Abstract
The innovative development of cancer therapies has led to an unprecedented improvement in survival outcomes and a wide array of treatment-related toxicities, including those that are cardiovascular in nature. Aging of the population further adds to the number of patients being treated for cancer, especially those with comorbidities. Such pre-existing and developing cardiovascular diseases pose some of the greatest risks of morbidity and mortality in patients with cancer. Addressing the complex cardiovascular needs of these patients has become increasingly important, resulting in an imperative for an intersecting discipline: cardio-oncology. Over the past decade, there has been a remarkable rise of cardio-oncology clinics and service lines. This development, however, has occurred in a vacuum of standard practice and training guidelines, although these are being actively pursued. In this council perspective document, the authors delineate the scope of practice in cardio-oncology and the proposed training requirements, as well as the necessary core competencies. This document also serves as a roadmap toward confirming cardio-oncology as a subspecialty in medicine.
Keywords: cancer, cardio-oncology, cardiotoxicity, training
The treatment of cancer has evolved dramatically over the past 2 to 3 decades such that the previous treatment triad of cytotoxic chemotherapy, radiation therapy, and surgery has been expanded to now include a plethora of targeted and immune-based therapies (1). Owing to the success of these therapies, the outcomes of patients with cancer have continued to improve to the point that there are now more cancer survivors than ever before, with projections to exceed 20 million in the United States alone in the near future (2). In particular, aging of the population (and other common risk factors) will contribute to the increase and clustering of cancer and cardiovascular (CV) disease (CVD) (3). The interplay between these 2 disease entities has gained increasing attention (4) and led to the proposition of a new subspecialty: cardio-oncology (CO) (5,6).
Over the past 15 years, there has been an exponential growth in the number of CO services, not only in the United States, but also worldwide (7–10), which has occurred without much guidance endorsed by professional societies (11). In the void of evidence-based standards, practice patterns likely vary across offices, clinics, and institutions. Moreover, there have been no universal qualifications for physicians to be regarded as a “cardio-oncology specialist” or “cardio-oncologist” (12). Despite this, there is an expectation that any physician providing CO care would possess the experience and knowledge to do so in a manner analogous to other subspecialties (13). Delineating the scope of the practice of CO and its knowledge base and core competencies, as well as defining the unique and complex training requirements in CO, are the objectives of this proposal (Central Illustration). This document may further serve as a roadmap toward CO as a focused new discipline (subspecialty) in medicine.
CENTRAL ILLUSTRATION. Central Elements of Cardio-Oncology Training.
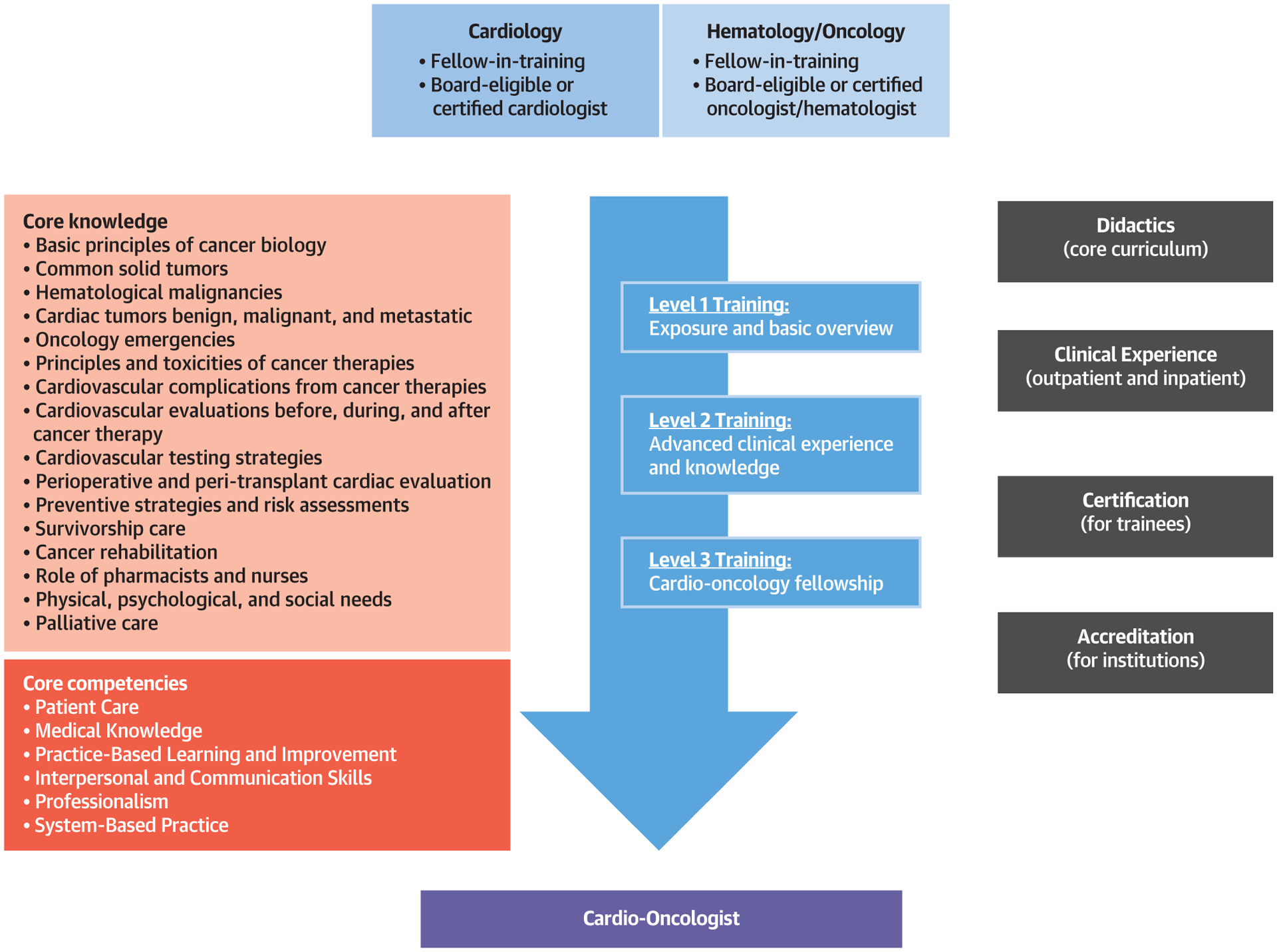
Cardiology and hematology/oncology trainees or specialists can aspire to different levels of cardio-oncology training and acquire core knowledge elements and core competencies as outlined in an environment that provides the appropriate learning environment.
DEFINITION OF THE SCOPE OF PRACTICE OF CO
CO addresses the CV needs of patients throughout the entire cancer journey: before (risk assessment), during (detection of CV toxicity), and after (survivorship) cancer treatment. It thus covers a broad spectrum of disease processes. Patients from various disciplines with various malignancies at various stages may be referred for a plethora of CV complications (9). Consequently, one might consider CO as an integration of the breadth and depth of knowledge in oncology, as well as cardiology (Figure 1). CO providers not only need to be well-rounded cardiologists, but they also must have a sound understanding of solid and hematologic malignancies, including an appreciation of the natural history, treatment options, and overall cancer prognosis. In general, the leading reasons for referral to the CO clinic are pretherapy evaluation for patients with high baseline risk and complications of cancer therapy, especially cardiomyopathy or heart failure, vascular disease, and arrhythmias (14,15). The guiding principle of the CO approach is to enable patients to complete the best possible cancer therapy with the lowest possible CV impact (16). This principle is applied across the entire continuum of cancer care (Table 1).
FIGURE 1. Cardio-Oncology as the Integration of Cardiology and Oncology/Hematology.
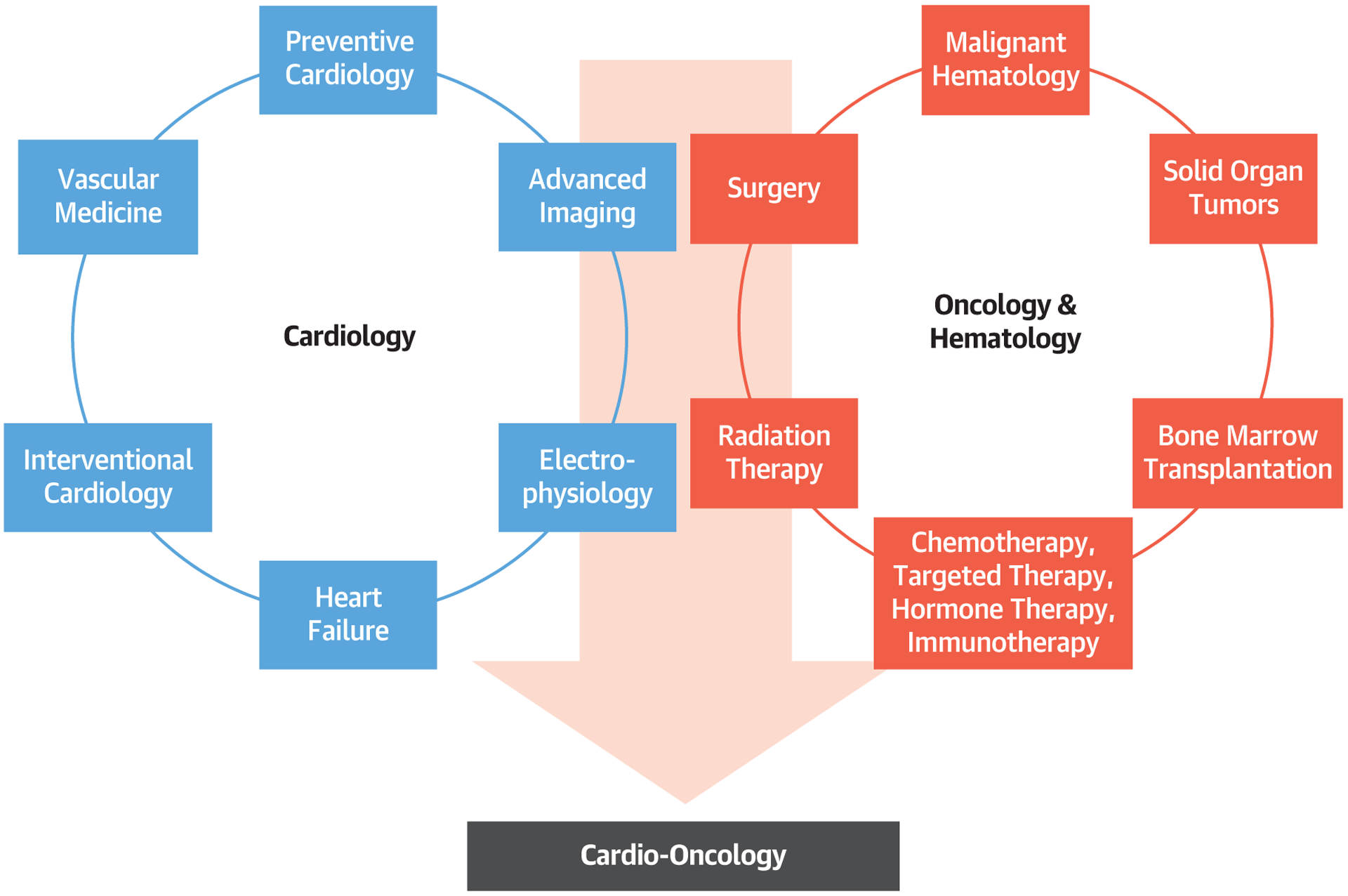
The specialty of cardio-oncology can be considered as the blend of both cardiology and oncology/hematology, with the large diversity in disease entities and subspecialty aspects, as illustrated.
TABLE 1.
Common Reasons for CO Referral Across the Continuum of Cancer Care
| Before cancer therapy | |
| High baseline cardiovascular risk (by history of risk factors or disease) |
|
| High cancer therapy-related risk |
|
| During cancer therapy | |
| Pre-existing compensated or decompensated CVD |
|
| New CVD as a complication of cancer therapy |
|
| After cancer therapy | |
| Pre-existing CVD |
|
| Complications of cancer therapy |
|
| High long-term CV risk of cancer therapy |
|
ADT = androgen deprivation therapy; BCR-ABL = breakpoint cluster region-Abelson murine leukemia; BTK = Bruton’s tyrosine kinase; CAR = chimeric antigen receptor; CO = cardio-oncology; CV = cardiovascular; CVD = cardiovascular disease; EGFR = endothelial growth factor receptor; HF = heart failure; ICI = immune checkpoint inhibitor; MI = myocardial infarction; PDGFR = platelet-derived growth factor receptor; VEGFR = vascular endothelial growth factor receptor.
KEY TOPICS AND CORE KNOWLEDGE ELEMENTS IN CO
The background of the CO provider may differ and needs to be accounted for in training and practice (Figure 2). For the cardiologist, becoming a cardio-oncologist requires collaboration with other disciplines in order to gain a proper perspective of oncology and malignant hematology. Education is needed in the general principles of oncology/hematology, including cancer biology, all forms of cancer therapy (chemotherapy, targeted therapy, hormone therapy, immunotherapy, radiation therapy, and surgery), bone marrow transplantation, and cancer survivorship with all its attendant CV considerations (17). Alternatively, those with an oncology/hematology background aspiring to become an expert in this field need to gain a perspective of CVD, imaging characteristics in all forms of CVD, and the general management of a broad range of CV conditions (Figure 2). Even though both a cardiology-focused and an oncology-focused training pathway can lead to subspecialty training in CO, the clinical practice patterns will likely diverge. Whereas a cardiologypathway cardio-oncologist will focus on first-hand prevention of CV events and management of active CV problems in these patients, the oncology-pathway cardio-oncologist will lean toward (early) recognition of these issues and integration of best practices in cancer treatments and their planning, with an emphasis on prevention, rather than direct treatment, of CV complications, and comanagement with a cardiologist.
FIGURE 2. Road to Cardio-Oncology Based on Prior Training Track.
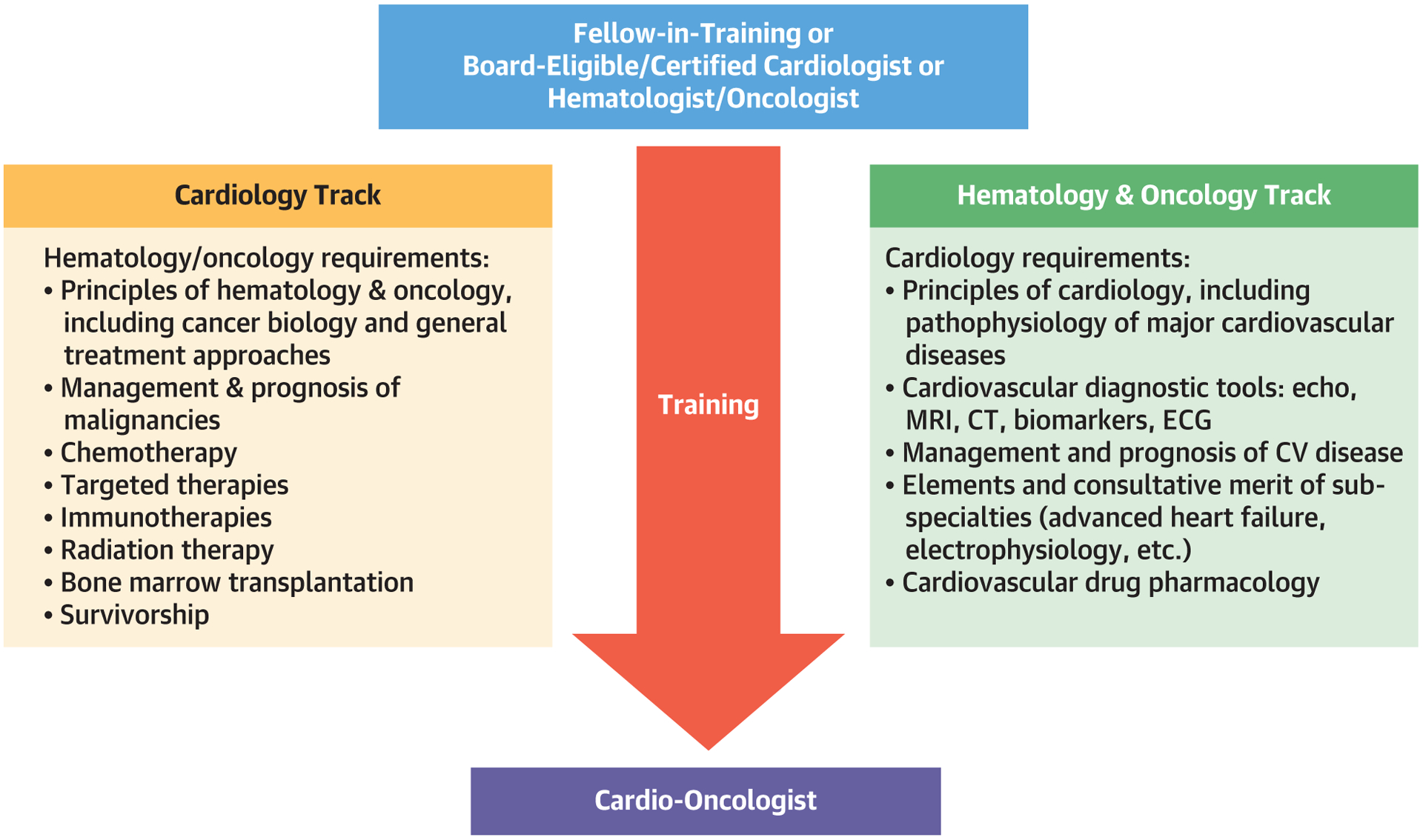
Both cardiology and hematology/oncology fellows are eligible for training in cardio-oncology. Their training needs, however, differ, emphasizing the learning of the knowledge of their counterpart discipline. CT = computed tomography; CV = cardiovascular; ECG = electrocardiography; MRI = magnetic resonance imaging.
Irrespective of the training background, all providers must maintain core knowledge in the CV toxicities of cancer therapies, in CV risk evaluation before, during, and after cancer treatment, and in the unique management aspects of CVD in patients diagnosed with cancer. Table 2 outlines the scope of knowledge for cardio-oncologists. In the following sections, 3 areas will be highlighted that represent key elements of the CO practice.
TABLE 2.
Specific Core Medical Knowledge Elements for Cardio-Oncology Training
|
CO = cardio-oncology; ECG = electrocardiogram.
CVD ASPECTS OF MALIGNANCIES
Commonly viewed as isolated entities, tumors and malignant disease processes can have a systemic component that affects the CV system. On the basis of the subject matter of CO, those seeking advanced training should have knowledge of these disease entities so that they are a resource to other providers in the care team for these patients. This is in light of the fact that all malignancies have broad CV effects, ranging from destabilizing previously controlled CVD to producing new CV risks or direct cancer involvement of the CV system (tumor spread). This includes (oncological) emergencies such as superior vena cava syndrome, cardiac tamponade, and fulminant myocarditis. Cardio-oncologists will be required to advise on the proper coordination of care for these patients, including selection of testing and treatment modalities. Further, CVD may influence the development and progression of malignancies, an aspect that has emerged in recent years and has become known as “reverse cardio-oncology.” Recognition of shared risk factors in CVD and cancer represents an opportunity for advancing research and understanding the interplay of these conditions (18,19).
CV TOXICITIES OF CANCER THERAPIES
The vast majority of CO practice focuses on the prevention and management of CV complications of cancer therapy (15): 1) treatment-based (what drugs, surgery, or radiation therapy are/were used?); 2) symptom/complication-based (what is/was the clinical manifestation i.e., dyspnea, chest pain, rhythm disturbance, etc.?); and 3) patient characteristic-based (what are/were the patient-specific CV risks or established disease?). Details of CV toxicity related to specific therapeutics have been widely published, and all classes of drugs seem to have some CV toxicity associated with their administration (20). Similarly, virtually every CV symptom or major adverse CV event has been the focus of some review. The major CV, oncological, and hematologic conditions to be familiar with are listed in Table 2. Cardiac-specific toxicities include myocardial diseases (cardiomyopathy, heart failure with preserved or reduced ejection fraction, asymptomatic left ventricular systolic dysfunction), hypertension, endothelial and vascular dysfunction, accelerated atherosclerosis, thrombosis and bleeding, pulmonary hypertension, pericardial disease, and QT prolongation and conduction disease/arrhythmias, as well as radiation-induced CV disease (myocardial, pericardial, valvular, arrhythmias and conduction, autonomic dysfunction) (21–26). A detailed listing of these studies is beyond the scope and purpose of this document.
CV EVALUATION OF CANCER PATIENTS
An integral part of providing optimal CV care to the patient being treated for cancer is the ability to select and perform CV testing. Accordingly, cardio-oncologists need to be familiar with and have a basic knowledge of all cardiac imaging and stress testing modalities, vascular studies, electrocardiography tools, and laboratory parameters. Over the past 2 decades, there has been a paradigm shift in the approach to the evaluation of CV toxicity in the oncology patient. Multimodality imaging is now an essential component and includes 2-dimensional and 3-dimensional echocardiography with strain imaging, cardiac magnetic resonance imaging with quantification of mechanical deformation, cardiac computed tomography (CT), and cardiac positron emission tomography (27–30). Hence, it is indispensable to collaborate with advanced CV imaging specialists in the care of these patients.
PROPOSED TRAINING IN CO
The aspects outlined in the preceding text are beyond what currently exists in the standard curriculum in cardiology or hematology/oncology fellowships, and thus there is a clear mandate for a more organized framework that properly conveys this content (31,32). An initial introduction into this topic must be present in general fellowship programs, both for cardiology and hematology/oncology. The integration of other specialties into cardiology fellowship training has been similarly proposed for other cardiology areas such as internal medicine and endocrinology for preventive medicine (33). Accordingly, as outlined in the general cardiology and subspecialty disciplines, we propose a level I to III training structure as summarized in Table 3 (34).
TABLE 3.
Essential Elements Required for Each Representative Level of Cardio-Oncology Training
| Level | Elements |
|---|---|
| Level I training | |
| Exposure and basic overview: basic core curriculum | Context
|
Goal
| |
Eligibility
| |
Format
| |
Duration
| |
| Level II training | |
| Advanced clinical experience and knowledge | Context
|
Goal
| |
Eligibility
| |
Format
| |
Duration
| |
| Level III training | |
| Cardio-oncology fellowship | Context
|
Goal
| |
Eligibility
| |
Format
| |
Duration
|
LEVELS OF TRAINING
LEVEL I TRAINING.
This level of training allows the trainee to obtain sufficient knowledge to understand the basic evaluation and management of the CO patient. The content can be delivered in a basic introductory “rotation” with a minimum of 2 to 4 weeks’ exposure depending on the volume of the program. This rotation may be offered to internal medicine residents, as well as fellows, in cardiology and hematology/oncology. Upon completion, the trainee should have the skill set to recognize the complexity of the CO patient, understand the basic evidence-based approaches to management, and to understand when referral to a cardio-oncologist is needed. Trainees who have completed level I training should not be considered able to practice as independent consultants in CO.
LEVEL II TRAINING.
This training level refers to the additional training in 1 or more areas that enables the cardiology or hematology/oncology specialist to provide more focused expert care for patients with specific CO conditions and to have an understanding regarding appropriate use of specific cardiac diagnostic testing, biomarkers, and interventional procedures in patients with cancer. This level of training is recognized in other subspecialties that have an available instrument or benchmark, such as a qualifying examination, to measure specific knowledge, skills, or competence. Level II training is appropriate for trainees who are seeking to join an existing CO practice or provide CO consultations to existing small practices without the resources of a large academic institution. This level of training is not sufficient to permit trainees to start their own CO programs.
On the basis of content delivery and educational experiences in this area, trainees seeking level II training should be exposed to 3 to 6 months of dedicated CO training. This includes a minimum of 20 unique half-day outpatient clinic sessions and participation in a minimum of 50 unique inpatient consultations (please see additional information under Level III Training). It is anticipated that during a standard 3-year cardiology or hematology/oncology fellowship training program, sufficient time will be available to receive level II training in CO. This can be accomplished through elective rotations with a cumulative minimum of 3 months of didactic and clinical experience. It is recommended that trainees participate in a minimum of 2 to 4 days in oncology clinics during the training period (12 weeks), but more exposure is encouraged based on the institution and the trainee’s basic understanding of oncology.
Trainees are encouraged, but not required, to contribute, start, and/or complete a research project in the area of CO. Level II trainees should obtain a fundamental understanding of the concepts outlined in the medical knowledge section of the core competencies in Table 4. Trainees should also have exposure to clinics in other complimentary areas. The goal is to obtain a basic understanding of the presentation, management, treatment duration, and outcome of a variety of malignancies, especially those likely to receive known cardiotoxic therapies. Those trainees joining the program from an oncology/hematology track should focus on the basic understanding of the presentation, management, treatment duration, and outcome of a variety of CV conditions, particularly those seen in patients being treated for cancer.
TABLE 4.
Core Competencies for Cardio-Oncology Training
| Patient care |
|
| Medical knowledge |
|
| Practice-based learning and improvement |
|
| Interpersonal and communication skills |
|
| Professionalism |
|
| System-based practice |
|
Abbreviations as in Table 1.
LEVEL III TRAINING.
This level of training is currently proposed for cardiologists who would like to have the most advanced education and experience in this area in order to become “expert subspecialists.” This translates to designation as a cardio-oncologist with the qualifications to start their own programs, qualify as director of a CO service line, and/or direct a research program.
Level III training cannot generally be obtained in the context of a standard 3-year CVD fellowship, but credit can be carried over from time spent during the first 3 years of fellowship. On the basis of volume and clinical availability, the recommended minimum duration for level III training is 6 months, and the average training time for most institutions will be 12 months. It is recommended that over time, completion of a 12-month program should become the reference standard for most institutions.
Similar to the common practice of the “continuity clinic,” the concept of the half-day clinic was maintained in the current recommendations, and considering a full academic year with allotted time for absences (e.g., vacation), 40 weeks of half-day continuity clinics are expected to be attainable within 1 full year of CO training dedication. Higher volume centers with 2 half-day clinics or 1 full-day clinic opportunity are likely able to accommodate the fulfillment of this recommendation even within a 6-month time frame. Accordingly, trainees seeking level III training should be exposed to 6 to 12 months of dedicated CO training, including a minimum of 40 unique half-day outpatient clinic sessions and participation in a minimum of 100 unique inpatient consultations. Volumes for minimum expectations for level II and level III training in CO are correlated by a factor 2.
Level III trainees are expected to complete at least 1 research project in the area of CO, not including case report publications. Currently, there are several unaccredited programs for advanced training in CO at institutions with a comprehensive cancer center as listed in Table 5. Trainees who have completed a level III training program should have mastery in the diagnosis, evaluation, workup, management, and follow-up of the CO patient as outlined in the medical knowledge section of Table 4. There is, as of yet, no formal level III certification process for this level of certification in CO.
TABLE 5.
List of Cardio-Oncology Fellowship Programs in the United States
| Name of Program | Location | Contact Information | Program Description |
|---|---|---|---|
| Mayo Clinic | Rochester, Minnesota | Martha Grogan, MD E-mail: grogan.martha@mayo.edu Phone: (507) 284–3667; education coordinator Kris Baldwin |
1-yr fellowship (board-eligible cardiology trainees/certified cardiologists) with focus on cardiac amyloidosis and cardio-oncology |
| Memorial Sloan Kettering Cancer Center | New York, New York | Sade Gibbons E-mail: gibbonss@mskcc.org Phone: (212) 639–5154 |
1- to 2-yr research and clinical fellowship in cardio-oncology for board-eligible/certified cardiologists |
| University of Alabama | Birmingham, Alabama | Carrie Lenneman, MD E-mail: clenneman@uabmc.edu Phone: (205) 975–7123 |
2-yr program with 1 yr dedicated to clinical cardio-oncology and additional year to complete a clinical research project on a T32 grant |
| University of Pennsylvania | Philadelphia, Pennsylvania | Joseph Carver, MD E-mail: jrc@mail.upenn.edu Phone: not available |
Either a 3-month rotation for cardiology or oncology fellows, or a 1-yr intensive training position for board-eligible/certified cardiologists or oncologists |
| University of Texas MD Anderson Cancer Center | Houston, Texas | Lauren Sutton E-mail: lmsutton1@mdanderson.org Phone: (713) 792–1958 |
1-yr clinical and research fellowship |
| University of South Florida & Moffitt Cancer Center | Tampa, Florida | Twyla Sumpter Fellowship coordinator E-mail: tsumpter@health.usf.edu Phone: (813) 259–0600 |
1-yr clinical and research fellowship |
| Vanderbilt University | Nashville, Tennessee | Javid Moslehi, MD E-mail: javid.moslehi@vanderbilt.edu Phone: not available |
1- or 2-yr clinical and research fellowship |
| Washington University School of Medicine | St. Louis, Missouri | Joshua Mitchell, MD E-mail: jdmitchell@wustl.edu Phone: (314) 273–2255 |
1-yr program designed to provide comprehensive exposure to all aspects of inpatient and outpatient cardio-oncology and cardiac amyloidosis |
Programs are listed in alphabetical order and are current as of June 2020; the most up-to-date status is available on the American College of Cardiology website.
TRAINING COMPONENTS
DIDACTICS.
Didactic instruction may take place in a variety of formats, including, but not limited to: lectures, conferences, tumor boards, journal clubs, grand rounds, clinical case presentations, and patient safety or quality improvement conferences. A recommended core lecture series should include, but is not be limited to: CV complications of specific cancers, cardiac tumors, anticipated or observed CV toxicities of cancer therapies, natural history and prognosis of common malignant diseases with and without treatment, and the comprehensive evaluation, treatment, and prognosis of CVD encountered in patients being treated for cancer. Beyond providing excellence in clinical care, a cardio-oncologist has the responsibility to educate other trainees and colleagues about important CO principles (35–37).
HANDS-ON EXPERIENCE, OUTPATIENT VISITS, AND INPATIENT CONSULTATION.
Hands-on experience is vitally important for training in CO and is necessary to acquire the core competencies for level I to III training as detailed in the preceding text. This experience includes exposure to structured CO clinics and inpatient consultative services. In addition, CV trainees should have observational experiences in oncology clinics. The goal is to obtain a basic understanding of the presentation, management, treatment duration, and outcome (prognosis) of a variety of malignancies across a broad spectrum of clinical situations. During rotations, trainees should review case histories, investigate the mechanisms of cancer therapies including type and amounts of therapy, clarify physical findings, interpret electrocardiograms, as well as review advanced cardiac imaging studies performed.
Likewise, hematology/oncology trainees should attend general cardiology and cardiology subspecialty clinics and practices. The goal for these trainees is to obtain a basic understanding of the presentation and management of CVD in patients with cancer and especially CV toxicities of cancer therapies, including prevention, treatment duration, and outcome. Similar to the CV trainee, they will review case histories, physical examination findings, and test results that help define CV side effects and the best mode of treatment and prevention.
To obtain advanced training (level II or III), programs should provide adequate exposure to allow the trainee to obtain the knowledge and skills required to manage the broad spectrum and complexities of the CO patient. Programs should be able to provide access and exposure to CV complications of a variety of cancer treatments including different systemic agents, immune and radiation therapies, and stem cell transplantation. At a minimum, the program should provide a structured CO clinic at least 2 half days per week, with the addition of inpatient consultation (Table 3) The latter type of hospital-based practice includes mainly patients with active cancer who require expert consultations for the management of CVD and toxicities. Training programs should provide the framework that enables CO trainees to obtain proficiency and meet requirements in all areas either on campus or by external rotations.
Trainees should log their experience in: 1) clinical consultations by disease and complication; and 2) interpretation of echocardiograms (including acquisition and troubleshooting of strain and 3-dimensional imaging techniques). Trainees should understand the basics in acquisition and interpretation of advanced cardiac imaging in the form of cardiac CT, cardiac magnetic resonance imaging, and if possible, cardiac positron emission tomography.
For all trainees, attendance at tumor boards and oncology-led morbidity and mortality case conferences with CV cases is highly encouraged, not only to provide expert opinion and guidance on patient management, but also to learn how to navigate the close collaboration needed between hematology/oncology and cardiology in the field. There is a need for cross-disciplinary communication and education, and regular joint conferences and programs are a very efficient and effective way to reach this common goal.
The complexity of the care of patients with cancer is evident in the outpatient setting and even more so in the hospital setting (Table 6). The latter provides exposure to a wide array of CV complications that are potentially life-threatening and thus have a direct and tangible impact on overall outcomes (21,38,39). Intuitively, the requirement of CV and even intensive care expertise in this setting is very compelling (40). There should be specific exposure for discerning the presence of CV emergencies that include major CV events requiring nuanced management such as acute coronary syndrome, pericardial tamponade, acute arrhythmias requiring treatment, and acute heart failure, as well as hypertensive emergency and other vascular events (41,42). In addition, hospitalized bone marrow transplant recipients are typically very vulnerable and require special considerations regarding the management of blood pressure, volume status, antiplatelet and anticoagulant medications, and in particular, drug-drug interactions that have an important impact on therapeutic choices. An understanding of the role and the goals of palliative care in the CO patients completes the spectrum.
TABLE 6.
The Importance of Inpatient and Outpatient CO Training Experience
| Outpatient training | |
| CV risk assessment and mitigation strategies before cancer therapy | Anticipation for high-risk cancer treatment
|
Optimization of pre-existing CVD
| |
| Evaluation and management of common CVDs in cancer patients on active therapy (pre-existing and newly arising) | Left ventricular dysfunction
|
CAD
| |
PAD
| |
VTE and PE
| |
Arrhythmias
| |
Cardiac masses
| |
| Survivorship |
|
| Inpatient training | |
| Evaluation and management of cardio-oncological emergencies | Cardiac tamponade
|
SVC syndrome
| |
ACS
| |
Cardiac arrhythmias
| |
VTE and PE
| |
Acute heart failure
| |
Myocarditis
| |
Cardiac masses
| |
| Evaluation and management of bone marrow transplant patients |
|
| Evaluation and management of the oncology patient in the intensive care unit |
|
ACS = acute coronary syndrome; CAD = coronary arterydisease; PAD = peripheral arterialdisease; PE = pulmonary embolism; SVC = superior vena cava; VTE = venous thromboembolism; other abbreviations as in Table 1.
FOCUS ON CO RESEARCH (CLINICAL, TRANSLATIONAL, AND/OR BASIC INVESTIGATION).
For level III training, it is expected that at least 1 clinical project is completed during the training period leading to publication (43,44). It is crucial to balance clinical demands with research productivity, especially in such a novel discipline in which more detailed observations are always necessary to improve patient care (45,46). Programs should, therefore, incorporate protected time for research and academic pursuits.
Upon completion of training, a cardio-oncologist should be able to evaluate patients receiving experimental therapies and identify potential CV adverse events from these therapies. Furthermore, a cardio-oncologist will need to be aware of ongoing clinical trials for which patients may be eligible and serve as a referring provider and/or collaborator.
SUMMARY OF INSTITUTIONAL AND FACULTY REQUIREMENTS
Successful training programs in CO need equal contributions from the institution as well as the faculty. The primary requirement is having accredited training programs in cardiology and hematology/oncology. Programs providing level III training must have a close relationship with an established high-volume, full-spectrum cancer program, for example, a National Cancer Institute-designated cancer program or equivalent with an adequate flow of patients. It is also critical that there are regularly scheduled, dedicated, and structured outpatient CO clinics and either a standing or an on-demand CO inpatient consultative service. Furthermore, for advanced training in CO, the program should be well equipped to evaluate the full spectrum of CV complications of cancer therapies and cardiac tumors. Institutions offering level III training are required to provide all aspects of an advanced cardiac imaging service. Identifying patients who may be eligible for advanced heart failure therapies, including heart transplantation and durable mechanical circulatory support, is of utmost importance. It has previously been demonstrated that patients with chemotherapy-induced cardiomyopathy have a survival after heart transplantation that is comparable to those who receive cardiac allografts for other cardiomyopathies (47,48). Thus, it is fundamental to understand the utility and limitations of these treatment options for cancer survivors.
Furthermore, it is critical to have at least 1 dedicated faculty member with experience and/or specific training in CO. This faculty member should have achieved the skills equivalent to level III training. Coupled with that expertise, this faculty physician should have adequate dedicated CO clinic time to provide clinical volume for training (49). In addition, there must be formal mentoring to provide didactic as well as clinical research exposure in CO. Didactic topics should include, but are not limited to, cancer therapy-related cardiac dysfunction and basic understanding of appropriate CV imaging strategies, as well as knowledge of CV risk assessment (risk stratification) and the short- and long-term consequences of cardiotoxic cancer therapy. Adjunctive faculty in cardiology with expertise in vascular medicine and atherosclerosis, heart failure physiology and clinical management, arrhythmias, valvular disease, pericardial disease, and advanced cardiac imaging should be available to participate and supplement the integration of the management of these conditions in the setting of cancer therapies. Training programs should also engage faculty in oncology for additional didactics and clinical experiences. Last, but not least, the training environment should be such that alternatives to face-to-face consultations are reviewed including virtual electronic consultations (e-consultations), and non-face-to-face phone and video visits (telemedicine). These provide opportunities for health care delivery in the setting of numerous potential restraints, including (timely) appointment accessibility due to limited provider or facility availability, for example due to geographic distances including rural areas or other environmental factors such as outbreaks of viral pandemics.
EVALUATION OF COMPETENCY
Under the tutelage of the program director, the faculty should obtain a record and verify each trainee’s experiences, assess performance, and document satisfactory achievement. A logbook that meets Accreditation Council for Graduate Medical Education reporting standards and summarizes pertinent clinical information (number of cases, diversity of referral sources, diagnoses, disease severity, outcomes, and disposition) for each encounter should be considered. The program director is responsible for confirming the experience and competence, and for reviewing the overall progress of individual trainees with the clinical competency committee to ensure achievement of selected training milestones and identify areas in which additional focused training may be required.
Program directors should evaluate the quality of care and follow-up, they should evaluate the judgment of the trainee and their ability to make appropriate decisions in the care of patients. Trainees receiving level II training should have a fundamental knowledge of the diagnosis, management, and follow-up of the CO patient, as well as when to refer to a level III-trained clinician and/or a more suitable facility. Trainees who have received level III training should be regarded by their program to have mastery in the diagnosis, management, and follow-up of the CO patient. In addition, trainees should have a fundamental knowledge in CO research. Trainees who have completed level III training are regarded as “safe to practice,” that is, competent to provide advanced care independently. It is the duty of the program director to document milestones showing mastery in these areas.
CERTIFICATION
Although critical evaluation of a trainee’s competency by the program is essential, an independent form of certification and accreditation is equally important. A universal knowledge base is a key step toward standardization and is to be expected from any provider who provides designated CO care. As outlined in another recent American College of Cardiology (ACC) council perspective, the American Board of Internal Medicine has traditionally been the accrediting and certifying body but has its own requirements and limitations that can hinder implementation (33). For an emerging discipline such as CO, similar to preventive cardiology, different modes of certification and accreditation need to be considered. For instance, the ACC and/or the International Cardio-Oncology Society could serve as a responsible body to facilitate a certification process and develop a competency exam. In fact, the International Cardio-Oncology Society has commenced such efforts and has completed a certification examination that tests competency in CO in a standardized manner. A score of 80% or higher is required to become certified.
CONCLUSIONS AND THE FUTURE
It has become increasingly clear that the involvement of a cardio-oncologist enables the cancer treatment team to provide the most effective cancer therapies while minimizing CV toxicity and improving the health of long-term survivors of cancer. As such, over the last 15 years, CO has begun establishing itself as an independent subspecialty of medicine and has already been recognized by most major cardiology and oncology societies as a distinct specialty area. Guideline and/or expert recommendations specific to CO have been published by the American Society of Clinical Oncology, European Society of Cardiology, European Society for Medical Oncology, Canadian Cardiovascular Society, and the National Comprehensive Cancer Network, among others. CO is increasingly represented at every national and international cardiology meeting, and there are currently 2 dedicated CO journals (the Cardio-Oncology Journal, and JACC: CardioOncology) with other journals incorporating CO sections. The ACC has established its own CO council as has the European Society of Cardiology with important contributions (7,50).
On a global scale, the number of dedicated training programs has been increasing, but this particular aspect has not yet received widespread attention, and there is currently no standardization. This document provides key recommendations to address critical gaps in education and to establish appropriate and consistent expectations for a CO-trained provider to meet the growing demand. This perspective is also a first crucial step toward an accreditation process in this area.
HIGHLIGHTS.
Cardiovascular comorbidities and toxicities are increasingly important aspects of cancer therapies and outcomes.
Enabling optimal cancer therapy at the lowest cardiovascular risk is a key goal of cardio-oncology.
Cardio-oncology, like other subspecialties, requires expert knowledge, specific skills, and dedicated training.
AUTHOR RELATIONSHIP WITH INDUSTRY
Dr. Zaha is supported by The Cancer Prevention Research Institute of Texas award RP18040. Dr. Mitchell is supported by the Children’s Discovery Institute and Longer Life Foundation. Dr. Herrmann is supported by the National Institutes of Health/National Cancer Institute grant R01CA233601 and the Miami/Florida Heart Research Foundation. Dr. Mitchell has received research funds from Pfizer. Dr. Barac has been on the advisory board of Takeda Ariad Pharmaceuticals. Dr. Dent has grant funding from Novartis US; and has received consultant fees from Novartis Canada. Dr. Lenihan has been a consultant to Lilly, Acorda, Bristol Myers Squibb, and Roche; and has received research funding from Myocardial Solutions. Dr. Herrmann has been a consultant to Amgen, Bristol Myers Squibb, and Takeda (Ariad) Pharmaceuticals. None of these are directly related to the current work.
ABBREVIATIONS AND ACRONYMS
- ACC
American College of Cardiology
- CO
cardio-oncology
- CT
computed tomography
- CV
cardiovascular
- CVD
cardiovascular disease
REFERENCES
- 1.McCune JS. Rapid advances in immunotherapy to treat cancer. Clin Pharmacol Ther 2018;103: 540–4. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 3.Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019;40:3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenihan DJ, Cardinale D, Cipolla CM. The compelling need for a cardiology and oncology partnership and the birth of the International CardiOncology Society. Prog Cardiovasc Dis 2010; 53:88–93. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL. Birth and maturation of cardio-oncology. J Am Coll Cardiol CardioOnc 2019;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancellotti P, Suter TM, Lopez-Fernandez T, et al. Cardio-oncology services: rationale, organization, and implementation. Eur Heart J 2019;40: 1756–63. [DOI] [PubMed] [Google Scholar]
- 7.Cardinale D, Martinoni A, Cipolla CM, et al. Atrial fibrillation after operation for lung cancer: clinical and prognostic significance. Ann Thorac Surg 1999;68:1827–31. [DOI] [PubMed] [Google Scholar]
- 8.Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 2000;36: 517–22. [DOI] [PubMed] [Google Scholar]
- 9.Lenihan DJ, Fradley M, Dent S, et al. Proceedings from the Global Cardio-Oncology Summit: the top 10 priorities to actualize for CardioOncology. J Am Coll Cardiol CardioOnc 2019;1:256–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovenaux L, Cautela J, Resseguier N, et al. Practices in management of cancer treatment-related cardiovascular toxicity: a cardio-oncology survey. Int J Cardiol 2017;241:387–92. [DOI] [PubMed] [Google Scholar]
- 11.Hayek SS, Ganatra S, Lenneman C, et al. Preparing the cardiovascular workforce to care for oncology patients: JACC review topic of the week. J Am Coll Cardiol 2019;73:2226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barac A, Murtagh G, Carver JR, et al. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J Am Coll Cardiol 2015;65:2739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenihan DJ, Hartlage G, DeCara J, et al. Cardio-oncology training: a proposal from the International Cardioncology Society and Canadian Cardiac Oncology Network for a new multidisciplinary specialty. J Card Fail 2016;22: 465–71. [DOI] [PubMed] [Google Scholar]
- 14.Pareek N, Cevallos J, Moliner P, et al. Activity and outcomes of a cardio-oncology service in the United Kingdom-a five-year experience. Eur J Heart Fail 2018;20:1721–31. [DOI] [PubMed] [Google Scholar]
- 15.Kappel C, Rushton M, Johnson C, et al. Clinical experience of patients referred to a multidisciplinary cardio-oncology clinic: an observational cohort study. Curr Oncol 2019;26:e322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller AP, Handberg EM, Rodgers GP. It takes a team to deliver optimal cardiovascular care. J Am Coll Cardiol 2018;72:948–51. [DOI] [PubMed] [Google Scholar]
- 17.Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol 2012;30: 3657–64. [DOI] [PubMed] [Google Scholar]
- 18.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaes AH, Shenoy C. Is it time to include cancer in cardiovascular risk prediction tools? Lancet 2019;394:986–8. [DOI] [PubMed] [Google Scholar]
- 20.Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31: 171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71: 1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates JE, Howell RM, Liu Q, et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the Childhood Cancer Survivor study. J Clin Oncol 2019;37:1090–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry ML, Niu J, Zhang N, Giordano SH, Chavez-MacGregor M. Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. J Am Coll Cardiol Img 2018;11: 1084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bijl JM, Roos MM, van Leeuwen-Segarceanu EM, et al. Assessment of valvular disorders in survivors of Hodgkin’s lymphoma treated by mediastinal radiotherapy +/− chemotherapy. Am J Cardiol 2016;117:691–6. [DOI] [PubMed] [Google Scholar]
- 26.Heidenreich PA, Hancock SL, Lee BK, Mariscal CS, Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol 2003;42:743–9. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort study. J Am Coll Cardiol 2015;65:2511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan JH, Todd RM, Vasu S, Hundley WG. Cardiovascular magnetic resonance in the oncology patient. J Am Coll Cardiol Img 2018;11: 1150–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2013;26:1013–32. [DOI] [PubMed] [Google Scholar]
- 30.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27: 911–39. [DOI] [PubMed] [Google Scholar]
- 31.Ganatra S, Hayek SS. Cardio-oncology for GenNext: a missing piece of the training puzzle. J Am Coll Cardiol 2018;71:2977–81. [DOI] [PubMed] [Google Scholar]
- 32.Weissman G, Auseon AJ, Arrighi JA, et al. Perceptions and utilization of the U.S. Core Cardiovascular Training Statement. J Am Coll Cardiol 2019;73:2896–9. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro MD, Maron DJ, Morris PB, et al. Preventive cardiology as a subspecialty of cardiovascular medicine: JACC council perspectives. J Am Coll Cardiol 2019;74:1926–42. [DOI] [PubMed] [Google Scholar]
- 34.Fuster V, Halperin JL, Williams ES, et al. COCATS 4 Task Force 1: training in ambulatory, consultative, and longitudinal cardiovascular care. J Am Coll Cardiol 2015;65:1734–53. [DOI] [PubMed] [Google Scholar]
- 35.Cullen MW. Cardiovascular education science: a worthy pursuit for fellows-in-training and early career cardiologists? J Am Coll Cardiol 2019;74: 2322–5. [DOI] [PubMed] [Google Scholar]
- 36.Dauerman HL. RESPONSE: cardiovascular education scholars: a new pathway to promotion. J Am Coll Cardiol 2019;74:2325. [DOI] [PubMed] [Google Scholar]
- 37.Scherrer-Crosbie M response: a call to action for established cardio-oncologists: time to train the future. J Am Coll Cardiol 2018;71:2980–1. [DOI] [PubMed] [Google Scholar]
- 38.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol 2019;74:3099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ball S, Ghosh RK, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol 2019;74:1714–27. [DOI] [PubMed] [Google Scholar]
- 40.Lyons PG, Klaus J, McEvoy CA, Westervelt P, Gage BF, Kollef MH. Factors associated with clinical deterioration among patients hospitalized on the wards at a tertiary cancer hospital. J Oncol Pract 2019;15:e652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Gulati R, Lennon RJ, et al. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Mayo Clin Proc 2016;91:1680–92. [DOI] [PubMed] [Google Scholar]
- 42.Tuzovic M, Herrmann J, Iliescu C, Marmagkiolis K, Ziaeian B, Yang EH. Arterial thrombosis in patients with cancer. Curr Treat Options Cardiovasc Med 2018;20:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gal D, Thijs B, Glanzel W, Sipido KR. A changing landscape in cardiovascular research publication output: bridging the translational gap. J Am Coll Cardiol 2018;71:1584–9. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor CM, Bristow MR. Changing the research culture in the United States. J Am Coll Cardiol HF 2018;6:344–5. [DOI] [PubMed] [Google Scholar]
- 45.Valentine CM. Tackling the quadruple aim: helping cardiovascular professionals find work-life balance. J Am Coll Cardiol 2018;71:1707–9. [DOI] [PubMed] [Google Scholar]
- 46.Marbach JA, Moreland R, Simard T. Effect of a formalized research curriculum on fellows-in-training and early career research productivity. J Am Coll Cardiol 2017;70:2723–6. [DOI] [PubMed] [Google Scholar]
- 47.Oliveira GH, Hardaway BW, Kucheryavaya AY, Stehlik J, Edwards LB, Taylor DO. Characteristics and survival of patients with chemotherapy-induced cardiomyopathy undergoing heart transplantation. J Heart Lung Transplant 2012;31: 805–10. [DOI] [PubMed] [Google Scholar]
- 48.Lenneman AJ, Wang L, Wigger M, et al. Heart transplant survival outcomes for adriamycin-dilated cardiomyopathy. Am J Cardiol 2013;111: 609–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow BJW, Alenazy A, Small G, et al. Competency-based medical education: do the cardiac imaging training guidelines have it right? J Am Coll Cardiol Img 2019;12:2505–13. [DOI] [PubMed] [Google Scholar]
- 50.Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–801. [DOI] [PubMed] [Google Scholar]


