Abstract
BACKGROUND:
A broad, comprehensive review of studies exploring associations between lifestyle factors and non-muscle invasive bladder cancer (NMIBC) outcomes is warranted to consolidate recommendations and identify gaps in research.
OBJECTIVE:
To summarize the literature on associations between lifestyle factors and clinical outcomes among patients with NMIBC.
METHODS:
PubMed was systematically queried for articles published through March 2019 regarding lifestyle factors and recurrence, progression, cancer-specific mortality, and all-cause mortality among patients with NMIBC.
RESULTS:
Notwithstanding many ambiguities, there is good-quality evidence suggesting a benefit of smoking avoidance/cessation, healthy body mass index (BMI), and type II diabetes mellitus prevention and treatment. Lactobacillus casei probiotic supplementation may reduce recurrence. There have been individual studies suggesting a benefit for uncooked broccoli and supplemental vitamin E as well as avoidance of supplemental vitamin B9, areca nut chewing, and a “Western diet” pattern high in fried foods and red meat. Additional studies do not suggest associations between NMIBC outcomes and use of fibrin clot inhibitors; insulin and other oral hypoglycemics; statins; supplemental selenium, vitamin A, vitamin C, and vitamin B6; fluid intake and intake of specific beverages (e.g., alcohol, coffee, green tea, cola); various dietary patterns (e.g., Tex-Mex, high fruit and vegetable, low-fat); and occupational and chemical exposures.
CONCLUSIONS:
Despite a myriad of publications on lifestyle factors and NMIBC, a need remains for research on unexplored associations (e.g., physical activity) and further studies that can elucidate causal effects. This would inform future implementation strategies for healthy lifestyle change in NMIBC patients.
Keywords: Urinary bladder neoplasms, lifestyle, body mass index, smoking, diet, diabetes mellitus, dietary supplements, prognosis, recurrence, non-invasive
INTRODUCTION
Bladder cancer is the 2nd most common cancer of the genitourinary system [1]. In the United States (US) alone, roughly 80,000 new cases and 18,000 deaths from bladder cancer are expected in 2019 [1]. Non-muscle invasive bladder cancer (NMIBC) comprises approximately 70% of new cases [2]. Bladder cancer-specific survival of high-grade NMIBC ranges from 70–85% at ten years, and rates are even higher among those with low-grade disease [3, 4]. Still, the five-year probability of recurrence and progression ranges from 31% to 78% and from less than 1% to 45%, respectively, depending on clinical and pathological severity [5]. Given the chronicity of NMIBC, it is essential to elucidate lifestyle factors that may reduce the risk of poor outcomes.
Previous systematic reviews and meta-analyses have consolidated available data regarding associations between lifestyle factors and NMIBC prognosis. For example, one meta-analysis reported an increased risk of recurrence and bladder cancer-specific mortality (CSM) among current and former smokers [6]. Another reported an increased risk of recurrence among obese and overweight patients [7]. The latter review also included a discussion of dietary factors and supplements. However, given the rapidly expanding literature on a growing number of lifestyle factors that may influence NMIBC outcomes, an updated review summarizing a wider variety of lifestyle factors is warranted. To this end, we aimed to compile a comprehensive overview of studies evaluating associations between lifestyle factors and recurrence, progression, CSM, and all-cause mortality (ACM) in patients with NMIBC.
MATERIALS AND METHODS
Evidence acquisition
We sought to include all studies that quantitatively evaluated associations between lifestyle factors and NMIBC outcomes. In March 2019, a PubMed literature search was conducted based on the following terms: (cancer OR carcinoma OR neoplas* OR tumor) AND (bladder OR urothelial OR “transitional cell”) AND (NMIBC OR non-muscle-invasive) AND (“risk factor” OR recur* OR progression OR death OR survival OR fatal OR prognos* OR outcome). The result was 2,009 articles for review. Articles were considered relevant if they: included participants with a diagnosis of NMIBC; assessed at least one lifestyle factor; and did so in relation to recurrence (reappearance of tumor following initial treatment), progression (upstaging to muscle-invasive, nodal, and/or metastatic disease and cystectomy for treatment-resistant disease), CSM, and/or ACM. Upon selection of 45 relevant articles, we reviewed the reference sections to identify additional relevant studies. We ultimately identified a total of 105 articles published between 1977–2019 for inclusion. Full article screening and inclusion criteria are outlined in Fig. 1. Study inclusion was consistent with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [8]. As a literature review, this study is exempt from any requirement for Institutional Review Board approval.
Fig. 1.
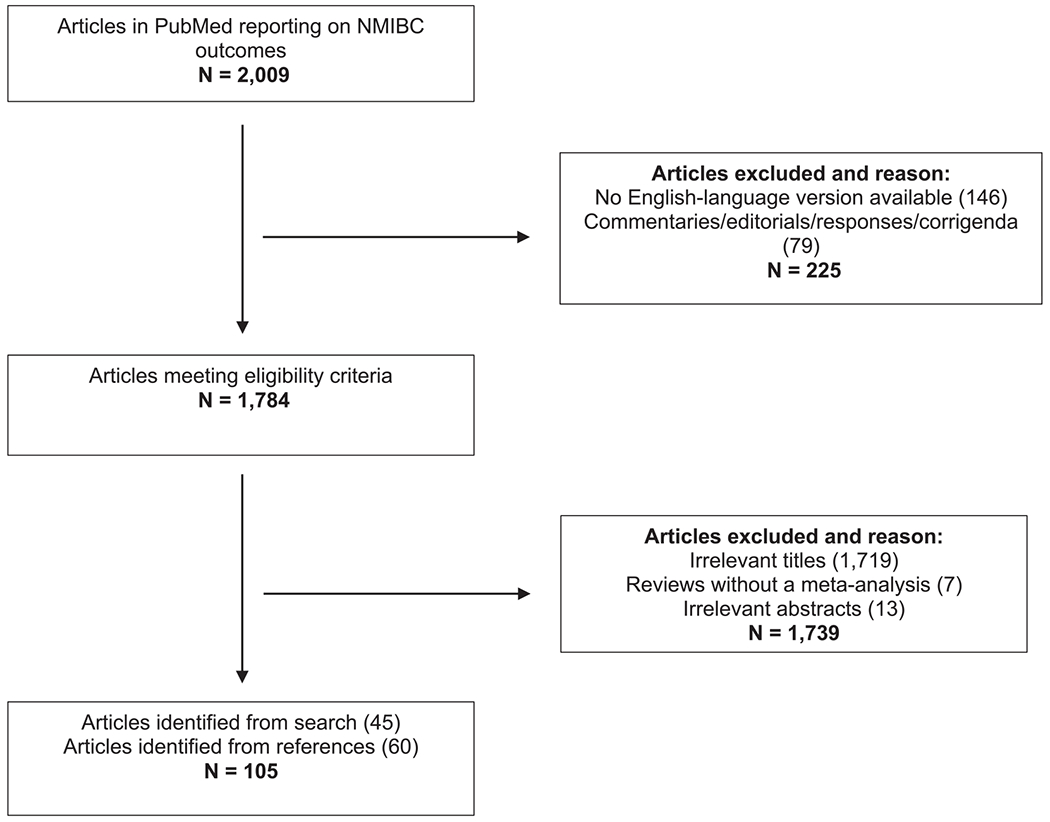
Flow diagram showing article identification, eligibility, and inclusion.
Evidence synthesis
Table 1 summarizes lifestyle factors with evidence of benefit relative to NMIBC outcomes. Supplementary Table 1 details the design and results of each included study.
Table 1.
Factors with evidence beneficial associations with NMIBC outcomes
| Factor | Evidence summary |
|---|---|
| Healthy BMI*** | Multiple studies have reported elevated BMI (≥25 kg/m2) is associated with increased risks of recurrence and progression, with more ambiguous results suggesting increased risks of CSM and ACM. Two studies reported that sarcopenia may be a better body composition metric for predicting outcomes than BMI. |
| Lactobacillus casei supplementation*** | Three RCTs reported supplementation reduces risk of recurrence. |
| Smoking avoidance/cessation*** | Multiple studies have reported that current smoking is associated with recurrence. Current and former smoking may be associated with CSM. Long-term smoking cessation may be associated with reduced recurrence and progression. |
| DMII prevention and treatment** | Associations are ambiguous; however, a few studies have reported associations with improved outcomes among patients with DMII treated with metformin. |
| Broccoli (uncooked)** | One study reported higher intake was associated with reduced CSM and ACM. |
| Supplemental vitamin B9 (folate) avoidance** | One study reported supplementation was associated with an increased risk of recurrence. |
| Supplemental vitamin E** | One study reported supplementation was associated with a reduced risk of recurrence. |
| “Western diet” avoidance** | One study reported a diet high in red meat and fried foods was associated with an increased risk of recurrence. |
| Areca nut chewing avoidance* | One study reported that heavy use (>10 nuts/day) was associated with increased risk of recurrence. |
| Glyburide avoidance* | One study reported use was associated with increased risk of CSM. |
ACM= All-cause mortality, BMI = Body mass index, CSM = Cancer-specific mortality, DMII = Diabetes Mellitus type II, NMIBC = Nonmuscle invasive bladder cancer, RCT = Randomized controlled trial.
Evidence from retrospective studies suggests benefit.
Evidence from prospective studies or non-randomized trial suggests benefit.
Evidence from meta-analyses and/or randomized controlled trials suggests benefit.
RESULTS
Smoking
Fifty-seven studies have assessed associations between smoking-related exposures and NMIBC outcomes [6, 9–64]. Multiple exposure types have been studied, including current versus never, [6, 9–59] former versus never, [6, 9–36, 60] current versus former, [9–15, 60–63] and cumulative exposure (e.g., pack-years, quantity/day, cigarette index) [9–13, 16–19, 37, 38, 60, 64].
The sum of existing evidence suggests that smoking may be moderately associated with recurrence [6, 9–12, 14, 16–35, 37, 39–53, 60–62, 64]. One meta-analysis of 11 studies of 6,908 NMIBC patients reported an increased risk of recurrence among current smokers (at the time of questionnaire completion, interview, or from patient record review) compared to nonsmokers (hazard ratio [HR] 1.27, 95% confidence interval [CI] 1.09–1.46). The association for former smokers relative to nonsmokers was attenuated (n = 5,382 NMIBC patients, HR 1.13, 95% CI 1.00–1.25) [20]. Another meta-analysis of 15 studies that included 10,192 patients with NMIBC or MIBC reported an increased risk of recurrence in both current (summary relative risk estimate [SSRE] 1.23, 95% CI 1.05–1.45) and former smokers (SSRE 1.22, 95% CI 1.09–1.37) relative to never smokers. The meta-analysis did not, however, find an association with progression among 5 studies with 3,979 NMIBC or MIBC patients [6]. Indeed, the evidence regarding smoking and disease progression has been ambiguous [6, 9–11, 13, 15, 16, 18–24, 39–46, 60, 61]. The majority of studies among patients with NMIBC alone have reported no association between smoking and CSM [13, 15, 20, 25, 39–41, 54] or ACM [11, 25, 26, 39, 40]. However, in studies with a mix of patients with NMIBC and MIBC, the evidence regarding an association between smoking and CSM [6, 18, 21, 36, 42, 43, 55, 63] or ACM [18, 21, 36, 38, 42, 43, 56–59, 63] has been mixed. In the latter aforementioned meta-analysis, the authors also reported an increased pooled risk of CSM for both current (SRRE 1.28, 95% CI 1.07–1.52) and former smokers (SRRE 1.20, 95% CI 1.03–1.41) in 5 studies with 4,372 patients with NMIBC and MIBC [6].
Regarding cumulative exposure, Rink et al. reported a substantially reduced risk of recurrence and progression among short-term (≤19.9 years) smokers and a reduced risk of progression among light (≤19 cigarettes per day) long-term smokers compared to long-term heavy smokers, albeit no difference in ACM [10]. Still, a case-control study by Leibovici et al. of 519 patients with NMIBC and 505 healthy controls examined pack-years as a continuous variable and reported no association with recurrence or progression [19].
Overall, compared to current smoking, long-term smoking cessation may be associated with improved outcomes compared to current smoking [9–11, 37, 38, 60, 61]. In an international retrospective cohort of 2,043 patients with NMIBC, Rink et al. explored the association of multiple types of smoking exposures on recurrence, progression, and ACM [10]. Compared to current smokers, patients who quit smoking ≥ 10 years prior to NMIBC diagnosis had a reduced risk of recurrence (HR 0.66, 95% CI 0.52–0.84) and progression (HR 0.42, 95% CI 0.22–0.83), albeit no difference in ACM. In contrast, a prospective cohort of 722 patients with NMIBC showed no difference in risk of recurrence in those who had quit smoking greater than 40 years prior to diagnosis and current smokers [12].
Body mass index
Eighteen studies have assessed the association between body mass index (BMI) and prognosis [7, 24, 25, 30, 34, 39, 59, 65–75]. One study assessed BMI at diagnosis, [24] 13 studies assessed BMI at time of treatment (e.g., transurethral resection of bladder tumor (TURBT), radical cystectomy), [25, 30, 34, 39, 59, 65–72] and three studies did not report timing of BMI measurement [73–75].
Among NMIBC patients, the majority of studies suggest that elevated BMI is associated with increased risks of recurrence [7, 24, 34, 65, 66, 73, 74] and progression [65, 66, 73, 74]. The association between BMI and recurrence is more conflicting in studies with both NMIBC and MIBC patients [7, 25, 30, 68, 70]. In a meta-analysis of three studies of 1,633 patients with NMIBC, Westhoff et al. reported that being overweight (BMI 25–29.9 kg/m2; pooled HR 1.29, 95% CI 1.05–1.58) or obese (BMI ≥ 30 kg/m2; pooled HR 1.82, 95% CI 1.12–2.95) was associated with recurrence [7]. Westhoff et al. also meta-analyzed the results of three studies among patients with NMIBC and MIBC and reported no association between BMI and recurrence. Although Westhoff et al. reported no association with progression, [7] multiple studies have suggested an increased risk [65, 66, 73, 74]. Lenis et al. investigated the association between metabolic syndrome (MetS) and outcomes in patients with NMIBC undergoing TURBT. MetS was not associated with recurrence or progression overall, but analyses of MetS components indicated that obesity was a strong predictor (HR 3.42, 95% CI 1.55–7.52) [73]. Wyszynski et al. reported that smoking may work synergistically with BMI to worsen outcomes among patients with NMIBC; [24] while being overweight was not associated with recurrence among never or former smokers, it was statistically significantly associated with recurrence risk among current smokers (HR = 2.24, 95% CI 1.15–4.34).
The association between BMI and CSM and ACM among patients with NMIBC remains unclear [39, 65, 74]. In studies among those with NMIBC and MIBC, studies of mortality outcomes primarily vary between no association [7, 25, 59, 67, 69, 71] and increased mortality risk [25, 30, 68, 70]. The largest study observing the association between BMI and outcomes consisted of 4,118 patients with NMIBC and MIBC treated with radical cystectomy. Multivariable analyses demonstrated an increased risk of both CSM (HR 1.43, 95% CI 1.24–1.66) and ACM (HR 1.81, 95% CI 1.60–2.05) comparing obese patients to those with BMI <25 kg/m2 [68]. In contrast, the meta-analysis by Westhoff et al. and a prospective cohort study by Gierth et al. reported no association between BMI and CSM [7, 75]. Gierth et al. did, however, report reduced ACM among obese patients (HR 0.60, 95% CI 0.39–0.92) compared to those with healthy weight [75].
Two studies hypothesized that sarcopenia rather than BMI is a better predictor of outcomes in patients undergoing cystectomy [59, 71]. Psutka et al. used computerized tomography to calculate fat mass index and skeletal muscle index among patients with NMIBC and MIBC. In models including BMI and sarcopenia, they found no association between BMI and ACM, while sarcopenia was associated with an increased risk of ACM (HR 1.67, 95% CI 1.11–2.50). Furthermore, in a sub-analysis of nonsarcopenic patients, the authors reported a 7% decreased risk of ACM for every 1 kg/m2 increase in BMI (p = 0.008) [59]. Future directions for studies on sarcopenia include examining its association with outcomes among patients with lower risk disease undergoing surveillance.
Diabetes and diabetic drugs
Eleven studies have investigated the association between type II diabetes mellitus (DMII) and/or diabetic drugs and NMIBC outcomes [25, 29, 40, 44, 45, 66, 72, 73, 76–78].
Among patients with NMIBC, there have been mixed reports on associations between DMII and both recurrence [29, 40, 44, 45, 66, 72, 73] and progression [40, 44, 45, 66, 72, 73]. Based on retrospective data on 1,117 patients with NMIBC, Reiken et al. reported that those with DMII not treated with metformin had a statistically significantly increased risk of recurrence (HR 1.39, 95% CI 1.04–1.86) and progression (HR 2.21, 95% CI 1.29–3.77) compared to those without DMII [40]. Those with DMII treated with metformin had a reduce risk of recurrence compared to those without DMII (HR 0.48, 95% CI 0.26–0.89). In regard to glycemic control, Ahn et al. reported that a higher proportion of those with HbA1c ≥ 7.0% experienced progression compared to those with HbA1c < 7.0% (28.8% versus 11.5%, p = 0.026). However, this association became non-significant upon multivariable analysis [72]. Studies among NMIBC patients have reported a null association between DMII and CSM [40, 76, 78]. However, a study of 13,811 patients with NMIBC aged 66 years or older reported DMII was associated with a slightly increased risk of ACM (HR 1.04, 95% CI 1.03–1.05) compared to no DMII [76]. The use of metformin, insulin, and other hypoglycemics does not appear to be associated with CSM or ACM, [40, 78] and glyburide may be associated with an increased risk of CSM [78].
In a study of NMIBC and MIBC patients undergoing radical cystectomy, DMII treated with metformin was not associated with recurrence, CSM, or ACM compared to no DMII. Patients with DMII not treated with metformin, however, did have an increased risk of CSM (HR 1.53, 95% CI 1.12–2.09) and ACM (HR 1.52, 95% CI 1.16–2.00) [25]. Nayan et al. reported that among patients with DMII, metformin use may be associated with a reduced risk of recurrence (HR 0.38, 95% CI 0.20–0.72) and CSM (HR = 0.57, 95% CI 0.35–0.91) [77]. Insulin and other hypoglycemic agents did not confer the same benefit.
Drugs for cardiovascular health
Eighteen studies have reported on associations between drugs for cardiovascular health and NMIBC outcomes [30, 32, 33, 39, 41, 76, 79–90]. Among them, 10 have investigated statins, [30, 33, 39, 41, 76, 79–83] and 10 have investigated fibrin clot inhibitors (FCIs) like aspirin, warfarin, and celecoxib [32, 33, 39, 84–90].
A study of 84 patients with NMIBC by Hoffman et al. raised concern that statins may be associated with increased odds of progression and cystectomy [81]. However, this early analysis was based on a small cohort of patients, among whom only 19 took statins during intravesical bacillus Calmette-Guérin (BCG) therapy. Most subsequent studies have not replicated these findings, with the vast majority reporting no association between statin use and recurrence, [30, 39, 41, 79–83] progression, [39, 41, 79, 80, 82, 83] CSM, [30, 39, 41, 76, 80, 83] or ACM [39, 80, 82, 83]. Furthermore, Richard et al. reported a potential reduced risk of ACM with statin use [76]. In their retrospective review of the records of 13,811 older patients (median age 76) with NMIBC, they analyzed the association between cumulative statin exposure (rather than current use at diagnosis) and both CSM and ACM. After a median 7.1 years of follow-up, there was no improvement in CSM, but there was a slight reduction in ACM according to increased length of use (HR 0.93 per year of use, 95% CI 0.91–0.96).
As with statin use, two early studies found positive associations between FCIs and risk of NMIBC recurrence. However, one study only had 45 patients, [88] neither study included multivariable analyses, [85, 88] and they did not distinguish between types of FCIs. Many studies since have reported null associations between most FCIs and recurrence, [33, 39, 84, 86, 87, 89, 90] progression, [32, 39, 79, 84, 86, 87] CSM, [39, 87] or ACM [39, 86, 87]. Two studies have demonstrated an association between aspirin use and a reduced risk of recurrence [32] and progression to cystectomy [84]. For example, in a retrospective cohort of 907 patients with NMIBC or MIBC, Boorjian et al. reported that there was a slight reduction in progression to cystectomy among those using aspirin at time of BCG (HR 0.70, 95% CI 0.52–0.96) [84]. Despite these results, the majority of studies have reported no association with aspirin and NMIBC outcomes [33, 39, 87, 90]. Regarding warfarin use, Boorjian et al. reported an association with an increased risk of progression to surgery (HR 1.89, 95% CI 1.31–2.74) [84]. The effect of celecoxib was tested in two randomized controlled trials (RCTs) among patients with NMIBC undergoing BCG therapy [86, 89]. Both reported no association with recurrence. The BOXIT trial also reported no effect on progression to invasive disease or mortality, but did report suggestive increased incidence of serious cardiovascular events in the celecoxib group (5.2%) compared to the placebo group (1.7%, p = 0.07) [86].
Diet and supplements
Twenty-five studies have reported on associations between diet and/or supplements and NMIBC outcomes [15, 29, 31, 47, 51, 52, 58, 91–108]. Five evaluated dietary patterns or individual food items, [29, 58, 94–96] five evaluated beverages, [15, 31, 47, 58, 97] 14 evaluated vitamins and minerals, [51, 91–94, 98–106] and three evaluated probiotics [52, 107, 108].
One retrospective cohort study of 239 patients with NMIBC assessed self-reported intake of multiple dietary items and CSM and ACM [95]. An intake of ≥ 1 serving of uncooked broccoli/month (mean 3.9 servings/month) was associated with a reduced risk of CSM (HR 0.43, 95% CI 0.25–0.74) and ACM (HR 0.57, 95% CI 0.39–0.83) compared to an intake of < 1 serving/month. This and two other studies reported no associations between fruit and/or vegetable consumption and recurrence, [94, 96] progression, [96] or mortality [95]. Another study reported that heavy areca nut chewing (>10 nuts/day), a practice common among individuals in South Asia, may be associated with an increased risk of recurrence (HR 2.18, 95% CI 1.37–3.47) compared to non-chewing [29].
Westhoff et al. grouped individual dietary items in order to observe associations of four dietary “patterns” with recurrence and progression in 595 patients with NMIBC [96]. The fruit and vegetable pattern, low-fat pattern (low-fat alternative foods like light salad dressing, low-fat dairy, etc.), and Tex-Mex pattern (Mexican food, barbecue, pizza) were not significantly associated with the outcomes of interest. However, the Western pattern, which consisted primarily of fried foods and red meat, was associated with an increased risk of recurrence (HR 1.48, 95% CI 1.06–2.06). The study did not investigate the individual dietary components that resulted in increased risk (e.g., red meat may confer a greater risk than fried foods or vice versa).
Two studies have reported a null association between total fluid intake and recurrence [31, 97]. Evidence leans toward no association between alcohol intake and NMIBC prognosis [15, 97]. Still, it is interesting to note that, among Japanese men with NMIBC or MIBC, Wakai et al. reported that moderate drinkers of < 2 gou/day (1 gou = 180 ml of Japanese sake) had a reduced risk of ACM (HR 0.41, 95% CI 0.22–0.77) compared to never drinkers [58]. Limited evidence suggests other beverages, such as coffee, green tea, or cola, and artificial sweetener do not appear to be associated with outcomes among patients with NMIBC [47, 58].
Among supplemental vitamins and minerals, one RCT reported a reduced risk of recurrence among those taking 400IU of supplemental vitamin E (RR 0.53, 95% CI 0.11–0.94) [100]. However, the sample was relatively small (n = 46) and the non-intervention group was not placebo-controlled. Another study reported that synthetic vitamin B9 (folate) may be associated with increased risk of recurrence (HR 1.80 in tertile 3 compared to tertile 1, 95% CI 1.14–2.84) [101]. Evidence leans toward questionable to no benefit from supplemental vitamin A, [51, 102–106] vitamin B6, [91, 92] selenium, [93] and megadose multivitamin formulations [98, 99]. Multiple RCTs have investigated the effect of synthetic retinoids like fenretinide [102, 103] and etretinate on NMIBC outcomes [104–106]. Effects on recurrence ranging from null [102–104, 106] to beneficial [105] have been reported, and high rates of ophthalmologic and dermatologic adverse effects may outweigh any indeterminate benefit for bladder cancer prognosis.
Finally, three RCTs have assessed the effect of probiotics containing Lactobacillus casei on prognosis. All reported a reduction in recurrence in the intervention group [52, 107, 108]. The largest trial with longest period of follow-up reported that, among 202 patients with NMIBC randomized to weekly intravesical epirubicin instillation with or without a daily oral probiotic supplement, those in the probiotic group had improved 3-year recurrence-free survival (74.6% versus 59.5%, p = 0.0234) [52]. Recurrence-free survival remained significant upon adjustment for tumor multiplicity, size, and stage (HR 0.57, 95% CI 0.35–0.93). However, there was no difference in progression, CSM, or ACM between groups.
Occupational/chemical exposures
Five studies have reported on the association between occupational [109] or chemical exposures and NMIBC outcomes [47, 58, 109–111]. None reported statistically significant associations.
DISCUSSION
In this review, we provided a broad overview of the relationship between various lifestyle factors and NMIBC prognosis. To our knowledge, this is the most comprehensive review of its kind, with over 100 publications on lifestyle factors included.
Among the risk factors discussed, smoking is most convincingly associated with prognosis [112]. Studies suggest that smoking avoidance may reduce recurrence and progression. However, some ambiguity exists regarding cessation, cumulative exposure, and mortality outcomes. This may be related to the “field cancerization” theory, suggesting that long-term carcinogenic insults to the bladder urothelium overshadow the benefit of cessation upon diagnosis [113]. Still, the possibility of harm in bladder cancer prognosis, coupled with the well-established harm to other organ systems, suggests that avoidance and cessation counseling is warranted [63, 114].
Compared to a healthy BMI, being overweight or obese appears to be associated with an increased risk of recurrence and progression. Multiple mechanisms that could explain the associations have been proposed. Elevated BMI could contribute to carcinogenesis through inflammation. Higher BMI is associated with high levels of insulin, insulin-like growth factor (IGF), steroid hormones, and cytokines, all of which can contribute to an increased rate of oncogenic transformation through chronic activation of metabolic signaling cascades [115]. Additionally, an increased neutrophil-to-lymphocyte ratio and elevated C-reactive protein levels, serologic markers of systemic inflammation, are more commonly found in patients with elevated BMI [116]. Finally, high BMI may also make procedures more technically challenging, limiting visualization and risking residual tumor following intravesical procedures [66, 74]. Despite these plausible mechanisms, it has also been proposed that higher BMI, and thus higher energy reserves, may improve rehabilitation following invasive procedures [75]. Regardless, there exists enough evidence to suggest that overweight and obese patients should be counseled on strategies to reduce BMI. Even given the conflicting evidence on bladder cancer prognosis, the known detrimental association between BMI and other health outcomes suggests that achieving a healthy BMI should be encouraged [117, 118]. Given the findings reported in studies on sarcopenia, further investigation of the negative impact of frailty and muscle mass is warranted.
Many studies suggest that DMII is associated with an increased risk of recurrence and progression among patients with NMIBC. Mixed NMIBC/MIBC studies (i.e., patients with more advanced disease) also suggest that DMII could be associated with increased risk of CSM and ACM. The mechanisms that may underlie associations with DMII overlap with those hypothesized for elevated BMI. Patients with DMII also have elevated insulin and IGF-I due to insulin resistance, which subsequently stimulates cell proliferation and inhibits apoptosis [119–121]. Increased circulating IGF-I may be associated with increased bladder cancer risk, although evidence from case-control studies is conflicting [122, 123]. More generally, hyperglycemia may dysregulate energy balance, which affects intracellular metabolism and impairs immune function, leading to increased risk of multiple different cancer types [124]. Metformin may also reduce recurrence and CSM, though no other antidiabetic agents have been shown to improve outcomes. Its use has been connected with a reduced risk of multiple cancer types [125]. Lin et al. reported that, in a large cohort of patients with DMII matched with control participants, metformin use was associated with a dramatically reduced overall cancer risk compared to those without DMII in a dose-dependent fashion [125]. Metformin indirectly reduces mammalian target of rapamycin (mTOR) signaling, which inhibits cancer cell growth and proliferation [126, 127]. Another group suggested that metformin may help prevent stem cell repopulation in bladder cancer, thereby preventing progression [128]. Given findings from basic science research, investigators are exploring potential synergistic effects between metformin and chemotherapeutics like gefitinib to treat multiple cancer types [129].
Statins have been proposed as anti-cancer agents given multiple potential antitumorigenic effects, including angiogenesis suppression, tumor growth inhibition, cell cycle arrest, and apoptosis induction [130–133]. The majority of studies, however, suggest that statins are not associated with bladder cancer-specific outcomes. Still, given their cardiovascular benefit, statins may reduce ACM among patients with bladder cancer [134].
It has been hypothesized that FCIs may reduce the effectiveness of BCG [135]. Studies in murine models have suggested that fibronectin is important for intravesical binding of BCG, meaning that disruption of fibronectin might reduce the ability of BCG to induce the immune response necessary for bladder cancer treatment [135, 136]. Research in humans has not supported the hypothesis. Studies over the past 25 years suggest that FCIs are not associated with bladder cancer outcomes. This understanding is critical given that many patients require drugs like aspirin for cardiovascular health or warfarin to combat cancer-induced hypercoagulability [137, 138].
Regarding dietary exposures, uncooked broccoli has been reported to have prognostic benefit. Broccoli is rich in isothiocyanates which have demonstrated chemopreventive effects in bladder cancer in cell lines and mouse models [139]. The diminished benefit of cooked broccoli is consistent with our understanding of the breakdown of beneficial phytochemicals when exposed to high temperatures [140]. Fluid intake does not appear to be associated with NMIBC outcomes; however, the literature supporting this conclusion is much less robust than the literature reporting a lack of association between fluid intake and bladder cancer risk [141]. The association between alcohol intake and worse NMIBC outcomes is weak, and its effect on other aspects of health is heterogeneous. Some cardiovascular benefit has been observed, but alcohol consumption has also been linked to multiple cancer types [142]. Vitamin E supplementation was beneficial in one study, perhaps through its anti-inflammatory properties [143]. Still, its harmful influence on other cancer types should caution routine supplementation recommendation [144].
Among dietary exposures, L. casei probiotic supplementation had the most promising effect on bladder cancer outcomes, with all three available RCTs reporting reduced risk of recurrence [52, 107, 108]. A significant amount of research is emerging regarding the association of the gastrointestinal and genitourinary microbiome with genitourinary malignancy [145]. A proposed mechanism of benefit that underlies L. casei probiotic supplementation is the improved balance of intestinal flora, which in turn prevents production and promotes elimination of carcinogens [146]. For example, L. casei may prevent urinary excretion of carcinogens that are generated following the consumption of fried ground beef [147]. Additionally, L. casei may enhance immune activity [148]. Future directions include trials among patients undergoing BCG therapy and among patients with MIBC. Additionally, there is a need for well-designed epidemiologic studies investigating the association between bladder cancer outcomes and dietary sources of L. casei (e.g., yogurt, fermented milk, certain cheeses).
Finally, despite the known increased risk of bladder cancer incidence with certain occupational and environmental exposures (e.g., aromatic hydrocarbons, N-nitrosamines), no study reported an association with outcomes among patients with NMIBC [149].
Although a multitude of lifestyle factors have been studied, there are limitations among individual studies, and gaps in our knowledge remain. For example, no studies have evaluated physical activity, which has shown substantial prognostic benefit in other cancer types [150, 151]. Furthermore, those lifestyle factors with retrospective observational evidence of benefit should be confirmed in additional rigorously conducted prospective studies and, if resources are available, RCTs. Evidence from RCTs can be especially useful for revealing true causal associations in factors with previously ambiguous associations. For example, despite prior evidence suggesting a potential benefit, vitamin E was found to increase risk of prostate cancer in the Selenium and Vitamin E Cancer Prevention Trial [144, 152]. Once a final consensus on lifestyle recommendations in bladder cancer is reached, implementation strategies should be investigated to help patients make long-lasting behavior changes. Lifestyle studies of other cancer types have begun to explore health monitoring technology and online behavior change resources for this purpose [153, 154]. Additionally, deficits in patient knowledge of the association between lifestyle and bladder cancer warrants improved methods of patient education. For example, Neider et al. prospectively surveyed patients presenting for urologic evaluation on their knowledge of risk factors for various cancers [155]. Only 36% reported smoking as a risk factor for bladder cancer compared to 98% reporting smoking as a risk factor for lung cancer. Patients were less likely to report smoking as a risk factor for bladder cancer if they were not college educated, were older (60–80 years old), or were male. Furthermore, Winters et al. reported that, among patients with bladder cancer in the Surveillance, Epidemiology, and End Results (SEER) database, there was no significant difference in the adjusted odds ratio of smoking cessation among patients with bladder cancer compared to noncancer controls [156]. This further demonstrates a need for the dissemination of educational resources for patients with bladder cancer as well as implementation strategies for lifestyle modifications. Finally, it is important to investigate the effect of lifestyle interventions on patient quality of life and functional status and, in turn, how improvements in these important patient-reported outcomes might affect prognosis.
A limitation of this review that warrants acknowledgment is the large number of studies identified via review of publication citations relative to those studies identified in our search. Regardless, the scope of this review is quite comprehensive and includes more studies on individual factors than many prior reviews. Still, it is possible that studies on some individual factors may not have been identified. Additionally, we did not search databases other than PubMed, and thus studies of individual factors not available in this database may have been overlooked.
CONCLUSION
Available literature on the associations between lifestyle factors and bladder cancer outcomes suggests that smoking avoidance/cessation, healthy BMI, DMII prevention and treatment, and L. casei supplementation may be beneficial. The use of most diabetic drugs, FCIs, and statins appears to be safe and does not affect treatment effectiveness. Further research is needed to support possible benefits of uncooked broccoli consumption; metformin use; supplemental vitamin E use; and possible harms from diets high in fried foods and red meat, supplemental vitamin B9 use, areca nut chewing, and glyburide use. Evidence did not otherwise suggest associations for other dietary items (individual or multimodal), beverages, or nutritional supplements; fluid intake; or occupational or chemical exposures.
Supplementary Material
ACKNOWLEDGMENTS
The authors have no acknowledgments.
FUNDING
This work was funded by the National Center for Complementary and Integrative Health (T32AT003997) and the Helen Diller Family Chair in Population Science for Urologic Cancer.
Footnotes
CONFLICT OF INTEREST
SPP is on the advisory board for BMS; consultant for Photocure.
SAK is on the advisory board and is a consultant for Mojo Enterprises Inc.
The other authors have no conflicts of interest to disclose.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/BLC-190249
REFERENCES
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- [2].Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6, Supplement 1):4–34. [DOI] [PubMed] [Google Scholar]
- [3].Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. The Journal of Urology. 1997;158(1):62–7. [DOI] [PubMed] [Google Scholar]
- [4].Palou J, Sylvester RJ, Faba OR, Parada R, Pena JA, Algaba F, et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guerin. European Urology. 2012;62(1):118–25. [DOI] [PubMed] [Google Scholar]
- [5].Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. European Urology. 2006;49(3):466–77. [DOI] [PubMed] [Google Scholar]
- [6].Hou L, Hong X, Dai M, Chen P, Zhao H, Wei Q, et al. Association of smoking status with prognosis in bladder cancer: Ameta-analysis. Oncotarget. 2017;8(1):1278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Westhoff E, Witjes JA, Fleshner NE, Lerner SP, Shariat SF, Steineck G, et al. Body Mass Index, Diet-Related Factors, and Bladder Cancer Prognosis: A Systematic Review and Meta-Analysis. Bladder cancer (Amsterdam, Netherlands). 2018;4(1):91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elab-oration. PLoS Medicine. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li HM, Azhati B, Rexiati M, Wang WG, Li XD, Liu Q, et al. Impact of smoking status and cumulative smoking exposure on tumor recurrence of non-muscle-invasive bladder cancer. International Urology and Nephrology. 2017;49(1):69–76. [DOI] [PubMed] [Google Scholar]
- [10].Rink M, Furberg H, Zabor EC, Xylinas E, Babjuk M, Pycha A, et al. Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. European Urology. 2013;63(4): 724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rink M, Xylinas E, Babjuk M, Hansen J, Pycha A, Comploj E, et al. Impact of smoking on outcomes of patients with a history of recurrent nonmuscle invasive bladder cancer. The Journal of Urology. 2012;188(6):2120–7. [DOI] [PubMed] [Google Scholar]
- [12].van Osch FHM, Jochems SHJ, Reulen RC, Pirrie SJ, Nekeman D, Wesselius A, et al. The association between smoking cessation before and after diagnosis and non-muscle-invasive bladder cancer recurrence: A prospective cohort study. Cancer Causes & Control : CCC. 2018;29(7):675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Korkes F, Juliano CAB, Bunduky MAP, Costa ACDM, Castro MGd. Amount of tobacco consumption is associated with superficial bladder cancer progression. Einstein (Sao Paulo). 2010;8:473–6. [DOI] [PubMed] [Google Scholar]
- [14].Serretta V, Altieri V, Morgia G, Di Lallo A, Carrieri G, Allegro R. Cigarette smoking status at diagnosis and recurrence in intermediate-risk non-muscle-invasive bladder carcinoma. Urology. 2013;81(2):277–81. [DOI] [PubMed] [Google Scholar]
- [15].Cheng L, Neumann RM, Weaver AL, Spotts BE, Bost- wick DG. Predicting cancer progression in patients with stage T1 bladder carcinoma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 1999;17(10):3182–7. [DOI] [PubMed] [Google Scholar]
- [16].Grotenhuis AJ, Ebben CW, Aben KK, Witjes JA, Vrieling A, Vermeulen SH, et al. The effect of smoking and timing of smoking cessation on clinical outcome in non-muscle-invasive bladder cancer. Urologic Oncology. 2015;33(2):65.e9–17. [DOI] [PubMed] [Google Scholar]
- [17].Lammers RJ, Witjes WP, Hendricksen K, Caris CT, Janzing-Pastors MH, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non-muscle-invasive bladder cancer. European Urology. 2011;60(4):713–20. [DOI] [PubMed] [Google Scholar]
- [18].Lee C, Kim KH, You D, Jeong IG, Hong B, Hong JH, et al. Smoking and survival after radical cystectomy for bladder cancer. Urology. 2012;80(6):1307–12. [DOI] [PubMed] [Google Scholar]
- [19].Leibovici D, Grossman HB, Dinney CP, Millikan RE, Lerner S, Wang Y, et al. Polymorphisms in inflammation genes and bladder cancer: From initiation to recurrence, progression, and survival. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2005;23(24):5746–56. [DOI] [PubMed] [Google Scholar]
- [20].van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Significant Role of Lifetime Cigarette Smoking in Worsening Bladder Cancer and Upper Tract Urothelial Carcinoma Prognosis: A Meta-Analysis. The Journal of Urology. 2016;195(4 Pt 1):872–9. [DOI] [PubMed] [Google Scholar]
- [21].Sfakianos JP, Shariat SF, Favaretto RL, Rioja J, Herr HW. Impact of smoking on outcomes after intravesical bacillus Calmette-Guerin therapy for urothelial carcinoma not invading muscle of the bladder. BJU International. 2011;108(4):526–30. [DOI] [PubMed] [Google Scholar]
- [22].Holz S, Albisinni S, Gilsoul J, Pirson M, Duthie V, Quackels T, et al. Risk factor assessment in high-risk, bacillus Calmette-Guerin-treated, non-muscle-invasive bladder cancer. Research and Reports in Urology. 2017;9:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chade DC, Shariat SF, Godoy G, Savage CJ, Cronin AM, Bochner BH, et al. Clinical outcomes of primary bladder carcinoma in situ in a contemporary series. The Journal of Urology. 2010;184(1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120(3):408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rieken M, Xylinas E, Kluth L, Crivelli JJ, Chrystal J, Faison T, et al. Effect of diabetes mellitus and metformin use on oncologic outcomes of patients treated with radical cystectomy for urothelial carcinoma. Urologic Oncology. 2014;32(1):49.e7–14. [DOI] [PubMed] [Google Scholar]
- [26].Michalek AM, Cummings KM, Pontes JE. Cigarette smoking, tumor recurrence, and survival from bladder cancer. Preventive Medicine. 1985;14(1):92–8. [DOI] [PubMed] [Google Scholar]
- [27].Lacombe L, Fradet V, Levesque E, Pouliot F, Larue H, Bergeron A, et al. Phase II Drug-Metabolizing Polymorphisms and Smoking Predict Recurrence of Non-Muscle-Invasive Bladder Cancer: A Gene-Smoking Interaction. Cancer Prevention Research (Philadelphia, Pa). 2016;9(2):189–95. [DOI] [PubMed] [Google Scholar]
- [28].Rink M, Xylinas E, Babjuk M, Pycha A, Karakiewicz PI, Novara G, et al. Smoking Reduces the Efficacy of Intravesical Bacillus Calmette-Guerin Immunotherapy in Non-muscle-invasive Bladder Cancer. European Urology. 2012;62(6):1204–6. [DOI] [PubMed] [Google Scholar]
- [29].Cao J, Xu R, Zhao X, Zhong Z, Zhang L, Zhu X, et al. Areca Nut Chewing and an Impaired Estimated Glomerular Filtration Rate as Significant Risk Factors for Non-Muscle-Invasive Bladder Cancer Recurrence. Scientific Reports. 2016;6:29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].da Silva RD, Xylinas E, Kluth L, Crivelli JJ, Chrystal J, Chade D, et al. Impact of statin use on oncologic outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy. The Journal of Urology. 2013;190(2):487–92. [DOI] [PubMed] [Google Scholar]
- [31].Donat SM, Bayuga S, Herr HW, Berwick M. Fluid intake and the risk of tumor recurrence in patients with superficial bladder cancer. The Journal of Urology. 2003;170(5):1777–80. [DOI] [PubMed] [Google Scholar]
- [32].Gee JR, Jarrard DF, Brnskewitz RC, Moon TD, Hedican SP, Leverson GE, et al. Reduced bladder cancer recurrence rate with cardioprotective aspirin after intravesical bacille Calmette-Guerin. BJU International. 2009;103(6): 736–9. [DOI] [PubMed] [Google Scholar]
- [33].Pastore A, Palleschi G, Fuschi A, Silvestri L, Al Salhi Y, Costantini E, et al. Can daily intake of aspirin and/or statins influence the behavior of non-muscle invasive bladder cancer? A retrospective study on a cohort of patients undergoing transurethral bladder resection. BMC Cancer. 2015;15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yonekura S, Terauchi F, Hoshi K, Yamaguchi T, Kawai S. Optimal body mass index cut-point for predicting recurrence-free survival in patients with non-muscle-invasive urothelial carcinoma of bladder. Oncology Letters. 2018;16(3):4049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ahirwar D, Kesarwani P, Manchanda PK, Mandhani A, Mittal RD. Anti- and proinflammatory cytokine gene poly-morphism and genetic predisposition: Association with smoking, tumor stage and grade, and bacillus Calmette-Guerin immunotherapy in bladder cancer. Cancer Genetics and Cytogenetics. 2008;184(1):1–8. [DOI] [PubMed] [Google Scholar]
- [36].Bostrom PJ, Mirtti T, Kossi J, Laato M, Nurmi M. Twenty-year experience of radical cystectomy for bladder cancer in a medium-volume centre. Scandinavian Journal of Urology and Nephrology. 2009;43(5):357–64. [DOI] [PubMed] [Google Scholar]
- [37].Ogihara K, Kikuchi E, Yuge K, Ito Y, Tanaka N, Matsumoto K, et al. Refraining from Smoking for 15 Years or More Reduced the Risk of Tumor Recurrence in Non-muscle Invasive Bladder Cancer Patients. Annals of Surgical Oncology. 2016;23(5):1752–9. [DOI] [PubMed] [Google Scholar]
- [38].Mitra AP, Castelao JE, Hawes D, Tsao-Wei DD, Jiang X, Shi SR, et al. Combination of molecular alterations and smoking intensity predicts bladder cancer outcome: A report from the Los Angeles Cancer Surveillance Program. Cancer. 2013;119(4):756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Singla N, Haddad AQ, Passoni NM, Meissner M, Lotan Y. Anti-inflammatory use may not negatively impact oncologic outcomes following intravesical BCG for high-grade non-muscle-invasive bladder cancer. World Journal of Urology. 2017;35(1):105–11. [DOI] [PubMed] [Google Scholar]
- [40].Rieken M, Xylinas E, Kluth L, Crivelli JJ, Chrystal J, Faison T, et al. Association of diabetes mellitus and metformin use with oncological outcomes of patients with non-muscle-invasive bladder cancer. BJU International. 2013;112(8):1105–12. [DOI] [PubMed] [Google Scholar]
- [41].Segal R, Yafi FA, Brimo F, Tanguay S, Aprikian A, Kassouf W. Prognostic factors and outcome in patients with T1 high-grade bladder cancer: Can we identify patients for early cystectomy? BJU International. 2012;109(7):1026–30. [DOI] [PubMed] [Google Scholar]
- [42].Carpenter AA. Clinical Experience with Transitional Cell Carcinoma of the Bladder with Special Reference to Smoking. The Journal of Urology. 1989;141(3, Part 1):527–8. [DOI] [PubMed] [Google Scholar]
- [43].Yafi FA, Aprikian AG, Chin JL, Fradet Y, Izawa J, Estey E, et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: A Canadian multicentre experience. BJU International. 2011;108(4):539–45. [DOI] [PubMed] [Google Scholar]
- [44].Rausch S, Hennenlotter J, Todenhofer T, Aufderklamm S, Schwentner C, Sievert KD, et al. Impaired estimated glomerular filtration rate is a significant predictor for non-muscle-invasive bladder cancer recurrence and progression-introducing a novel prognostic model for bladder cancer recurrence. Urologic Oncology. 2014;32(8):1178–83. [DOI] [PubMed] [Google Scholar]
- [45].Hwang EC, Kim YJ, Hwang IS, Hwang JE, Jung SI, Kwon DD, et al. Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: A retrospective cohort study. International Journal of Urology : Official Journal of the Japanese Urological Association. 2011;18(11):769–76. [DOI] [PubMed] [Google Scholar]
- [46].Kashif Khan M, Ahmed I, Raza SJ. Factors effecting recurrence and progression of high grade non invasive bladder cancer treated by intravesical BCG. Pakistan Journal of Medical Sciences. 2014;30(2):326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Koch M, Hill GB, McPhee MS. Factors affecting recurrence rates in superficial bladder cancer. Journal of the National Cancer Institute. 1986;76(6):1025–9. [PubMed] [Google Scholar]
- [48].Nerli RB, Ghagane SC, Shankar K, Sanikop AC, Hiremath MB, Dixit NS, et al. Low-Grade, Multiple, Ta Non-muscle-Invasive Bladder Tumors: Tumor Recurrence and Worsening Progression. Indian journal of Surgical Oncology. 2018;9(2):157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Raitanen MP, Tammela TL. Impact of tumour grade, stage, number and size, smoking habits and sex on the recurrence rate and disease-free interval in patients with transitional cell carcinoma of the bladder. Annales Chirurgiae et Gynaecologiae. 1995;84(1):37–41. [PubMed] [Google Scholar]
- [50].Allard P, Fradet Y, Tetu B, Bernard P. Tumor-associated antigens as prognostic factors for recurrence in 382 patients with primary transitional cell carcinoma of the bladder. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 1995;1(10):1195–202. [PubMed] [Google Scholar]
- [51].Michalek AM, Cummings KM, Phelan J. Vitamin A and tumor recurrence in bladder cancer. Nutrition and Cancer. 1987;9(2–3):143–6. [DOI] [PubMed] [Google Scholar]
- [52].Naito S, Koga H, Yamaguchi A, Fujimoto N, Hasui Y, Kuramoto H, et al. Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. The Journal of Urology. 2008;179(2):485–90. [DOI] [PubMed] [Google Scholar]
- [53].Thompson IM, Peek M, Rodriguez FR. The impact of cigarette smoking on stage, grade and number of recurrences of transitional cell carcinoma of the bladder. The Journal of Urology. 1987;137(3):401–3. [DOI] [PubMed] [Google Scholar]
- [54].Lopez-Beltran A, Croghan GA, Croghan I, Huben RP, Mettlin C, Gaeta JF. Prognostic factors in survival of bladder cancer. Cancer. 1992;70(4):799–807. [DOI] [PubMed] [Google Scholar]
- [55].Raitanen MP, Nieminen P, Tammela TL. Impact of tumour grade, stage, number and size, and smoking and sex, on survival in patients with transitional cell carcinoma of the bladder. British Journal of Urology. 1995;76(4):470–4. [DOI] [PubMed] [Google Scholar]
- [56].Takashi M, Murase T, Mizuno S, Hamajima N, Ohno Y. Multivariate evaluation of prognostic determinants in bladder cancer patients. Urologia Internationalis. 1987;42(5):368–74. [DOI] [PubMed] [Google Scholar]
- [57].Yu GP, Ostroff JS, Zhang ZF, Tang J, Schantz SP, Smoking history and cancer patient survival: A hospital cancer registry study. Cancer Detection and Prevention. 1997;21(6):497–509. [PubMed] [Google Scholar]
- [58].Wakai K, Ohno Y, Obata K, Aoki K. Prognostic significance of selected lifestyle factors in urinary bladder cancer. Japanese Journal of Cancer Research : Gann. 1993;84(12):1223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Psutka SP, Boorjian SA, Moynagh MR, Schmit GD, Frank I, Carrasco A, et al. Mortality after radical cystictomy: Impact of obesity versus adiposity after adjusting for skeletal muscle wasting. The Journal of Urology. 2015;193(5):1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen CH, Shun CT, Huang KH, Huang CY, Tsai YC, Yu HJ, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU International. 2007;100(2):281–6; discussion 6. [DOI] [PubMed] [Google Scholar]
- [61].Fleshner N, Garland J, Moadel A, Herr H, Ostroff J, Trambert R, et al. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer. 1999;86(11):2337–45. [PubMed] [Google Scholar]
- [62].Yuruk E, Tuken M, Colakerol A, Serefoglu EC. The awareness of patients with non - muscle invasive bladder cancer regarding the importance of smoking cessation and their access to smoking cessation programs. Int Braz J Urol. 2017;43(4):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Koshiaris C, Aveyard P, Oke J, Ryan R, Szatkowski L, Stevens R, et al. Smoking cessation and survival in lung, upper aero-digestive tract and bladder cancer: Cohort study. Br J Cancer. 2017;117(8):1224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ajili F, Kourda N, Karay S, Darouiche A, Chebil M, Boubaker S. Impact of smoking intensity on outcomes of patients with non muscle invasive bladder cancer treated by BCG immunotherapy. Ultrastructural Pathology. 2013;37(4):273–7. [DOI] [PubMed] [Google Scholar]
- [65].Ferro M, Vartolomei MD, Russo GI, Cantiello F, Farhan ARA, Terracciano D, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World Journal of Urology. 2019;37(3):507–14. [DOI] [PubMed] [Google Scholar]
- [66].Xu T, Zhu Z, Wang X, Xia L, Zhang X, Zhong S, et al. Impact of body mass on recurrence and progression in Chinese patients with Ta, T1 urothelial bladder cancer. International Urology and Nephrology. 2015;47(7):1135–41. [DOI] [PubMed] [Google Scholar]
- [67].Bachir BG, Aprikian AG, Izawa JI, Chin JL, Fradet Y, Fairey A, et al. Effect of body mass index on the outcomes of patients with upper and lower urinary tract cancers treated by radical surgery: Results from a Canadian multicenter collaboration. Urologic Oncology. 2014;32(4):441–8. [DOI] [PubMed] [Google Scholar]
- [68].Chromecki TF, Cha EK, Fajkovic H, Rink M, Ehdaie B, Svatek RS, et al. Obesityis associated with worse oncological outcomes in patients treated with radical cystectomy. BJU International. 2013;111(2):249–55. [DOI] [PubMed] [Google Scholar]
- [69].Maurer T, Maurer J, Retz M, Paul R, Zantl N, Gschwend JE, et al. Influence of body mass index on operability, morbidity and disease outcome following radical cystectomy. Urologia Internationalis. 2009;82(4):432–9. [DOI] [PubMed] [Google Scholar]
- [70].Dabi Y, Rouscoff Y, Anract J, Delongchamps NB, Sibony M, Saighi D, et al. Impact of body mass index on the oncological outcomes of patients treated with radical cystectomy for muscle-invasive bladder cancer. World Journal of Urology. 2017;35(2):229–35. [DOI] [PubMed] [Google Scholar]
- [71].Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: Impact on cancer-specific and all-cause mortality. Cancer. 2014;120(18):2910–8. [DOI] [PubMed] [Google Scholar]
- [72].Ahn JH, Jung SI, Yim SU, Kim SW, Hwang EC, Kwon DD. Impact of Glycemic Control and Metformin Use on the Recurrence and Progression of Non-Muscle Invasive Bladder Cancer in Patients with Diabetes Mellitus. Journal of Korean Medical Science. 2016;31(9):1464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lenis AT, Asanad K, Blaibel M, Donin NM, Chamie K. Association between Metabolic Syndrome and Recurrence of Nonmuscle Invasive Bladder Cancer following bacillus Calmette-Guerin Treatment. Urology Practice. 2018;5(2):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kluth LA, Xylinas E, Crivelli JJ, Passoni N, Comploj E, Pycha A, et al. Obesity is associated with worse outcomes in patients with T1 high grade urothelial carcinoma of the bladder. The Journal of Urology. 2013;190(2): 480–6. [DOI] [PubMed] [Google Scholar]
- [75].Gierth M, Zeman F, Denzinger S, Vetterlein MW, Fisch M, Bastian PJ, et al. Influence of Body Mass Index on Clinical Outcome Parameters, Complication Rate and Survival after Radical Cystectomy: Evidence from a Prospective European Multicentre Study. Urologia Internationalis. 2018;101(1):16–24. [DOI] [PubMed] [Google Scholar]
- [76].Richard PO, Ahmad AE, Bashir S, Hamilton RJ, Nam RK, Leao R, et al. Effect of statins as a secondary chemopreventive agent among individuals with non-muscle-invasive bladder cancer: A population-based analysis. Urologic Oncology. 2017;35(6):342–8. [DOI] [PubMed] [Google Scholar]
- [77].Nayan M, Bhindi B, Yu JL, Hermanns T, Mohammed A, Hamilton RJ, et al. The effect of metformin on cancer-specific survival outcomes in diabetic patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Urologic Oncology. 2015;33(9):386.e7–13. [DOI] [PubMed] [Google Scholar]
- [78].Richard PO, Ahmad AE, Bashir S, Zlotta A, Bhindi B, Leao R, et al. Impact of oral hypoglycemic agents on mortality among diabetic patients with non-muscle-invasive bladder cancer: A population based analysis. Canadian Urological Association Journal=Journal de l’Association Des Urologues Du Canada. 2018;12(6):203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Berglund RK, Savage CJ, Vora KC, Kurta JM, Cronin AM. An analysis of the effect of statin use on the efficacy of bacillus calmetteguerin treatment for transitional cell carcinoma of the bladder. The Journal of Urology. 2008;180(4):1297–300; discussion 300. [DOI] [PubMed] [Google Scholar]
- [80].Crivelli JJ, Xylinas E, Kluth LA, da Silva RD, Chrystal J, Novara G, et al. Effect of statin use on outcomes of non-muscle-invasive bladder cancer. BJU International. 2013;112(2):E4–12. [DOI] [PubMed] [Google Scholar]
- [81].Hoffmann P, Roumeguere T, Schulman C, van Velthoven R. Use of statins and outcome of BCG treatment for bladder cancer. The New England Journal of Medicine. 2006;355(25):2705–7. [DOI] [PubMed] [Google Scholar]
- [82].Kamat AM, Wu X. Statins and the effect of BCG on bladder cancer. The New England Journal of Medicine. 2007;356(12):1276; authorreply −7. [DOI] [PubMed] [Google Scholar]
- [83].Skolarus TA, Lee EW, Virgo KS, Katz MD, Hudson MA, Kibel AS, et al. Intravesical bacille Calmette-Guerin therapy for non-muscle-invasive bladder cancer: Effects of concurrent statin therapy. Journal of the American College of Surgeons. 2009;209(2):248–53. [DOI] [PubMed] [Google Scholar]
- [84].Boorjian SA, Berglund RK, Maschino AC, Savage CJ, Herr HW. Fibrin clot inhibitor medication and efficacy of bacillus Calmette-Guerin for bladder urothelial cancer. The Journal of Urology. 2009;182(4):1306–12. [DOI] [PubMed] [Google Scholar]
- [85].Hudson MA, Yuan JJ, Catalona WJ, Ratliff TL. Adverse impact of fibrin clot inhibitors on intravesical bacillus Calmette-Guerin therapy for superficial bladder tumors. The Journal of Urology. 1990;144(6):1362–4. [DOI] [PubMed] [Google Scholar]
- [86].Kelly JD, Tan WS, Porta N, Mostafid H, Huddart R, Protheroe A, et al. BOXIT-A Randomised Phase III Placebo-controlled Trial Evaluating the Addition of Celecoxib to Standard Treatment of Transitional Cell Carcinoma of the Bladder (CRUK/07/004). European Urology. 2019;75(4):593–601. [DOI] [PubMed] [Google Scholar]
- [87].Lipsky MJ, Badalato GM, Motamedinia P, Hruby GW, McKiernan JM. The effect of fibrin clot inhibitors on the immunomodulatory efficacy of Bacillus Calmette-Guerin therapy for non-muscle-invasive bladder cancer. Urology. 2013;81(6):1273–8. [DOI] [PubMed] [Google Scholar]
- [88].P’Ng K B, Walsh MD, Seymour GJ, Lavin MF, Gardiner RA. The adverse effect of fibrin-clot inhibiting drugs on intravesical bacillus Calmette-Guerin efficacy for superficial bladder cancer. The Australian and New Zealand Journal ofSurgery. 1993;63(2):127–30. [DOI] [PubMed] [Google Scholar]
- [89].Sabichi AL, Lee JJ, Grossman HB, Liu S, Richmond E, Czerniak BA, et al. A randomized controlled trial of celecoxib to prevent recurrence of nonmuscle-invasive bladder cancer. Cancer Prevention Research (Philadelphia, Pa). 2011;4(10):1580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Witjes JA, vd Meijden AP, Doesburg W, Debruyne FM. Influence of fibrin clot inhibitors on the efficacy of intravesical Bacillus Calmette-Guerin in the treatment of superficial bladder cancer. The Dutch South-east Cooperative Urological Group. European Urology. 1993;23(3):366–70. [DOI] [PubMed] [Google Scholar]
- [91].Byar D, Blackard C. Comparisons of placebo, pyridoxine, and topical thiotepa in preventing recurrence of stage I bladder cancer. Urology. 1977;10(6):556–61. [DOI] [PubMed] [Google Scholar]
- [92].Newling DW, Robinson MR, Smith PH, Byar D, Lock-wood R, Stevens I, et al. Tryptophan metabolites, pyridoxine (vitamin B6) and their influence on the recurrence rate of superficial bladder cancer. Results of a prospective, randomised phase III study performed by the EORTC GU Group. EORTC Genito-Urinary Tract Cancer Cooperative Group. European Urology. 1995;27(2):110–6. [DOI] [PubMed] [Google Scholar]
- [93].Goossens ME, Zeegers MP, van Poppel H, Joniau S, Ackaert K, Ameye F, et al. Phase III randomised chemoprevention study with selenium on the recurrence of non-invasive urothelial carcinoma. The SELEnium and BLAdder cancer Trial. European Journal of Cancer (Oxford, England : 1990). 2016;69:9–18. [DOI] [PubMed] [Google Scholar]
- [94].Jochems SHJ, van Osch FHM, Reulen RC, van Hensbergen M, Nekeman D, Pirrie S, et al. Fruit and vegetable intake and the risk of recurrence in patients with nonmuscle invasive bladder cancer: A prospective cohort study. Cancer causes & control: CCC. 2018;29(6):573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer epidemiology, biomarkers & prevention : A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(7):1806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Westhoff E, Wu X, Kiemeney LA, Lerner SP, Ye Y, Huang M, et al. Dietary patterns and risk of recurrence and progression in non-muscle-invasive bladder cancer. International Journal of Cancer. 2018;142(9):1797–804. [DOI] [PubMed] [Google Scholar]
- [97].Jochems SHJ, van Osch FHM, Reulen RC, van Hensber- gen M, Nekeman D, Pirrie SJ, et al. Total Fluid Intake and the Risk of Recurrence in Patients With Non-Muscle Invasive Bladder Cancer: A Prospective Cohort Study. Bladder Cancer. 2018;4(3):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lamm DL, Riggs DR, Shriver JS, vanGilder PF, Rach JF, DeHaven JI. Megadose vitamins in bladder cancer: A double-blind clinical trial. The Journal of Urology. 1994;151(1):21–6. [DOI] [PubMed] [Google Scholar]
- [99].Nepple KG, Lightfoot AJ, Rosevear HM, O’Donnell MA, Lamm DL. Bacillus Calmette-Guerin with or without interferon alpha-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. The Journal of Urology. 2010;184(5):1915–9. [DOI] [PubMed] [Google Scholar]
- [100].Mazdak H, Zia H. Vitamin e reduces superficial bladder cancer recurrence: A randomized controlled trial. International Journal of Preventive Medicine. 2012;3(2): 110–5. [PMC free article] [PubMed] [Google Scholar]
- [101].Tu H, Dinney CP, Ye Y, Grossman HB, Lerner SP, Wu X. Is folic acid safe for non-muscle-invasive bladder cancer patients? An evidence-based cohort study. The American Journal of Clinical Nutrition. 2018;107(2):208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Decensi A, Torrisi R, Bruno S, Costantini M, Curotto A, Nicolo G, et al. Randomized trial of fenretinide in superficial bladder cancer using DNA flow cytometry as an intermediate end point. Cancer Epidemiol Biomarkers Prev. 2000;9(10):1071–8. [PubMed] [Google Scholar]
- [103].Sabichi AL, Lerner SP, Atkinson EN, Grossman HB, Car-away NP, Dinney CP, et al. Phase III prevention trial of fenretinide in patients with resected non-muscle-invasive bladder cancer. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2008;14(1):224–9. [DOI] [PubMed] [Google Scholar]
- [104].Pedersen H, Wolf H, Jensen SK, Lund F, Hansen E, Olsen PR, et al. Administration of a retinoid as prophylaxis of recurrent non-invasive bladdertumors. Scandinavian Journal of Urology and Nephrology. 1984;18(2):121–3. [DOI] [PubMed] [Google Scholar]
- [105].Alfthan O, Tarkkanen J, Grohn P, Heinonen E, Pyrhonen S, Saila K. Tigason (etretinate) in prevention of recurrence of superficial bladder tumors. A double-blind clinical trial. European Urology. 1983;9(1):6–9. [DOI] [PubMed] [Google Scholar]
- [106].Studer UE, Jenzer S, Biedermann C, Chollet D, Kraft R, von Toggenburg H, et al. Adjuvant treatment with a vitamin A analogue (etretinate) after transurethral resection of superficial bladdertumors. Final analysis of a prospective, randomized multicenter trial in Switzerland. European Urology. 1995;28(4):284–90. [DOI] [PubMed] [Google Scholar]
- [107].Aso Y, Akazan H. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urologia Internationalis. 1992;49(3):125–9. [DOI] [PubMed] [Google Scholar]
- [108].Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. European Urology. 1995;27(2):104–9. [DOI] [PubMed] [Google Scholar]
- [109].Selinski S, Burger H, Blaszkewicz M, Otto T, Volk- ert F, Moormann O, et al. Occupational risk factors for relapse-free survival in bladder cancer patients. Journal of toxicology and environmental health Part A. 2016;79(22-23):1136–43. [DOI] [PubMed] [Google Scholar]
- [110].Carta A, Pavanello S, Mastrangelo G, Fedeli U, Arici C, Porru S. Impact of Occupational Exposures and Genetic Polymorphisms on Recurrence and Progression of Non-Muscle-Invasive Bladder Cancer. International Journal of Environmental Research and Public Health. 2018; 15(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lukas C, Selinski S, Prager HM, Blaszkewicz M, Hengstler JG, Golka K. Occupational bladder cancer: Polymorphisms of xenobiotic metabolizing enzymes, exposures, andprognosis. Journal of Toxicology and Environmental Health Part A. 2017;80(7-8):439–52. [DOI] [PubMed] [Google Scholar]
- [112].Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, et al. Cigarette smoking and bladder cancer in men: A pooled analysis of 11 case-control studies. International Journal of Cancer. 2000;86(2):289–94. [DOI] [PubMed] [Google Scholar]
- [113].Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Research. 2003;63(8):1727–30. [PubMed] [Google Scholar]
- [114].Barbaresko J, Rienks J, Nothlings U. Lifestyle Indices and Cardiovascular Disease Risk: A Meta-analysis. American Journal of Preventive Medicine. 2018;55(4):555–64. [DOI] [PubMed] [Google Scholar]
- [115].Hopkins BD, Goncalves MD, Cantley LC. Obesity and Cancer Mechanisms: Cancer Metabolism. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2016;34(35):4277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Mbeutcha A, Shariat SF, Rieken M, Rink M, Xylinas E, Seitz C, et al. Prognostic significance of markers of systemic inflammatory response in patients with non-muscle-invasive bladder cancer. Urologic Oncology. 2016;34(11):483.e17–.e24. [DOI] [PubMed] [Google Scholar]
- [117].Zaccardi F, Dhalwani NN, Papamargaritis D, Webb DR, Murphy GJ, Davies MJ, et al. Nonlinear association of BMI with all-cause and cardiovascular mortality in type 2 diabetes mellitus: A systematic review and meta-analysis of 414,587 participants in prospective studies. Diabetologia. 2017;60(2):240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhong S, Yan X, Wu Y, Zhang X, Chen L, Tang J, et al. Body mass index and mortality in prostate cancer patients: A dose-response meta-analysis. Prostate Cancer and Pro-static Diseases. 2016;19(2):122–31. [DOI] [PubMed] [Google Scholar]
- [119].Macaulay VM. Insulin-like growth factors and cancer. British Journal of Cancer. 1992;65(3):311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bach LA, Rechler MM. Insulin-like growth factors and diabetes. Diabetes/metabolism reviews. 1992;8(3):229–57. [DOI] [PubMed] [Google Scholar]
- [121].Insulin Giovannucci E., insulin-like growth factors and colon cancer: A review of the evidence. The Journal of Nutrition. 2001;131(11 Suppl):3109s–20s. [DOI] [PubMed] [Google Scholar]
- [122].Zhao H, Grossman HB, Spitz MR, Lerner SP, Zhang K, Wu X. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. The Journal of Urology. 2003;169(2):714–7. [DOI] [PubMed] [Google Scholar]
- [123].Lin C, Travis RC, Appleby PN, Tipper S, Weider- pass E, Chang-Claude J, et al. Pre-diagnostic circulating insulin-like growth factor-I and bladder cancer risk in the European Prospective Investigation into Cancer and Nutrition. International Journal of Cancer. 2018;143(10):2351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocrine-Related Cancer. 2009;16(4):1103–23. [DOI] [PubMed] [Google Scholar]
- [125].Lin HC, Kachingwe BH, Lin HL, Cheng HW, Uang YS, Wang LH. Effects of metformin dose on cancer risk reduction in patients with type 2 diabetes mellitus: A 6-year follow-up study. Pharmacotherapy. 2014;34(1):36–45. [DOI] [PubMed] [Google Scholar]
- [126].Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prevention research (Philadelphia, Pa). 2008;1(5):369–75. [DOI] [PubMed] [Google Scholar]
- [127].Makhlin I, Zhang J, Long CJ, Devarajan K, Zhou Y, Klein- Szanto AJ, et al. The mTOR pathway affects proliferation and chemosensitivity of urothelial carcinoma cells and is upregulated in a subset of human bladder cancers. BJU International. 2011;108(2 Pt 2):E84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Liu Q, Yuan W, Tong D, Liu G, Lan W, Zhang D, et al. Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget. 2016;7(19):28235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Deng J, Peng M, Wang Z, Zhou S, Xiao D, Deng J, et al. Novel application of metformin combined with targeted drugs on anticancer treatment. Cancer Science. 2019;110(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lee SJ, Lee I, Lee J, Park C, Kang WK. Statins, 3-hydroxy- 3-methylglutaryl coenzyme A reductase inhibitors, potentiate the anti-angiogenic effects of bevacizumab by suppressing angiopoietin2, BiP, and Hsp90alpha in human colorectal cancer. Br J Cancer. 2014;111(3):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tapia-Perez JH, Preininger R, Kirches E, Reinhold A, Butzmann J, Prilloff S, et al. Simultaneous Administration of Statins and Pioglitazone Limits Tumor Growth in a Rat Model of Malignant Glioma. Anticancer Research. 2016;36(12):6357–65. [DOI] [PubMed] [Google Scholar]
- [132].Keyomarsi K, Sandoval L, Band V, Pardee AB. Synchro-nization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Research. 1991;51(13):3602–9. [PubMed] [Google Scholar]
- [133].Qi XF, Zheng L, Lee KJ, Kim DH, Kim CS, Cai DQ, et al. HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death & Disease. 2013;4:e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. New England Journal of Medicine. 2016;374(21):2021–31. [DOI] [PubMed] [Google Scholar]
- [135].Hudson MA, Brown EJ, Ritchey JK, Ratliff TL. Modulation of fibronectin-mediated Bacillus Calmette-Guerin attachment to murine bladder mucosa by drugs influencing the coagulation pathways. Cancer Research. 1991;51(14):3726–32. [PubMed] [Google Scholar]
- [136].Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. The Journal of Clinical Investigation. 1990;85(1):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Becker RC, Meade TW, Berger PB, Ezekowitz M, O’Connor CM, Vorchheimer DA, et al. The Primary and Secondary Prevention of Coronary Artery Disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6, Supplement):776S–814S. [DOI] [PubMed] [Google Scholar]
- [138].Akl EA, Kahale LA, Barba M, Neumann I, Labedi N, Terrenato I, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database of Systematic Reviews. 2014(7). [DOI] [PubMed] [Google Scholar]
- [139].Abbaoui B, Lucas CR, Riedl KM, Clinton SK, Mortazavi A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Molecular Nutrition & Food Research. 2018;62(18):1800079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Oliviero T, Verkerk R, Dekker M. Isothiocyanates from Brassica Vegetables-Effects of Processing, Cooking, Mastication, and Digestion. Molecular Nutrition & Food Research. 2018;62(18):e1701069–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bai Y, Yuan H, Li J, Tang Y, Pu C, Han P. Relationship between bladder cancer and total fluid intake: A meta-analysis of epidemiological evidence. World J Surg Oncol. 2014;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physiccal activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians. 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- [143].Jiang Q Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radical Biology & Medicine. 2014;72:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama. 2011;306(14): 1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Markowski MC, Boorjian SA, Burton JP, Hahn NM, Inger- sol MA, Maleki Vareki S, et al. The Microbiome and Genitourinary Cancer: A Collaborative Review. European Urology. 2019;75(4):637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Fuller R Probiotics in man and animals. The Journal of Applied Bacteriology. 1989;66(5):365–78. [PubMed] [Google Scholar]
- [147].Hayatsu H, Hayatsu T. Suppressing effect of Lactobacillus casei administration on the urinary mutagenicity arising from ingestion of fried ground beef in the human. Cancer Letters. 1993;73(2-3):173–9. [DOI] [PubMed] [Google Scholar]
- [148].Nagao F, Nakayama M, Muto T, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Bioscience, Biotechnology, and Biochemistry. 2000;64(12):2706–8. [DOI] [PubMed] [Google Scholar]
- [149].Cumberbatch MGK, Cox A, Teare D, Catto JWF. Contemporary Occupational Carcinogen Exposure and Bladder Cancer: A Systematic Review and Meta-analysis Contemporary Occupational Carcinogen Exposure and Bladder Cancer Contemporary Occupational Carcinogen Exposure and Bladder Cancer. JAMA Oncology. 2015;1(9):1282–90. [DOI] [PubMed] [Google Scholar]
- [150].Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2011;29(6):726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Buffart LM, Kalter J, Sweegers MG, Courneya KS, New-ton RU, Aaronson NK, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treatment Reviews. 2017;52:91–104. [DOI] [PubMed] [Google Scholar]
- [152].Taylor PR, Albanes D. Selenium, Vitamin E, and Prostate Cancer— Ready for Prime Time? JNCI: Journal of the National Cancer Institute. 1998;90(16):1184–5. [DOI] [PubMed] [Google Scholar]
- [153].Kenfield SA, Van Blarigan EL, Ameli N, Lavaki E, Cedars B, Paciorek AT, et al. Feasibility, Acceptability, and Behavioral Outcomes from a Technology-enhanced Behavioral Change Intervention (Prostate 8): A Pilot Randomized Controlled Trial in Men with Prostate Cancer. European Urology. 2019. [DOI] [PubMed] [Google Scholar]
- [154].VanBlarigan EL, Chan H,VanLoon K, Kenfield SA, Chan JM, Mitchell E, et al. Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): A pilot randomized controlled trial. BMC Cancer. 2019;19(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Nieder Alan M, John S, Messina Catherine R, Granek Iris A, Adler Howard L. Are Patients Aware of the Association Between Smoking and Bladder Cancer? Journal of Urology. 2006;176(6):2405–8. [DOI] [PubMed] [Google Scholar]
- [156].Winters BR, Wen L, Holt SK, Dash A, Gore JL, Schade GR, et al. Does the Diagnosis of Bladder Cancer Lead to Higher Rates of Smoking Cessation? Findings from the Medicare Health Outcomes Survey. The Journal of Urology. 2019;202(2):241–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


