Introduction
Chronic kidney disease (CKD) is a common chronic disease, affecting 15.23% of the U.S. population [1]. CKD is especially common in older adults, with an estimated prevalence of 46% in adults over the age of 70 years [2]. Unfortunately, older adults are at risk for other health problems and consequences related to having CKD. One comorbidity commonly experienced by older adult patients with CKD is cognitive impairment. The exact etiology of cognitive impairment in patients with CKD is not fully understood but is likely a combination of factors related to the CKD process itself and micro-vascular changes [3–5]. CKD is thought to confer a specific and unique risk factor for the development of cognitive impairment. Through the complex interaction of clinical factors and the internal vascular milieu, CKD is considered a stimulus for accelerated aging that causes changes in the cerebrovascular-renal axis that leads to cognitive impairment [5]. The readers are referred to a recent review by Bronas et al. [5].
Cognitive impairment has a reported prevalence that ranges anywhere from 13% to 70% in patients with CKD [6,7]. Cognitive impairment in patients with CKD becomes most apparent when a patient’s estimated glomerular filtration rate (GFR) is less than 60ml/min/1.73m2 (CKD Stage 3) [8]. As the GFR decreases, cognitive function also appears to progressively worsen [9,10]. Cognitive decline escalates even more dramatically when a patient begins dialysis, which makes it critically important that cognitive impairment is identified and addressed prior to the initiation of dialysis [11]. Cognitive impairment occurs in patients with CKD of all ages but is especially common in older adults with CKD [12]. Although cognitive impairment occurs in patients with CKD of all ages, it occurs more commonly in older adults and older age has been associated with high risk of progression of cognitive impairment [12]. It is unclear in current state of the science whether the mechanisms leading to cognitive impairment in patients CKD and the manifestations are similar in adults under 65 years old, but the physiologic processes that are considered to play a prominent role in the development of cognitive impairment are present in adult patients with CKD of all ages [5]. Older adults with CKD have a 37% higher risk of developing dementia than older adults without CKD [13]. Older adults with CKD and cognitive impairment are at increased risk for hospitalization, morbidity, and mortality [14–16]; therefore, it is essential that cognitive impairment is identified and addressed in this at risk population.
For any patient with suspected cognitive impairment, it is essential that an appropriate and thorough cognitive assessment is conducted. Cognitive function is often evaluated through the assessment of one or more of the six neurocognitive domains- complex attention, executive function, learning and memory, language, perceptual motor, and social cognition [17]. Cognitive function can be assessed with a clinical assessment or, more ideally, with standardized tests to quantify and objectively assess cognitive function [17,18]. There are numerous cognitive function tests available to assess the domains of cognitive function. Examples of common cognitive function tests and their associated domains are displayed in Table 1. In clinical practice when a patient is noted to have a deficit on a cognitive test, they may be diagnosed with mild cognitive impairment (MCI). MCI is a clinical syndrome characterized by a person being at a level of cognitive function between changes normally attributed to aging and those that are characteristic of dementia, and with the impairment not being significant in more than one cognitive domain [18,19,20]. The clinical concept of MCI has assisted providers in identifying patients in the predementia stage. MCI has been found to progress to Alzheimer’s disease and dementia with an incidence as high as 42% at 5 years [18,21]. A person is diagnosed with overt dementia, or a major neurocognitive disorder per the DSM V nomenclature, when they are noted to have a substantial decline in at least one cognitive domain that interferes with independence in activities of daily life [18].
Table 1-.
Examples of Cognitive Tests and their Domain of Assessment
| Cognitive Test | Domain |
|---|---|
| Mini Mental State Exam (MMSE) | Composite Test (Global cognition) |
| Trail Making Test A (TMT-A) | Complex Attention |
| Trail Making Test B (TMT-B) | Executive Function |
| California Learning Test (CVLT) | Learning and Memory |
| Hopkins Verbal Learning Test (HVLT) | Language |
| Number Comparison | Perceptual Motor |
There is a critical need to identify cognitive impairment in older adults with CKD, so patients can be managed appropriately prior to the initiation of dialysis, when cognitive impairment is expected to dramatically worsen. Unfortunately, cognitive assessment utilizing a standardized cognitive test is not part of the routine care for patients with CKD [22]. This likely contributes to the large variability in prevalence rates of cognitive impairment reported for patients with CKD [23], which suggests that the true depth and breadth of cognitive impairment in this population is still not clear. The current practice of cognitive assessment in patients with CKD needs to be fully evaluated, so recommendations can be made on the ideal method to assess cognitive function in this population. Therefore, the purpose of this integrative review is to explore how cognitive function is currently assessed with cognitive function tests in older adult patients with CKD.
Methods
Given the range of research methods utilized to explore cognitive impairment in CKD, the integrative review methodology of Whittemore and Knafl was applied to capture all data related to the study purpose [24]. A research librarian was consulted regarding database utilization and preferred search language, after a preliminary review of the literature was conducted. Search terms were selected to obtain the broadest scope of literature about evaluation of cognitive function in patients with CKD (Table 2). Five electronic databases were searched to locate studies that evaluated cognitive function in patients with CKD: Medline via Ovid, CINAHL, Embase, Scopus, and PsychInfo. The searches included all available literature from the start of the databases through the final search date of December 2, 2017.
Table 2-.
Search terms
| Renal insufficiency, chronic kidney disease, kidney failure, chronic renal disease, chronic renal insufficiency AND Cognitive function, cognition, cognitive impairment, cognitive dysfunction, mental status, cognitive disorder, mild cognitive impairment |
The inclusion criteria were articles that a) evaluated patients with a mean age greater than 65 years old, since cognitive impairment is more common in older adults with CKD [12,25,26] and the World Health Organization and Healthy People 2020 define “older adult” as those over 65years [27,28] b) included patients with CKD Stage 3–5 or the average GFR of the sample being less than 60ml/min/1.73m2, which is where cognitive impairment becomes most apparent and occurs most frequently [8] c) conducted cognitive assessment via a standardized test d) and were available in English. Studies were excluded if they a) only evaluated patients with end stage renal disease (ESRD), since the cognitive impairment experienced by patients with ESRD is influenced by unique factors related to the dialysis procedure [29,30] b) only evaluated patients who had received a kidney transplant, since cognitive impairment in this population is influenced by unique factors, including immunosuppressant medications [31], c) or only included a patient’s self-rated cognitive function.
Results
Through the search and analysis strategies, 36 studies were identified that were published in a 12-year period, from 2005 to 2017 (Appendix A). The final search strategy is outlined in the flow diagram [32] displayed in Figure 1.
Figure 1-.
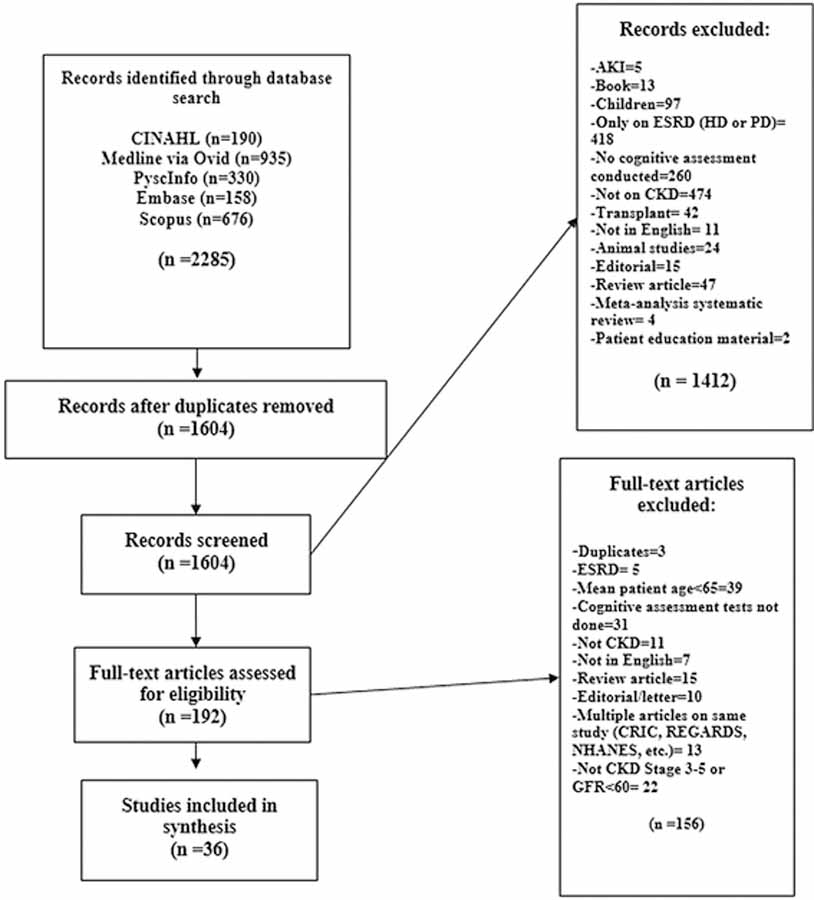
Search strategy
Purpose
The majority of the included studies had the primary purpose of exploring associations between CKD and cognitive function. The purpose of 26 studies was to explore the relationship between kidney function and cognitive function [12,25,33–56]. Four studies sought to explore the relationship between cognitive function and kidney function in patients with CKD, as well as another diagnosis (stroke, atrial fibrillation, depression, etc.) [57–60]. Two studies evaluated associations between neuroimaging findings and cognitive impairment in patients with CKD [61,62]. The remaining studies each had a unique purpose in their evaluation of patients with CKD. One study examined the utilization of the Mini-Addenbrooke Cognitive Examination (M- ACE) [63], one examined the influence of uric acid on cognitive function,[64] one evaluated the influence of cognitive impairment on decision making [47] and one evaluated the impact of medication dosage on cognitive impairment [65].
Study Design
The two most commonly utilized designs in the included studies were prospective cohort designs and comparative cross-sectional designs. The design of 15 studies was a prospective cohort design, and these studies sought to evaluate changes in cognitive function over time in patients with CKD [12,25,34,36, 39,41,46,47,49,50,54,59–62,66]. The comparative cross- sectional studies compared patients with CKD with another patient group, and this method was utilized in 15 studies [33,35,37, 40,42–44,48,51–53,55,56,63,64]. Of these studies, 12 utilized patients with a GFR greater than 60ml/min/1.73m2 as a comparison group [33,35,40,43,44,48,51–53,55,63,64], one study utilized patients on hemodialysis (HD) as a comparison group [56], and one study utilized both patients with a GFR greater than 60ml/min/1.73m2 and patients on renal replacement therapy (HD, peritoneal dialysis, and transplant) as a comparison group [42]. Of the remaining studies, one study utilized a cross- sectional design without a comparison group [58], one study utilized a case report [65], one study utilized a retrospective comparative study [57], one study conducted a post hoc analysis of a randomized controlled trial [38], and one study utilized a population based observational survey [45].
Sample
The samples utilized by the included studies were varied. The mean age of the participants in the included studies was 74.5 years, with a range of patients from age 65 [64] to 83 [53] years. The sample size of the studies ranged from 2 [65] to 33654 [66] (Appendix A). There were 16 studies that provided the mean estimated GFR for their samples of patients with CKD [12,34,35,37,38,42,43,46,53,57,59,60,62–64,67]. The mean GFR for the samples in these studies was 43.85ml/min/1.73m2 (range eGFR 17–59.8ml/min/1.73m2). The remaining studies reported kidney function as stage of CKD (Stage 3, 4, 5) [47,49,58,65] or as a range of GFR (eGFR 15–30, eGFR 30–45, eGFR > or <60, etc.) [25,33,36,39,41,44,45,48,50–52,61,66].
Quality of Studies
Study quality was assessed with a process for studies of varied designs in an integrative review [68]. The mean quality rating of the studies was 9.75 out of 13 (SD=.55). All studies utilized quantitative methods and included a thorough description of the methods. The sampling methods in the studies were convenience, purposive, and cohort. The most prevalent sampling method was purposive sampling or utilization of a cohort, which was utilized in 28 studies. The full quality assessment is displayed in Table 3.
Table 3-.
Quality Assessment
| Author | Year | Study typea | Samplingb | Methodc | Statistical analysis | Score |
|---|---|---|---|---|---|---|
| Afsar et al. | 2011 | 4 | 1 | 1 | 3 | 9 |
| Amano et al. | 2013 | 4 | 1 | 1 | 2 | 8 |
| Antunes et al. | 2014 | 4 | 1 | 1 | 3 | 9 |
| Auriel et al. | 2016 | 4 | 2 | 1 | 3 | 10 |
| Buchman et al. | 2009 | 4 | 2 | 1 | 3 | 10 |
| Chen et al. | 2017 | 4 | 2 | 1 | 3 | 10 |
| Corrao et al. | 2015 | 4 | 2 | 1 | 3 | 10 |
| Darsie et al. | 2014 | 4 | 2 | 1 | 3 | 10 |
| Elias et al. | 2009 | 4 | 2 | 1 | 3 | 10 |
| Etgen et al. | 2009 | 4 | 2 | 1 | 3 | 10 |
| Feng et al. | 2013 | 4 | 2 | 1 | 3 | 10 |
| Foster et al. | 2016 | 4 | 2 | 1 | 3 | 10 |
| Hobson et al. | 2016 | 4 | 2 | 1 | 3 | 10 |
| Iyasere et al. | 2017 | 4 | 1 | 1 | 3 | 9 |
| Jovanovich et al. | 2014 | 4 | 2 | 1 | 3 | 10 |
| Khatri et al. | 2009 | 4 | 2 | 1 | 3 | 10 |
| Kurella, Chertow et al. | 2005 | 4 | 2 | 1 | 3 | 10 |
| Kurella, Yaffe et al. | 2005 | 4 | 2 | 1 | 3 | 10 |
| Kuriyam et al. | 2013 | 4 | 2 | 1 | 3 | 10 |
| Lai et al. | 2016 | 4 | 1 | 1 | 3 | 9 |
| Lee, Chin et al. | 2011 | 4 | 2 | 1 | 3 | 10 |
| Lee, Shimada et al. | 2015 | 4 | 2 | 1 | 3 | 10 |
| Lin et al. | 2009 | 4 | 2 | 1 | 3 | 10 |
| Murray et al. | 2016 | 4 | 2 | 1 | 3 | 10 |
| Post et al. | 2010 | 4 | 1 | 1 | 3 | 9 |
| Pulignano et al. | 2014 | 4 | 1 | 1 | 3 | 9 |
| Rodriguez Angarita et al. | 2016 | 4 | 3 | 1 | 3 | 11 |
| Rodriguez Villareal et al. | 2014 | 4 | 2 | 1 | 3 | 10 |
| Romijn et al. | 2015 | 4 | 1 | 1 | 3 | 9 |
| Sasaki et al. | 2011 | 4 | 2 | 1 | 3 | 10 |
| Slinin et al. | 2008 | 4 | 2 | 1 | 3 | 10 |
| Srivanitchapoom et al. | 2013 | 4 | 2 | 1 | 3 | 10 |
| Umemura et al. | 2013 | 4 | 2 | 1 | 3 | 10 |
| Wang et al. | 2016 | 4 | 2 | 1 | 3 | 10 |
| Weng et al. | 2012 | 4 | 1 | 1 | 3 | 9 |
| Zammit et al. | 2015 | 4 | 2 | 1 | 3 | 10 |
| Mean | 4 | 1.778 | 1 | 2.972 | 9.75 | |
| Range | 4 | 1 to 3 | 1 | 2 to 3 | 8 to 11 |
Study type: 3=qualitative design; 4=quantitative descriptive design; 5=mixed qualitative and quantitative descriptive; 6=quantitative experimental and quasi-experimental
Sampling: 0=not explained; 1=convenience; 2=purposive or case matching/cohort; 3=random or 100%
Method: 1=methods and tools explained; 0=not explained
Analysis:1=narrative statistics; 2=descriptive statistics; 3= inferential statistics10
Most Common Test
In the included studies, 36 different types of cognitive screening tests were utilized at varying frequencies (Figure 2). The full details of which tests were utilized in each study are displayed in Appendix B. The most commonly utilized cognitive assessment test was the Mini Mental State Exam (MMSE) which was utilized in 16 of the 36 studies [12,33,44,47–49,51– 53,56,59,60,62–65]. The MMSE was used as a singular test to evaluate cognitive function in eight studies [33,47–49,51,53,59,64]. The second most commonly utilized tests were the digit symbol substitution test, which was utilized in eight of the 36 studies [25,34,35,42,45, 46,52,56], and evaluation of verbal fluency [34,38,40,43,55,56,60,62]. The third most commonly utilized tests were each utilized in studies seven times, which were the Trail Making Test B (TMT-B) [35,40,42, 43,50,55,56]. The number of tests utilized in each study to assess cognitive function were varied. The number of tests utilized in each study ranged from one [33,36,37,41,45,47– 49,51,53,54,57–59,64,66–67] to 12 [35] tests. (Figure 3)
Figure 2-.

Number of Tests Utilized in Each Study
Figure 3-.

Number of Times Each Test Utilized
The tests utilized in the included studies evaluated various domains of cognitive function. The tests were identified with the domain most evaluated by the test (Appendix B) [69,70]. The most common area assessed in the included studies was global cognition, which was assessed by 13 of the utilized tests (Figure 4). The second most common domain of assessment was tests that evaluated memory, which were 9 of 36 tests utilized in the included studies (Figure 4).
Figure 4-.

Number of Cognitive Tests per Doman
Psychometrics
The authors of some of the included studies reported the psychometrics of the cognitive tests utilized in their study. Sixteen studies provided citations for the validity of the cognitive test utilized in their study, which supported that the tests had adequate validity in assessing cognitive function in the general population [36–39,43,44,46,48,56–61,64,66]. Only Hobson and colleagues assessed test validity of their utilized test, the M-ACE, with their sample of patients with diabetes and CKD through the assessment of criterion validity with a DSM diagnosis of dementia and convergent validity with the MMSE [63]. Two studies provided citations of previously measured reliability for the utilized cognitive tests [44,64]. Hobson and colleagues assessed the reliability of the M-ACE with assessment of internal consistency via Cronbach alpha (.81) [63]. Three studies cited previously reported sensitivity of the cognitive tests [43,50,53], and two studies cited previously reported specificity related to cognitive impairment [50,53]. Hobson and colleagues assessed the sensitivity and specificity of their utilized test in identifying cognitive impairment in their sample [63].
Findings
Of the 36 studies included in the review, 30 studies explored the relationship between kidney disease and cognitive impairment. Of these studies, 29 found significant relationships between CKD and cognitive impairment [25,33–37,39–46,48–50,52–56,58–62,66,67]. Of these studies, 14 evaluated changes in cognitive function over time and found significant changes over time through the cognitive test utilized in their study [12,25,34,36,39,41,46,49,50,54,60–62,66]. One study did not find a significant relationship between CKD and cognitive impairment with logistic regression, but the authors reported a nonsignificant trend for increased odds of cognitive impairment in patients with GFR less than 48ml/min [51]. The remaining seven studies had findings that supported their study purpose. (Appendix A)
Discussion
Through this review, it has been identified that the cognitive assessment of older adult patients with CKD is vast and varies. There were 36 different tests administered to assess cognitive function in the reviewed articles, with authors utilizing anywhere from one [33,36,37,41,45,47–49,51,53,54,57–59,64,66–67] to 12 [35] tests in each study. This has been similarly found in studies that sought to synthesize associations between CKD and cognitive decline [10] and in studies that evaluated cognitive function in patients on hemodialysis [11].
Research and clinical findings suggest that the domains affected in the cognitive impairment of CKD are unique and may be related to deficits seen in vascular dementia, which are different than the domains of impairment seen in patients with Alzheimer’s disease [5]. The cognitive impairment seen in patients with vascular dementia is non-progressive, slowed cognitive performance that primarily affects executive function and may cause mild impairments in memory [20]. Similarly, patients with CKD are primarily noted to have deficits in executive function [9,40,50,71,72], but have also been found to have deficits in global cognition [9,40,72], memory [40,72], attention and concentration [71,72], and language and naming [40,72]. Patients with Alzheimer’s disease primarily have deficits in episodic memory while other domains are conserved [20]. Cognitive decline is considered to be a part of the normal aging process in all older adults, particularly with deficits noted in novel situations that utilize memory, spatial, and executive function [20]. The cognitive impairment noted in patients with CKD is also different than the decline noted with normal aging. This is specifically related to vascular dementia and impaired executive function. These differences emphasize the critical need to assess patients with CKD with cognitive tests that evaluate the appropriate domains. From this review, it was found that composite tests of global cognition are frequently utilized but not every test of global cognition evaluates the domains most affected in patients with CKD, such as executive function [70]. Therefore, it is critically important that patients with CKD are evaluated for cognitive impairment with tests that assess the domains where they most commonly experience cognitive impairment, in addition to evaluating the domains affected by Alzheimer’s disease. With an appropriate assessment, there is a greater potential that the full magnitude of cognitive impairment in patients with CKD is understood.
Most Frequently Utilized Tests
The most commonly utilized test identified in this review was the MMSE, which was used in 16 of the 36 studies. When including the utilization of the 3MS, which is a modified version of the MMSE with more items and a revised grade scoring system [73], versions of the MMSE were utilized in 21 studies. The frequent utilization of the MMSE has similarly been found when cognitive function is assessed in patients on hemodialysis [11]. The MMSE has advantages in its relatively strong sensitivity and specificity in detecting cognitive impairment in the general population, as well as the ability of the test to evaluate five cognitive dimensions- orientation, memory, attention, language, and visuoconstruction [69,70]. The MMSE has limitations in that it is copyrighted and has poor sensitivity in detecting MCI (.18) [69,70,74].
The low sensitivity of the MMSE in detecting MCI is concerning because it may not detect cognitive impairment in its mildest stages, including the deficits found in older adult patients with CKD. The utilization of the MMSE in patients with CKD is also concerning because the MMSE does not assess executive function, which is the cognitive domain where patients with CKD and patients with vascular dementia are often noted to have deficits [9,40,50,71,72]. Eight of the reviewed studies utilized the MMSE as the only test to evaluate cognitive function [33,47– 49,51,53,59,64]. It is possible that these studies did not capture all patients with cognitive impairment because executive function was not assessed. Of the 30 studies that sought to find an association between CKD and cognitive impairment, only one study did not find a significant relationship between cognitive impairment and lower GFR [51]. Interestingly, this study only utilized the MMSE to evaluate cognitive function. Given the potential limitations of the utilization of the MMSE in older adult patients with CKD, it is possible that this study did not find a significant relationship because of the limited scope of evaluation of cognitive function with the MMSE.
There were two tests that were utilized in eight studies. The digit symbol substitution test, which is a 90 second test of how many appropriate symbols a participant can place next to a corresponding number according to a key [75], was one of the second most commonly utilized tests. Digit symbol substitution tests evaluate processing speed, which requires the utilization of the cognitive abilities of attention, memory, and visual scanning [42,76]. Digit symbol substitution tests are often used in research and practice because they are easy to administer in a consistent way [42,75]. One example of this test is the Digit Symbol Substitution Test (DSST) from the Wechsler Adult Intelligence Scale, which has been utilized for over 15 years and has scores that are significantly correlated with the age of the person being evaluated while being independent of educational level [75]. Unfortunately, digit symbol substitution tests have weaknesses. For a person to be accurately evaluated with digit symbol substitution tests, visual acuity, motor coordination, and motor speed need to be intact [77]. Therefore, digit symbol substitution tests cannot be administered to obtain a true assessment of cognitive function if a person has deficits in any of these areas. Symbol familiarity also plays a role in the performance on digital substitution tests for older adults, which can alter the ability of the test to be a factual representation of the patient’s cognitive ability [76]. The use of digit symbol substitution tests has limitations in the assessment of cognitive function for older adult patients with CKD. Unfortunately, digit symbol substitution tests do not assess executive function. The digit symbol substitution test was utilized as the singular cognitive test in only one study of this review [45]. Given the lack of assessment of executive function, the use of digit symbol substitution tests as a singular assessment of cognitive function in patients with CKD should be utilized with caution, as it may not evaluate all potential domains of deficit.
Tests of verbal fluency were also utilized in eight of the evaluated studies. Tests of verbal fluency are an assessment of a person’s ability to name words beginning with a certain letter or belonging to a certain category [78]. Tests of verbal fluency evaluate language, in addition to semantic memory and working memory, which is considered a component of executive function [40]. Deficits in verbal fluency have been found frequently in patients with vascular dementia [72]. Unfortunately, tests of verbal fluency have limitations in that there is limited normative data related to gender, age, educational level, and intelligence [78]. In addition, the norms that are available are only applicable to people that are fluent in English [78]. A test of verbal fluency was not utilized as the singular cognitive assessment in any of the included studies, which is appropriate given the limited cognitive domains that are assessed by tests of verbal fluency.
The TMT-B was utilized in seven of the studies of the review. The TMT-B evaluates executive function, as well as mental flexibility, in its assessment of the speed at which a person can complete a number and letter sequence in the correct order [70]. The TMT-B has shown strength as a cognitive test in determining loss of cognitive function in patients at all stages of kidney disease, even those with only minor deficits in cognition [70]. In addition, when utilized in one sample of older adult patients with ESRD, many patients were unable to complete a cognitive battery that included the TMT-B due to visual impairment, lack of motivation, or motor difficulties [79]. The complexity and time limit of this cognitive assessment needs to be considered when it is utilized in older adults with CKD.
Recommendations and Future Research Directions
It is critical that older adult patients with CKD have an appropriate assessment to identify cognitive impairment, so this population can receive care and manage the risks associated with this complication of CKD. Since executive function is commonly affected in patients with CKD [9,40,50,71,72], cognitive function tests that evaluate executive function should be utilized in practice and research that seeks to fully understand cognitive impairment of older adults with CKD. To assure that multiple aspects of cognitive function are evaluated, it is reasonable that patients with CKD are assessed for cognitive impairment with either a composite test or multiple tests that evaluate different domains of cognitive function, including executive function and other domains commonly affected in patients with CKD [9,40,50,71,72]. This is particularly important when evaluating patients with CKD that have varying levels of GFR, since patients at different levels of kidney impairment may experience deficits in different cognitive domains. As described by Yaffe and colleagues in the Chronic Renal Insufficiency Cohort (CRIC) study, patients with an estimated GFR below 30ml/min/1.73m2 were found to have deficits in global cognition, naming, attention, executive function, and delayed memory but had relatively intact category fluency and immediate memory, as compared to patients with estimated GFR above 30ml/min/1.73m2 [72]. These findings emphasize that older adults with CKD should be evaluated across multiple cognitive domains, particularly when evaluating groups of patients with different levels of GFR.
Further research is needed on the appropriate way to assess cognitive function in older adult patients with CKD. A large priority is the determination of the ideal cognitive assessment test to utilize with older adult patients with CKD. In the articles reviewed, there were limited reports on the reliability, validity, sensitivity, and specificity of the cognitive assessment tests utilized. Unfortunately, this has led to the assessment of cognitive function in patients with CKD being done in various ways utilizing different tests. Therefore, it is essential that thorough psychometric evaluation of cognitive tests is conducted with older adult patients with CKD to establish an ideal test to utilize with this at-risk population.
Clinical Implications
The findings of this review have very important clinical implications. The reviewed studies highlight the large burden of cognitive impairment experienced by older adult patients with CKD. Assessment of cognitive function should be done for all patients with CKD, and this review has highlighted the complexity associated with doing this assessment. With many cognitive screening tests available, it is important that clinicians choose appropriate cognitive screening tests for older adults with CKD. Clinical assessments that aim to assess cognitive function in patients with CKD should utilize more than a singular test to assess multiple cognitive domains, including those most commonly affected in patients with CKD. In practice and research, assessing a single cognitive domain in a patient with CKD is likely not sufficient. Executive function should be one of the domains assessed in all older adult patients with CKD with suspected cognitive impairment, since there is support that assessment of executive function is a necessary and sensitive indicator of cognitive impairment in patients with CKD [9].
In clinical practice, it is critically important that older adult patients with CKD are evaluated with cognitive tests that consider the patient’s other comorbidities and deficits. As previously described, tests such as the digit symbol substitution test require visual acuity, motor coordination, and motor speed [77]. It is necessary to choose appropriate cognitive tests that consider the patient’s other comorbidities and sensory deficits to avoid missing cases and fully capture the magnitude of the problem of cognitive impairment in older adult patients with CKD.
Limitations
Although this review was rigorous and thorough, it does have some limitations. The articles evaluated were those that were available in English, which may have excluded relevant international studies. Although the review was integrative in nature, a full review of grey literature was not conducted, which may have omitted relevant citations [80]. There were some limitations related to the evaluation of tests utilized to assess cognitive function. Every test was not counted individually if it was a derivation of the same test, such as the different versions of digit symbol substitution tests. In addition, composite tests of global cognition were counted as one test, although they evaluate different cognitive domains. This has similarly been done in other studies that evaluated cognitive assessment in patients with CKD [10]. This may have led to misrepresentation of the number of cognitive tests utilized in each study because if a composite was used, less tests per study may have been utilized. In addition, this may have led to certain cognitive domains not being fully counted. This review also has limitations in that it did not evaluate the full breadth of studies that evaluated cognitive function in patients with CKD, since studies were only evaluated if the mean age of participants was greater than 65 years old to capture studies focused on older adults. The utilization of this cutoff may have eliminated relevant studies of cognitive impairment in patients with CKD. It is possible with the inclusion of studies with patients with a mean age less than 65 years old that the utilization of cognitive function assessments would have been different. However, the purpose of this study was to focus on older adults with a mean age of greater than 65 years of age due to the higher prevalence of cognitive impairment in this population. Future studies should investigate cognitive function assessment in midlife and younger adults. Despite these limitations, a thorough review was conducted that revealed the breadth of assessment tests currently being utilized to evaluate cognitive function in older adult patients with CKD.
Conclusion
Through this integrative review, it has been elucidated that the assessment of cognitive function in older adults with CKD is done in many, inconsistent ways. In the reviewed articles, there were wide variations in the tests chosen, the number of tests utilized, and the cognitive domains assessed. Since there is not a test of cognitive function validated for use in older adult patients with CKD, it is critical in research and practice that assessment of cognitive function in older adult patients with CKD is done thoroughly by assessing various cognitive domains. With a more comprehensive assessment of cognitive function, the true magnitude of cognitive impairment in older adults with CKD can be understood and interventions can be developed to address this debilitating consequence of CKD.
Supplementary Material
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Compliance with Ethical Standards
-On behalf of all authors, the corresponding author states that there is no conflict of interest.
-Human subjects were not utilized in this research. For this type of study formal consent is not required.
-This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Centers for Disease Control and Prevention (CDC). Chronic kidney disease (CKD) surveillance project 2016; https://nccd.cdc.gov/ckd/. [Google Scholar]
- 2.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: Current prevalence, future projections, and clinical significance. Adv Chronic Kidney D 2010;17(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura MK, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: The Frequent Hemodialysis Network Trials. Clin J Am Soc Nephrol 2010;5(8):1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helmer C, Stengel B, Metzger M, et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 2011;77(23):2043–2051. [DOI] [PubMed] [Google Scholar]
- 5.Bronas UG, Puzantian H, & Hannan M (2017). Cognitive Impairment in Chronic Kidney Disease: Vascular Milieu and the Potential Therapeutic Role of Exercise. BioMed Research International, 2017. doi: 10.1155/2017/2726369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray A, Tupper DE, Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology 2006;67(2):216–223. [DOI] [PubMed] [Google Scholar]
- 7.Tamura MK, Yaffe K, Hsu CY, et al. Cognitive Impairment and Progression of CKD. Am J Kidney Dis 2016;68(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurella Tamura M. Kidney function and cognitive impairment in US adults: The reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis 2008;52(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 2004;52(11):1863–1869. [DOI] [PubMed] [Google Scholar]
- 10.Etgen T, Chonchol M, Förstl H, Sander D. Chronic kidney disease and cognitive impairment: A systematic review and meta-analysis. Am J Nephrol 2012;35(5):474–482. [DOI] [PubMed] [Google Scholar]
- 11.O’Lone E, Connors M, Masson P, et al. Cognition in people with end-stage kidney disease treated With hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 2016;67(6):925–935. [DOI] [PubMed] [Google Scholar]
- 12.Weng S-C, Shu K-H, Tang Y-J, et al. Progression of cognitive dysfunction in elderly chronic kidney disease patients in a veteran’s institution in central Taiwan: a 3-Year longitudinal study. Internal Medicine 2012;51(1):29–35. [DOI] [PubMed] [Google Scholar]
- 13.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004;15(7):1904–1911. [DOI] [PubMed] [Google Scholar]
- 14.Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial 2008;21(1):29–37. [DOI] [PubMed] [Google Scholar]
- 15.Murray A. Cognitive impairment in CKD: no longer an occult burden. Am J Kidney Dis 2010;56(4):615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molnar MZ, Sumida K, Gaipov A, et al. Pre-ESRD dementia and post-ESRD mortality in a large cohort of incident dialysis patients. Dement Geriatr Cogn Disord 2017;43(5–6):281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and statistical manual of mental disorders | DSM Library 5th ed. Washington, DC: 2017. [Google Scholar]
- 18.Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol 2014;10(11):634. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 1999;56(3):303–308. [DOI] [PubMed] [Google Scholar]
- 20.Lindeboom J, Weinstein H. Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease, and vascular cognitive impairment. Eur Pharmacol 2004;490(1):83–86. [DOI] [PubMed] [Google Scholar]
- 21.Roberts RO, Knopman DS, Mielke MM, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 2014;82(4): 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Kidney Foundation (NKF). KDOQI Guideline 6- Association of level of GFR with complications Adults 2002; http://www2.kidney.org/professionals/kdoqi/guidelines_ckd/p6_comp_g11.htm.
- 23.Bugnicourt J-M, Godefroy O, Chillon J-M, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol 2013;24(3):353–363. [DOI] [PubMed] [Google Scholar]
- 24.Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs 2005;52(5):546–553. [DOI] [PubMed] [Google Scholar]
- 25.Darsie B, Shlipak MG, Sarnak MJ, Katz R, Fitzpatrick AL, Odden MC. Kidney function and cognitive health in older adults: the Cardiovascular Health Study. Am J Epidemiol 2014;180(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Renal Data System (USRDS). USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States 2005; https://www.usrds.org/2005/pdf/06_morb_and_mort_05.pdf.
- 27.World Health Organization. Proposed working definition of an older person in Africa for the MDS Project 2016; http://www.who.int/healthinfo/survey/ageingdefnolder/en/. Accessed October 30, 2017.
- 28.Office of Disease Prevention and Health PRomotion. Older Adults: Healthy People 2020 2017; https://www.healthypeople.gov/2020/topics-objectives/topic/older-adults. Accessed October 30, 2017.
- 29.Kurella Tamura M, Vittinghoff E, Hsu C-y, et al. Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray A. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv Chronic Kidney Dis 2008;15(2):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta A, Mahnken JD, Johnson DK, et al. Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC Nephrol 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Tetlzlaff J, Altman DG, The Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antunes JPV, Bulhões C, Fonte P, Abreu MJ, Oliveira R. Renal function and cognitive Dysfunction: Cross-sectional study of users enrolled at Ponte-Family Health Unit. J Bras Nefrol 2015;37(1). [DOI] [PubMed] [Google Scholar]
- 34.Buchman AS. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 2009;73(12):920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant 2009;24(8):2446–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etgen T, Sander D, Chonchol M, et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrol Dial Transplant 2009;24(10):3144–3150. [DOI] [PubMed] [Google Scholar]
- 37.Foster R, Walker S, Brar R, et al. Cognitive impairment in advanced chronic kidney disease: The Canadian Frailty Observation and Interventions Trial. Am J Nephrol 2016:473–480. [DOI] [PubMed] [Google Scholar]
- 38.Jovanovich AJ, Chonchol M, Brady CB, et al. 25-vitamin D, 1,25-vitamin D, parathyroid hormone, fibroblast growth factor-23 and cognitive function in men with advanced CKD: a veteran population. Clin Nephrol 2014;82(5):S1–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khatri M. CKD associates with cognitive decline. J Am Soc Nephrol 2009;20(11):2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis 2005;45(1):66–76. [DOI] [PubMed] [Google Scholar]
- 41.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 2005;16(7):2127–2133. [DOI] [PubMed] [Google Scholar]
- 42.Lai S, Mecarelli O, Pulitano P, et al. Neurological, psychological, and cognitive disorders in patients with chronic kidney disease on conservative and replacement therapy. Medicine 2016;95(48):e5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JJ, Chin HJ, Byun MS, et al. Impaired frontal executive function and predialytic chronic kidney disease. J Am Geriatr Soc 2011;59(9):1628–1635. [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Shimada H, Park H, et al. The association between kidney function and cognitive decline in community-dwelling, elderly Japanese people. J Am Med Dir Assoc 2015;16(4):349.e341. [DOI] [PubMed] [Google Scholar]
- 45.Lin C-Y, Lin J-W, Lin L-Y, Kuo H-K. Chronic kidney disease, atherosclerosis, and cognitive and physical function in the geriatric group of the National Health and Nutrition Survey 1999–2002. Atherosclerosis 2009;202(1):312–319. [DOI] [PubMed] [Google Scholar]
- 46.Murray AM, Bell EJ, Tupper DE, et al. The Brain in Kidney Disease (BRINK) cohort study: Design and Baseline Cognitive Function. Am J Kidney Dis 2016;67(4):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez Villarreal I, Ortega O, Hinostroza J, et al. Geriatric assessment for therapeutic decision-making regarding renal replacement in elderly patients with advanced chronic kidney disease. Nephron Clin Pract 2014;128(1–2):73–78. [DOI] [PubMed] [Google Scholar]
- 48.Romijn MDM, van Marum RJ, Emmelot-Vonk MH, Verhaar HJJ, Koek HL. Mild chronic kidney disease is associated with cognitive function in patients presenting at a memory clinic: Association between chronic kidney disease and cognitive function. Int J Geriatr Psychiatry 2015;30(7):758–765. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki Y, Marioni R, Kasai M, Ishii H, Yamaguchi S, Meguro K. Chronic kidney disease: A risk factor for dementia onset: A population‐ based study. The Osaki‐ Tajiri Project. J Am Geriatr Soc 2011;59(7):1175–1181. [DOI] [PubMed] [Google Scholar]
- 50.Slinin Y, Osteoporotic Fractures in Men Study G. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc 2008;56(11):2082–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivanitchapoom P, Senanarong V. Estimated creatinine clearance and cognitive impairment in Thai older adults: a pilot study from the dementia and disability project in Thailand. J Med Assoc Thai 2013;96 Suppl 2:S47. [PubMed] [Google Scholar]
- 52.Umemura T, Kawamura T, Umegaki H, et al. Association of chronic kidney disease and cerebral small vessel disease with cognitive impairment in elderly patients with type 2 diabetes. Dement Geriatr Cogn Dis Extra 2013;3(1):212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Liu T, Cai Y-y, Jiang H, Liu H-x, Lin C. Kidney function and cognitive impairment in people aged 80 years and over with untreated hypertension: A cross- sectional survey. Kidney Blood Press Res 2016;41(1):70–77. [DOI] [PubMed] [Google Scholar]
- 54.Khatri M, Nickolas TL, Moon YP, et al. CKD associates with cognitive decline. J Am Soc Nephrol 2009;20(11): 2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zammit AR, Katz MJ, Lai JY, Zimmerman ME, Bitzer M, Lipton RB. Association between renal function and cognitive ability domains in the Einstein aging study: a cross- sectional analysis. J Gerontol A Biol Sci 2015;70(6):764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Post JB, Jegede AB, Morin K, Spungen AM, Langhoff E, Sano M. Cognitive profile of chronic kidney disease and hemodialysis patients without dementia. Nephron Clin Pract 2010;116(3):c247–c255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corrao S, Argano C, Nobili A, et al. Brain and kidney, victims of atrial microembolism in elderly hospitalized patients? Data from the REPOSI study. Eur J Int Med 2015;26(4):243–249. [DOI] [PubMed] [Google Scholar]
- 58.Rodríguez-Angarita CE, Sanabria-Arenas RM, Vargas–Jaramillo JD, Ronderos–Botero I. Cognitive impairment and depression in a population of patients with chronic kidney disease in Colombia: A prevalence Sstudy. Can J Kidney Health Dis 2016;3(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng L, Yap KB, Ng TP. Depressive symptoms in older adults with chronic kidney disease: mortality, quality of life outcomes, and correlates. Am J Geriatr Psychiatry 2013;21(6):570. [DOI] [PubMed] [Google Scholar]
- 60.Pulignano G, Del Sindaco D, Di Lenarda A, et al. Chronic renal dysfunction and anaemia are associated with cognitive impairment in older patients with heart failure. J Cardiovasc Med (Hagerstown) 2014;15(6):481–490. [DOI] [PubMed] [Google Scholar]
- 61.Auriel E, Kliper E, Shenhar-Tsarfaty S, et al. Impaired renal function is associated with brain atrophy and poststroke cognitive decline. Neurology 2016;86(21):1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuriyama N, Mizuno T, Ohshima Y, et al. Intracranial deep white matter lesions (DWLs) are associated with chronic kidney disease (CKD) and cognitive impairment: A 5-year follow-up magnetic resonance imaging (MRI) study. Arch Gerontol Geriatr 2013;56(1):55–60. [DOI] [PubMed] [Google Scholar]
- 63.Hobson P, Rohoma Kamel H, Wong Stephen P, Kumwenda Mick J. The utility of the Mini-Addenbrooke’s Cognitive Examination as a screen for cognitive impairment in elderly patients with chronic kidney disease and diabetes. Dement Geriatr Cogn Dis Extra 2016;6(3):541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afsar B, Elsurer R, Covic A, Johnson RJ, Kanbay M. Relationship between uric acid and subtle cognitive dysfunction in chronic kidney disease. Am J Nephrol 2011;34(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amano C, Ito T, Egawa M, et al. Effects of different administration protocols on the plasma concentration of donepezil hydrochloride in dementia patients with stage 5 chronic kidney disease. Nephron Extra 2013;3(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y-C, Weng S-C, Liu J-S, Chuang H-L, Hsu C-C, Tarng D-C. Severe decline of estimated glomerular filtration rate associates with progressive cognitive deterioration in the elderly: A community-based cohort study. Scientific Reports 2017;7:42690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iyasere O, Okai D, Brown E. Cognitive function and advanced kidney disease: longitudinal trends and impact on decision-making. Clin Kid J 2017:sfw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olsen J, Baisch MJ. An integrative review of information systems and terminologies used in local health departments. J Am Med Inform Assoc 2014;21(e1):e20–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurella Tamura M, Yaffe K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 2011;79(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider SM, Kielstein JT, Braverman J, Novak M. Cognitive function in patients with chronic kidney disease: Challenges in neuropsychological assessments. Semin Nephrol 2015;35(4):304. [DOI] [PubMed] [Google Scholar]
- 71.Weiner DE, Gaussoin SA, Nord J, et al. Cognitive function and kidney disease: Baseline data from the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 2017;70(3):357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yaffe K, Chronic Renal Insufficiency Cohort I. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 2010;58(2):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48(8):314. [PubMed] [Google Scholar]
- 74.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief Sscreening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 75.Hoyer WJ, Stawski RS, Wasylyshyn C, Verhaeghen P. Adult age and digit symbol substitution performance: A meta-analysis. Psychol Aging 2004;19(1):211–214. [DOI] [PubMed] [Google Scholar]
- 76.Stephens R, Kaufman A. The role of long-term memory in digit-symbol test performance in young and older adults. Neuropsychol Dev Cogn 2009;16(2):219. [DOI] [PubMed] [Google Scholar]
- 77.Wechsler D The measurement and appraisal of adult intelligence 4th ed. Baltimore, MD.: Williams & Wilkins; 1958. [Google Scholar]
- 78.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14(2):167–177. [PubMed] [Google Scholar]
- 79.Neumann D, Robinski M, Mau W, Girndt M. Cognitive testing in patients with CKD: The problem of missing cases. Clin J Am Soc Nephrol 2017;12(3):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nurs Res 2003;52(4):256–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


