Abstract
Background
Altered mental status (AMS) is a common neurological manifestation of COVID-19 infection in hospitalized patients. The principal causes of AMS have yet to be determined. We aimed to identify the common causes of AMS in patients with COVID-19 presenting to the emergency department with AMS on arrival.
Methods
We conducted a retrospective observational study of patients presenting with AMS to three New York hospitals, from March 1 to April 16, 2020. Underlying causes of AMS on arrival to the emergency department (ED) were categorized as (1) neurological causes (stroke, seizure, encephalitis); (2) metabolic encephalopathy; (3) indeterminant. Multivariable analysis was used to assess independent predictors.
Results
Overall, 166 patients presented to the ED with AMS. Metabolic encephalopathy was diagnosed as the cause in 154 (92.8%), with 118 (71.1%) categorized as multifactorial ME and 36 (21.7%) with single-cause ME. Hypoxia 103 (62.0%) and renal failure 75 (45.2%) were the most common underlying mechanisms. Neurological causes of AMS occurred in a total 20 patients (12%) and as the sole factor in 5 (3.0%); 10 (6.0%) cases were seizure related and 10 (6.0%) were cerebrovascular events. Of the 7 patients with indeterminant causes, only 1 was suspicious for encephalitis (0.6%). Age, pre-existing dementia and cerebrovascular disease, and impaired renal function were independent predictors of AMS.
Conclusion
In patients with COVID-19, AMS on presentation to the ED is most frequently caused by metabolic encephalopathy (delirium). Seizures and cerebrovascular events contribute to a lesser degree; encephalitis appears rare.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-021-10623-5.
Keywords: COVID-19, Encephalopathy, Delirium, Encephalitis
Introduction
The neurological manifestations of Coronavirus disease 2019 (COVID-19) infection are in their initial phase of exploration and description. Recent reports from China and Spain suggest up to 36–57% of hospitalized patients had neurological manifestations [1, 2]. Within this group of patients, 20% exhibited altered mental status (AMS). To date, the principal cause of AMS in COVID-19 infection has yet to be investigated.
The term AMS refers to any change from a patient’s baseline mental status. The root basis of AMS is an impairment of consciousness, which may be caused by a wide spectrum of conditions that induce acute brain dysfunction [3]. There are two interrelated domains of neurologic function that govern conscious behavior: the level of consciousness and the content of consciousness [3, 4]. The level of consciousness refers to the state of arousal (or wakefulness) and responsiveness to the surrounding environment and stimuli [3, 4]. The content of consciousness refers to the totality of thought and behavior during wakefulness, and is comprised several components such as orientation, attention, perception, memory and executive function [3, 4]. AMS manifests as a change in level of consciousness, content of consciousness, or both.
Broadly speaking, viral infection can precipitate AMS in two ways: by causing diffuse brain dysfunction from severe systemic illness (otherwise known as encephalopathy or delirium) or from more direct invasive effects on the brain (i.e., encephalitis) [5, 6]. To better understand the causes of AMS in COVID-19, we conducted a retrospective observational analysis of patients with COVID-19 that presented with AMS to the emergency department (ED) during the height of the COVID-19 pandemic in New York City. We also compared patients with AMS as a presenting feature to those without, to identify clinical characteristics associated with AMS in patients with COVID-19.
Methods
Study design, participants and data collection
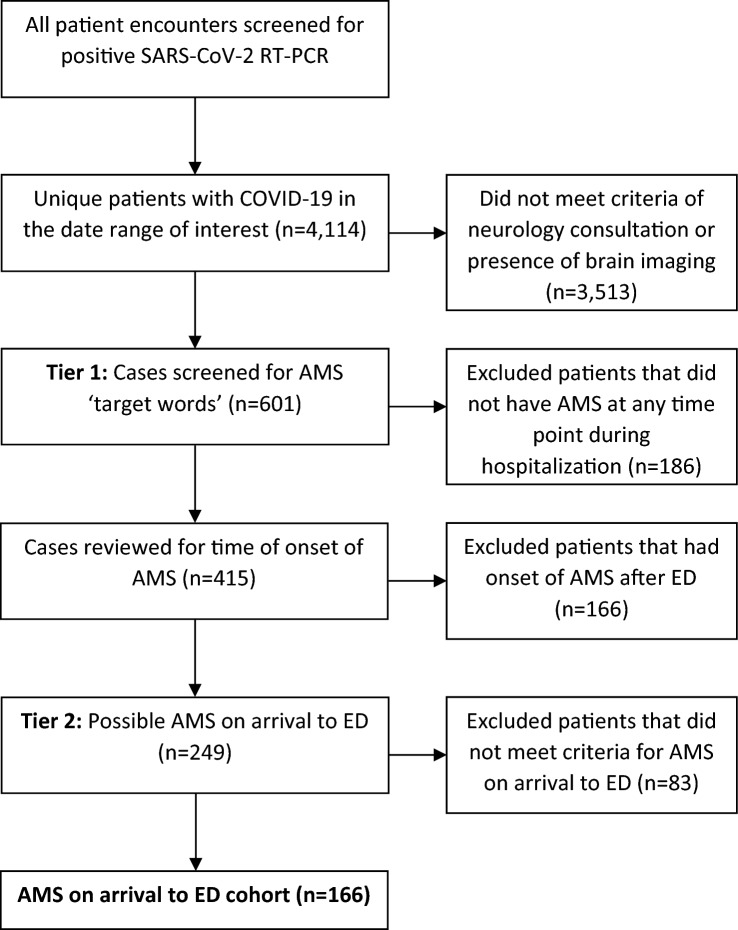
We included patients presenting or admitted to three Montefiore Health System hospitals in the Bronx, New York, between March 1, 2020, and April 16th, 2020. Baseline demographics, comorbidities, medications, imaging studies, final disposition and laboratory variables, including COVID-19 status, were systematically extracted using automated data extraction tools. Glomerular filtration rate was estimated using age, sex and creatinine without a black race coefficient [7, 8]. COVID-19 status was defined by a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay by nasopharyngeal swab using FDA‐approved assays (Abbott, Luminex Aries, Cepheid Xpert Xpress SARS‐CoV‐2, Hologic Panther Fusion real‐time RT‐PCR SARS‐CoV‐2 assay). A total of 4114 unique patients with a positive SARS-CoV-2 RT-PCR were identified during the period of interest. Of these, there were 601 patients with presumed neurological manifestations defined by having a neurologic consultation or imaging of the brain including computerized tomography (CT) or magnetic resonance imaging (MRI).
AMS designation
The electronic medical record of all 601 patients was manually reviewed and screened for AMS (Fig. 1). Abstractors were provided with ‘trigger words’ or phrases that may be used to indicate AMS, such as ‘mental status change’, ‘disoriented’, ‘lethargic’, ‘encephalopathic’, ‘delirious’, and that prompted the screener to look for details of episodes that might indicate AMS [9]. A total of 415 patients were found to have AMS at some point during their hospitalization. Of these, 249 were flagged as AMS that may have been present on arrival to the ED.
Fig. 1.
Flow diagram for inclusion and exclusion criteria
For the second-tier review, these 249 cases were then reviewed in detail by two board-certified neurologists (DA and MM). The “AMS on arrival to the ED” designation was based on the initial ED clinical assessment: the initial history of present illness and physical examination performed by physicians and nurses, as documented in their respective notes. All of the physical examinations included a mental status examination which, at minimum, consisted of an assessment of the patient’s level of arousal and assessment of the patient’s level of orientation to person, place and time.
To meet criteria for “AMS on arrival to the ED” patients had to have a clearly documented (1) change in level consciousness, (2) change in content of consciousness, or (3) both [4]. Criteria for change in the level of consciousness were defined as reduced wakefulness on examination. This included the entire spectrum of arousal deficits from mild to severe: somnolence, drowsiness, lethargy, obtundation, stupor, coma [3]. Change in content of consciousness was defined as any abnormality in the following cognitive domains: orientation (disoriented to situation, time or place), attention (inattentive, confused, delirious, encephalopathic, disorganized thinking), perception (hallucinations), psychomotor behavior (agitation) [10].
Additionally, to meet AMS criteria there needed to be a clear change from baseline and therefore each patient’s cognitive and functional baseline was ascertained by chart review for history of dementia, cognitive impairment, relevant underlying neurologic or psychiatric conditions, and nursing home residence. These underlying general medical conditions are not only important for determining whether the patient had a change from baseline but they are also well known to predispose patients to AMS and lower the threshold of metabolic insult that may cause AMS [9, 11, 12]. Of the 249 cases, 166 met the criteria for “AMS on arrival to the ED.”
AMS causation determination
To identify the potential underlying cause of the AMS, second-tier reviewers (DA and MM) determined if an abnormality was specific and sufficient enough to produce AMS according to the following rules: (Supplemental data).
Neurologic causes: (a) acute lesions found on CT or MRI consistent with clinical presentation; (b) seizure related (post-ictal or status epilepticus); (c) recrudescence of prior deficit [12]; (d) encephalitis/suspected encephalitis [5].
Metabolic derangements (metabolic encephalopathy): one of the following major abnormalities represented a level that by itself may produce AMS [13]. Hyperglycemia (glucose > 500 mg/dL); hypoglycemia (glucose < 60 mg/dL); hypernatremia (sodium > 150 mmol/L); hyponatremia (sodium < 130 mmol/L); acidosis (pH < 7.20); alkalosis (pH > 7.6); hypercarbia PaCO2 above 70 mmHg [14]. Hypoxia < 94% pulse oximetry on room air (SpO2) (the lowest SpO2 needed to support aerobic metabolism has never been identified) [15]; temperature (> 100.4°F); severe sepsis (SIRS criteria). Kidney failure encompassed 2 designations—acute renal failure defined as BUN > 4× upper limit of normal or acute kidney injury (AKI), which was defined as creatinine > 1.5× baseline or AKI clinically diagnosed by the treating team [16, 17]. Acute hepatic injury was defined as an elevation in aspartate aminotransferase or alanine aminotransferase of more than 15 times the upper limit of normal. Toxic exposure was defined as intoxication or withdrawal from an identifiable psychoactive substance.
Indeterminant: if a cause could not be identified by the above criteria, the case was labeled as indeterminant. All indeterminant cases were then re-adjudicated by reviewers for a consensus best fit retrospective diagnosis.
Statistical analysis
For baseline data, mean was used for continuous variables and counts and percentages for categorical variables. In Table 1, comparing COVID positive patients who had AMS on ED presentation and those that did not, continuous variables were tested for normality using Kolmogorov–Smirnov, D’Agostino and Pearson, Shapiro–Wilk, and Anderson–Darling tests. No normality test was passed for any of the continuous variable. For this reason, comparisons of continuous variables were done using Mann–Whitney U tests. Categorical variables were compared by χ2 analysis. All demographic, comorbid, clinical and biomarker variables listed in Table 1, except for mortality, were included in the multivariable regression. None of the variables in the multivariable regression were multicollinear using a VIF threshold of 2.0. The significance threshold was set at a two-sided p value less than 0.05. All analyses were performed in Microsoft Excel (Microsoft Corporation), Graphpad Prism and MATLAB (MathWorks). This study was approved by the Albert Einstein College of Medicine/Montefiore Medical Center Institutional Review Board.
Table 1.
Clinical characteristics
| Variable | AMS on presentation n = 166 |
No AMS on presentation n = 3948 |
p value |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years ± SD) | 74.0 ± 13.1 | 61.6 ± 16.7 | < 0.0001 |
| Age > 70 years | 111 (66.9%) | 1364 (34.6%) | < 0.0001 |
| Male Sex | 90 (54.2%) | 2120 (53.7%) | 0.8955 |
| Black | 83 (50.0%) | 1386 (35.1%) | < 0.0001 |
| Non-Hispanic White | 17 (10.2%) | 294 (7.5%) | 0.1776 |
| Mortality | 76 (45.8%) | 1003 (25.4%) | < 0.0001 |
| Medical history | |||
| Diabetes mellitus | 69 (41.6%) | 1397 (35.4%) | 0.1033 |
| Congestive heart failure | 42 (25.3%) | 554 (14.0%) | < 0.0001 |
| Cerebrovascular disease | 51 (30.7%) | 451 (11.4%) | < 0.0001 |
| Dementia | 56 (33.7%) | 328 (8.3%) | < 0.0001 |
| Hepatic disease | 13 (7.8%) | 328 (8.3%) | > 0.9999 |
| Renal disease | 67 (40.4%) | 850 (21.5%) | < 0.0001 |
| Biomarkers on admission | |||
|
O2 saturation (% ± SD) (% missing value) |
92.9 ± 8.1 3.0 |
92.5 ± 8.7 3.6 |
0.3750 |
|
Temperature (°C ± SD) (% missing value) |
37.1 ± 0.9 4.2 |
37.4 ± 0.9 4.0 |
< 0.0001 |
|
Erythrocyte sedimentation rate (mm/h ± SD) (% missing value) |
83.7 ± 41.4 72.3 |
78.0 ± 32.7 78.4 |
0.5608 |
|
C-reactive protein (mcg/mL ± SD) (% missing value) |
14.0 ± 10.9 18.7 |
13.6 ± 11.1 27.4 |
0.8054 |
|
d-Dimer (quartile ± SD) (% missing value) |
2.5 ± 0.8 23.5 |
2.3 ± 0.7 33.4 |
0.0018 |
|
White blood cell count (k/μL ± SD) (% missing value) |
9.3 ± 4.6 3.0 |
8.4 ± 7.5 7.2 |
0.0003 |
|
Glomerular filtration rate (mL/min ± SD) (% missing value) |
38.7 ± 31.4 < 1.0 |
66.0 ± 38.4 < 1.0 |
< 0.0001 |
|
Aspartate aminotransferase > 40 (% missing value) |
84 (50.6%) 7.2 |
1841 (46.6%) 10.7 |
0.3151 |
|
Blood glucose < 60 mg/dL (% missing value) |
2 (1.2%) 6.6 |
22 (0.6%) 16.1 |
0.2522 |
|
Blood glucose > 500 mg/dL (% missing value) |
12 (7.2%) 6.6 |
108 (2.7%) 16.1 |
0.0030 |
|
Serum albumin < 3.5 g/dL (% missing value) |
61 (36.8%) 4.2 |
934 (23.7%) 9.3 |
0.0003 |
|
Serum sodium < 130 mEq/L (% missing value) |
5 (3.0%) 3.0 |
254 (6.4%) 7.6 |
0.0997 |
|
Serum sodium > 150 mEq/L (% missing value) |
34 (20.5%) 3.0 |
171 (4.3%) 7.6 |
< 0.0001 |
|
Systolic blood pressure < 100 mmHg (% missing value) |
26 (15.7%) < 1.0 |
288 (7.3%) < 1.0 |
< 0.0001 |
Results
We identified 166, or 4% of patients with COVID-19, who met criteria for AMS on arrival to the ED. The patient demographics are listed in Table 1. Notably, patients with AMS were significantly older compared to those without AMS (74.0 vs. 61.6%, p < 0.001) and more likely to be black (50.0 vs. 35.1%, p < 0.001). Nearly 46% of patients with AMS died, compared to 25.4% in the non-AMS cohort (p < 0.001) (Table 1). Seventy-six percent (126/166) of these patients had an underlying general medical condition that predisposed them to AMS, including at least one of the following: pre-existing dementia, cerebrovascular disease, neurologic condition (other than dementia or cerebrovascular disease), schizophrenia, developmental intellectual disability, or current residence in a nursing home (Table 2).
Table 2.
Underlying general medical conditions that are predisposing vulnerabilities to AMS
| Underlying general medical condition | No. (% of patients) (n = 166) |
|---|---|
| At least one vulnerability | 126 (75.9) |
| Dementia | 66 (39.8) |
| Nursing home residence | 61 (36.7) |
| Cerebrovascular disease | 50 (30.1) |
| Neurological conditiona | 9 (5.4) |
| Schizophrenia | 9 (5.4) |
| Developmental intellectual disability | 2 (1.2) |
aOther than dementia or cerebrovascular disease
Causes of AMS
The underlying cause of AMS was identified as a metabolic encephalopathy in 92.8% (154/166), single neurologic cause in 3.0% (5/166) and indeterminant in 4.2% (7/166) (Table 3). Of those patients with metabolic encephalopathy, 71.1% (118/166) were multifactorial (> 1 major metabolic derangement or 1 metabolic derangement plus a neurologic event) and 21.7% (36/166) were caused by a single factor. The metabolic derangements causative of AMS are listed in Table 3, the most common causes were hypoxia 62.0% (103/166) and renal failure 45.2% (75/166). Of the single cause group (n = 36), the most common causes were hypoxia 50.0% (18/36) and fever 22.2% (8/36); 86% (31/36) had at least one underlying general medical condition predisposing them to AMS (Supplemental data). Of the multifactorial group, 14 patients had an additional neurological factor, which included 8 seizure related (6 post-ictal, 2 status-epilepticus), and 7 cerebrovascular events (Table 3).
Table 3.
Altered mental status causes
| Diagnosis | No. (%) (n = 166) |
|---|---|
| AMS from metabolic encephalopathy | 154 (92.8) |
| Single cause | 36 (21.7) |
| Multifactorial | 118 (71.1) |
| AMS from single neurologic factor | 5 (3.0) |
| Ischemic stroke | 2 (1.2) |
| Post-ictal | 2 (1.2) |
| Subarachnoid hemorrhage | 1 (0.6) |
| AMS from indeterminant cause | 7 (4.2) |
| Possible seizure/possible encephalitis | 1 (0.6) |
| Panic attack | 1 (0.6) |
| Probable metabolic | 5 (3.0) |
| Total metabolic derangements | |
| Hypoxia | 103 (62.0) |
| Renal failure | 75 (45.2) |
| Fever | 50 (30.1) |
| Hypernatremia | 34 (20.4) |
| Sepsis | 22 (13.2) |
| Acid–base disturbance | 21 (12.6) |
| Hyperglycemia | 15 (9.0) |
| Hypoglycemia | 7 (4.2) |
| Hyponatremia | 5 (3.0) |
| Hypercapnea | 3 (1.8) |
| Total neurological diagnoses | 20 (12.0) |
| Seizure related | 10 (6.0) |
| Ischemic stroke | 6 (3.6) |
| Intracerebral hemorrhage | 1 (0.6) |
| Subarachnoid hemorrhage | 1 (0.6) |
| Multi-compartment hemorrhage | 1 (0.6) |
| Hypertensive encephalopathy | 1 (0.6) |
In total, 20 patients had neurological diagnoses contributing to AMS. Neurologic cause as the sole factor producing AMS, without metabolic derangement, occurred in 5 patients (3.0%): 2 post-ictal, 2 acute ischemic strokes, and 1 subarachnoid hemorrhage. In total, 10 (6.0%) patients had seizure-related contribution to their AMS (post-ictal or status): 7 had a history of epilepsy, 1 had a newly diagnosed brain mass, 1 had a multi-compartment hemorrhage, and 1 had an alcohol withdrawal seizure (Table 3).
Seven patients did not meet pre-specified criteria and thus were categorized as indeterminant cause (4.2%) (Supplementary data). Consensus best fit diagnosis was metabolic encephalopathy in 5, panic attack in 1, and possible seizure/possible encephalitis in 1 (Supplementary data).
After adjusting for demographics, comorbidities and clinical variables, we found increasing age (p < 0.01), history of dementia (p < 0.001), history of cerebrovascular disease (p < 0.01), and decreased renal function on arrival (p < 0.05) to be independent predictors of AMS (Table 4).
Table 4.
Multivariate analysis of variables significant in univariate analysis
| Variable | Odds ratio (95% confidence interval) | p value |
|---|---|---|
| Cerebrovascular disease history | 1.82 (1.15–2.83) | 0.0063 |
| Dementia history | 2.35 (1.44–3.78) | 0.0006 |
| Glomerular filtration rate (mL/min) | 0.99 (0.98–1.00) | 0.0164 |
| Age > 70 | 1.79 (1.16–2.79) | 0.0100 |
Discussion
The overwhelming majority of patients with COVID-19 that presented to the ED with AMS had a metabolic encephalopathy as the underlying cause, with hypoxia and kidney failure being the most frequent etiologies. Patients with a presentation suspicious for encephalitis were rare < 1% (1/166). Furthermore, we show that age, a history of dementia and cerebrovascular disease, and impaired renal function are independent predictors of AMS on arrival to the ED.
Thus, delirium from metabolic derangements secondary to hypoxia or a systemic inflammatory response is the most common cause of AMS in our cohort. Twenty patients (12%) had an acute neurologic diagnosis contributing to their AMS, half of which were seizure related (7 patients with known epilepsy, 3 with clear provoking factors) and half of which were acute cerebrovascular events. These results align with what has been previously established in patients with SARS-CoV-1 and MERS-CoV: AMS caused by direct viral invasion of the CNS is possible, but occurs infrequently [18].
Moreover, these results highlight a synergism between two sets of overlapping vulnerabilities: vulnerability to encephalopathy and vulnerability to COVID-19 disease severity. With regard to vulnerability to encephalopathy, in which age, dementia, and cerebrovascular disease are well-established risk factors [11, 19, 20], our findings independently validate these prior studies. These underlying factors not only raise the chance of a patient developing encephalopathy, but they also lower the threshold of metabolic insult needed to induce it. [3] Consistent with this, we found at least one underlying general medical condition that is a predisposing vulnerability to AMS present in 86% (31/36) of patients who had metabolic encephalopathy caused by a single factor. Moreover, AMS as a prodrome that occurs days prior to hypoxia and inflammation likely accounted for at least 57% (4/7) of the AMS indeterminant cases. This phenomenon of prodromal AMS heralding overt infectious signs is well described in patients with dementia and infection, and more recently described in dementia patients with COVID-19 infection [21].
With regard to vulnerability to COVID-19 illness severity, renal impairment emerged as an independent predictor of AMS in our multivariate analysis. AKI is common in severe COVID-19 infection [22] and our results show that AKI can be a key trigger for the cascade of physiologic disturbances that produce metabolic encephalopathy in vulnerable COVID-19 patients.
This study has several limitations. During the New York City COVID-19 surge, threshold for ED admission was skewed toward severe infection by pre-ED admission screening. This may have affected the data in 2 ways. (1) Patients with mild-to-moderate symptoms (afebrile, no hypoxia) with unappreciated AMS may have been triaged home instead of admitted to ED, and thus not been captured in this cohort. (2) Given the combination of patient acuity and limited hospital resources, coincident direct neurologic effects of COVID-19 may have gone under-investigated and thus underdiagnosed in this cohort. This study did not track patients that developed AMS after their time in the ED. Patients who developed in-hospital delirium or ICU-delirium were not studied. Aside from in-hospital contributions to delirium (psychoactive medicine, immobilization, mechanical ventilation) it is unlikely this group of patients had a vastly different set of causes of AMS [23]. Lastly, another limitation is the high number of missing values of some biomarkers, like sedimentation rate, which thereby limits that biomarker’s reliability and clinical utility. Since none of these biomarkers were found to be significant in the fully adjusted model future studies are needed to determine if any are indeed associated with COVID-19-associated AMS.
In conclusion, AMS upon ED presentation in COVID-19 patients is most frequently caused by metabolic encephalopathy and is associated with age and pre-existing dementia and cerebrovascular disease. Similar to other acute illnesses, frail older adults are less able to tolerate the stressors of COVID-19 infection and a frequent manifestation of this is delirium. This detailed elaboration of the principal causes of AMS can help guide differential diagnosis and management of COVID-19 patients with AMS.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Mao L, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:E1–E8. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero-Sanchez C, et al. Neurologic manifestations in hospitalized patients with COVID-19. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Posner J, et al. Plum and Posner's diagnosis of stupor and coma. 4. Oxford University Press; 2007. [Google Scholar]
- 4.Smith A, Han J. Altered mental status in the emergency department. Semin Neurol. 2019;39:5–19. doi: 10.1055/s-0038-1677035. [DOI] [PubMed] [Google Scholar]
- 5.Cho T, Mckendall R. Clinical approach to the syndromes of viral encephalitis, myelitis, and meningitis. Handb Clin Neurol. 2014;123:89–123. doi: 10.1016/B978-0-444-53488-0.00004-3. [DOI] [PubMed] [Google Scholar]
- 6.Ellul M, et al. Defining causality in COVID-19 and neurological disorders. J Neurol Neurosurg Psychiatry. 2020;91(8):811–812. doi: 10.1136/jnnp-2020-323667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey A, et al. Using standardized serum creatinine values in the modification of diet in renal disease equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Eneanya N, Yang W, Reese P. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322(2):113–114. doi: 10.1001/jama.2019.5774. [DOI] [PubMed] [Google Scholar]
- 9.Sacynski J, et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62:518–524. doi: 10.1111/jgs.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ropper A, et al. Adams and Victor's principles of neurology. 11. McGraw-Hill Education; 2019. [Google Scholar]
- 11.Inouye S, Westendrop R, Sacsynski J. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topcuouglu M, et al. Recrudescense of deficits after stroke: clinical and imaging phenotype, triggers, and risk factors. JAMA Neurol. 2017;9:1048–1055. doi: 10.1001/jamaneurol.2017.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan B, Leung L, Caplan L. A neurologist's approach to delirium: diagnosis and management of toxic metabolic encephalopathies. Eur J Intern Med. 2013;25(2):112–116. doi: 10.1016/j.ejim.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Eidelman L, et al. The spectrum of septic encephalopathy: definitions etiologies and mortalities. JAMA. 1996;275(6):470–473. doi: 10.1001/jama.1996.03530300054040. [DOI] [PubMed] [Google Scholar]
- 15.Marino P. The ICU book. 4. New York: Lippincott Williams & Wilkens; 2014. [Google Scholar]
- 16.Ronco C, Bellomo R, Kellum J. Acute kidney injury. Lancet. 2019;394:1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 17.Lu R, et al. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11:707–719. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 18.Zubair A, et al. Neuropathogenesis and neurologic manifestations of the Coronaviruses in the age of Coronavirus disease of 2019. JAMA Neurol. 2020;77:1018. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolph J, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcantanio E. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poloni T, et al. Prevalence and prognostic value of delirium as the initial presentation of COVID-19 in the elderly with dementia: an Italian retrospective study. EClin Med. 2020;26:1–8. doi: 10.1016/j.eclinm.2020.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson S, et al. Presenting characteristics, comorbidities, and outcomes among patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsiech J, Ely E, Gong M. Can intensive care unit delirium be prevented and reduced? Ann Am Thorac Soc. 2013;10(6):648–656. doi: 10.1513/AnnalsATS.201307-232FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.