve diagnosis and management in a high-risk population: a prospective study. Open Forum Infect Dis. 2019 doi: 10.1093/ofid/ofz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson H, Vindebro R, von Pawel-Rammingen U. The streptococcal cysteine protease SpeB is not a natural immunoglobulin-cleaving enzyme. Infect Immun. 2013;81(6):2236–2241. doi: 10.1128/IAI.00168-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anbalagan S, McShan WM, Dunman PM, Chaussee MS. Identification of Rgg binding sites in the Streptococcus pyogenes chromosome. J Bacteriol. 2011;193(18):4933–4942. doi: 10.1128/JB.00429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


