Abstract
Background:
Kawasaki disease (KD) is the most common cause of acquired heart disease in developed countries. KD is increasingly being reported from India; however, studies involving the large number of patients are few.
Methods:
All children presenting to the center from January 2017 to December 2019, diagnosed to have KD, were retrospectively included in the study. Clinical and laboratory profiles, including echocardiograms, were reviewed. Factors contributing to intravenous immunoglobulin (IVIg) refractoriness and the development of coronary artery abnormalities (CAA) were assessed.
Results:
A total of 39 children with KD presented to the center during the study. While 32 received initial treatment at our center, seven were referred after the initial IVIg infusion. The age range was 2 months to 11 years (mean 42.15 ± 38.51 months). More than two-thirds of the cohort was male (n = 27/39). Mucosal involvement was the commonest clinical abnormality for the group, followed by rash. Hemoglobin was significantly lower in the group with coronary artery involvement (P = 0.001). CAA (61.5%), incomplete KD, and atypical features were much more common in infants compared to the rest. Refractoriness to treatment was significantly more common in infants (P = 0.029).
Conclusions:
A significant proportion of infants with KD had cardiac involvement. Infants were more likely to have IVIg-resistant disease.
Keywords: Coronary artery abnormality, Kawasaki disease, South India, infants
INTRODUCTION
Kawasaki disease (KD) is a self-limiting, medium vessel vasculitis with predilection for coronary arteries.[1] Over the last decade, KD has been increasingly reported from various centers of the Indian subcontinent;[2] however, studies involving large number of patients are few.[3,4] In the current study, we describe the clinical profile and management of children who presented to a tertiary care center in India over a 3-year period.
METHODS
This is a retrospective study involving all patients with KD that were managed at Aster CMI hospital, Bangalore, from January 2017 to December 2019. The following were documented: demographic and clinical features, laboratory values, echocardiogram findings, treatment received, and for those with coronary artery involvement, follow-up echocardiograms. Those patients who received initial treatment for KD at other centers were grouped separately and not included in the statistical analysis of clinical or laboratory features on presentation.
Diagnosis of KD was based on the standard diagnostic criteria as per the American Heart Association (AHA) statement.[5] Children who had persistence or recrudescence of fever 36 h to 7 days after completion of first intravenous immunoglobulin (IVIg) infusion were considered to be refractory.
Echocardiograms were recorded using Philips Epiq 7 machine with S12-4 and S8-3 transducers. The presence of coronary artery abnormality, valvular regurgitation, aortic root dilation (defined by a Z score of >2), left ventricular dysfunction, or pericardial effusion was documented. Z scores were derived using data from Dallaire and Dahdah.[6] Coronary artery abnormalities (CAA) were classified according to AHA 2017 recommendations,[5] as follows:
No involvement: Z score always <2
Dilation only: Z score 2 to <2.5; or if initially <2, a decrease in Z score during follow-up ≥1
Small aneurysm: Z score ≥2.5 to <5
Medium aneurysm: Z score ≥5 to <10, and absolute dimension <8 mm
Large or giant aneurysm: Z score ≥10, or absolute dimension ≥8 mm.
For analysis, continuous variables were reported as mean, SD and range and categorical data as percentage. Pearson's Chi-squared test was used to compare distribution between groups for qualitative data analysis. Fisher's exact test was used when cell samples were very small. Student's t-test was used to find the significance between two groups for normally distributed continuous variables; Mann–Whitney U-test was used for nonparametric data. A P < 0.05 was considered statistically significant.
RESULTS
A total of 39 children presented to the center over a 3-year period. The age range was 2 months to 11 years (mean 42.15 ± 38.51 months). More than two-thirds of the cohort was male (n = 27/39). Seven children had received initial treatment elsewhere and were referred for refractoriness to treatment, persistence of CAA or, in one case, preexisting X-linked agammaglobulinemia. These children were not included in the analysis of clinical and laboratory features on presentation.
The clinical features on presentation of 32 children are given in Table 1. Mucosal involvement was the most common clinical abnormality for the group, followed by rash. Eighteen children had complete KD. Nine of the 14 incomplete KD cases were infants, of whom two-thirds had coronary artery abnormality (CAA).
Table 1.
Clinical features in the 32 newly diagnosed Kawasaki disease cases
| Clinical feature | Number of patients |
|---|---|
| Mucosal changes | 29 (90.6) |
| Rash | 28 (87.5) |
| Eye signs | 23 (71.8) |
| Extremity signs | 19 (59.4) |
| Lymphadenopathy | 12 (37.5) |
| Irritability | 10 (31.2) |
| Perianal peeling | 5 (15.6) |
| Diarrhea | 3 (9.4) |
| Cough | 2 (6.2) |
| Shock | 2 (6.2) |
| Seizure | 2 (6.2) |
| BCG scar reactivation | 1 (3.1) |
BCG: Bacillus Calmette–Guérin
A total of 17 out of 39 children (43.6%) had CAA. The left main was the commonest coronary artery to be involved (n = 14), followed by left anterior descending (n = 9) and right coronary artery (n = 5). Valvular regurgitation was seen in five children, involving the mitral (n = 2) and aortic valves (n = 3). The mechanism of aortic regurgitation (AR) was aortic root dilation in one child, noncoronary-cusp prolapse in another child and valvulitis in the third. One child had pericardial effusion. No left ventricular dysfunction was seen in this cohort.
Infants had a more severe disease profile. Cardiac involvement was significantly higher in infants (P = 0.029), with eight of thirteen infants having CAA and one infant developing aortic root enlargement with AR. Infants manifested atypical features such as upper limb arterial thrombosis and liver derangement, in addition to CAA in a 2-month-old [Figure 1a–d]. A 10-month-old child had lower motor neuron facial palsy with coronary involvement. Only two children in the entire cohort had deranged liver enzymes, and both were infants with CAA. Refractoriness to treatment was significantly more common in infants as compared to older children (P = 0.029). 40% of infants had persistence of CAA.
Figure 1.
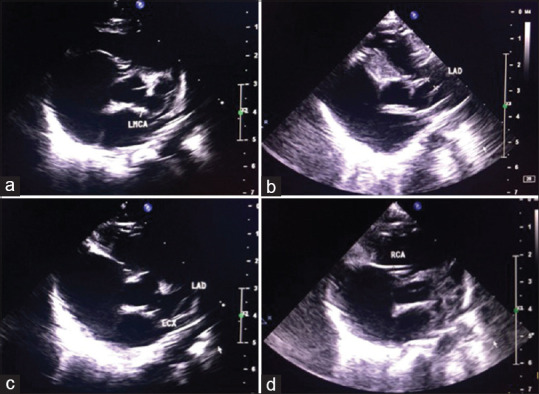
Large aneurysms of the (a) LMCA- Left main coronary artery (4.64 mm, Z score 10.98), (b) LAD- left anterior descending (5.35 mm, Z score 15.32), (c) LCx- left circumflex (6.73 mm, Z score 20.47) with (d) normal RCA- right coronary artery (1.47 mm, Z score 1.31) in a 2 months old child with moderate mitral valve regurgitation, and associated left upper limb arterial thrombosis and liver derangement
The association of clinical features and laboratory values on presentation with coronary artery involvement, is summarized in Table 2. The hemoglobin was significantly lower in the group with CAA than without.
Table 2.
Comparison of clinical features and laboratory parameters in Kawasaki disease children with and without coronary artery abnormality
| With CAA (n=12) | Without CAA (n=20) | P | |
|---|---|---|---|
| Clinical features, n (%) | |||
| Age <12 months | 8/12 (75) | 5/20 (25) | 0.006 |
| Gender | |||
| Male | 7/12 (58.3) | 14/20 (70) | 0.403 |
| Female | 5/12 (41.6) | 6/20 (30) | |
| Eye signs present | 10/12 (83.3) | 13/20 (65) | 0.178 |
| Mucosal changes present | 12/12 (100) | 17/20 (85) | 0.629 |
| Lymphadenopathy present | 5/12 (41.7) | 7/20 (35) | 0.720 |
| Extremity signs present | 5/12 (41.7) | 14/20 (70) | 0.281 |
| Rash present | 11/12 (91.7) | 17/20 (85) | 0.629 |
| Mean days of fever | 11.16±4.59 | 9.65±4.71 | 0.343 |
| Laboratory values, mean±SD | |||
| CRP (mg/L) | 56.18±47.72 | 67.28±78.09 | 0.621 |
| ESR (mm at end of 1st h) | 78.7±36.34 | 66.3±34.17 | 0.381 |
| Platelet counts (x 109/L) | 769±540 | 515±211 | 0.142 |
| TLC (×109/L) | 16.321±5.697 | 14.244±6.882 | 0.562 |
| Hemoglobin (g/L) | 93.5±13.5 | 113.8±12.4 | 0.001 |
| Liver function tests | With CAA (available in n=7/12) | Without CAA (available in n=8/20) | P |
| AST | 69.43±81.21 | 26.88±9.58 | 0.216 |
| ALT | 91±179 | 18.9±10.2 | 0.33 |
| Albumin | 3.47±0.71 | 3.85±0.35 | 0.23 |
CAA: Coronary artery abnormality, CRP: C reactive protein, ESR: Erythrocyte sedimentation rate, TLC: Total leukocyte counts, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, SD: Standard deviation
No difference was seen in the time interval from onset of disease to IVIg treatment in those who developed CAA as compared to those who did not. Treatment was initiated at a mean of 10.1 (±4.1) days in those with CAA and in 11 (±5) days in those without CAA (P = 0.608). Three children were not offered treatment and these included two cases whose symptoms had resolved by presentation and one whose family denied treatment.
A total of nine children in the cohort were refractory to treatment (23%). Of the nine, CAA was present in 7, and the association of CAA with refractoriness was statistically significant (P = 0.025). Of those who received initial treatment at our center, there was no significant difference in refractoriness between those who received early IVIg and those with IVIg treatment beyond 10 days of fever. Of the children refractory to IVIg, 5 received infliximab, 3 received methylprednisolone, while 1 received both infliximab and methylprednisolone.
Follow-up for children with cardiac involvement is summarized in Table 3. All children with dilation or small aneurysms had resolution of coronary abnormality on follow-up, while large and the majority of medium-sized aneurysms did not resolve. All those with the persistence of coronary artery aneurysms were male. Computed tomography coronary angiogram was performed for one child with refractory KD, to confirm the finding of medium aneurysm of RCA that persisted despite two doses of IVIg and Infliximab. There was no difference between the regression of CAA at different coronary arterial sites in this group. A 2-year-old who had IVIg-refractory KD (treated with two doses of IVIg and intravenous infliximab) with moderate coronary artery aneurysms, was noted to have severe AR due to noncoronary cusp prolapse at 5 months of follow-up, and continued to have AR with preserved cardiac function at 1.5 years of follow-up.
Table 3.
Cardiac outcomes on follow-up in children with coronary artery abnormality
| Classification | Total number | Lost to follow-up/aneurysm at follow up of <1 year duration | Number of resolved by 1 year, n (%) | Time to resolution (range) |
|---|---|---|---|---|
| Ectasia | 3 | 0 | 3 (100%) | 2-3 days |
| Small aneurysm | 7 | 0 | 7 (100%) | 2 days-3 months |
| Medium aneurysm | 5 | 1 (diagnosed less than 1 year back) | 1 (20%) | 2 months |
| Large aneurysm | 2 | 1 (denied therapy) | 0 | - |
CAA: Coronary artery abnormality
DISCUSSION
KD is the most common cause of acquired heart disease affecting children in the developed world.[1,7] Current literature suggests that KD may soon replace rheumatic heart disease as the most common cause of acquired heart disease in India as well.[8,9] In this report, we describe the clinical features and cardiac outcomes of 39 children with KD.
One-third of our patients were infants, which is unlike other cohorts reported from India[10] and this might be attributed to the rising awareness of pediatricians and availability of Pediatric Rheumatology services at our center. KD in infants is often incomplete[11] and presents a diagnostic dilemma.[12] Infants in our cohort had significantly higher IVIg resistance and CAA. Singh et al. reported 17 children with KD in young infants, below 6 months of age,[13] of whom 35% developed CAA and 2 children died. A significant delay in diagnosis and therapy was noted in their cohort.
Although some studies have reported the time between fever onset and diagnosis[14] or the time between the onset of disease to IVIg treatment to be associated with CAA[15,16] we did not find a significant association. Harpe et al.[17] reported a higher incidence of changes in coronary arteries' diameter in patients with atypical ages for KD (<1 year or >10 years' age). Similar to McCrindle et al.,[15] we found that an age <1 year was significantly associated with the development of CAA.
Certain laboratory features have been shown to be associated with CAA, including abnormal levels of hemoglobin[14,18] and elevated neutrophil/band counts.[18] We too, found lower hemoglobin to be significantly associated with the development of CAA. Although the platelet count did not differ significantly between groups in our study, thrombocytopenia is a known risk factor for the development of CAA.[5] An increased risk of CAA has been seen in children with elevated CRP levels.[16] We found a higher CRP in the CAA group, though not statistically significant.
IVIg resistance has been found to be a risk factor for CAA development, and we had similar observations.[16] Infliximab has been reported to be effective in IVIg refractory patients in many studies[19,20] and was the most common second-line therapy chosen in our patients with IVIg resistant disease.
Friedman et al.[21] reported nonregression of CAA to be associated with larger CAA Z-score at diagnosis, and bilateral CAA. In our study, all the large and majority of medium-sized aneurysms did not regress. Many studies have mentioned infants to have a greater chance of resolution of CAA.[18] However, 40% of the infants with CAA in our study did not have resolution on follow-up at 1 year.
Valvular regurgitation is seen in approximately 25% of patients of KD,[5] with the mitral and aortic valves being commonly involved. The mechanism of early valvular regurgitation may include pancarditis or, in the case of mitral regurgitation, myocardial ischemia.[5] The mechanism of AR in KD has mostly been linked with aortic root enlargement.[5] Interestingly, the mechanism of AR in a child in this cohort was noncoronary-cusp prolapse and occurred at the time of relapse of KD, 5 months after the initial illness. This may be attributed to late-onset valvulitis and structural damage to the valve cusp itself. A similar case was previously reported by Fuse et al.,[22] where the damaged left coronary cusp was found to have “folded” upon itself, leading to AR. Furthermore, the delayed occurrence of AR after 18 months of illness, in the absence of aortic root dilation, has been reported by Fukunaga et al.[23] resulting from thickening of the valve cusps, and in their case, histology showed sequelae of valvulitis.
One of the strengths of our study is the availability of long-term follow-up, including repeat echocardiograms in all of our patients. The study has the limitation of being a retrospective study and as it involves a single referral center, it may not be representative of the general population.
CONCLUSION
Infants are more likely to present with incomplete KD and have a higher risk of IVIg resistance and coronary damage. Of the laboratory parameters, low hemoglobin is significantly associated with the development of coronary artery aneurysms. Valvular regurgitation constitutes an important cardiac association and may be due to pancarditis in the acute stage or aortic root dilation or valvulitis in case of late-onset involvement. Hence, once a diagnosis of KD is made, a thorough echocardiographic study is warranted to evaluate for signs of myocarditis, besides evaluation for coronary artery aneurysms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Son MB, Sundel RP. Kawasaki disease. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn L, editors. Textbook of Pediatric Rheumatology. 7th ed. Philadelphia: Elsevier Saunders; 2016. pp. 467–83. [Google Scholar]
- 2.Jiao F, Jindal AK, Pandiarajan V, Khubchandani R, Kamath N, Sabui T, et al. The emergence of Kawasaki disease in India and china. Glob Cardiol Sci Pract. 2017;2017:e201721. doi: 10.21542/gcsp.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Aulakh R, Bhalla AK, Suri D, Manojkumar R, Narula N, et al. Is Kawasaki disease incidence rising in Chandigarh, north India? Arch Dis Child. 2011;96:137–40. doi: 10.1136/adc.2010.194001. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Bhattad S. Kawasaki disease incidence at Chandigarh, north India, during 2009-2014. Rheumatol Int. 2016;36:1391–7. doi: 10.1007/s00296-016-3543-y. [DOI] [PubMed] [Google Scholar]
- 5.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A Scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–99. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 6.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24:60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the committee on rheumatic fever, endocarditis, and Kawasaki disease, Council on cardiovascular disease in the young, American Heart Association. Pediatrics. 2004;114:1708–33. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 8.Kumar RK, Tandon R. Rheumatic fever and rheumatic heart disease: The last 50 years. Indian J Med Res. 2013;37:643–58. [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: A global update. Arch Dis Child. 2015;100:1084–8. doi: 10.1136/archdischild-2014-307536. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Gupta MK, Bansal A, Kumar RM, Mittal BR. A comparison of the clinical profile of Kawasaki disease in children from northern India above and below 5 years of age. Clin Exp Rheumatol. 2007;25:654–7. [PubMed] [Google Scholar]
- 11.Mastrangelo G, Cimaz R, Calabri GB, Simonini G, Lasagni D, Resti M, et al. Kawasaki disease in infants less than one year of age: An Italian cohort from a single center. BMC Pediatr. 2019;19:321. doi: 10.1186/s12887-019-1695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller MG. Kawasaki disease in infancy. Adv Emerg Nurs J. 2019;41:222–8. doi: 10.1097/TME.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Agarwal S, Bhattad S, Gupta A, Suri D, Rawat A, et al. Kawasaki disease in infants below 6 months: A clinical conundrum? Int J Rheum Dis. 2016;19:924–8. doi: 10.1111/1756-185X.12854. [DOI] [PubMed] [Google Scholar]
- 14.Uysal F, Bostan OM, Celebi S, Uysal B, Hamitoglu S, Cil E. Outcomes of Kawasaki Disease: A Single-Center Experience. Clin Pediatr. 2015;54:579–84. doi: 10.1177/0009922814561594. [DOI] [PubMed] [Google Scholar]
- 15.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, et al. Coronary artery involvement in children with Kawasaki disease: Risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–9. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 16.Yan F, Pan B, Sun H, Tian J, Li M. Risk factors of coronary artery abnormality in children with Kawasaki disease: A Systematic review and meta-analysis. Front Pediatr. 2019;7:374. doi: 10.3389/fped.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Harpe M, Bernardo S, Hofer M, Sekarski N. Thirty years of Kawasaki disease: A single-center study at the university hospital of Lausanne. Front Pediatr. 2019;7:11. doi: 10.3389/fped.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micallef ES, Attard MS, Grech V. A case of atypical Kawasaki disease with giant coronary artery aneurysm containing thrombus. Images Paediatr Cardiol. 2016;18:9–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Sharma D, Suri D, Gupta A, Rawat A, Rohit MK, et al. Infliximab is the new kid on the block in Kawasaki disease: A single-centre study over 8 years from north India. Clin Exp Rheumatol. 2016;34:S134–8. [PubMed] [Google Scholar]
- 20.Tremoulet AH. Adjunctive therapies in Kawasaki disease. Int J Rheum Dis. 2018;21:76–9. doi: 10.1111/1756-185X.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman K, Gauvreau K, Hamaoka-Okamoto A, Tang A, Berry E, Tremoulet A. Coronary artery aneurysms in Kawasaki disease: Risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5:e003289. doi: 10.1161/JAHA.116.003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuse S, Tomita H, Ohara T, Iida K, Takamuro M. Severely damaged aortic valve and cardiogenic shock in an infant with Kawasaki disease. Pediatr Int. 2003;45:110–3. doi: 10.1046/j.1442-200x.2003.01666.x. [DOI] [PubMed] [Google Scholar]
- 23.Fukunaga S, Egashira A, Arinaga K, Akasu I, Kai E, Higashi T, et al. Aortic valve replacement for aortic regurgitation due to Kawasaki disease. Report of two cases. J Heart Valve Dis. 1996;5:231–4. [PubMed] [Google Scholar]


