Abstract
Introduction:
Triple-negative breast cancer (TNBC) is an aggressive type of breast cancer associated with poor prognosis and limited treatment options. Validated prognostic and predictive biomarkers are needed to guide treatment decisions and prognostication.
Areas covered:
In this review, we discuss established and developing prognostic and predictive biomarkers in TNBC and associated emerging and approved therapies. Biomarkers reviewed include epidermal growth factor receptor (EGFR), vascular endothelial growth factors (VEGF), fibroblast growth factor receptor (FGFR), human epidermal growth factor receptor 2 (HER2), androgen receptor, NOTCH signaling, oxidative stress/redox signaling, microRNAs, TP53 mutation, breast cancer susceptibility gene 1 or 2 (BRCA1/2) mutation/homologous recombination deficiency (HRD), NTRK gene fusion, PI3K/AKT/mTOR, immune biomarkers (programmed death-ligand 1 (PDL1), tumor-infiltrating lymphocytes (TILs), tumor mutational burden (TMB), neoantigens, defects in DNA mismatch repair proteins (dMMR)/microsatellite instability-high (MSI-H)), circulating tumor cells/cell-free DNA, novel targets of antibody-drug conjugates, and residual disease.
Expert opinion:
Biomarker-driven care in the management of TNBC is increasing and has helped expand options for patients diagnosed with this subtype of breast cancer. Research efforts are ongoing to identify additional biomarkers and targeted treatment options with the ultimate goal of improving clinical outcomes and survivorship.
Keywords: Biomarkers, breast cancer, predictive, prognostic, triple-negative
1. Introduction
Triple-negative breast cancer (TNBC) represents approximately 15% of invasive breast cancer (BC). TNBC is defined by the absence of estrogen receptor/progesterone receptor and human epidermal growth factor receptor 2 (HER2) expression. TNBC is more prevalent in premenopausal women, African American women, and deleterious breast cancer susceptibility gene 1 or 2 (BRCA 1/2) mutation carriers. TNBC has a poor prognosis in relation to other BC subtypes. Patients present with a more aggressive clinical course, including advanced stage at initial diagnosis, earlier recurrence with metastatic spread, and decreased overall survival (OS) [1,2].
Patients with TNBC have diverse clinical outcomes, including heterogeneous rates of pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT) in early-stage disease as well as varying response to therapy and subsequent survival in the metastatic setting [3]. Genetic tumor heterogeneity may largely attribute to this phenomenon [4,5]. Major genomic sequencing efforts have increased our insight into the molecular heterogeneity of TNBC. Approximately 70% of TNBC overlaps with the basal-like intrinsic subtype. Molecular subtypes of TNBC have also been identified by Lehmann et al., which include basal-like 1 (BL1), basal-like 2 (BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL), and luminal androgen receptor (LAR) [4]. These were further classified into four subtypes by Burstein et al.: androgen receptor (AR) positive, mesenchymal, basal-like immune suppressed, and basal-like immune activated [5]. Several studies have shown these subtypes may be able to predict response to targeted therapy. However, these subtypes are not routinely used in clinical practice and cytotoxic chemotherapy remains the main therapeutic option.
Further understanding of the molecular and transcriptonomic characterization of TNBC may lead to new molecularly targeted therapy in TNBC. In this review, we describe clinically important mechanisms of tumorigenesis (Figure 1), and associated predictive and prognostic biomarkers (Table 1). We further discuss associated therapeutic targets if applicable, both emerging and approved (Figure 2). Ultimately, these classifications will pave the way for more precise therapies and effective personalized medicine for patients diagnosed with TNBC. We will highlight such clinically significant pathways involving protein, RNA, and DNA targets.
Figure 1.
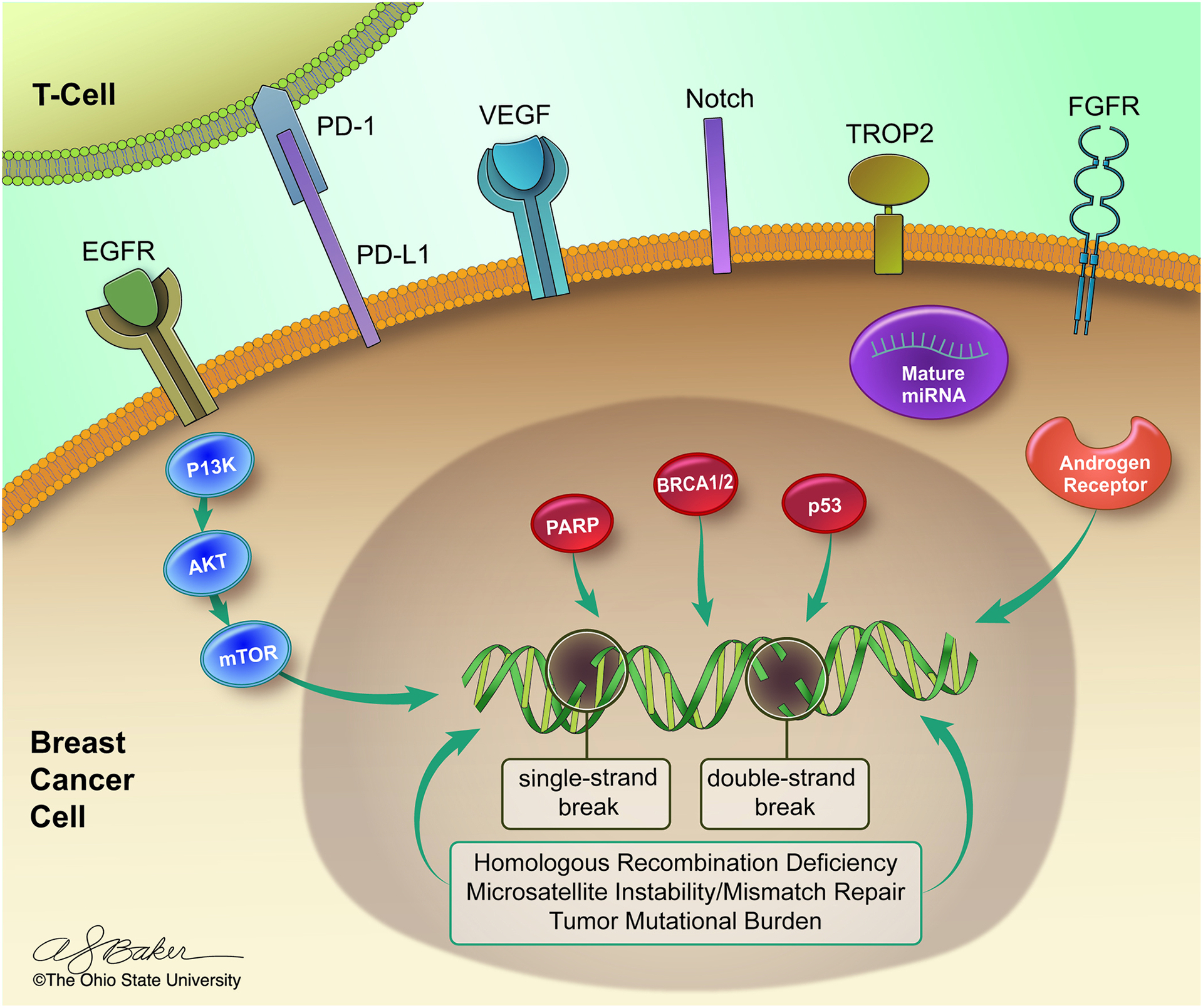
Key mechanisms of signal transduction and tumorigenesis in triple-negative breast cancer.
Table 1.
Prognostic and Predictive Biomarkers in Triple-Negative Breast Cancer.
| Biomarker | Approximate Prevalence in TNBC | Mechanism | Targeted Therapy | Prognostic /Predictive Significance |
|---|---|---|---|---|
| BRCA 1/2 germline mutation | 10–20% | Homologous recombination and DNA double strand break repair | PARP inhibitor | Higher response to platinum and predictor of response to PARP inhibitors |
| Elevated HRD Score | 45–70% | Homologous recombination and DNA double strand break repair | No clinically beneficial targeted therapy | Predictor of pCR to neoadjuvant platinum therapy |
| PDL1 | Variability (immune vs tumor), disease stage, antibody: 40% on immune cells (SP142 antibody) in metastatic disease 80% by CPS ≥1 (22C3 antibody) in primary disease |
Evasion of tumor immune surveillance | Immune checkpoint inhibitors | Improved pCR and survival in trials with immunotherapy |
| Tumor Infiltrating Lymphocytes | Variability (intra-tumoral vs stromal, primary vs metastatic) | Stromal lymphocytic infiltration of tumor microenvironment | No clinically beneficial targeted therapy | Improved pCR, DFS, and OS in early TNBC; Predictor of increased response to neoadjuvant CT |
| High Tumor Mutational Burden | 3–11% | Somatic mutations per megabase of DNA | Pembrolizumab | Predictor of response to pembrolizumab |
| MSI-H/ dMMR |
<2% of all breast cancer | Defect in DNA replication associated errors | Pembrolizumab | Predictor of response to pembrolizumab |
| AR | 30–35% | Steroid nuclear transcription factor | Abiraterone Acetate Bicalutamide Enzalutamide |
Improved DFS; Maybe associated with chemoresistance |
| EGFR | 13–76% | Receptor tyrosine kinase involved in cell proliferation/survival | No clinically beneficial targeted therapy | Poor prognostic factor associated with worse DFS |
| VEGF | 30–60% | Bind to receptor tyrosine kinases and promote angiogenesis | No clinically beneficial targeted therapy | High expression is associated with disease progression and metastases |
| FGFR | FGFR1 over-expression: 18% FGFR1 gene amplification: 33% FGFR2 gene amplification: < 5% |
Receptor tyrosine kinase involved in cell proliferation |
*TKIs (FGFR-selective or multi-targeted) *FGFR isoform antibodies *Fibroblast growth factor ligand inhibitors |
FGFR1 amplification: unclear FGFR2 amplification: poor OS |
| HER2 | 45–55% of all BC with HER2 IHC 1+ or 2+ 2% of BC with HER mutation |
Low HER2 protein expression with undetectable ERBB2 gene amplification |
*Antibody-Drug Conjugates *Vaccines *anti-HER2 TKIs, for HER2-mutated |
Possible predictor of response to HER2 antibody-drug conjugate and TKIs |
| TP53 Mutation | 80% | Encodes transcription factor protein that promotes cell cycle arrest | No clinically beneficial targeted therapy | Conflicting reports on prognostic significance |
| Micro RNAs | N/A | non-coding RNAs which regulate post-transcription expression of genes involved in the carcinogenesis | No clinically beneficial targeted therapy | Particular signatures associated with worse DFS, worse OS and chemoresistance |
| PI3K/AKT/mTOR pathway | PI3K 7–9% PTEN 30–50% |
PI3K- intracellular lipid kinases in signaling cascade that promotes cell proliferation/survival PTEN- tumor suppressor genes that downregulate signaling cascade |
Alpelisib Ipatasertib Capivasertinib |
Possible predictors of response to PI3K/AKT/mTOR inhibitors |
| NTRK gene fusion | <1% | Gene fusion results in constitutively active TRK proteins which promote tumor growth | Larotrectinib Entrectinib |
Predictor of response to tropomysin receptor kinase inhibitors |
| NOTCH signaling pathway | 10% | Oncogene involved in cell proliferation, cell death, cell differentiation, and stem cell maintenance | *AL101 | Poor prognostic factor with decreased DFS and OS |
treatment under investigation
Key: AKT: protein kinase B; AR: androgen receptor; BRCA1/2: breast cancer susceptibility gene 1 or 2; CPS: combined positive score; CT: chemotherapy; dMMR: deficient mismatch repair; DFS: disease-free survival; EGFR: epidermal growth factor receptor; FGFR: fibroblast growth factor receptor; HER2: human-epidermal growth factor receptor 2; HRD: homologous recombination deficiency; MSI-H: microsatellite instability-high; MTOR: mammalian target of rapamycin; NTRK: neurotrophic tyrosine kinase; OS: overall survival; PARP: poly ADP-ribose polymerase; pCR: pathologic complete response; PD-L1: programmed death-ligand 1; PI3K: phosphoinositide 3-kinase; PTEN: phosphatase and tensin homolog; TKIs: tyrosine kinase inhibitor; TNBC: triple-negative breast cancer; TRK: tropomyosin receptor kinase; VEGF: vascular endothelial growth factor
Figure 2.
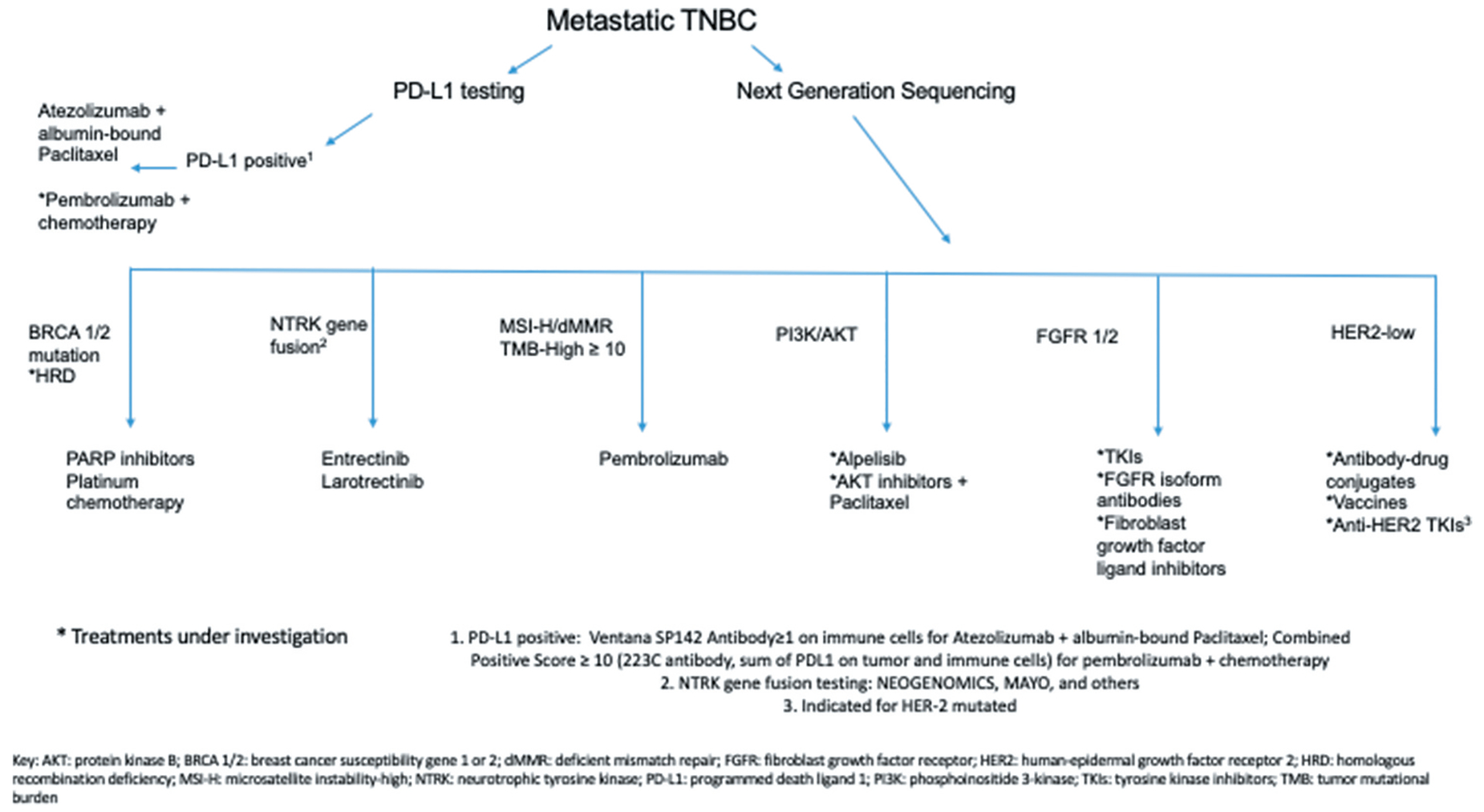
Diagram summarizing emerging and approved therapeutic options for triple-negative breast cancer based on biomarkers.
1. Protein expression as prognostic biomarkers in TNBC
A series of protein biomarkers have been evaluated in TNBC. We discuss the pertinent protein targets and their potential druggable strategies.
1.1. Epidermal growth factor receptor
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that belongs to the ErbB family and is involved in angiogenesis, cell proliferation, metastases as well as inhibition of apoptosis. Studies have demonstrated that EGFR protein expression is more frequently overexpressed in TNBC compared to other subtypes. The frequency of overexpression of EGFR can vary greatly from 13% to 76% [6]. EGFR expression has been shown to be an independent prognostic indicator of worse disease-free survival (DFS) and OS [7,8]. Due to the high frequency of EGFR expression in TNBC, EGFR tyrosine kinase inhibitors (TKI) as well as anti-EGFR monoclonal antibodies have been studied alone and in combination with chemotherapy and the results were underwhelming with no benefit seen in the early stage [9,10] or metastatic setting [11–13].
1.2. Vascular endothelial growth factors
Vascular endothelial growth factors (VEGFs) are expressed in 30–60% of TNBC [3]. VEGF promotes angiogenesis by stimulating endothelial cell proliferation and migration, inhibiting endothelial cell apoptosis, and supporting newly formed blood vessels [14]. One study found that patients with TNBC had significantly higher rates of intra-tumoral VEGF compared with non-TNBC patients [15]. In this study, patients with TNBC had shorter recurrence-free survival, BC corrected survival, and OS.
Given that angiogenesis is thought to be a key component driving tumor cell proliferation and survival, bevacizumab, a monoclonal antibody that binds VEGF-A, has been studied as a target for the treatment of TNBC [16]. In the neoadjuvant setting, the addition of bevacizumab was found to increase pCR [17]; however, no improvement in DFS or OS was seen in the adjuvant setting [18]. The addition of bevacizumab was also evaluated in several phase III studies to first- or second-line chemotherapy treatment in metastatic BC [19–21]. Each of these studies demonstrated improved PFS with the addition of bevacizumab; however, there was no difference in OS.
1.3. Fibroblast growth factor receptor
The fibroblast growth factor receptors (FGFR) are a family of transmembrane receptors that play an important role in regulating cellular functions including differentiation, proliferation, and angiogenesis. FGFR 1–4 comprises receptor tyrosine kinases, and abnormal signaling contributes to oncogenesis via multiple mechanisms of gene alteration, including point and activating mutations, fusions/rearrangements, and amplifications. In BC cells, FGFR1 amplification is the most frequent aberrancy implicated in tumorigenesis [22]. The prevalence of FGFR1 over-expression was estimated to be 18%, with FGFR1 gene amplification approximately 33%, in a cross-sectional study of TNBC specimens [23]. FGFR2 amplification is relatively less common and occurs in less than 5% of TNBC [22]. While in hormone receptor-positive BC the presence of FGFR1 amplification is consistently associated with a worse prognosis [24], its role in TNBC is more controversial. Some studies indicate no association with prognosis [23,25], while other literature suggests an inferior OS [26]. FGFR2 expression has been correlated with a poor OS [27].
FGFR signaling inhibition is an encouraging pharmacologic target [28]. The majority of these compounds are small-molecule TKIs. This includes favorable results observed in clinical trials for both multi-targeted TKIs [29] and FGFR-selective TKIs [30]. Distinct from TKIs, there are also preclinical data to suggest the efficacy of antibodies against FGFR isoforms [31] and inhibitors of fibroblast growth factor ligands [32], the latter of which has shown promise in phase I clinical trial for solid tumors including BC [32].
1.4. HER2
HER2-positive BC represents 15% of all BCs in which gene amplification of ERBB2 leads to over-expression of the HER2 protein. There is an emphasis to establish a new classification of BC in tumors with low HER2 protein expression but undetectable gene amplification (Immunohistochemistry (IHC) 1 + or IHC 2+ with negative in situ hybridization (ISH)), referred to as HER2-low. These tumors comprise subtypes that are classically referred to as HER2 negative. HER2-low BC represents approximately 45–55% of all BCs [33]. The mechanism for HER2 protein expression in BC cells that lack gene amplification is not completely elucidated; however, multiple mechanisms have been implicated, including activation of the NF-kB pathway by chemotherapy or radiotherapy as well as epigenetic alterations [33]. HER2-low as a prognostic biomarker remains less clear, with conflicting results in retrospective analyses [33–35]. While HER2-low BC has not been shown to significantly respond to well-established anti-HER2 therapies including trastuzumab [36], it has shown efficacy in relation to many novel anti-HER2 targeted agents [33]. There are numerous antibody–drug conjugates (ADC) under evaluation [33]. The anti-HER2 ADC Trastuzumab deruxtecan (DS-8201a) showed a favorable response in a phase 1 trial of advanced HER2-low solid tumors, including in BC (NCT02564900) [37]. It is being studied further in the phase III setting (NCT03734029) as well as in phase I trials in combination with checkpoint inhibitors (NCT04042701, NCT03523572).
Multiple anti-HER2 vaccines are also under evaluation in HER2-low BC, with some displaying favorable results in the TNBC sub-population [33,38]. In addition, HER2 gene mutations, present in approximately 2% of all BCs, can be found in HER2-low tumors, and data suggest a response to anti-HER2 TKIs. Neratinib has shown efficacy in metastatic HER2-mutated, HER2-low BC [39], and other anti-HER2 TKIs, including poziotinib (NCT02544997) and pyrotinib (NCT03412383), are being examined. Further clinical validation of the aforementioned compounds may increase treatment options for many patients with her2-low TNBC.
1.5. Androgen receptor
The AR is part of the steroid receptor family and functions as a nuclear transcription factor. The AR normally resides in the cytoplasm waiting to be bound by a ligand. Upon ligand binding, the AR translocates to the nucleus where it binds to androgen-related elements and promotes cell proliferation [40]. While AR signaling is more common in HR-positive BC, the prevalence in TNBC is approximately 30–35% [41–43]. AR positivity is associated with the LAR subtype, low tumor grade, lower risk of nodal involvement, and older age at diagnosis [41–43]. AR-positive TNBC has a lower Ki-67 index than AR-negative TNBC and could be less sensitive to chemotherapy, which is in accordance with the LAR subtype having a lower pCR rate relative to other subtypes [44,45]. Several recent meta-analyses have found AR expression is associated with improved DFS in TNBC, while the impact on OS is less established [46,47].
Multiple studies have evaluated the role of anti-androgen medications in the treatment of locally advanced or metastatic BC [48,49]. Two phase II studies investigating the use of the nonsteroidal AR inhibitors, bicalutamide and enzalutamide, found a clinical benefit ratio of approximately 20–25% [48,49]. Additionally, there are several ongoing clinical trials in the metastatic setting to evaluate the use of AR blockade in combination with various targeted therapies including CDK4/6 inhibitors, and PI3K inhibitors (NCT 03090165, NCT 02457910).
1.6. NOTCH signaling pathway
The NOTCH signaling pathway may be a promising biomarker in TNBC. The Notch signaling pathway activates many genes associated with cell differentiation, proliferation, and cell death [50]. The NOTCH signaling pathway consists of four receptors (Notch-1, Notch-2, Notch-3, Notch-4) which interact with five ligands (Delta-like 1, Delta-like 3, Delta-like 4, Jagged-1, and Jagged-2) [51]. Notch gain of function mutations are present in approximately 10% of TNBC [52]. Studies have showed a correlation between Notch-1 and positive lymph node status and Jagged-1 and larger tumor size [51]. It has also been shown that increased expression of Notch-1, Notch-4, or Jagged-1 is considered a poor prognostic factor associated with decreased survival [51]. Notch inhibitors have been developed to target this pathway, including, AL101, a pan-Notch gamma secretase inhibitor and future studies investigating the use of notch inhibitors are being planned.
1.7. Oxidative stress/redox signaling
Reactive oxygen species are a group of small reactive molecules and free radicals that are derived from oxygen and continuously produced in the body [53]. Appropriate amounts of reactive oxygen species are critical for cell functioning and survival. However, oxidative stress occurs when there is an imbalance between reactive oxygen species and antioxidants. Oxidative stress can result in DNA damage as well as disrupt signaling pathways involved in cell proliferation, apoptosis, and angiogenesis among others. The role of oxidative stress in BC initiation and progression as well as its utility as a prognostic marker remains controversial [54]. However, a recent study found increased reactive oxygen species in TNBC cells as well as increased dependency on ROS for cell survival compared to hormone receptor-positive BC cells [54]. While initial studies evaluating the use of dietary antioxidants have not shown benefit in the prevention of cancer, the potential role of redox-based anticancer therapies in TNBC has shown promise and remains an area of active investigation [53,55].
2. RNA expression as prognostic biomarkers in TNBC
Data on RNA and microRNA (miRNA) biomarkers are evolving in TNBC. Listed below are some emerging work in this area.
2.1. MicroRNAs
MiRNAs are circulating non-coding RNA molecules 17–27 nucleotides in length which regulate post-transcriptional expression of genes involved in the oncogenic pathway such as oncogenes and tumor suppressor genes [56]. High-throughput sequencing techniques have identified 28,000 mature miRNAs. Due to their stability, miRNAs may be advantageously studied in noninvasive samples such as blood, serum, and urine as prognostic biomarkers. Studies have shown that dysregulated miRNAs are involved in the carcinogenesis of BC. Specific miRNA signatures are unique and appear to be prognostic to TNBC [56–58]. For example, decreased expression of miR-155 has been shown to be predictive of poor OS in TNBC patients, while elevated levels of miR-21, miR-27a/b, miR-210, and miR-454 were associated with shorter OS. Similarly, decreased expression of miR-374a/b and increased level of miR-454 correlated with shorter DFS. Other panels of miRNAs were found to be associated with chemoresistance; expression of miR-181a was elevated in TNBC tissue samples from patients who did not respond to neoadjuvant chemotherapy. Also, a pilot study with bloodborne miRNA signatures from 21 basal-like TNBC cases treated with neoadjuvant therapy highlighted 321 miRNAs including miR-34a that were deregulated when comparing expression pre- and post-treatment and found that that complete responders had a tendency to have higher miRNA levels after platinum-based chemotherapy [59]. Also, the GeparSixto trial demonstrated that certain miRNA signatures may predict a pCR in TNBC [60].
Long non-coding RNAs (lncRNA) are transcripts with lengths exceeding 200 nucleotides that may not be translated into proteins. Like miRNAs, they also perform regulatory functions in various hallmarks of cancer biology. LncRNAs are disordered in many cancer types, including TNBC. The lncRNA known as highly up-regulated in liver cancer (HULC) has been found to be upregulated in TNBC tissues and has been shown to correlate with poorer clinical outcomes. Also, the metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a highly conserved lncRNA and recent preclinical studies have identified MALAT1 as a potential biomarker in TNBC, helping to predict prognosis and metastasis.
3. DNA expression as prognostic biomarkers in TNBC
The arena of DNA and genetic-based biomarkers is rapidly expanding in TNBC and this is an exciting area of development. We discuss here the importance of understanding the genetic pathways that drive TNBC.
3.1. TP53 mutation
TP53 is a gene located on chromosome 17 that encodes the p53 transcription factor protein [3]. When DNA damage occurs, p53 transcription is increased, promoting cell cycle arrest and allowing for DNA repair or apoptosis [3]. TP53 is one of the most frequently mutated genes in BC and is mutated in approximately 80% of TNBC [61,62]. TP53 mutations result in genetic instability and a higher probability of loss of heterozygosity and the p53 protein expression may vary due to the type of mutation. Several studies have attempted to determine the impact of TP53 mutation on TNBC prognosis, but due to the variable expression of p53, the value of TP53 status as a prognostic biomarker is unclear [61,62].
Since TP53 is mutated in a majority of TNBC cases, it is an attractive candidate for antitumor therapies. While mutated TP53 has previously been viewed as ‘non-druggable,’ more recently several compounds that target mutated p53 have been created [61,62]. These compounds including PRIMA-1, APR-246, PK11007, and COTI-2, and have been shown to reactivate mutant p53 and restore wildtype properties [62].
3.2. BRCA 1/2 germline mutation and homologous recombination deficiency (HRD)
BRCA1/2 code for tumor suppressor proteins involved in DNA repair via homologous recombination and therefore play a critical role in genetic integrity. BRCA mutations lead to homologous recombination deficiency (HRD). BRCA1/2 germline mutation is present in approximately 10–20% of TNBC. Several studies have shown no difference in outcomes between BRCA1/2 carriers and non-carriers. Interestingly, the POSH study showed that at 10 years’ OS was 78% in gBRCA carriers compared to 69% in BRCA-negative carriers [63]. The improvement in OS in gBRCA TNBC might be caused by better sensitivity of gBRCA carriers to chemotherapy as a result of defects in HRD or higher immune activation resulting in better survival.
Patients with gBRCA 1/2 mutations should be more susceptible to DNA-damaging agents like platinums and poly (ADP’-ribose) polymerase (PARP) inhibitors. Two phase III trials demonstrated the significant prolonged PFS of PARP inhibitors as monotherapy compared with standard chemotherapy in the metastatic setting for gBRCA 1/2 mutated BC [64,65]. Multiple trials have also evaluated combination regimens of PARP inhibitors with platinum agents [66,67], but these trials have had inconsistent results. Furthermore, there is interest to study the combination of immunotherapy with PARP inhibitors, given that DNA repair deficiency can lead to increase immunogenicity [68] and PARP inhibition is associated with up-regulation of PDL1 expression [69]. Studies combining immunotherapy with PARP inhibitors are ongoing and show promise.
However, a larger proportion of patients have been reported to harbor HRD. HRD can also be identified in tumors that do not carry BRCA1/2 mutation, defining a subgroup of patients referred to as BRCAness. BRCAness has emerged to describe a phenotype common in TNBC which shares similar molecular characteristics and resulting clinical features to BRCA-mutated patients [70]. Patients with a BRCAness phenotype have DNA repair defects through a variety of different mechanisms, including epigenetic inactivation of BRCA as well as germline or somatic mutations in other key genes involved in the homologous recombination system. Examples of such important genes include, but are not limited to, BARD1, ATR, PALB2, RAD51, RAD51D, ATM, CHK1, PLK1, and WEE1 [70,71].
A HRD score has been developed as a tool to further identify TNBC tumors which encompass a BRCAness phenotype and is estimated to be present in approximately 45–70% of TNBC [72]. High HRD score is significantly associated with improved pCR rate with standard NACT in TNBC [73]. Understanding which TNBC tumors have HRD may further elucidate the patients that would benefit therapeutically from platinum agents [74]. In a study by Telli et al., the HRD score was evaluated as a biomarker to predict response to therapy in early TNBC and predicted the likelihood of response to platinum-containing therapy in three neoadjuvant clinical trials [73]. This benefit has not been demonstrated in the metastatic setting, as the TNT trial showed no difference in PFS or OS between carboplatin and docetaxel when stratified according to HRD status [75].
3.3. NTRK gene fusion
Chromosomal translocations are well-known oncogenic drivers in malignancies, and targeting gene fusions have become a highly effective strategy to treat rearrangement-driven cancers. Somatic chromosomal rearrangements involving the NTRK1, NTRK2, or NTRK2 genes occur in approximately 1% of all solid tumors [76]. TRK gene fusion events result in over-expression of the proteins and constitutive downstream activation which promotes tumor growth. The LOXO-101 trial evaluated the efficacy of larotrectinib, a tropomysin receptor kinase inhibitor, which showed an overall response rate of 71% and led to FDA approval. More recently, a second tropomysin receptor kinase inhibitor, entrectinib, has been shown to be efficacious for patients with NTRK-fusion-positive solid tumors [77] and was recently approved by the FDA. While the incidence of NTRK gene fusions in BC is extremely rare at <1% [78], this is a highly effective treatment option for patients with NTRK gene fusions.
3.4. PI3K/AKT/mTOR
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR)-dependent pathway is associated with cell metabolism, proliferation, differentiation, and survival [79]. In many cancers, this pathway is overactive due to gain-of-function mutations of phosphatidylinositol-4, 5-bisphosphate 3-kinase, catalytic subunit, alpha (PIK3CA), loss-of-function alterations of the tumor suppressor phosphatase and tensin homolog (PTEN), deregulation of receptor tyrosine kinase signaling, and amplification and mutations of receptor tyrosine kinases. In TNBC patients approximately 10% have an activating mutation in PIK3CA and 30–50% with PTEN alterations [79].
Different subtypes of TNBC have specific PI3K pathway mutations/alterations; for example, PIK3CA and AKT1 mutations are more likely to be found in AR-positive TNBC [79]. PIK3CA mutations have proven to have a predictive value for treatment with α-selective and β-sparing PI3K inhibitor, Alpelisib, in the advanced setting for hormone receptor-positive, HER2-negative BC [80]. There are ongoing clinical trials to evaluate the use of alpelisib in other BC subtypes including TNBC [NCT04216472, NCT03207529].
In addition to PI3K inhibitors, AKT inhibitors have also been developed and demonstrated promising activity in TNBC [81,82]. Ipatasertib, a small molecule inhibitor of AKT, demonstrated improved PFS compared to placebo when combined with paclitaxel for first-line metastatic TNBC [81]. Capivasertib, another oral small molecular AKT inhibitor, demonstrated improved PFS and OS when combined with paclitaxel for first-line treatment of TNBC with the effects being most pronounced in patients with tumors harboring mutations of PIK3CA, AKT, or PTEN [82]. Phase III trials with AKT inhibitors are ongoing.
4. Biomarkers of immunotherapy in TNBC
4.1. PDL1 and TILs
The immune system normally occupies an important role in preventing tumorigenesis, and immunologic evasion through multiple mechanisms is a critical process in the development of malignancy. There has been tremendous development in immunotherapy to improve outcomes across multiple solid tumor types. However, we have only recently begun to better understand its therapeutic role in BC, which has not traditionally been considered immunogenic [83]. TNBC is more immunogenic, and the presence of multiple components of the immune microenvironment has been linked with positive prognostic features, when compared with HR-positive BC [84]. There is therefore emerging interest to study the effect of immune-modulating therapies in this BC subtype.
Programmed cell death protein 1 (PD1) is a transmembrane receptor protein on the surface of cells in the adaptive immune system, including T cells, which bind to a ligand, programmed death-ligand 1 (PDL1) or Programmed death-ligand 2 (PDL2), both present on tumor cells and tumor-infiltrating immune cells. This interaction induces T cell inhibition and thereby normally mediates self-tolerance and evasion of the immune microenvironment by the tumor. PDL1 is commonly expressed in approximately 20% of TNBC and is associated with poor prognostic features such as young age, higher grade, ER-negative status, HER2-positive status, and larger tumor size [85].
PDL1 can be measured and quantified on tumor or immune cells. PDL1 expression in TNBC has been variable when quantified by IHC across studies and institutions. This range may be related to cell measured (immune vs. tumor), stage of TNBC (primary vs advanced), site of metastatic disease, variation in antibody clones, and numerical cutoff used to define positivity [85–87]. Monoclonal antibodies directed against PD1 and PDL1 effectively release the down-regulation of the immune system, leading to immune-mediated response against the tumor. PDL1 expression was also found to be associated with improved pCR rate [88], metastatic-free survival, and OS [85].
Multiple trials have demonstrated the potential beneficial role of checkpoint inhibitors in early-stage TNBC [86,89]. Keynote-522 is the first phase III trial which showed an improved outcome in this setting. Patients were randomized to receive NACT with or without pembrolizumab. Pembrolizumab resulted in a significant increase in the pCR rate with an absolute difference of 13.6% (64.8% vs 51.2%). PDL1 expression was measured by IHC using the combined positive score (CPS) (22C3 antibody) which quantifies the sum of PDL1 on tumor and immune cells. PDL1 positivity was defined as a CPS ≥1 and was present in about 80% of the patients. The benefit of pembrolizumab was observed independent of PDL1 status [86]. The benefit of neoadjuvant checkpoint inhibitor seen in Keynote-522 is similar to that seen in other phase II studies [89]. In early-stage TNBC, PDL1 does not appear to predict response to immunotherapy, and response to checkpoint inhibitors can be observed in tumors negative for PDL1 expression.
The IMPassion 130 study led to the first FDA approval of atezolizumab as first-line therapy in metastatic TNBC with a PDL1 (PDL1 ≤ 1%) positive tumor. Patients were randomized to receive nab-paclitaxel with or without atezolizumab, and there was a significant PFS (7.5 vs. 5 months) and OS (25 vs. 15.5 months) benefit in the PDL1 positive subgroup. PDL1 positivity was defined as PDL ≥1% by IHC based on PDL1 expressing immune cells (Ventana SP142 antibody) and was present in 40% of the patients [87]. The Keynote 355 trial evaluating the combination of Pembrolizumab plus chemotherapy as first line treatment for metastatic disease showed improvement in PFS in patients whose tumors expressed PDL1 (CPS ≥ 10) [90]. However, the phase 3 Impassion131 study, evaluating atezolizumab with paclitaxel, did not meet the primary endpoint of improvement in PFS. The differences observed in PFS between IMPassion 130 and IMPassion 131 could be due to the chemotherapy backbone or steroid premedication. Based on these results, nab-paclitaxel and atezolizumab should still be considered as first-line therapy for patients with metastatic TNBC with a PDL1 positive tumor.
TNBC are also characterized by high mutation rate and greater tumor-infiltrating lymphocytes (TILs) which are important cells of the adaptive immune system involved in the tumor microenvironment. TILs are highly expressed in approximately 20% of the TNBC cases. TILs are both present intra-tumorally and in adjacent tissue stroma and the presence of intra-tumoral and stromal TILs has a prognostic and predictive role. Assessed in tumor samples of many large clinical trials, an increase in TILs is linked with improved DFS, OS, and pCR rate with NACT in early TNBC [91,92]. As suggested in the analysis of two phase III adjuvant trials, there is approximately a 15% reduction in recurrence and death for every 10% increment increase in TILs [92]. TILS have also been found to potentially predict response to immunotherapy. KEYNOTE-086 showed that higher TILs were associated with significantly improved ORR to pembrolizumab [93]. TILs have also been studied as a biomarker in metastatic TNBC, with higher levels associated with better prognosis. Its potential to predict response to immunotherapy with pembrolizumab in this setting has been demonstrated in KEYNOTE-119 in patients with TILs ≥5% [94]. However, the data for this are less mature compared with the primary setting.
Given the association of high TILs with improved long-term outcomes in early TNBC, future studies should include their use as a prognostic biomarker to guide de-escalation of therapy, including the potential for chemotherapy-sparing regimens. Based on promising prognostic data, the 2019 St Gallen International Consensus guidelines recommend TILs be routinely characterized in TNBC [95]. However, this practice has not yet been adopted as standard of care measure.
4.2. Tumor mutational burden and MSI-H/dMMR
Evaluating other prognostic/predictive markers, in addition to PDL1, may identify additional patients who could benefit from immunotherapy. Tumor mutational burden (TMB) refers to the number of somatic mutations per megabase (mut/Mb) of DNA measured using whole exome or gene panel sequencing. A high TMB has been associated with increased T cell infiltration, high neoantigen burden, clinical response, and increased survival after immunotherapy in patients with melanoma, lung, and colorectal cancer. However, there are limited data regarding TMB in BC [96]. In BC, there are limited data regarding TMB and its predictive role is controversial. In primary BC, high TMB is present in up to 3% of the tumors, whereas in metastatic disease the frequency is as high as 11% [97]. BC tumors with high TMB appear to be more sensitive to checkpoint inhibitors; however, no difference in OS was seen in BC patients with high TMB treated with immunotherapy [98]. In June 2020, the FDA approved pembrolizumab for TMB high (≥10 mut/Mb), unresectable or metastatic solid tumors which have progressed following prior therapy or without alternative treatment options making this a treatment option for TNBC patients with TMB high.
Defects in DNA mismatch repair proteins (dMMR) and a subsequent microsatellite instability-high (MSI-H) characteristic leads to dysfunction in DNA replication and promotion of mutations leading to oncogenesis. This process stimulates the immune microenvironment of the tumor, and consequently, there is high interest in the study of immunotherapy in MSI-H/dMMR cancer [99]. PDL1 expressing tumors are correlated with MSI-H/dMMR, particularly in certain solid tumors including endometrial and colorectal cancer, and checkpoint inhibitors have shown efficacy in this setting [100]. Pembrolizumab is approved in adults with MSI-H/dMMR unresectable or metastatic solid tumors that have progressed or without alternative treatment options [101]. As the frequency of MSI-H/dMMR in BC is estimated at <2%, its use as a prognostic biomarker is unclear [102].
4.3. Neoantigens and vaccines
Somatic mutations in cancer result in tumor-specific novel proteins, termed neoantigens [103]. These neoantigen signatures are associated with greater antitumor T cell response, and there is evidence this response can be augmented with immunotherapy. Neoantigens are an exceptionally appealing immune target given their selective representation of tumor cells without expression on normal cells, resulting in minimal immune self-tolerance [104]. TNBC has a greater degree of neoantigens compared with other BC subtypes [105], and BC vaccines may be one of the potential mechanisms to target this [106]. These vaccines present neoepitopes to T cells, increasing the ability of the immune system to recognize and destroy aberrant cancer cells. Two phase I trials are currently evaluating the efficacy of a neoantigen vaccine with or without durvalumab in patients with early TNBC with residual disease (NCT03199040) or metastatic TNBC (NCT03606967). In these studies, an individual approach to neoantigen identification is used via gene sequencing panels and complex algorithms to predict epitopes. However, the efficacy of these vaccines is yet to be known and the optimal tumor neoantigens for clinical use are not well established.
5. Circulating tumor cells and cell-free DNA as prognostic biomarkers in TNBC
In recent years, there has been increasing focus on the incorporation of liquid biopsies including circulating tumor cells (CTC) and cell-free DNA (cfDNA) or circulating tumor DNA (ctDNA) into clinical practice [107]. CTC are nucleated cancer cells that have entered the vasculature and are found in the bloodstream. Multiple different methods are utilized to detect CTC including immunohistochemistry, flow cytometry, and RT-PCR [108]. CtDNA are fragments of DNA that are released from tumor cells that have undergone apoptosis or necrosis and are able to be collected from plasma [107]. Both CTC and ctDNA/cfDNA have been investigated as potential prognostic biomarkers in a variety of BC settings [108].
The use of ctDNA/cfDNA has been evaluated as a biomarker for monitoring metastatic BC. A study by Dawson et al. compared radiographic imaging of tumors with ctDNA, CA 15–3, and CTC in 30 women actively receiving treatment for metastatic BC [109]. The study found ctDNA had the greatest correlation with changes in tumor burden and provided the earliest measure of treatment response in 53% of the women. A retrospective cohort study of 164 patients with metastatic TNBC found that the presence of a cfDNA fraction greater than 10% was associated with worse outcomes, regardless of clinicopathological data [110].
In addition to ctDNA/cfDNA, the evaluation of CTCs at the start of a new treatment regimen for metastatic BC and several weeks afterward has prognostic significance. In one study, CTCs ≥5 per 7.5 mL were associated with decreased PFS and OS. Additionally, patients who had an increase in CTC counts 3–5 weeks after the start of treatment and/or 6–8 weeks after the start of treatment had decreased PFS and OS [111]. A recent expert consensus paper by Cristofanilli et al. determined that number of CTCs could be used to stratify patients with metastatic BC into two subgroups with prognostic significance [112]. This study classified patients with ≥5 CTCs per 7.5 mL as Stage IV aggressive and those with <5 CTCs per 7.5 mL as Stage IV Indolent. The Stage IV Indolent group had longer median OS compared to the Stage IV aggressive group (36.3 Vs. 16.0 months, p < 0.0001). However, making therapy changes based on these CTC cutpoints has not been showed to improve overall survival outcomes.
The prognostic significance of ctDNA has also been evaluated during and after NACT for TNBC [113,114]. Riva et al. found that ctDNA was positive in 75% of the patients prior to NACT and continued positivity after one cycle of chemotherapy was associated with shorter DFS (p < 0.001) and OS (p = 0.006) [113]. Radovich et al. found that detection of ctDNA in patients with early-stage TNBC with residual disease after NACT was an independent prognostic risk factor for disease recurrence [114]. Out of 148 patients, ctDNA was detected in 64% and at a median follow-up of 16.7 months, detection was associated with inferior distant DFS (32.5 mos. vs. NR, p = 0.003). As a next step, the investigators will soon be opening BRE18–334, also known as the PERSEVERE trial [115]. In this phase II trial, TNBC patients with minimal residual disease at the time of surgery after NACT will be tested for ctDNA. Patients with ctDNA will undergo genomic sequencing to help determine post-neoadjuvant genomically targeted treatment. Patients without a targetable mutation or patients without ctDNA will receive standard of care. While the use of liquid biopsies, including CTCs and ctDNA, may represent an important stratification tool to help identify patients most likely to benefit from additional treatment options, there are ongoing efforts to improve the sensitivity of these methods as a tool for detecting minimal residual disease [116]. Whether the use of these liquid bipsies to guide treatment decisions actually impacts and improves disease course is yet to be determined.
6. New targets of antibody-drug conjugates (ADCs) in TNBC
ADCs are a new class of anticancer drugs that are designed as a monoclonal antibody which is conjugated to a potent cytotoxin. The monoclonal antibody is directed against an antigen on the surface of the cancer cell. For ADCs to be effective, the target antigen must be expressed on the cancer cell. Several promising antigens have been identified in TNBC: 1) trophoblast cell-surface antigen (Trop-2), 2) the glycoprotein nonmetastatic b (GPNMB), 3) LIV, and 4) the mucin 1-attached sialogycotype CA6 [117–119].
Trop-2 is a type I transmembrane glycoprotein, with a relevant role in migration, cell proliferation, cell cycle progression, and metastasis [117]. Sacituzumab govitecan (IMMU-132) is an antibody targeting Trop-2, linked to the topoisomerase-I inhibitor SN-38, the active metabolite of irinotecan that induces DNA damage [120]. IMMU-132–01 (NCT01631552), a phase I/II clinical trial, showed the efficacy of Sacituzumab Govitecan-hziy, with 33.3% response rate in heavily pretreated TNBC patients. Based on these results, on 22 April 2020, the FDA granted accelerated approval to Sacituzumab Govitecan-hziy for patients with metastatic TNBC who received at least two prior therapies for metastatic disease [120]. GPNMB is involved in processes like cell migration, invasion, angiogenesis, or epithelial-mesenchymal transition, highly overexpressed in TNBC, and a biomarker of poor prognosis in BC [118]. LIV-1 is a zinc transporter protein downstream target of STAT3, implicated in cell adhesion and epithelial-to-mesenchymal transition [119]. CA6 is selectively expressed on solid tumors, and is therefore, an ideal target for ADC therapy. Additional ADCs are currently under investigation such as, SAR566658 which is an ADC directed against CA6 which carries DM4, a maytansine-derived anti-microtubule agent, as payload (NCT01156870).
7. Residual disease as a prognostic biomarker in TNBC
Residual disease is defined as the presence of invasive cancer in the tumor or lymph nodes of the resected pathologic specimen after neoadjuvant therapy. Approximately one-third of patients with TNBC who are treated with neoadjuvant chemotherapy are most likely to have a pCR compared with other BC subtypes [121]. The presence of residual disease has been associated with increased risk of relapse [121] and adjuvant capecitabine has been shown to improve PFS and OS in patients with residual disease. Several studies comparing pretreatment and posttreatment biopsy samples found significant genomic changes which could be the reason for resistance to conventional chemotherapies. Molecular analysis of posttreatment biopsy samples may be necessary in TNBC patients who do not experience pCR after NACT in order to tailor adjuvant therapy to better improve outcomes.
8. Conclusion
Tremendous progress has been made to better understand the biology of TNBC. We now recognize TNBC as having distinct molecular subgroups that are driven by the activation/inactivation of distinct pathways. By further understanding, these pathways, promising prognostic and predictive biomarkers have been identified. With the approval of immunotherapy for PDL1 positive metastatic TNBC and PARP inhibitors for BRCA positive metastatic TNBC, personalized medicine options are currently available to select patients. However, the mainstay of treatment remains chemotherapy. A critical need remains for the development of more modern NGS-based biomarker to continue to improve outcomes in patients with TNBCs. Current promising biomarkers include FGFR, HER2, PI3K/AKT/mTOR pathway, AR receptors, and ADC therapies.
9. Expert opinion
Given the heterogeneity of TNBC, it is very unlikely that there can be a single approach to the management of these tumors, and evaluating the heterogeneity of TNBC is of particular importance to identify patients who may benefit from targeted therapy. One way to evaluate and define this heterogeneity is to identify somatic mutations by sequencing tumor DNA. Next-generation sequencing (NGS) is a commonly used method to sequence a panel of oncogenes and evaluate for actionable mutations. NGS may have implications for patient classification, prognosis, treatment, and evaluation of drug resistance. NGS can be performed on tumor tissue or ctDNA from blood samples. Studies have shown high concordance rates between mutations detected in solid tumor tissue biopsies and those detected in ctDNA [122]. There are several different NGS platforms commercially available including FoundationOne, MSK-IMPACT, Guadant360, and Caris Molecular Intelligence Tumor Profiling. These NGS tests vary in both the specific genes tested as well as the overall number of genes. A recent study found that the use of next-generation sequencing in metastatic BC patients frequently identified potential treatment options [123]. A recent study on patient perspectives of genomic testing in patients with metastatic BC found patients had limited genomic knowledge and highlights the importance of patient education as the use of these tests becomes more prevalent [124].
In regards to targeting the breast immune microenvironment, there are many emerging biomarkers under investigation. A major challenge will be to learn the optimal approach in integrating these diverse biomarkers together to represent multiple aspects of the immune system and further improve the precision of immunotherapy in TNBC. Implications include their inclusion for prognostication and staging, and also to guide treatment escalation or de-escalation. Advanced knowledge in immune biomarkers may also pave a better understanding of the role of immunotherapy in PDL1 negative metastatic TNBC, which represents the majority of cases, and as therapy beyond the first-line setting.
Future directions may include the use of artificial intelligence technologies including computer-generated advanced algorithms to further integrate multilayered data related to tumor heterogeneity. Particularly, it may provide an opportunity to study greater nuances in molecular phenotypes and biologic mechanisms, which could guide prognostication and tailor an even more personalized approach to therapy. We believe that artificial intelligence should complement, but not take the place of, provider-based individualized care and the use of current evidenced-based biomarkers.
In all these efforts, we strongly recommend that patients be an integral part of therapeutic decision-making. Ongoing research is focusing on how to improve understanding of genetic and genomic biomarkers in shared decision-making. Partnerships with patients are key as the field rapidly evolves with new findings that impact treatment outcomes, toxicity profiles and long-term prognosis. One such effort is the Count Me In organization, which is an excellent model of patient-partnered research and includes over 5000 patients with metastatic BC who have joined the effort since 2015.
In summary, we believe the next decade will usher in a new era of multiple new biomarkers which will hopefully improve outcomes in TNBC. Continued team science collaborations are key as we delve further into central clinical dilemmas and the biology of these very aggressive cancers.
Article highlights.
Triple-negative breast cancer is a heterogeneous subtype of breast cancer associated with poor prognosis
Identification of both prognostic and predictive biomarkers to guide treatment decisions is increasingly important
Many different protein targets (EGFR, VEGF, FGFR, HER2, and Androgen Receptor) are under investigation, but currently, direct clinical application is limited
RNA expression has been identified as a prognostic biomarker, but there are currently no RNA targeted therapies available
Sequencing of DNA can help identify targetable therapy options
Immunotherapy is emerging as a potential treatment option based on immune biomarkers (PD-L1, TMB, MSI-H/ddMR)
The role of circulating tumor cells and circulating tumor DNA continues to be an area of active investigation
Detection of somatic mutations by DNA sequencing (next-generation/whole-genome sequencing) of metastatic tumor tissue is imperative to identify potential targeted treatment options
Funding
This paper received no funding.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–12. [DOI] [PubMed] [Google Scholar]
- 3.da Silva JL, Cardoso Nunes NC, Izetti P, et al. Triple negative breast cancer: a thorough review of biomarkers. Crit Rev Oncol Hematol. 2020;145:102855. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann BD, Pietenpol JA. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast. 2015;24(Suppl 2):S36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This research described six molecular subtypes of TNBC, including basal-like 1 (BL1), basal-like 2 (BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL), and luminal androgen receptor (LAR).
- 5.Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This research described four distinct molecular subtypes of TNBC, including AR-positive, mesenchymal, basal-like immune suppressed, and basal-like immune activated.
- 6.Park HS, Jang MH, Kim EJ, et al. High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod Pathol. 2014;27(9):1212–1222. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Conchas GA, Rodriguez-Romo L, Hernandez-Barajas D, et al. Epidermal growth factor receptor overexpression and outcomes in early breast cancer: a systematic review and a meta-analysis. Cancer Treat Rev. 2018;62:1–8. [DOI] [PubMed] [Google Scholar]
- 8.Abdelrahman AE, Rashed HE, Abdelgawad M, et al. Prognostic impact of EGFR and cytokeratin 5/6 immunohistochemical expression in triple-negative breast cancer. Ann Diagn Pathol. 2017;28:43–53. [DOI] [PubMed] [Google Scholar]
- 9.Bernsdorf M, Ingvar C, Jörgensen L, et al. Effect of adding gefitinib to neoadjuvant chemotherapy in estrogen receptor negative early breast cancer in a randomized phase II trial. Breast Cancer Res Treat. 2011;126(2):463–470. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda N, Wang X, Lim B, et al. Safety and efficacy of panitumumab plus neoadjuvant chemotherapy in patients with primary HER2-negative inflammatory breast cancer. JAMA Oncol. 2018;4 (9):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler M, Awada A, Harter P, et al. A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2012;134(3):1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30 (21):2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yardley DA, Ward PJ, Daniel BR, et al. Panitumumab, gemcitabine, and carboplatin as treatment for women with metastatic triple-negative breast cancer: a Sarah Cannon Research Institute phase II trial. Clin Breast Cancer. 2016;16(5):349–355. [DOI] [PubMed] [Google Scholar]
- 14.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23(4):792–799. [DOI] [PubMed] [Google Scholar]
- 15.Linderholm BK, Hellborg H, Johansson U, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20(10):1639–1646. [DOI] [PubMed] [Google Scholar]
- 16.Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366(4):299–309. [DOI] [PubMed] [Google Scholar]
- 18.Miller KD, O’Neill A, Gradishar W, et al. Double-blind phase III trial of adjuvant chemotherapy with and without bevacizumab in patients with lymph node-positive and high-risk lymph node-negative breast cancer (E5103. J Clin Oncol. 2018;36 (25):2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. [DOI] [PubMed] [Google Scholar]
- 20.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. [DOI] [PubMed] [Google Scholar]
- 21.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29 (32):4286–4293. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Garcia J, Muñoz-Couselo E, Soberino J, et al. Targeting FGFR pathway in breast cancer. Breast. 2018;37:126–133. [DOI] [PubMed] [Google Scholar]
- 23.Jafarian AH, Kooshkiforooshani M, Farzad F, et al. The relationship between fibroblastic growth factor receptor-1 (FGFR1) gene amplification in triple negative breast carcinomas and clinicopathological prognostic factors. Iran J Pathol. 2019;14(4):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomiguchi M, Yamamoto Y, Yamamoto-Ibusuki M, et al. Fibroblast growth factor receptor-1 protein expression is associated with prognosis in estrogen receptor-positive/human epidermal growth factor receptor-2-negative primary breast cancer. Cancer Sci. 2016;107(4):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ, Seo AN, Park SY, et al. Low prognostic implication of fibroblast growth factor family activation in triple-negative breast cancer subsets. Ann Surg Oncol. 2014;21(5):1561–1568. [DOI] [PubMed] [Google Scholar]
- 26.Cheng CL, Thike AA, Tan SY, et al. Expression of FGFR1 is an independent prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat. 2015;151(1):99–111. [DOI] [PubMed] [Google Scholar]; • Although the role of FGFR1 amplification in TNBC is controversial, this study suggests its role as a prognostic biomarker associated with an inferior OS.
- 27.Sun S, Jiang Y, Zhang G, et al. Increased expression of fibroblastic growth factor receptor 2 is correlated with poor prognosis in patients with breast cancer. J Surg Oncol. 2012;105(8):773–779. [DOI] [PubMed] [Google Scholar]
- 28.André F, Bachelot T, Campone M, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19(13):3693–3702. [DOI] [PubMed] [Google Scholar]
- 29.Soria JC, DeBraud F, Bahleda R, et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014;25(11):2244–2251. [DOI] [PubMed] [Google Scholar]
- 30.Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol. 2017;35 (2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai A, Meetze K, Vo NY, et al. GP369, an FGFR2-IIIb-specific antibody, exhibits potent antitumor activity against human cancers driven by activated FGFR2 signaling. Cancer Res. 2010;70 (19):7630–7639. [DOI] [PubMed] [Google Scholar]
- 32.Tolcher AW, Papadopoulos KP, Patnaik A, et al. A phase I, first in human study of FP-1039 (GSK3052230), a novel FGF ligand trap, in patients with advanced solid tumors. Ann Oncol. 2016;27 (3):526–532. [DOI] [PubMed] [Google Scholar]
- 33.Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38 (17):1951–1962. [DOI] [PubMed] [Google Scholar]; • This review article provides an algorithm to define HER2-low BC, defines its prognostic value, and describes treatment approaches with novel HER2 targeted therapies.
- 34.Camp RL, Dolled-Filhart M, King BL, et al. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res. 2003;63(7):1445–1448. [PubMed] [Google Scholar]
- 35.Eggemann H, Ignatov T, Burger E, et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer. 2015;22(5):725–733. [DOI] [PubMed] [Google Scholar]
- 36.Fehrenbacher L, Cecchini RS, Geyer CE, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38(5):444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modi S, Tsurutani J, Tamura K. Abstract P6-17-02: trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer: updated results of a large phase 1 study. Cancer Res. 2019;79(4 Suppl):Abstract nr P6–17–02. [Google Scholar]
- 38.Mittendorf EA, Ardavanis A, Litton JK, et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016;7(40):66192–66201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554 (7691):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kono M, Fujii T, Lim B, et al. Androgen receptor function and androgen receptor-targeted therapies in breast cancer: a review. JAMA Oncol. 2017;3(9):1266–1273. [DOI] [PubMed] [Google Scholar]
- 41.Safarpour D, Pakneshan S, Tavassoli FA. Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am J Cancer Res. 2014;4(4):353–368. [PMC free article] [PubMed] [Google Scholar]
- 42.Park S, Koo J, Park HS, et al. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21(3):488–492. [DOI] [PubMed] [Google Scholar]
- 43.Collins LC, Cole KS, Marotti JD, et al. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24(7):924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santonja A, Sánchez-Muñoz A, Lluch A, et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget. 2018;9(41):26406–26416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barton VN, D’Amato NC, Gordon MA, et al. Androgen receptor biology in triple negative breast cancer: a case for classification as AR+ or quadruple negative disease. Horm Cancer. 2015;6 (5–6):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Pan B, Zhu H, et al. Prognostic value of androgen receptor in triple negative breast cancer: a meta-analysis. Oncotarget. 2016;7(29):46482–46491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu Q, Mao Y, Fei XC, et al. The impact of androgen receptor expression on breast cancer survival: a retrospective study and meta-analysis. PLoS One. 2013;8(12):e82650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19(19):5505–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This phase II study showed the use of bicalutamide was beneficial in select hormone receptor-negative, AR-positive metastatic BC patients.
- 49.Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrated clinical activity of enzalutamide in patients with advanced AR-positive TNBC.
- 50.Giuli MV, Giuliani E, Screpanti I, et al. Notch signaling activation as a hallmark for triple-negative breast cancer subtype. J Oncol. 2019;8707053:2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Speiser JJ, Erşahin C, Osipo C. The functional role of Notch signaling in triple-negative breast cancer. Vitam Horm. 2013;93:277–306. [DOI] [PubMed] [Google Scholar]
- 52.Broner E, Alpert G, Gluschnaider U. abstract AL101: mediated tumor inhibition in notch-altered TNBC PDX models. J clin oncol. 2019;37:15_suppl, 1064–1064. [Google Scholar]
- 53.Hecht F, Pessoa CF, Gentile LB, et al. The role of oxidative stress on breast cancer development and therapy. Tumour Biol. 2016;37 (4):4281–4291. [DOI] [PubMed] [Google Scholar]
- 54.Sarmiento-Salinas FL, Delgado-Magallón A, Montes-Alvarado JB, et al. Breast cancer subtypes present a differential production of reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Front Oncol. 2019;9:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatem E, Azzi S, El Banna N, et al. Auranofin/vitamin C: a novel drug combination targeting triple-negative breast cancer. J Natl Cancer Inst. 2019;111(6):597–608. [DOI] [PubMed] [Google Scholar]
- 56.Paszek S, Gabło N, Barnaś E, et al. Dysregulation of microRNAs in triple-negative breast cancer. Ginekol Pol. 2017;88(10):530–536. [DOI] [PubMed] [Google Scholar]
- 57.Sugita BM, Pereira SR, de Almeida RC, et al. Integrated copy number and miRNA expression analysis in triple negative breast cancer of Latin American patients. Oncotarget. 2019;10(58):6184–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang F, Zhang W, Shen Y, et al. Identification of dysregulated microRNAs in triple-negative breast cancer (review). Int J Oncol. 2015;46(3):927–932. [DOI] [PubMed] [Google Scholar]
- 59.Kahraman M, Röske A, Laufer T, et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci Rep. 2018;8(1):11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevic I, Müller V, Weber K, et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018;16(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Synnott NC, Murray A, McGowan PM, et al. Mutant p53: a novel target for the treatment of patients with triple-negative breast cancer? Int J Cancer. 2017;140(1):234–246. [DOI] [PubMed] [Google Scholar]
- 62.Duffy MJ, Synnott NC, Crown J. Mutant p53 in breast cancer: potential as a therapeutic target and biomarker. Breast Cancer Res Treat. 2018;170(2):213–219. [DOI] [PubMed] [Google Scholar]
- 63.Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. [DOI] [PubMed] [Google Scholar]; •• OlympiAD led to approval of single agent olaparib for metastatic BC and germline BRCA 1/2 mutation.
- 65.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• EMBRACA led to approval of single agent talazoparib for metastatic BC and germline BRCA 1/2 mutation.
- 66.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19 (4):497–509. [DOI] [PubMed] [Google Scholar]
- 67.Wolf DM, Yau C, Sanil A, et al. DNA repair deficiency biomarkers and the 70-gene ultra-high risk signature as predictors of veliparib/carboplatin response in the I-SPY 2 breast cancer trial. NPJ Breast Cancer. 2017;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pantelidou C, Sonzogni O, De Oliveria Taveira M, et al. PARP inhibitor efficacy depends on CD8. Cancer Discov. 2019;9 (6):722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16 (2):110–120. [DOI] [PubMed] [Google Scholar]
- 71.Lin PH, Chen M, Tsai LW, et al. Using next-generation sequencing to redefine BRCAness in triple-negative breast cancer. Cancer Sci. 2020;111(4):1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Timms KM, Abkevich V, Hughes E, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014;16(6):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Telli ML, Hellyer J, Audeh W, et al. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer. Breast Cancer Res Treat. 2018;168(3):625–630. [DOI] [PubMed] [Google Scholar]; • This study illustrates high HRD score is associated with improved response to neoadjuvant chemotherapy, independent of gBRCA mutation.
- 74.Jin J, Zhang W, Ji W, et al. Predictive biomarkers for triple negative breast cancer treated with platinum-based chemotherapy. Cancer Biol Ther. 2017;18(6):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2--mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24(5):628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This trial led to FDA approval of larotrectinib for adult and pediatric solid tumors with NTRK gene fusion.
- 77.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross J, Chung J, Elvin J, et al. Abstract P2-09-15: NTRK fusions in breast cancer: clinical, pathologic and genomic findings. (SABCS 2017). [Google Scholar]
- 79.Ellis H, Ma CX. PI3K inhibitors in breast cancer therapy. Curr Oncol Rep. 2019;21(12):110. [DOI] [PubMed] [Google Scholar]
- 80.André F, Ciruelos E, Rubovszky G, et al. Alpelisib for. N Engl J Med. 2019;380(20):1929–1940. [DOI] [PubMed] [Google Scholar]
- 81.Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study showed the combination of ipatasertib, an AKT small molecule inhibitor, and paclitaxel improved PFS in metastatic BC.
- 82.Schmid P, Abraham J, Chan S, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38 (5):423–433. [DOI] [PubMed] [Google Scholar]
- 83.Wagner J, Rapsomaniki MA, Chevrier S, et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell. 2019;177(5):1330–1345.e1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desmedt C, Haibe-Kains B, Wirapati P, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14(16):5158–5165. [DOI] [PubMed] [Google Scholar]
- 85.Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6 (7):5449–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. [DOI] [PubMed] [Google Scholar]; •• Keynote-522 is the first phase III trial to show improved outcomes with neoadjuvant checkpoint inhibitor in early TNBC.
- 87.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. [DOI] [PubMed] [Google Scholar]; •• IMpassion 130 led to first FDA approval of a checkpoint inhibitor as first-line therapy in metastatic TNBC in PDL1-positive tumors.
- 88.Cerbelli B, Pernazza A, Botticelli A, et al. PD-L1 expression in TNBC: a predictive biomarker of response to neoadjuvant chemotherapy? Biomed Res Int. 2017;2017:1750925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279–1288. [DOI] [PubMed] [Google Scholar]
- 90.Casta J, Guo Z, Karantza V. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (PBO) + chemo for previously untreated, locally recurrent, inoperable or metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2017;28(10):x25. [Google Scholar]
- 91.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. [DOI] [PubMed] [Google Scholar]; • This study showed increased TIL concentration in TNBC tumors predicted a more favorable response to NACT.
- 92.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32 (27):2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397–404. [DOI] [PubMed] [Google Scholar]
- 94.Loi S, Winer E, Lipatov O. Abstract PD5–03: relationship between tumor-infiltrating lymphocytes (TILs) and outcomes in KEYNOTE-119 study of pembrolizumab vs chemotherapy for previously treated metastatic triple-negative breast cancer (mTNBC). In: Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 Dec 10–14; San Antonio, TX. Philadelphia (PA): AACR. Cancer Res. 2020;80(4 Suppl):Abstract nr PD5–03. [Google Scholar]
- 95.Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–1557. [DOI] [PubMed] [Google Scholar]
- 96.Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bertucci F, Ng CKY, Patsouris A, et al. Genomic characterization of metastatic breast cancers. Nature. 2019;569(7757):560–564. [DOI] [PubMed] [Google Scholar]
- 98.Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wen YH, Brogi E, Zeng Z, et al. DNA mismatch repair deficiency in breast carcinoma: a pilot study of triple-negative and non-triple-negative tumors. Am J Surg Pathol. 2012;36(11):1700–1708. [DOI] [PubMed] [Google Scholar]
- 101.Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452–478. [DOI] [PubMed] [Google Scholar]; • Pembrolizumab is approved in adults with MSI-H/dMMR unresectable or metastatic solid tumors which have progressed or without alternative treatment options.
- 102.Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017: PO.17.00073.doi: 10.1200/PO.17.00073.Epub 2017 Oct 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68(3):889–892. [DOI] [PubMed] [Google Scholar]
- 104.Yarchoan M, Johnson BA, Lutz ER, et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17(4):209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luen S, Virassamy B, Savas P, et al. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–250. [DOI] [PubMed] [Google Scholar]
- 106.Liu XS, Mardis ER. Applications of immunogenomics to cancer. Cell. 2017;168(4):600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Y, Sheng M, Zheng L, et al. Application of circulating tumor DNA in breast cancer. Breast J. 2020;26:1797–1800. [DOI] [PubMed] [Google Scholar]
- 108.Lustberg MB, Stover DG, Chalmers JJ. Implementing liquid biopsies in clinical trials: state of affairs, opportunities, and challenges. Cancer J. 2018;24(2):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368 (13):1199–1209. [DOI] [PubMed] [Google Scholar]; • This study demonstrated the utility of ctDNA as a biomarker for metastatic BC.
- 110.Stover DG, Parsons HA, Ha G, et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol. 2018;36 (6):543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrated that cfDNA tumor fraction is a negative prognostic biomarker in TNBC.
- 111.Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15 (4):406–414. [DOI] [PubMed] [Google Scholar]; • This study demonstrated the prognostic significance of CTCs on PFS and OS in metastatic BC.
- 112.Cristofanilli M, Pierga JY, Reuben J, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol. 2019;134:39–45. [DOI] [PubMed] [Google Scholar]
- 113.Riva F, Bidard FC, Houy A, et al. Patient-specific circulating tumor DNA detection during neoadjuvant chemotherapy in triple-negative breast cancer. Clin Chem. 2017;63(3):691–699. [DOI] [PubMed] [Google Scholar]
- 114.Radovich M, Jiang G, Chitambar C, et al. Abstract GS5–02: detection of circulating tumor DNA (ctDNA) after neoadjuvant chemotherapy is significantly associated with disease recurrence in early-stage triple-negative breast cancer (TNBC): preplanned correlative results from clinical trial BRE12–158. (SABCS, 2019). [Google Scholar]
- 115.Harrison P Outcomes in TN breast cancer predicted by blood biomarkers. 2019. [Google Scholar]
- 116.Parsons HA, Rhoades J, Reed SC, et al. Sensitive detection of minimal residual disease in patients treated for early-stage breast cancer. Clin Cancer Res. 2020;26(11):2556–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ambrogi F, Fornili M, Boracchi P, et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9(5):e96993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rose AA, Grosset AA, Dong Z, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16 (7):2147–2156. [DOI] [PubMed] [Google Scholar]
- 119.Sussman D, Smith LM, Anderson ME, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014;13(12):2991–3000. [DOI] [PubMed] [Google Scholar]
- 120.Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–751. [DOI] [PubMed] [Google Scholar]; •• This study led to FDA approval of sacituzumab govitecan for adult patients with TNBC who received at least two prior therapies for metastatic disease.
- 121.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]; • In this pooled analysis of 12 large trials, about 50% of patients with TNBC who had residual disease following NACT developed recurrence, demonstrating the prognostic significance of pCR.
- 122.Chung JH, Pavlick D, Hartmaier R, et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with estrogen receptor-positive metastatic breast cancer. Ann Oncol. 2017;28(11):2866–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study showed high concordance rates between mutations detected in tissue and ctDNA.
- 123.Stover D, Reinbolt R, Adam E, et al. Prospective decision analysis study of clinical genomic testing in metastatic breast cancer: impact on outcomes and patient perceptions. JCO Precis Oncol. 2019;1–11. DOI: 10.1200/PO.19.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrated that DNA sequencing of tumors in patients with metastatic BC frequently identified potential treatment options.
- 124.Adams EJ, Asad S, Reinbolt R, et al. Metastatic breast cancer patient perceptions of somatic tumor genomic testing. BMC Cancer. 2020;20(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is the first study to evaluate patient perceptions of genomic tumor testing in metastatic BC patients.


