Abstract
Background and Aims:
Ultrasonography (USG) is used to evaluate gastric residual volume (GRV); however, this technique may have inter-assessor variability. This study aimed to measure GRV in three groups of fasted patients 2 h after they received 200 mL of water, clear apple juice or apple-flavoured oral rehydration solution (ORS) and to determine inter-assessor reliability of USG-guided GRV measurement.
Methods:
We randomised 90 adult patients planned for elective cancer surgery, with no risk factors for delayed gastric emptying, to receive 200 mL of water, clear apple juice or apple-flavoured ORS after overnight fasting. Two hours later, two blinded assessors (a trained anaesthesiologist and a radiologist) independently determined USG-guided GRV. The primary outcome was GRV measured by the radiologist. The secondary outcome was inter-assessor correlation and agreement in GRV measurements.
Results:
There was no statistically significant difference in median GRV between groups (apple-flavoured ORS 74.8 mL, apple juice 63.7 mL, and water 62.1 mL, P = 0.11). We found poor correlation between measurements of radiologist and anaesthesiologist (Intra-class correlation coefficient 0.3, 95% confidence intervals 0.09 to 0.48, P value 0.002). The average (mean) bias was 5.4 mL (standard deviation 42.3 mL) and the 95% limits of agreement were -79.2 ml to +90 ml.
Conclusion:
Patients receiving 200 mL of water, clear apple juice or apple-flavoured ORS had comparable GRV after 2 h. There was poor correlation and agreement between GRV measurements of different assessors, indicating that more training may be required for anaesthesiologists to attain proficiency in the quantitative assessment of GRV.
Keywords: Anaesthesiologists, pyloric antrum, radiologists, residual volume, ultrasonography
INTRODUCTION
Current pre-operative fasting guidelines recommend the use of clear fluids up to 2 h before anaesthesia in patients scheduled for elective surgery, who are not at risk for delayed gastric emptying or aspiration. This is in order to minimise peri-operative aspiration risk while avoiding prolonged fasting and dehydration.[1,2]
The use of ultrasonography (USG) for estimating gastric residual volume (GRV) has been described by various researchers.[3,4,5] Studies have validated both quantitative and qualitative versions of Perlas technique in adult patients.[6,7] Initial studies looking at USG measurement of GRV included certified sonographers or anaesthesiologists with experience of more than 50 gastric ultrasound examinations.[4,6] However, a subsequent study estimated that anaesthesiologists will achieve a 95% success rate in bedside qualitative USG assessment after performing approximately 33 examinations.[7]
At our tertiary-referral cancer centre, patients posted for elective surgery who have no risk factors for delayed gastric emptying or aspiration received 200 mL of plain water or clear apple juice 2 h before surgery. Recently, a clear apple-flavoured oral rehydration solution (ORS) has been introduced in the market. The aim of our study was to compare the effects of plain water, clear apple juice (Appy®) and apple-flavoured ORS (ORS Apple®) on GRV.
METHODS
We conducted a randomised trial in a tertiary-care cancer hospital between April 2018 and October 2018 after the Institutional Review Board approval and registration at the Clinical Trials Registry of India. We included adult patients (more than 18 years) of either gender who were planned for elective surgery. We excluded patients with comorbidities that delay gastric emptying (e.g., diabetics, morbid obesity with body mass index greater than 40, pregnancy, patients on opioids, pyloric stenosis, chronic renal failure, history of acid peptic disease or gastric reflux, intestinal obstruction, gastric or oesophageal malignancy or pathology), patients on antacids or prokinetics, and patients at risk of aspiration (GCS less than 13/15). To avoid problems with delay or cancellation of surgery in case the GRV was found to be high, we chose patients who were not posted for surgery on that particular day. Written informed consent was obtained from all participants. The study was conducted as per good clinical research practice in accordance with the Declaration of Helsinki.
The participants were allowed solid foods until 10 pm and clear liquids until midnight and were randomised to receive 200 mL of one of three study drinks – water or clear apple juice or apple-flavoured ORS– at 6.30 am the next morning. The fluids used were clear liquids with differing carbohydrate content and osmolarity. Clear apple juice (Appy®) has 16 grams of carbohydrate per 100 mL with osmolarity around 700 mOsmol per litre. Apple-flavoured ORS (ORS Apple®) has 1.35 gram of carbohydrate per 100 mL with osmolarity of 245 mOsmol per litre. We used a computer-generated random sequence prepared by a statistician from a Central Research Secretariat, with sequentially numbered opaque sealed envelopes for allocation concealment. We originally planned to include a control group of 30 patients who would fast overnight and not receive any fluid in the morning. However, during the study, revised fasting guidelines were uniformly adopted across the hospital, and all the patients started to receive clear fluids in the morning. As it would have been unethical to continue the practice of prolonged fasting, we omitted the control arm.
Patient accrual, consenting, randomisation and administration of study drink was performed by a member of the research team who was not involved in study assessments. For the apple juice and apple-flavoured ORS arms, the patients were blinded to the type of drink they were receiving (by wrapping the tetra pack in brown paper). Two hours later (window of 1.5 to 2.5 h), two assessors independently performed ultrasound to determine GRV. The assessors included one of two radiologists with expertise in gastric ultrasound and one of two anaesthesiologists who had performed 30 supervised USG-guided GRV examinations before study commencement. The assessors were blinded to randomisation arm and to each other's measurements and performed the assessments in the holding area of the OT complex sequentially with no time lag. The measurement made by the radiologist was considered the gold standard to calculate the final GRV. The patients receiving apple juice and apple-flavoured ORS were verbally asked to rate the palatability of the liquid they consumed on an ordinal scale (bad, average, good, excellent), in the language best understood by them.
For all GRV measurements, we used the same USG machine (Sonosite™ M Turbo). A curved array low-frequency transducer (2–5 MHz) with standard abdominal settings was used to identify relevant anatomic landmarks. The curvilinear probe was placed in the epigastrium in the paramedian plane with the patient in the right lateral decubitus (RLD) position. The probe was slowly moved laterally till the left lobe of the liver, and the gastric antrum below it was visualised. Two perpendicular diameters – antero-posterior (AP) and cranio-caudal (CC) – were measured from serosa to serosa between peristaltic contractions. The antral cross-sectional area (ACSA) was calculated using the formula of the area of an ellipse: ACSA = (AP × CC × pi)/4. GRV was determined by a formula based on a study by Perlas.[4]
Stomach volume (mL) = 27 + 14.6 ACSA (in RLD) (cm2) − 1.28 age (years)
The primary outcome was to compare GRV in the three groups of patients, 2 h after they received the study drink. The secondary outcome was to determine inter-assessor correlation and agreement in GRV measurement. The exploratory outcome was to compare the palatability of clear apple juice and apple-flavoured ORS.
Studies have shown that the average GRV in an individual fasted for 2 h after consumption of 200 mL of clear liquid is around 50 mL.[8] By assuming that the apple juice and ORS groups would have a difference of 20 mL from the water group, at a two-sided level of significance of 1% (Type 1 error for each comparison taken as 0.01 to account for multiple comparisons) and power of 80%, we needed 25 patients in each group. We accrued 30 patients in each group to account for protocol deviations. Data were entered into statistical software (SPSS 20.0) for analysis on an intention-to-treat basis. Categorical data were expressed as percentages and continuous data as median {inter-quartile range (IQR)}. The comparisons between groups were done using the Kruskal Wallis test for non-parametric data and the Chi-square test for categorical data. Intra-class correlation coefficients and Bland Altman limits of agreement were used to determine the agreement between assessors.
RESULTS
Between April and October 2018, we screened 105 participants; of these 90 were accrued in the study and included in the final analysis. There were no protocol violations. The baseline demographic characteristics of the patients were comparable [Table 1].
Table 1.
Demographic characteristics of the patients
| Water (n=30) | Clear apple juice (n=30) | Apple-flavoured electrolyte solution (n=30) | |
|---|---|---|---|
| Age (years) | 43.6±11.9 | 43.1±14.9 | 42.5±15.0 |
| Height (cm) | 161.8±9.0 | 161.2±9.0 | 158.7±9.7 |
| Weight (kg) | 59.2±11.4 | 58.6±13.3 | 62.4±16.5 |
| BMI (kg/m2) | 22.6±4.3 | 22.5±4.5 | 24.7±5.5 |
| Gender (Male/Female)* | 20/10 | 21/9 | 10/20 |
cm=Centimetre, kg=Kilogram, BMI=Body mass index, m=metre, Data represent mean with standard deviations for continuous data and actual numbers for categorical data*
GRV was higher in the apple-flavoured ORS group with a median of 74.8 mL (IQR 50.2 to 99 mL) as compared to apple juice group (median 63.7 mL, IQR 34.8 to 95.1 mL) and water (median 62.1 mL, IQR 49.0 to 87.0 mL) [Figure 1]. This difference was not statistically significant (P = 0.11). The median GRVs (mL/kg) in the apple-flavoured ORS, apple juice and water groups were 1.1 (IQR 0.8 to 1.7), 1.1 (IQR 0.7 to 1.7), and 1.1 (IQR 0.9 to 1.5), respectively. This difference was not statistically significant (P = 0.67). There was poor correlation between the measurements of the radiologist and anaesthesiologist. (Intra-class correlation coefficient 0.3, 95% confidence intervals 0.09; 0.48, P value 0.004). The Bland Altman plot showed that the average (mean) bias was 5.4 mL (standard deviation 42.3 mL) with 95% limits of agreement -79.2 ml to +90 ml, meaning that in 95% of cases, the difference between the two measurements ranged from -79 mL to + 90 mL [Figure 2].
Figure 1.
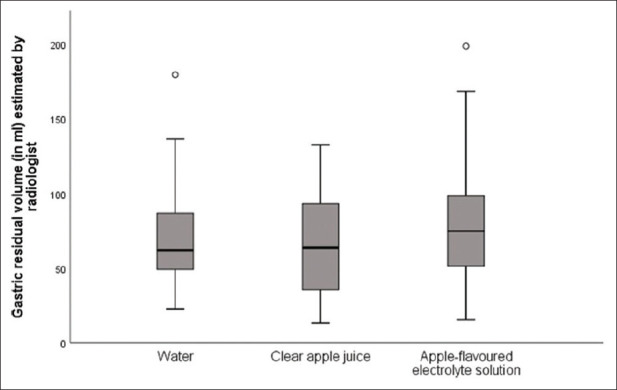
Box and whiskers plot showing median GRV in the three groups, as measured by the radiologist
Figure 2.
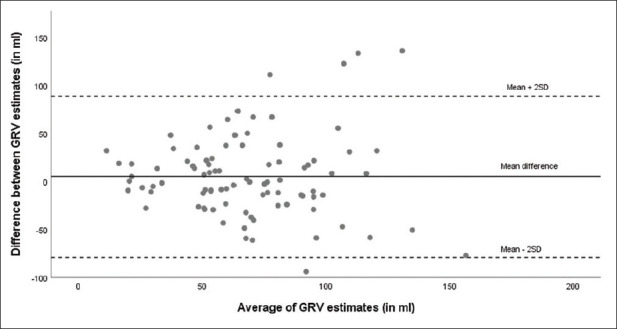
Bland –Altman plot showing agreement in GRV measurements made by radiologist and anaesthesiologist
GRV of more than 1.5 mL/kg carries a higher risk of aspiration.[9] Of the 90 patients, 26 (28.9%) had GRV greater than 1.5 mL/kg. As a post-hoc analysis, we compared the patients between groups, who had GRV higher than 1.5 mL per kg body weight; this difference was not statistically significant (P = 0.35) [Figure 3].
Figure 3.
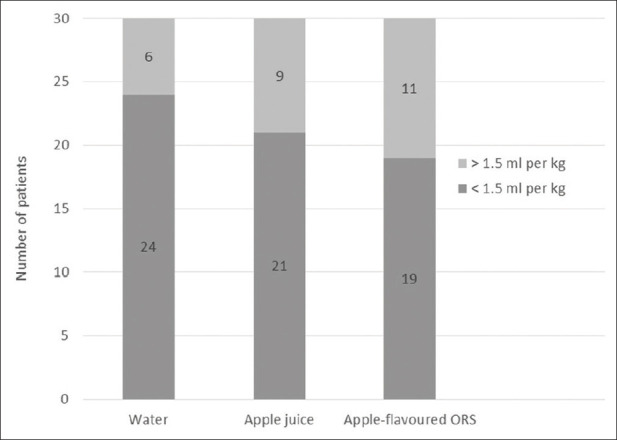
GRV distribution across groups as per body weight
Among 30 patients in the apple-flavoured ORS group, 14 (47%) rated the taste as excellent and 16 (53%) rated it as good, whereas in the clear apple juice group, 9 of 30 patients (30%) found the taste to be excellent, 16 (53%) rated it as good and 5 (17%) rated it as average (P value < 0.001) [Figure 4].
Figure 4.
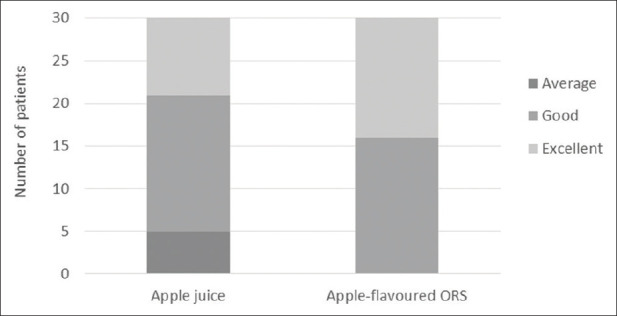
Rating of the palatability of apple juice and apple-flavoured ORS
DISCUSSION
In our study, we found no difference in GRV measured 2 h after consumption of water, clear apple juice or apple-flavoured ORS. There was poor correlation and agreement between GRV measurements made by the anaesthesiologist and radiologist. More patients rated the palatability of apple-flavoured ORS as excellent when compared to clear apple juice.
Several studies have described the use of USG for GRV assessment, with advantages such as performance at the bedside, lack of radiation exposure and availability of real-time data.[3,4,5,6,7,8,9,10,11,12,13,14] The GRV in these studies, even after following uniform fasting guidelines, has been variable. Sharma studied 100 patients presenting for elective surgery who had fasted for at least 6 h; 42 patients had GRV between 40 and 80 mL.[11] Bisinotto assessed GRV in 80 volunteers who were either fasting or had ingested 200 or 500 mL of isotonic solution 2 h earlier; the median GRV of 60 mL was similar across the groups.[8] The results from Sharma and Bisinotto are similar to the GRV of 60 to 70 mL found in our study. Perlas allowed patients to have clear liquids up to 5 h before surgery and found that mean GRV measured before surgery ranged from 0 to 16 mL.[4] van de Putte found a large range of GRV from 18 to 138 mL in fasted patients presenting for surgery.[14] The variations in GRV between these studies probably relate to differences in the patient population, type of last meal consumed, duration of fasting, and inter-individual variations in gastric emptying; for example, van de Putte found that some of the patients with high GRV had associated factors such as dyspepsia, prolonged fasting and consumption of a high-fat content meal before fasting.[14] It has been suggested that rather than absolute GRV values, GRV greater than 1.5 mL per kg body weight is a high risk for aspiration.[9,15] In our study, almost one-third of patients had GRV greater than 1.5 mL per kg despite following standard fasting guidelines. In other studies, this proportion has ranged between 2.7% to 28%, with higher values seen in studies that included patients with risk factors for delayed gastric emptying.[8,11,12,14,15] The clinical implication of this finding is unknown as none of these studies were powered to look at the incidence of aspiration under anaesthesia, which is a very rare event.
Another important finding from our study is the poor correlation and agreement between GRV measurements made by the radiologist and anaesthesiologist. There is very little data on the learning curve for USG measurement of GRV, and studies have used varying definitions of competence.[6,7,11,13] Recently published fasting guidelines by the Indian Society of Anaesthesiologists suggest that gastric ultrasound is a weak recommendation as a bedside tool for qualitative and quantitative assessment of gastric contents in the preoperative period.[2] Arzola's study concluded that 33 ultrasound examinations were adequate to achieve 95% competence; this was based on qualitative assessment and did not involve actual measurement of volumes.[7,16] In another study, anaesthesiologists performed USG-based GRV assessments after training under the supervision of a radiologist for 20 cases; they did not do a head-to-head comparison of agreement between measurements by the radiologist and anaesthesiologist.[11] Kruisselbrink found that inter-rater concordance between sonographers and anaesthesiologists was between 0.96 to 0.99; however, participating anaesthesiologists had completed at least 50 gastric examinations before the study.[6] Recently, Kruisselbrink and colleagues concluded that bedside USG has high sensitivity and specificity to differentiate solid, liquid, and empty stomach and to differentiate GRV more or less than 1.5 mL per kg; in this study, the anaesthesiologist had completed more than 100 gastric ultrasound examinations.[17] Similarly, in Kaydu's study, the assessor was a trained sonographer with experience of more than 50 gastric examinations.[13] However, the authors performed the assessment in the supine position and used a different formula for GRV calculation. The accuracy of their findings may be doubtful as the level of evidence for qualitative and quantitative assessment of gastric volume is strong only when performed in the RLD position.[2]
Our study found that more patients rated the palatability of apple-flavoured ORS as good to excellent compared to clear apple juice. However, as each patient received only one of the drinks, this could be attributed to inter-individual preferences. We chose clear apple juice and apple-flavoured ORS as these fluids have different carbohydrate content and osmolarity, which could affect GRV.[1,2] Ingestion of liquid with a carbohydrate level of more than 6% is the most important factor slowing gastric emptying.[18] However, we found no significant difference in GRV between groups. The volume of 200 mL was chosen as that is the usual practice at our institute and is the standard available tetra pack size, which facilitated blinding.
The strengths of our study are that it was a randomised, blinded study. To minimise bias, we standardised all study procedures and used the same ultrasound machine and technique for all measurements. We ensured that the anaesthesiologists involved in assessment had trained in ultrasound measurement of GRV. We included patients presenting for a variety of surgeries, which ensured generalisability. We blinded assessors to the type of fluid received by the participants, and to each other's readings.
Our study also had some limitations. First, for logistic reasons, we had to keep a window of 1.5 to 2.5 h for GRV estimation after ingestion of liquid. Gastric emptying may have been variable; however, the radiologist and anaesthesiologist performed assessments within 10 min of each other, and this was unlikely to affect agreement. Also, as it was a randomised study, it would affect all groups equally. Second, our study was not powered to look at outcomes such as aspiration during anaesthesia. Third, we took GRV measurements by the radiologist as the gold standard and did not use any other technique of validation. However, both the radiologists in the study had more than 10 years of experience in abdominal sonography, and it is reasonable to assume that their measurements were accurate. Fourth, we excluded paediatric patients, patients presenting for emergency surgery and those with risk factors for delayed gastric emptying. The results of this study cannot be extrapolated to those populations. The estimation of GRV was not performed on the day of the surgery. In patients posted for surgery, GRV could be slightly higher due to anxiety and delayed gastric emptying as seen in the study by van de Putte.[14] Finally, we did not do a subgroup analysis to evaluate the performance of a particular radiologist or anaesthesiologist as this was not the objective of the study.
In conclusion, we found no difference in GRV in fasted patients receiving three types of clear pre-operative drinks. However, we found large inter-assessor variations in USG-guided GRV measurements, suggesting the need for adequate training to gain proficiency in quantitative estimation of GRV.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the american society of anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126:376–93. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 2.Dongare PA, Bhaskar SB, Harsoor SS, Garg R, Kannan S, Goneppanavar U, et al. Perioperative fasting and feeding in adults, obstetric, paediatric and bariatric population: Practice Guidelines from the Indian Society of Anaesthesiologists. Indian J Anaesth. 2020;64:556–84. doi: 10.4103/ija.IJA_735_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114:1086–92. doi: 10.1097/ALN.0b013e31820dee48. [DOI] [PubMed] [Google Scholar]
- 4.Perlas A, Davis L, Khan M, Mitsakakis N, Chan VW. Gastric sonography in the fasted surgical patient: A prospective descriptive study. Anesth Analg. 2011;113:93–7. doi: 10.1213/ANE.0b013e31821b98c0. [DOI] [PubMed] [Google Scholar]
- 5.Perlas A, Mitsakakis N, Liu L, Cino M, Haldipur N, Davis L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–63. doi: 10.1213/ANE.0b013e318274fc19. [DOI] [PubMed] [Google Scholar]
- 6.Kruisselbrink R, Arzola C, Endersby R, Tse C, Chan V, Perlas A. Intra- and interrater reliability of ultrasound assessment of gastric volume. Anesthesiology. 2014;121:46–51. doi: 10.1097/ALN.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 7.Arzola C, Carvalho JC, Cubillos J, Ye XY, Perlas A. Anesthesiologists' learning curves for bedside qualitative ultrasound assessment of gastric content: A cohort study. Can J Anaesth. 2013;60:771–9. doi: 10.1007/s12630-013-9974-y. [DOI] [PubMed] [Google Scholar]
- 8.Bisinotto FMB, Naves AA, Lima HM, Peixoto ACA, Maia GC, Resende Junior PP, et al. Use of ultrasound for gastric volume evaluation after ingestion of different volumes of isotonic solution. Rev Bras Anestesiol. 2017;67:376–82. doi: 10.1016/j.bjan.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12–22. doi: 10.1093/bja/aeu151. [DOI] [PubMed] [Google Scholar]
- 10.Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82–9. doi: 10.1097/ALN.0b013e3181a97250. [DOI] [PubMed] [Google Scholar]
- 11.Sharma G, Jacob R, Mahankali S, Ravindra MN. Preoperative assessment of gastric contents and volume using bedside ultrasound in adult patients: A prospective, observational, correlation study. Indian J Anaesth. 2018;62:753–8. doi: 10.4103/ija.IJA_147_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi Y, Walker JC, Zhang F, Prindiville FE, Hanrahan JP, Mendelson R, et al. Preoperative gastric residual volumes in fasted patients measured by bedside ultrasound: A prospective observational study. Anaesth Intensive Care. 2018;46:608–13. doi: 10.1177/0310057X1804600612. [DOI] [PubMed] [Google Scholar]
- 13.Kaydu A, Gokcek E. Preoperative assessment of ultrasonographic measurement of antral area for gastric content. Med Sci Monit. 2018;24:5542–8. doi: 10.12659/MSM.908520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van de Putte P, Vernieuwe L, Jerjir A, Verschueren L, Tacken M, Perlas A. When fasted is not empty: A retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth. 2017;118:363–71. doi: 10.1093/bja/aew435. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Deo AS, Raman P. Effectiveness of standard fasting guidelines as assessed by gastric ultrasound examination: A clinical audit. Indian J Anaesth. 2018;62:747–52. doi: 10.4103/ija.IJA_54_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umesh G, Tejesh CA. Probing the future -Can gastric ultrasound herald a change in perioperative fasting guidelines? Indian J Anaesth. 2018;62:735–7. doi: 10.4103/ija.IJA_669_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruisselbrink R, Gharapetian A, Chaparro LE, Ami N, Richler D, Chan VWS, et al. Diagnostic accuracy of point-of-care gastric ultrasound. Anesth Analg. 2019;128:89–95. doi: 10.1213/ANE.0000000000003372. [DOI] [PubMed] [Google Scholar]
- 18.Leiper JB. Fate of ingested fluids: Factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr Rev. 2015;73:57–72. doi: 10.1093/nutrit/nuv032. [DOI] [PubMed] [Google Scholar]


