Abstract
BACKGROUND:
Although guidelines recommend focusing primarily on stroke risk to recommend anticoagulants in atrial fibrillation (AF), physicians report that geriatric syndromes (e.g., falls and disability) are important when considering anticoagulants. Little is known about the prevalence of geriatric syndromes in older adults with AF or the association with anticoagulant use.
METHODS:
We performed a cross-sectional analysis of the 2014 Health and Retirement Study, a nationally representative study of older Americans. Participants were asked questions to assess domains of aging, including function, cognition, and medical conditions. We included participants 65 years and older with 2 years of continuous Medicare enrollment who met AF diagnosis criteria by claims codes. We examined five geriatric syndromes: one or more falls within the last 2 years, receiving help with activities of daily living (ADLs) or instrumental ADLs (IADL), experienced incontinence, and cognitive impairment. We determined the prevalence of geriatric syndromes and their association with anticoagulant use, adjusting for ischemic stroke risk (i.e., CHA2DS2-VASc score [congestive heart failure, hypertension, age, diabetes mellitus, stroke, vascular disease, and sex]).
RESULTS:
In this study of 779 participants with AF (median age = 80 years; median CHA2DS2-VASc score = 4), 82% had one or more geriatric syndromes. Geriatric syndromes were common: 49% reported falls, 38% had ADL impairments, 42% had IADL impairments, 37% had cognitive impairments, and 43% reported incontinence. Overall, 65% reported anticoagulant use; guidelines recommend anticoagulant use for 97% of participants. Anticoagulant use rate decreased for each additional geriatric syndrome (average marginal effect = −3.7%; 95% confidence interval = −1.4% to −5.9%). Lower rates of anticoagulant use were reported in participants with ADL dependency, IADL dependency, and dementia.
CONCLUSION:
Most older adults with AF had at least one geriatric syndrome, and geriatric syndromes were associated with reduced anticoagulant use. The high prevalence of geriatric syndromes may explain the lower than expected anticoagulant use in older adults.
Keywords: atrial fibrillation, anticoagulants, geriatric syndromes, epidemiology
INTRODUCTION
The burden of atrial fibrillation (AF) is concentrated in older adults—80% of adults with AF are 65 years and older, many with comorbid conditions that affect functioning and quality of life.1,2 Whereas clinical guidelines recommend clinicians focus primarily on ischemic stroke risk when considering anticoagulants for thromboprophylaxis, physicians report that geriatric syndromes are important when considering anticoagulants for older adults.3–8
Geriatric syndromes are clinical conditions resulting from impairments in multiple organ systems and are common among older adults.9–12 Geriatric syndromes have a substantial impact on the well-being of older adults; they are dominant determinants of death, disability, and quality of life.12,13 Examples of geriatric syndromes include falls, impairments in activities of daily living (ADLs) and instrumental ADLs (IADLs), cognitive impairment, and incontinence. Given their significance, geriatric syndromes are increasingly acknowledged and incorporated into clinical guidelines that inform both common and complex clinical decisions, ranging from diabetes mellitus management to cancer treatment to cardiac intensive care unit care.14–16
Geriatric syndromes complicate anticoagulant use in AF. Geriatric syndromes are associated with a higher risk of ischemic stroke, suggesting greater anticoagulant benefit.17,18 However, geriatric syndromes may magnify harm by both increasing the risk of anticoagulant-associated hemorrhage and limiting patientsʼ ability to recover from major bleeding.18–21 Additionally, geriatric syndromes are associated with reduced life expectancy, thereby limiting the potential benefit of anticoagulants.13,22 Further, anticoagulant use can trigger additional clinic visits, laboratory testing, medication interactions, dietary restrictions, so-called nuisance bleeding, and out-of-pocket expenses—each of which may be particularly burdensome to older adults and their caregivers.23 This phenomenon of increased benefit and increased harm complicates anticoagulant use and has been most clearly demonstrated in patients with a history of falls and frailty.18,19,24,25
Despite their importance to the treatment context, little is known about the prevalence of geriatric syndromes in older adults with AF. Although the prevalence of frailty has been described in multiple studies, we know little about a broad array of geriatric syndromes.26 Geriatric characteristics were not collected during the randomized trials that inform current practice, and these features are rarely ascertained in prospective cohort studies. In this study, we describe the prevalence of geriatric syndromes among participants with AF in a nationally representative cohort of older Americans and then estimate the associations between geriatric syndromes and anticoagulant use.
METHODS
Design and Cohort
We performed a cross-sectional analysis to examine the prevalence of geriatric syndromes among older adults with AF. We used data from the 2014 wave of the Health and Retirement Study (HRS), a nationally representative, longitudinal study of older Americans.27 Participants were aged 50 years and older and interviewed every 2 years to measure changes in disability, health, and wealth as they transitioned from work to retirement. The HRS has conducted in-person and telephone interviews of more than 37,500 older adults since 1992, amounting to 350,000 person-years of observation. The study interviews participants related to four broad areas of aging: income and wealth; health, function, and cognition; work and retirement; and family connections. If a participant was unable to complete an interview because of physical or cognitive impairment, the interview was conducted with a proxy, usually a family member. For the participants who provided consent, the HRS also provides linkages to administrative data, including Medicare insurance claims and Social Security benefits. Through sampling methods, the HRS is nationally representative of the community-dwelling and nursing home population of older Americans.28 Population estimates of mortality, education, and nursing home residence from the HRS match estimates from the U.S. Census.29
To create the study cohort, we included participants who completed the 2014 interview, were 65 years or older at the time of the interview, agreed to Medicare claims linkage, and were continuously enrolled in Medicare fee for service (Parts A and B) in the 24 months before their 2014 interview (Supplementary Figure S1). We included participants who had one inpatient or two outpatient claims for AF (427.31 from the International Classification of Diseases, Ninth Revision, Clinical Modification) in the 24 months before their 2014 interview.30 The 2014 wave is the most contemporary wave for which claims data are available.
Measures
Sociodemographics and Clinical Comorbidities
We used HRS interview data to characterize participantsʼ age, sex, race, ethnicity, education, marital status, whether they lived alone, and nursing home residence. Health status was obtained through self-report. We report clinical comorbidities routinely considered in the treatment of patients with AF and those associated with significant morbidity and mortality in older adults. Specifically, we classified participants as having congestive heart failure, hypertension, prior stroke, diabetes mellitus, prior myocardial infarction, angina, lung disease, or cancer (excluding skin cancer), if the participant reported that a physician had ever told them they had that condition. Prior studies have examined the validity of self-reported cardiovascular comorbidities, such as those used in the CHA2DS2-VASc score (congestive heart failure, hypertension, age, diabetes mellitus, stroke, vascular disease, and sex), finding self-reported diagnoses to accurately reflect medical charts and population-level estimates.31–33 We classified participants as having depressive symptoms if they scored 4 or more on the Center for Epidemiological Studies-Depression scale.34,35
We calculated ischemic stroke risk for each participant via a CHA2DS2-VASc score.36 Guidelines contemporary with the study period recommended anticoagulation treatment for all patients with a CHA2DS2-VASc score of 2 or more.4 Current U.S. consensus guidelines recommend anticoagulation treatment for patients with CHA2DS2-VASc scores of 2 or more for men and 3 or more for women.3
Geriatric Syndromes
We evaluated the following geriatric syndromes: falls, impairment in ADLs, impairment in IADLs, cognitive impairment, and incontinence. We chose these syndromes based on a review of the literature to identify syndromes relevant in the clinical management of AF5–8 and those associated with death and disability in older adults.13,37
Participants were classified as having experienced no falls, noninjurious falls, or injurious falls. Falls were assessed by asking, “Have you fallen down in the last 2 years?” Those who answered “yes” were then asked, “In that fall (or any of these falls), did you injure yourself seriously enough to need medical treatment?” If they answered “yes,” we classified them as having had an injurious fall, and if “no” to have had only noninjurious falls.
Functional impairment was assessed by inquiring if participants had difficulty with or received help with ADLs (bathing, getting out of bed, dressing, eating, toileting, and walking) and IADLs (shopping for groceries, preparing hot meals, taking medications, making telephone calls, and managing money). For both ADLs and IADLs, participants were classified in hierarchical and mutually exclusive categories: no impairment (i.e., no difficulty or help), difficulty with one or more activities, and dependency (i.e., receiving help with one or more activities).
Cognitive impairment was assessed using the Langa-Weir score that has been validated against neuropsychiatric testing.38 For nonproxy interviews, the score is based on tasks of memory, working memory, and mental processing speed. For proxy interviews, the Langa-Weir score is based on the proxyʼs assessment of the participantʼs memory, impairments in IADLs, and the interviewerʼs assessment of cognitive impairment. The Langa-Weir score classifies participants as intact; having cognitive impairment, but not dementia; and having dementia.
Incontinence was assessed by asking, “This might not be easy to talk about, but during the last 12 months, have you lost any amount of urine beyond your control?”
Anticoagulant Use
To determine if they used anticoagulants, participants were asked, “Do you regularly take prescription medications other than aspirin to thin your blood or to prevent blood clots?” We relied on self-report data because prescription claims are not universally available and do not necessarily reflect ongoing use. In the subset of HRS participants with 12 months of continuous enrollment in Medicare Part D before their interview date, we performed a sensitivity analysis to examine the concordance of self-reported anticoagulant use with anticoagulant claims. We detail the results of this sensitivity analysis in Supplementary Table S7.
Analysis
We estimated the prevalence of baseline sociodemographics, clinical characteristics, and geriatric syndromes, having accounted for the complex survey design of the HRS (i.e., sampling strata, clusters, and weights). Separate log-binomial models regressed anticoagulant use onto each geriatric syndrome indicator as well as the count of geriatric syndromes. We assessed various functional forms of the association of the count of geriatric syndromes with anticoagulant use, finding a linear relation to be the best fit (Supplementary Table S3). All models adjusted for stroke risk using the CHA2DS2-VASc score. From these results, we report the predicted population rates of anticoagulant use and the average marginal effect (AME).39 We excluded less than 1% of participants missing data on individual geriatric syndromes or anticoagulant use (Supplementary Figure S1). We did not use significance testing to determine which confounders to include in the regression models, consistent with epidemiologic best practices.40 We report all results with 95% confidence intervals (CIs). We performed analyses using SAS 9.4 and R 3.4.4. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist can be found in Supplementary Table S8.
RESULTS
Participants
Among the 779 participants, the median age was 80 years, 50% were women, 93% identified as White and 4% identified as Black (Table 1). A total of 9% of interviews were conducted with the aid of a proxy. Cardiovascular comorbidities were common—76% reported hypertension, 32% reported congestive heart failure, and 25% reported a prior stroke. A total of 45% of participants reported their health as fair or poor.
Table 1.
Characteristics of Adults 65 Years and Older with Atrial Fibrillation, Weighted to Estimate National Prevalence, 2014
| Characteristic | Prevalence (95% CI) (n = 779) |
|---|---|
| Sociodemographics | |
| Age, median (IQR), y | 80 (74–86) |
| Women, % | 50 (46–54) |
| Married or partnered, % | 51 (47–55) |
| Lives alone, % | 35 (31–39) |
| Education, % | |
| Less than high school | 17 (13–20) |
| Completed high school | 54 (50–59) |
| More than high school | 29 (25–33) |
| Race, % | |
| White | 93 (91–95) |
| Black | 4 (2–5) |
| Other | 4 (2–5) |
| Hispanic ethnicity, % | 2 (1–5) |
| Proxy interview, % | 9 (7–12) |
| Nursing home residence, % | 6 (7–9) |
| Medical comorbidities, % | |
| Self-reported health (fair or poor) | 45 (41–48) |
| Depressive symptoms | 14 (11–17) |
| Congestive heart failure | 32 (29–36) |
| Hypertension | 76 (72–80) |
| Stroke | 25 (21–29) |
| Diabetes mellitus | 33 (29–38) |
| Myocardial infarction | 30 (26–33) |
| Angina | 29 (26–33) |
| Lung disease | 19 (16–22) |
| Cancer, excluding skin cancer | 28 (25–32) |
| Atrial fibrillation | |
| CHA2DS2-VASc score, %a | |
| 1 | 1 (0–3) |
| 2 | 8 (6–11) |
| 3 | 19 (16–22) |
| 4 | 21 (17–25) |
| 5 | 21 (18–24) |
| 6 | 16 (14–19) |
| 7 | 9 (6–11) |
| 8 | 4 (2–5) |
| 9 | 1 (0–2) |
| CHA2DS2-VASc score, median (IQR) | 4 (3–5) |
| 2014 Guidelines recommend anticoagulant use, %b | 99 (97–99) |
| 2019 Guidelines recommend anticoagulant use, %c | 97 (96–99) |
| Anticoagulant use, % | 65 (61–70) |
Note: Percentages shown are weighted using the Health and Retirement Study–RAND survey weights to estimate prevalence among adults 65 years and older with atrial fibrillation in the United States.
Abbreviations: CHA2DS2-VASc score, congestive heart failure/hypertension/age/diabetes mellitus/stroke/vascular disease; CI, confidence interval; IQR, interquartile range.
None with CHA2DS2-VASc score of 0.
Based on 2014 American Heart Association/American College of Cardiology/Health and Retirement Study consensus guidelines: recommended anticoagulation for all with a CHA2DS2-VASc score of 2 or greater.
Based on 2019 American Heart Association/American College of Cardiology/Health and Retirement Study consensus guidelines: recommended anticoagulation for men with CHA2DS2-VASc score of 2 or greater and women with CHA2DS2-VASc score of 3 or greater.
The median CHA2DS2-VASc score in the cohort was 4 (interquartile range = 3–5). Based on the 2014 American Heart Association (AHA)/American College of Cardiology (ACC)/HRS guidelines, anticoagulation therapy would be recommended for 99% (95% CI, 97%–99%) of study participants; current 2019 guidelines, would be recommended therapy for 97% (95% CI, 96%–99%) of participants. Among study participants, 65% (95% CI, 61%–70%) reported using anticoagulants.
Geriatric Syndromes
Most participants had one or more geriatric syndromes; 18% (95% CI, 14%–21%) had no geriatric syndromes (Table 2). Many participants fell in the preceding 2 years: 29% (95% CI, 25%–33%) reported a noninjurious fall, and 20% (95% CI, 17%–24%) reported an injurious fall. Functional impairments were common: 15% (95% CI, 13%–18%) reported difficulty with ADLs, and 23% (95% CI, 20%–27%) reported receiving help with ADLs. Similarly, 14% (95% CI, 12%–17%) reported difficulty with IADLs, and 28% (95% CI, 25%–31%) reported receiving help with IADLs. Regarding cognition, 23% (95% CI, 19%–27%) were classified as cognitively impaired but without dementia, and 14% (95% CI, 11%–17%) were classified as having dementia. Finally, 43% (95% CI, 39%–47%) of participants reported urinary incontinence. Impairment in individual ADLs and IADLs can be found in Supplementary Table S2.
Table 2.
Prevalence of Geriatric Syndromes in Adults 65 Years and Older with Atrial Fibrillation in a Nationally Representative Sample, 2014
| Variable | Prevalence (95% CI) |
|---|---|
| Falls in last 2 y, %a | |
| No falls | 51 (45–56) |
| Noninjurious fall | 29 (25–33) |
| Injurious fall | 20 (17–24) |
| ADLs, %b | |
| No impairment (neither difficulty nor help with ADLs) | 62 (58–65) |
| Difficulty (difficulty with ≥1 ADLs, does not require help with any ADLs) | 14 (11–16) |
| Dependency (help with ≥1 ADLs) | 25 (21–29) |
| IADLs, %c | |
| No impairment (neither difficulty nor help with IADLs) | 58 (55–62) |
| Difficulty (difficulty with ≥1 IADLs, does not require help with any IADLs) | 14 (12–17) |
| Dependency (help with ≥1 IADLs) | 28 (25–31) |
| Cognition, %d | |
| Intact | 63 (59–68) |
| Cognitively impaired, not dementia | 23 (19–27) |
| Dementia | 14 (11–17) |
| Incontinence, %e | 43 (39–47) |
| Count of geriatric syndromes, %f | |
| 0 | 18 (14–21) |
| 1 | 23 (20–27) |
| 2 | 21 (18–25) |
| 3 | 18 (15–20) |
| 4 | 10 (8–13) |
| 5 | 9 (7–12) |
Note: Percentages shown are weighted using the Health and Retirement Study–RAND survey weights to estimate prevalence among adults 65 years or older with atrial fibrillation in the United States.
Abbreviations: ADL, activity of daily living; CI, confidence interval; IADL, instrumental ADL.
Categories are hierarchical and mutually exclusive. If a participant reported multiple falls and any fall that resulted in an injury requiring medical attention, the participant was categorized as having had an injurious fall. Excludes three participants with missing fall data.
Categories are hierarchical and mutually exclusive. Difficulty defined as participant reported difficulty completing one or more ADLs and not requiring help with any ADL. ADLs include bathing, getting out of bed, dressing, eating, toileting, and walking. Excludes three participants with missing ADL data.
Categories are hierarchical and mutually exclusive. Difficulty defined as participant reported difficulty completing one or more IADLs and not requiring help with any IADL. IADLs include shopping for groceries, preparing hot meals, taking medications, making telephone calls, and managing money.
Categories are hierarchical and mutually exclusive. Cognitive status defined using the Langa-Wier score.38
Excludes three participants missing incontinence data.
Count of syndromes is the sum of impairment in each of the five syndromes defined in the table. Impairments defined as noninjurious fall or injurious fall, ADL difficulty or dependency, IADL difficulty or dependency, cognitive impairment or dementia, and incontinence. Excludes nine participants missing any geriatric syndrome data.
Geriatric Syndromes and Use of Anticoagulants
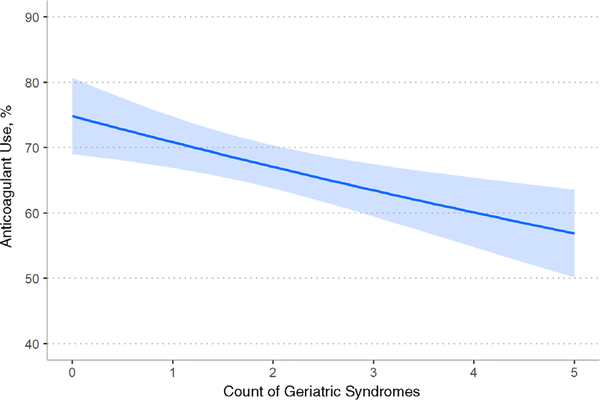
Participants with more geriatric syndromes were less likely to report anticoagulant use (Figure 1). For each additional geriatric syndrome, reported anticoagulant use decreased (AME = −3.7%; 95% CI, −5.9% to −1.4%).
Figure 1.
Association of anticoagulant use with count of geriatric syndromes. Count of syndromes is the sum of impairment in each of the five syndromes defined in the table. Impairments defined as noninjurious fall or injurious fall, activity of daily living (ADL) difficulty or dependency, instrumental ADL (IADL) difficulty or dependency, cognitive impairment or dementia, and incontinence. Analysis based on 768 participants; we excluded 8 with missing geriatric syndrome data, 2 with missing data on anticoagulant use, and 1 missing data on both. The slope represented the marginal effect of one-unit change in count of geriatric syndromes on anticoagulant use, adjusting for stroke risk (CHA2DS2-VASc score). The shaded area represents the 95% confidence interval. The slope is −3.7% (95% confidence interval = − 5.9% to −1.4%). Different functional forms were assessed—a linear relationship produced the best fit (see Supplementary Table S3).
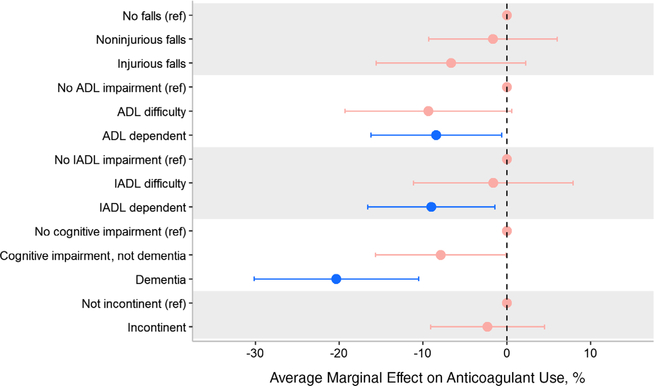
Anticoagulant use was significantly associated with some, but not all, geriatric syndromes (Figure 2). ADL-dependent participants were less likely to report anticoagulant use compared with those without ADL impairment (predicted population anticoagulant use = 61% vs 70%, respectively; AME = −9.1%; 95% CI = −17.1% to −1.2%). Similarly, IADL-dependent participants were less likely to report anticoagulant use relative to those unimpaired (predicted population anticoagulant use = 60% vs 69%, respectively; AME = −9.0%; 95% CI = −16.6% to −1.4%). The largest association was seen in participants with dementia; participants with dementia were the least likely to report anticoagulant use when compared with those cognitively intact (predicted population anticoagulant use = 51% vs 71%, respectively; AME = −20.3%; 95% CI = −30.1% to −10.5%). Although participants who reported injurious falls and incontinence were also less likely to report anticoagulant use, these differences were not statistically significant (Supplementary Table S5).
Figure 2.
Average marginal effect of individual geriatric syndromes on anticoagulant use adjusted for stroke risk. A negative marginal effect indicates lower use of anticoagulants. Models adjusted for stroke risk using CHA2DS2-VASc score. Red point estimates and confidence intervals denote values that are not, statistically, different from the reference group. Blue point estimate and confidence intervals denote values that are statistically different from the reference group. All levels within a syndrome are hierarchical and mutually exclusive. We present tabular results in Supplementary Table S5. Falls: If a participant reported multiple falls and any fall that resulted in an injury requiring medical attention, the participant was categorized as having had an injurious fall. Analysis on 773 participants; excludes 3 missing falls data and 3 missing anticoagulation data. Activity of daily living (ADL): Difficulty defined as participant reported difficulty completing one or more ADLs and not requiring help with any ADL. ADLs include bathing, getting out of bed, dressing, eating, toileting, and walking. Analysis on 773 participants; excludes 3 missing ADL data and 3 missing anticoagulation data. Instrumental ADL (IADL): Difficulty defined as participant reported difficulty completing one or more IADLs and not requiring help with any IADL. IADLs include shopping for groceries, preparing hot meals, taking medications, making telephone calls, and managing money. Analysis on 776 participants; excludes 3 missing anticoagulation data. Cognitive status: defined using the Langa-Wier score.38 Analysis on 776 participants; excludes 3 missing anticoagulation data. Incontinence analysis on 774 participants; excludes 2 missing incontinence data, 2 missing anticoagulation data, and 1 missing both.
We performed sensitivity analyses limiting the population to those for whom the 2014 AHA/ACC/HRS guidelines recommended anticoagulants (Supplementary Tables S4 and S6). We found no substantive difference when compared with the results presented above.
DISCUSSION
In a nationally representative cohort of older adults with AF, we find geriatric syndromes are common and are associated with lower rates of anticoagulant use. Among older adults with AF, 82% had one or more geriatric syndromes—this includes 49% with a fall, 38% with ADL impairment, 42% with IADL impairment, 37% with cognitive impairment, and 43% with incontinence. With each additional geriatric syndrome, the rate of anticoagulant use decreased by 3.7%. We observed variation in the associations between specific geriatric syndromes and anticoagulant use. Although clinicians cite falls as a concern when prescribing anticoagulants, we did not find a significant association with anticoagulant use. Among the one in seven participants with dementia, anticoagulation rates were the lowest.
These findings add to a growing literature on the prevalence of geriatric syndromes in older adults with AF and their association with anticoagulant use. Some prior studies examined one specific geriatric syndrome, frailty, often finding lower rates of anticoagulant use.41,42 Recent studies have examined a broader set of geriatric syndromes. Saczynski et al found that geriatric syndromes are common among older adults with AF but were not statistically associated with anticoagulant use.43 Although this important study sheds light on the burden of geriatric syndromes, the investigators recruited participants exclusively from cardiology clinics, and therefore, the population may not reflect the experience of typical older adults with AF. For instance, that study reported 86% anticoagulant use even among frail and cognitively impaired patients. Kapoor et al examined geriatric syndromes among adults with AF in long-term care facilities.44 In that population, they found geriatric syndromes were common and associated with higher rates of anticoagulant discontinuation. Like the results presented here, they found ADL dependency and cognitive impairment are associated with lower rates of anticoagulant use. Kapoor et al also found a statistically significant association with falls, whereas the corresponding association in this cohort was not statistically significant.
The results of this study make clear that in older adults with AF, complex geriatric comorbidities are the rule rather than the exception. Although geriatric syndromes have a substantial effect on quality of life and play a significant role in clinical decision-making, their impact on the care of persons with AF has seldom been considered. Although many randomized trials have shown anticoagulants are effective at preventing thromboembolic events in patients with AF,45,46 patients with geriatric syndromes were often excluded or underrepresented in those trials. The high prevalence of geriatric syndromes found in the current investigation calls into question whether those trial results are generalizable to most older adults with AF. The randomized trials establishing the efficacy of warfarin did not report the presence of geriatric syndromes let alone examine their impact on outcomes; in fact, geriatric syndromes were often criteria for exclusion. For instance, trials routinely excluded patients with dementia and often excluded patients with falls or unsteady gait, syndromes experienced by 14% and 49% of this nationally representative cohort, respectively.47–50 Although recent randomized trials of direct-acting anticoagulants did not exclude patients with geriatric syndromes, such patients were dramatically underrepresented. For example, among those enrolled in the randomized trial comparing apixaban and warfarin, 4% reported falling in the last year.51 Even after considering the different retrospective time periods, it is materially different from the 49% reporting a fall in the last 2 years in this national cohort.
Although trial data provide limited insights, observational studies of older adults with AF and geriatric syndromes highlight the complexity of anticoagulant use in this population. When examining falls and frailty, observational studies show that these syndromes are associated with an increase in both the risk of ischemic stroke and hemorrhage.18,19,24,25 Given the significant overlap between geriatric syndromes, and the high likelihood that those with geriatric syndromes have high levels of frailty, it stands to reason that the geriatric syndromes examined in this study may also be associated with an increased risk of ischemic stroke and hemorrhage. The high prevalence of geriatric syndromes described in this study underscores the urgent need to carefully study anticoagulant efficacy in older adults with AF and geriatric syndromes.
The study design and data have limitations that are important to consider when interpreting the results. We relied on self-report data to identify geriatric syndromes. Some older adults may not report difficulty because of cognitive impairment or social desirability. To that end, the prevalence measures may be an underestimate. Notably, we designed this study as a cross-sectional analysis to measure the prevalence of geriatric syndromes; this design may underestimate the association of geriatric syndromes and anticoagulant use because of survivor bias.52–54 Specifically, within levels of a geriatric syndrome, those who are healthier may be both more likely to use anticoagulants and to survive long enough to be included in a prevalent cohort. In this situation, the net effect would be to bias the effect estimate toward the null (i.e., to diminish the association between a geriatric syndrome and anticoagulant use). Additionally, although the HRS is nationally representative, findings from this study may not generalize to populations that are typically underrepresented in survey studies, such as patients receiving hospice care or racial and ethnic minorities, such as Asian Americans.
In conclusion, in a nationally representative cohort of older adults, we determined that most older adults with AF have one or more geriatric syndromes. Additionally, the presence of geriatric syndromes was associated with lower rates of anticoagulant use. The high prevalence of geriatric syndromes may explain the lower than expected anticoagulant use in older adults. Because randomized trials excluded or underenrolled older adults with geriatric syndromes and observational studies show geriatric syndromes are associated with an increased risk of ischemic stroke and hemorrhage, there is a pressing need for evidence to guide the optimal use of anticoagulants in older adults with geriatric syndromes.
Supplementary Material
Supplemental Figure S1: Cohort flow diagram.
Supplementary Table S2: Prevalence of individual activities of daily living, use of assistive devices, and instrumental activities of daily living in adults 65 years and older with atrial fibrillation in a nationally representative sample, 2014.
Supplemental Table S3: Functional form of the association between the count of geriatric syndromes and anticoagulant use.
Supplemental Table S4: Count of geriatric syndromes and anticoagulant use, sensitivity analysis limiting population to those where guidelines recommend anticoagulant use.
Supplemental Table S5: Average marginal effect of individual geriatric syndromes on anticoagulant use adjusted for stroke risk, tabular format.
Supplemental Table S6: Individual geriatric syndromes and anticoagulant use, sensitivity analysis limiting population to those where guidelines recommend anticoagulant use.
Supplemental Table S7: Concordance of self-reported anticoagulant use and claims-based anticoagulant use: Sensitivity analysis on self-reported anticoagulant use.
Supplemental Table S8: STROBE Statement—Checklist of items that should be included in reports of cross-sectional studies.
ACKNOWLEDGMENTS
Financial Disclosure:
This study was supported by the National Center for Advancing Translational Sciences (KL2TR001870), the National Institute on Aging (P30AG044281 and P30AG015272), and the National Heart, Lung, and Blood Institute (K24HL141354).
Conflict of Interest:
All authors have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). M.C.F. reports grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute during the conduct of the study. S.E.G. and K.E.C. report grants from the NIH/National Institute on Aging during the conduct of the study. S.J.S. reports grants from the NIH/National Center for Advancing Translational Sciences during the conduct of the study. No financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Sponsorʼs Role:
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
ETHICAL APPROVAL
The Human Research Protection Program Institutional Review Board at the University of California, San Francisco, approved this study (Institutional Review Board No. 16-19185).
DATA SHARING
Researchers can apply to use the Health and Retirement Study (hrs.isr.umich.edu/) for access to the data use in this study. Code used to generate the cohort and perform the analyses can be found on Github (https://github.com/sachinjshah).
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Wilke T, Groth A, Mueller S, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15(4):486–493. 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21): e1–e76. 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.McGrath ER, Go AS, Chang Y, et al. Use of oral anticoagulant therapy in older adults with atrial fibrillation after acute ischemic stroke. J Am Geriatr Soc. 2017;65(2):241–248. 10.1111/jgs.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashburner JM, Atlas SJ, Khurshid S, et al. Electronic physician notifications to improve guideline-based anticoagulation in atrial fibrillation: a randomized controlled trial. J Gen Intern Med. 2018;33:2070–2077. 10.1007/s11606-018-4612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OʼBrien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2014;167(4):601–609.e1. 10.1016/j.ahj.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Madhavan M, Holmes DN, Piccini JP, et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am Heart J. 2019;211:77–89. 10.1016/j.ahj.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med. 2000;132(5):337–344. 10.7326/0003-4819-132-5-200003070-00002. [DOI] [PubMed] [Google Scholar]
- 10.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–1353. 10.1001/jama.1995.03520410042024. [DOI] [PubMed] [Google Scholar]
- 11.Flacker JM. What is a geriatric syndrome anyway? J Am Geriatr Soc. 2003; 51(4):574–576. 10.1046/j.1532-5415.2003.51174.x. [DOI] [PubMed] [Google Scholar]
- 12.Inouye SK, Studenski S, Tinetti ME, Kuchel GA . Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane RL, Shamliyan T, Talley K, Pacala J. The association between geriatric syndromes and survival. J Am Geriatr Soc. 2012;60(5):896–904. 10.1111/j.1532-5415.2012.03942.x. [DOI] [PubMed] [Google Scholar]
- 14.Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295(16):1935–1940. 10.1001/jama.295.16.1935. [DOI] [PubMed] [Google Scholar]
- 15.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damluji AA, Forman DE, van Diepen S, et al. Older adults in the cardiac intensive care unit: factoring geriatric syndromes in the management, prognosis, and process of care: a scientific statement from the American Heart Association. Circulation. 2020;141(2):e6–e32. 10.1161/CIR.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 17.Flaker GC, Pogue J, Yusuf S, et al. Cognitive function and anticoagulation control in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3(3):277–283. 10.1161/CIRCOUTCOMES.109.884171. [DOI] [PubMed] [Google Scholar]
- 18.Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118(6):612–617. 10.1016/j.amjmed.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Rao MP, Vinereanu D, Wojdyla DM, et al. Clinical outcomes and history of fall in patients with atrial fibrillation treated with oral anticoagulation: insights from the ARISTOTLE trial. Am J Med. 2018;131(3):269–275.e2. 10.1016/j.amjmed.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Dodson JA, Petrone A, Gagnon DR, Tinetti ME, Krumholz HM, Gaziano JM. Incidence and determinants of traumatic intracranial bleeding among older veterans receiving warfarin for atrial fibrillation. JAMA Cardiol. 2016;1(1):65–72. 10.1001/jamacardio.2015.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Deelen BAJ, van den Bemt PMLA, Egberts TCG, vanʼt Hoff A, Maas HAAM. Cognitive impairment as determinant for sub-optimal control of oral anticoagulation treatment in elderly patients with atrial fibrillation. Drugs Aging. 2005;22(4):353–360. 10.2165/00002512-200522040-00007. [DOI] [PubMed] [Google Scholar]
- 22.Shah SJ, Singer DE, Fang MC, Reynolds K, Go AS, Eckman MH. Net clinical benefit of oral anticoagulation among older adults with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2019;12(11):e006212. 10.1161/CIRCOUTCOMES.119.006212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals–directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1(1):9–10. 10.1001/jamacardio.2015.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg GM, Silverstone FA, Rangu S, Leventer SL. Outcomes of long-term anticoagulation in frail elderly patients with atrial fibrillation. Clin Drug Investig. 1999;17(6):483–488. 10.2165/00044011-199917060-00009. [DOI] [Google Scholar]
- 25.Gugganig R, Aeschbacher S, Leong DP, et al. Frailty to predict unplanned hospitalization, stroke, bleeding, and death in atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2020;qcaa002. 10.1093/ehjqcco/qcaa002. [DOI] [PubMed] [Google Scholar]
- 26.Villani ER, Tummolo AM, Palmer K, et al. Frailty and atrial fibrillation: a systematic review. Eur J Intern Med. 2018;56:33–38. 10.1016/j.ejim.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Juster FT, Suzman R. An overview of the health and retirement study. J Hum Resour. 1995;30:S7. 10.2307/146277. [DOI] [Google Scholar]
- 28.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the health and retirement study (HRS). Int J Epidemiol. 2014;43(2):576–585. 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnega A The Health and Retirement Study: an introduction, the view from 38000 feet. Presented at the: The Health and Retirment Study Workshop; June p2, 2017; University of Michigan Insitute for Social Research. [Google Scholar]
- 30.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: whatʼs the optimal approach? Am J Med Qual. 2004;19(5):201–206. 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 31.Glymour MM, Avendano M. Can self-reported strokes be used to study stroke incidence and risk factors? evidence from the health and retirement study. Stroke. 2009;40(3):873–879. 10.1161/STROKEAHA.108.529479. [DOI] [PubMed] [Google Scholar]
- 32.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79(11):1554–1556. 10.2105/AJPH.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Steffick D Documentation of Affective Functioning Measures in the Health and Retirement Study. Institute for Social Research, Ann Arbor, Michigan: University of Michigan; 2000. https://hrs.isr.umich.edu/publications/biblio/5411. Accessed August 13, 2019 [Google Scholar]
- 35.Zivin K, Llewellyn DJ, Lang IA, et al. Depression among older adults in the United States and England. Am J Geriatr Psychiatry. 2010;18(11):1036–1044. 10.1097/JGP.0b013e3181dba6d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 37.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the health and retirement study. Ann Intern Med. 2007;147 (3):156–164. 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 38.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66B(suppl 1):i162–i171. 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norton EC, Dowd BE, Maciejewski ML. Marginal effects—quantifying the effect of changes in risk factors in logistic regression models. JAMA. 2019; 321:1304–1305. 10.1001/jama.2019.1954. [DOI] [PubMed] [Google Scholar]
- 40.Sun G-W, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996; 49(8):907–916. 10.1016/0895-4356(96)00025-X. [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre M-CD, St-Onge M, Glazer-Cavanagh M, et al. The effect of bleeding risk and frailty status on anticoagulation patterns in octogenarians with atrial fibrillation: the FRAIL-AF study. Can J Cardiol. 2016;32(2):169–176. 10.1016/j.cjca.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Papakonstantinou PE, Asimakopoulou NI, Papadakis JA, et al. Frailty status affects the decision for long-term anticoagulation therapy in elderly patients with atrial fibrillation. Drugs Aging. 2018;35(10):897–905. 10.1007/s40266-018-0587-6. [DOI] [PubMed] [Google Scholar]
- 43.Saczynski JS, Sanghai SR, Kiefe CI, et al. Geriatric elements and oral anticoagulant prescribing in older atrial fibrillation patients: SAGE-AF. J Am Geriatr Soc. 2020;68(1):147–154. 10.1111/jgs.16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoor A, Foley G, Zhang N, et al. Geriatric conditions predict discontinuation of anticoagulation in long-term care residents with atrial fibrillation. J Am Geriatr Soc. 2020;68:717–724. 10.1111/jgs.16335. [DOI] [PubMed] [Google Scholar]
- 45.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 46.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288(19):2441–2448. 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 47.Mant J, Hobbs FR, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 48.Mant JW, Richards SH, Hobbs FR, et al. Protocol for Birmingham Atrial Fibrillation Treatment of the Aged study (BAFTA): a randomised controlled trial of warfarin versus aspirin for stroke prevention in the management of atrial fibrillation in an elderly primary care population [ISRCTN89345269]. BMC Cardiovasc Disord. 2003;3(1):9. 10.1186/1471-2261-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Stroke Prevention in Atrial Fibrillation Investigators. Design of a multicenter randomized trial for the Stroke Prevention in Atrial Fibrillation Study. Stroke. 1990;21(4):538–545. 10.1161/01.STR.21.4.538. [DOI] [PubMed] [Google Scholar]
- 50.Stroke Prevention in Atrial Fibrillation Investigators. Stroke prevention in atrial fibrillation study: final results. Circulation. 1991;84(2):527–539. 10.1161/01.CIR.84.2.527. [DOI] [PubMed] [Google Scholar]
- 51.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 52.Feinstein AR. XI: sources of “chronology bias” in cohort statistics. Clin Pharmacol Ther. 1971;12(5):864–879. 10.1002/cpt1971125864. [DOI] [PubMed] [Google Scholar]
- 53.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 54.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Cohort flow diagram.
Supplementary Table S2: Prevalence of individual activities of daily living, use of assistive devices, and instrumental activities of daily living in adults 65 years and older with atrial fibrillation in a nationally representative sample, 2014.
Supplemental Table S3: Functional form of the association between the count of geriatric syndromes and anticoagulant use.
Supplemental Table S4: Count of geriatric syndromes and anticoagulant use, sensitivity analysis limiting population to those where guidelines recommend anticoagulant use.
Supplemental Table S5: Average marginal effect of individual geriatric syndromes on anticoagulant use adjusted for stroke risk, tabular format.
Supplemental Table S6: Individual geriatric syndromes and anticoagulant use, sensitivity analysis limiting population to those where guidelines recommend anticoagulant use.
Supplemental Table S7: Concordance of self-reported anticoagulant use and claims-based anticoagulant use: Sensitivity analysis on self-reported anticoagulant use.
Supplemental Table S8: STROBE Statement—Checklist of items that should be included in reports of cross-sectional studies.