Abstract
Cardiotoxicity leading to cardiovascular dysfunction and ultimately cardiac failure remains a major global health issue irrespective of race, age and country. Several factors including lipotoxicity, oxidative imbalance, exacerbated angiotensin-converting enzyme (ACE) activity and altered bioenergetics have been implicated in the pathophysiology of cardiovascular diseases. Turbina oblongata (E. Mey. ex Choisy) A. Meeuse is among the medicinal plants commonly used traditionally in the treatment and management of various ailments including cardiovascular dysfunctions in South Africa. In the present study, T. oblongata was investigated for its cardioprotective mechanism on oxidative-mediated cardiotoxicity by determining its effect on redox imbalance, purinergic and cholinergic dysfunction, and ACE activity as well as lipid dysmetabolism and pathways in iron-induced oxidative cardiac injury. Oxidative injury was induced ex vivo in freshly isolated heart by incubating with 0.1 mM FeSO4. Treatment was done by co-incubating with T. oblongata extract or gallic acid which served as the standard antioxidant. Induction of oxidative cardiac injury led to significant depleted levels of glutathione, triglyceride, HDL-cholesterol, superoxide, catalase and ENTPDase activities, with concomitant elevated levels of malondialdehyde, cholesterol, LDL-cholesterol, ACE, acetylcholinesterase, ATPase and lipase activities. These levels and activities were significantly reversed following treatment with T. oblongata. Induction of oxidative injury also caused alterations in lipid metabolites, with concomitant activation of beta oxidation of very long chain fatty acids, plasmalogen synthesis and mitochondrial beta-oxidation of long chain saturated fatty acids pathways. Some of the altered metabolites were restored following treatment with T. oblongata, with concomitant inactivation of beta oxidation of very long chain fatty acid pathway. These results indicate the cardioprotective effect of T. oblongata against oxidative-mediated cardiotoxicity. This is evidenced by its ability to mitigate lipotoxicity and modulate dysregulated cardiometabolic activities as portrayed by its antioxidative activity and suppressive effects on ACE, acetylcholinesterase and lipase activities, while modulating cardiac lipid dysmetabolism.
Keywords: cardiotoxicity, lipid dysmetabolism, metabolomics, turbina oblongata, cardiometabolism
Introduction
Cardiovascular dysfunctions leading to cardiovascular failure has been linked to global early mortalities, with oxidative stress arising from disordered cardiac bioenergetics playing a triggering role (Poulter, 2003; Wold et al., 2005; Li et al., 2019). This is often depicted by cardiac lipid dysmetabolism which is characterized by hyperlipidemia as well as defects in beta-oxidation pathway and mitochondrial oxidative phosphorylation (Antozzi and Zeviani, 1997). Fatty acids (FAs) have been reported as a major energy source for the heart, as they are estimated to meet over 70% of the heart’s energy needs (Drosatos and Schulze, 2013). Alteration in cardiac FAs and their metabolism leading to lipotoxicity has been implicated among the pathomechanism of cardiovascular dysfunctions (Erukainure et al., 2020b; Salau et al., 2020b). Exacerbated activities of cardiac lipase and angiotensin-converting enzyme (ACE), as well as altered cardiac supply of triglycerides from the liver and accumulation of cholesterol in the heart are major contributing factors of lipotoxicity. Lipotoxicity has been linked to increased production and accumulation of free radicals leading to oxidative stress, when the latter overwhelms the cardiac endogenous antioxidant system (Jiménez-González et al., 2020). This crosstalk between lipotoxicity and oxidative stress presents pathogenic mechanisms and progression of cardiotoxicity and other cardiovascular dysfunctions, ultimately leading to heart failure.
Turbina oblongata is among the medicinal plants commonly used traditionally in the treatment and management of various ailments in South Africa. It is a perennial herb belonging to the Convolvulaceae family and widely distributed in Botswana, Swaziland, Malawi, Tanzania, Zimbabwe and South Africa (Srivastava and Rauniyar, 2020). In South Africa, the plant is commonly found in all provinces except the Western Cape and it is locally referred to as ubhoqo (Semenya et al., 2018; Srivastava and Rauniyar, 2020). Its other synonyms are Ipomoea atherstonei, Ipomoea lambtoniana, Ipomoea oblongata and Ipomoea randii (Foden and Potter, 2005). The plant leaves have been reported for their wound healing properties and anticancer activities (Hutchings et al., 1996; Kose et al., 2015). The plant is also traditionally used in the treatment of diarrhea, respiratory infections, hypertension, impotency, renal disease, and sexually transmitted infections (Srivastava and Rauniyar, 2020). These properties can be attributed to its phytochemical constituents which have been reported to consist of flavonoids, terpenoids, glycosides, steroids, anthocyanins, and alkaloids.
The present study investigated the cardioprotective mechanism of T. oblongata on oxidative-mediated cardiotoxicity by reporting its effect on redox imbalance, purinergic and cholinergic dysfunction, ACE activity and lipid dysmetabolism in iron-induced oxidative cardiac injury.
Materials and Methods
Plant Material and Extraction
Turbina oblongata was collected from Eastern Cape Province, South Africa (GPS coordinates: 32°44′20.1″S 26°54′58.3″E). The plant sample was identified and authenticated at the Geo Potts herbarium (BLFU), University of the Free State (specimen voucher number: BLFU MGM0019).
The plant sample was air-dried after washing and was blended to fine powder. The powdered sample (100 g in a 1:5 mass:solvent ratio in ml) was subjected to sequential extraction using solvents of increasing polarity vis-à-vis hexane, dichloromethane (DCM), methanol and dichloromethane (MeOH:DCM; 1:1, v/v) and methanol (MeOH). Each extraction was carried out at ambient room temperature for 24 h. After extraction, each extract was filtered and subsequently concentrated in vacuo with an R–215 rotary evaporator (Buchi, Switzerland). Concentrated samples were collected into glass vials and stored at 4 C.
Enzyme Inhibitory Activity
The concentrated extract samples were investigated for their anti-hypertensive activities by determining their ability to inhibit the activity of ACE in vitro (Shalaby et al., 2006). Based on the ACE inhibitory activities, the DCM extract was selected for further evaluation for its anti-hypertensive potential by determining its ability to inhibit the activity of renin using modified standard method (Udenigwe et al., 2009). The DCM extract, based on its activity on ACE and Renin, was chosen for further ex vivo studies.
Induction of Oxidative Cardiac Injury Ex Vivo
Hearts harvested from Sprague Dawley rats were homogenized in 50 mM sodium phosphate buffer (pH 7.5; with 1% Triton X-100). The homogenates were then centrifuged at 15,000 rpm at 4°C for 10 min to obtain the tissue supernatants.
A 100 µl of the cardiac tissue supernatant was incubated with 0.1 mM FeSO4 (pro-oxidant) and different concentrations (30, 60, 120 and 240 μg/ml) of the DCM extract of T. oblongata at 37°C for 30 min according to previously published methods (Erukainure et al., 2020a; Salau et al., 2020b). Reaction samples without the extract served as the negative control (untreated), while reaction sample without the pro-oxidant and extract served as the normal control. Gallic acid was used as the standard antioxidative drug.
The study was conducted under the approved guidelines of the Animal Ethics Committee of the University of KwaZulu-Natal, Durban, South Africa (Protocol approval number: AREC/020/017D).
Determination of Oxidative Stress Parameters
Reduced Glutathione Level
This was determined according to Ellman’s method (Ellman, 1959). Briefly, 100 μl of the reaction samples was deproteinized with 300 μl of 10% TCA and centrifuged at 3,500 rpm for 5 min. 200 μl of the mixture was added to 50 μl of Ellman’s reagent in a 96-well plate and incubated for 5 min. Absorbance was measured at 415 nm and GSH level was extrapolated from a GSH standard curve.
Superoxide Dismutase Enzyme Activity
This was determined with slight modification of a previously established method (Kakkar et al., 1984). Briefly, 15 μl of the reaction samples was mixed with 170 μl of 0.1 mM diethylenetriaminepentaacetic acid (DETAPAC) in a 96-well plate. Then 15 μl of 1.6 mM 6-hydroxydopamine (6-HD) was added to the mixture and swirled. Absorbance was immediately read at 492 nm for 3 min at 1 min interval.
Catalase Activity
Catalase activity was determined using a previously established protocol (Aebi, 1984). Briefly, 100 μl of the reaction samples was mixed with 340 μl of 50 mM phosphate buffer (pH 7.0). Thereafter, 150 μl of 2 M hydrogen peroxide was added to the mixture and incubated for 5 min. Absorbance was measured at 240 nm at 1 min interval for 3 min.
Lipid Peroxidation
Lipid peroxidation was carried out by measuring the malondialdehyde (MDA) level of the reaction samples using a previously established method (Chowdhury and Soulsby, 2002). In brief, a reaction mixture made up of equal volumes (100 μl) of the reaction samples and 8.1% SDS solution, 1 ml of 0.25% thiobarbituric acid (TBA) and 375 μl of 20% acetic acid was boiled for 1 h. Absorbance was measured at 532 nm and MDA levels were extrapolated from a MDA standard curve.
Determination of ACE Activity
ACE activity was determined spectrophotometrically by a slight modification of Holmquist’s method (Holmquist et al., 1979), using N-[3-(2-Furyl) acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG) as substrate. Briefly, 200 μl of the reaction samples were incubated with 1 ml of 0.5 mM FAPGG for 10 min at 37°C. Absorbance was measured at 345 nm at 2 min intervals. ACE activity was measured as the rate of reaction (ΔA/min) as expressed below:
Where AI = initial absorbance; AF = final absorbance.
Determination of Cholinergic Enzymes Activity
Cholinergic enzyme activity was determined by analyzing the acetylcholinesterase activity of the reaction samples using the Ellman’s procedure (Ellman et al., 1961). A reaction containing 20 μl of the reaction samples, 10 μl of Ellman’s reagent (3.3 mM, pH 7.0) and 50 μl of phosphate buffer (0.1 M, pH 8) was incubated for 20 min at 25°C. Then 10 μl of 0.05 M acetylcholine iodide was added to the reaction samples and absorbance was measured at 412 nm at 3 min intervals.
Determination of Purinergic Enzymes Activities
The purinergic enzyme activities of the reaction samples were determined by analyzing the activities of adenylpyrophosphatase (ATPase) (Adewoye et al., 2000; Erukainure et al., 2017) and ecto-nucleoside triphosphate diphosphohydroase (ENTPDase) (Akomolafe et al., 2017) respectively.
ATPase Activity
Briefly, a reaction mixture containing 100 μl of the reaction samples, 100 μl of 5 mM KCl, 650 μl of 0.1 M Tris-HCl buffer, and 20 μl of 50 mM ATP was incubated in a shaker for 30 min at 37°C. Then 1.25% ammonium molybdate and 500 μl of a distilled water were added to terminate the reaction. Thereafter, a 500 μl of a freshly prepared 9% ascorbic acid was added to the mixture and allowed to stand for 30 min. Absorbance was measured at 660 nm.
ENTPDase Activity
Briefly, 20 μl of the reaction samples was incubated with a mixture containing 200 μl of the reaction buffer (1.5 mM CaCl2, 5 mM KCl, 0.1 mM EDTA, 10 mM glucose, 225 mM sucrose and 45 mM Tris-HCl) for 10 min at 37°C. 20 μl of 50 mM ATP was then added to the reaction mixture and further incubated in a shaker for 20 min at 37°C. Reaction was halted by adding 200 μl of 10% TCA, followed by 200 μl of 1.25% ammonium molybdate and a freshly prepared 9% ascorbic acid. The mixture was allowed to stand on ice for 10 min. Absorbance was read at 600 nm.
Determination of Lipase Activity
The lipase activity of the reaction samples was determined by a little modification of a previously established method (Kim et al., 2010). A 100 μl of the reaction samples was incubated with 169 μl of Tris buffer (100 mM Tris–HC1 and 5 mM CaCl2, pH 7.0) at 37°C for 15 min 5 μl of 10 mM p-NPB (p-nitrophenyl butyrate in dimethyl formamide) was added to the mixture and further incubated for 15 min at 37°C. Absorbance was measured at 405 nm at 1 min interval. Lipase activity was expressed as the rate of reaction (ΔA/min).
Determination of Cardiac Lipid Profile
Cardiac tissue supernatants were incubated overnight (12 h) with 0.1 mM FeSO4 and 240 μg/ml of T. oblongata DCM extract or gallic acid as described above. The samples were centrifuged at 15,000 rpm for 10 min at 4°C. The supernatants were immediately assayed for lipid profile levels including, total cholesterol, triglycerides, and HDL-cholesterol using an Automated Chemistry Analyzer (Labmax Plenno, Labtest Co. Ltd., Lagoa Santa, Brazil) with commercial assay kits according to manufacturer’s manual.
Metabolite Extraction and Profiling
As described above, the reaction mixture was prepared and incubated overnight (12 h). Lipid metabolites were then extracted from the samples and subjected to GC-MS metabolic profiling (Ralston-Hooper et al., 2011) with slight modification. Briefly, cold chloroform was mixed with the reaction samples in a ratio of 5:1 and vortexed for 1 min. The mixture was incubated on ice for 20 min and centrifuged at 15,000 rpm for 10 min at 4°C to yield two liquid phases. The lower phase (chloroform) layer containing the lipid metabolites and other non-polar metabolites was collected and subsequently profiled with GC-MS.
GC-MS Analysis of Lipid Metabolites
The chloroform layer which contains the lipid and other non-polar metabolites were analyzed with GC-MS (Agilent technologies 6,890 series GC coupled with (an Agilent) 5,973 Mass Selective Detector and driven by Agilent Chemstation software). The operating parameters include: Column: HP-5MS capillary column (30 m × 0.25 mm ID, 0.25 μm film thickness, 5% phenylmethylsiloxane); Carrier gas: ultra-pure helium; Flow rate: 1.0 ml min−1 and a linear velocity of 37 cm s−1; Injector temperature: set at 250°C. Initial oven temperature: 60°C, programmed to 280°C at the rate of 10°C min−1. Injection: 1 μl made in split mode at a split ratio of 20:1; Electron ionization mode: 70 eV; Electron multiplier voltage: at 1859 V; Ion source temperature: 230°C; Quadrupole temperature: 150°C; Solvent delay: 4 min; Scan range: 50–70 amu. Lipid metabolites were identified using an inbuilt NIST library.
Metabolic Pathway Analysis
To identify the relevant pathways involved in lipid metabolism in the protective role of T. oblongata on oxidative cardiotoxicity, the identified lipid metabolites were subjected to pathway enrichment analysis using the MetaboAnalyst 4.0 online server (Chong et al., 2018).
High-Performance Liquid Chromatography
HPLC-diode array detection analysis was performed on the DCM extract using an Agilent 1,100 series (Agilent, Waldbronn, Germany) instrument equipped with photo diode array, autosampler, column thermostat and degasser. A Phenomenex: Luna 5 µm C18 (2) (150 × 4.6 mm; 5 μm particle size) column was used as the stationary phase. Water containing 0.1% of formic acid (A) and acetonitrile (B) served as mobile phases at a flow rate of 1 ml/min. Gradient elution was applied as follows: Initial ratio 95% A: 5% B, keeping for 10 min, changed to 90% A: 10% B in 10 min, changed to 70% A: 30% B in 10 min, to 50% A: 50% B in 10 min, maintaining for 0.5 min and back to initial ratio in 0.5 min. Temperature was set to 30°C. Extract or standards were dissolved in HPLC grade methanol (2 mg/ml) and the injection volume was 20.0 μl. Chromatograms were recorded at 254 nm.
Statistical Analysis
One-way analysis of variance (ANOVA) was used in analyzing the data and presented as mean ± SD. Significant differences between means at p < 0.05 were obtained with the Tukey’s HSD-multiple range post-hoc test. Statistical analyses were done using IBM Statistical Package for the Social Sciences (SPSS) for Windows, version 23.0 (IBM Corp., Armonk, NY, United States). The GC-MS identified metabolites were subjected to statistical analysis (distinct changes and distribution) using the MetaboAnalyst 4.0 online server (Chong et al., 2018). Graphs were prepared using Microsoft Excel Spreadsheet and MetaboAnalyst 4.0 online server.
Results
Except for MEOH:DCM and DCM extracts, the extracts had no inhibitory effect on ACE activity as shown in Supplementary Figure S1A. The MEOH:DCM and DCM extracts significantly (p < 0.05) inhibited the enzyme activity in a dose dependent manner. This is further depicted by their IC50 values of 167 and 81.1 μg/ml, respectively (Table 1) and compared favourably with that of the standard ACE-inhibitor captopril oil (Supplementary Figure S1B).
TABLE 1.
IC50 values for ACE and renin inhibitory activities of T. oblongata extracts.
| Activities | HEX | MEOH | DCM:MEOH (1:1) | DCM | Aqueous | Captopril | Aliskiren |
|---|---|---|---|---|---|---|---|
| (µg/ml) | |||||||
| ACE inhibition | ND | 364 | 167 | 81.1 | ND | 1.31 | ND |
| Renin inhibition | ND | ND | ND | 127 | ND | ND | 13.4 |
HEX, hexane extract; MEOH, methanol extract; DCM:MEOH, dichloromethane and methanol extract; DCM, dichloromethane extract. ND = not determined.
The DCM extract having displayed the best ACE inhibitory activity, and was recommended for further investigated for its renin inhibitory activity. The extract significantly (p < 0.05) inhibited the ACE activity dose dependently (Supplementary Figure S2A), with an IC50 value of 127 μg/ml (Table 1). This activity compared favorably with that of Aliskiren (Supplementary Figure S2B and Table 1).
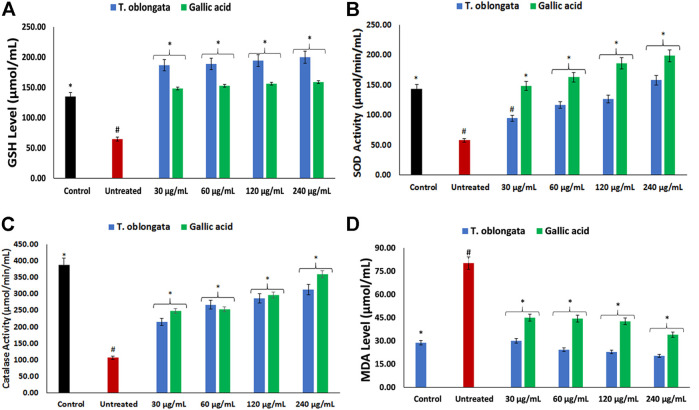
Induction of oxidative cardiac injury led to significant (p < 0.05) decreased levels of GSH, SOD and catalase activity, with concomitant elevated level of MDA as depicted in Figures 1A–D. Following treatment with the DCM extract, these levels and activities were significantly (p < 0.05) reversed to near normal. Except for GSH level, these reversals were dose-dependent and compared favourably with gallic acid.
FIGURE 1.
Effect of T. oblongata on (A) GSH level, (B) SOD, (C) catalase and (D) MDA level in oxidative cardiotoxicity. Data = mean ± SD; n = 3. *Statistically significant compared to untreated cardiac tissues; #statistically significant compared to normal cardiac tissues.
ACE activity was significantly (p < 0.05) elevated in cardiac tissues following the induction of oxidative cardiac injury as shown in Figure 2A. Treatment with the DCM extract led to significant (p < 0.05) depletion in the activity dose-dependently, depicting an inhibitory effect.
FIGURE 2.
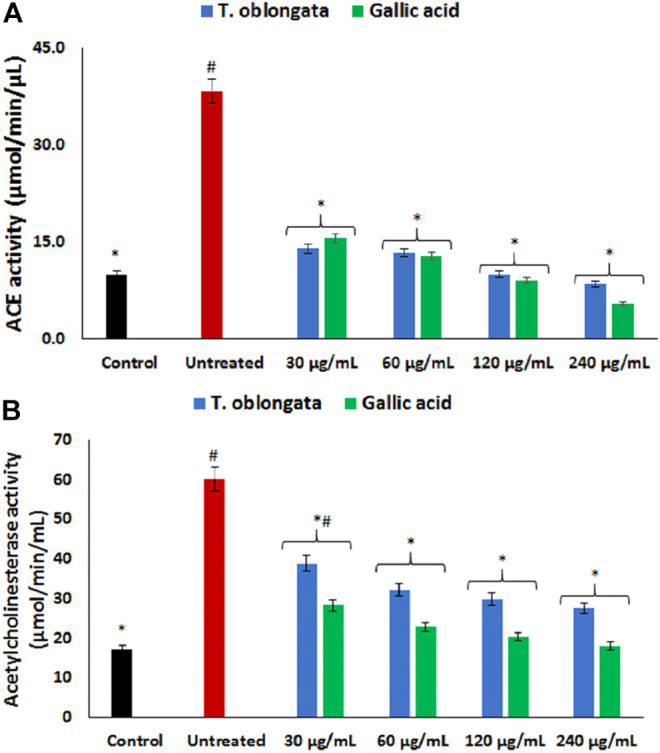
Effect of T. oblongata on (A) ACE and (B) acetylcholinesterase activities in oxidative cardiotoxicity. Data = mean ± SD; n = 3. *Statistically significant compared to untreated cardiac tissues; #statistically significant compared to the control (normal cardiac tissue).
As shown in Figure 2B, induction of oxidative cardiac injury led to significant (p < 0.05) elevation of cardiac acetylcholinesterase activity. The activity was significantly (p < 0.05) reversed dose-dependently to near noel following treatment with the DCM extract. The reversed activity compared favorably with that of gallic acid.
As depicted in Figures 3A,B, there was a significant (p < 0.05) elevation in cardiac ATPase activity, with concomitant decreased ENTPDase activity following the induction of oxidative cardiac injury. Treatment with the DCM extract led to significant (p < 0.05) reversion of these activities to near normal dose-dependently and compared favorably with gallic acid.
FIGURE 3.
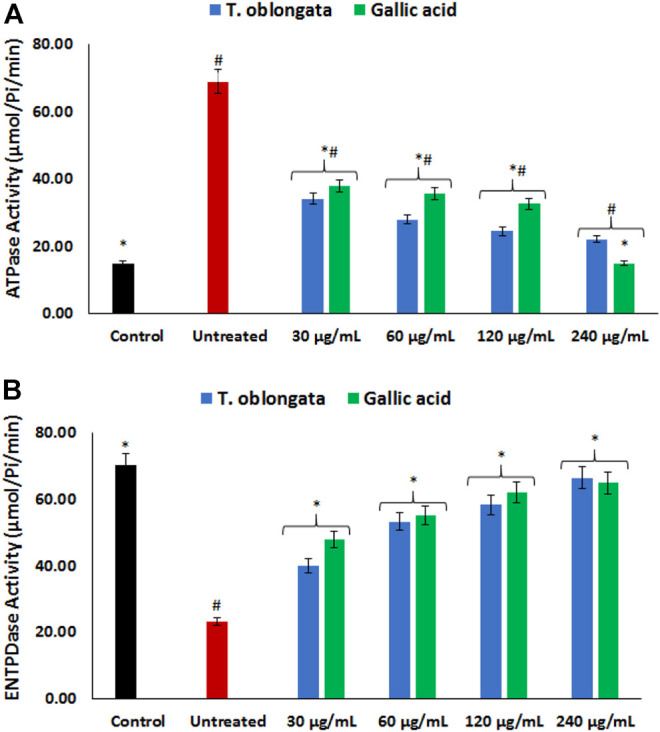
Effect of T. oblongata on (A) atpase and (B) entpdase activities in oxidative cardiotoxicity. Data = mean ± SD; n = 3. *Statistically significant compared to untreated cardiac tissues; #statistically significant compared to the control (normal cardiac tissues).
There was a significant (p < 0.05) elevation in cardiac lipase activity following the induction of oxidative cardiac injury as shown in Figure 4A. The activity was significantly (p < 0.05) inhibited dose-dependently after treatment with the DCM extract, and compared favorably with gallic acid.
FIGURE 4.
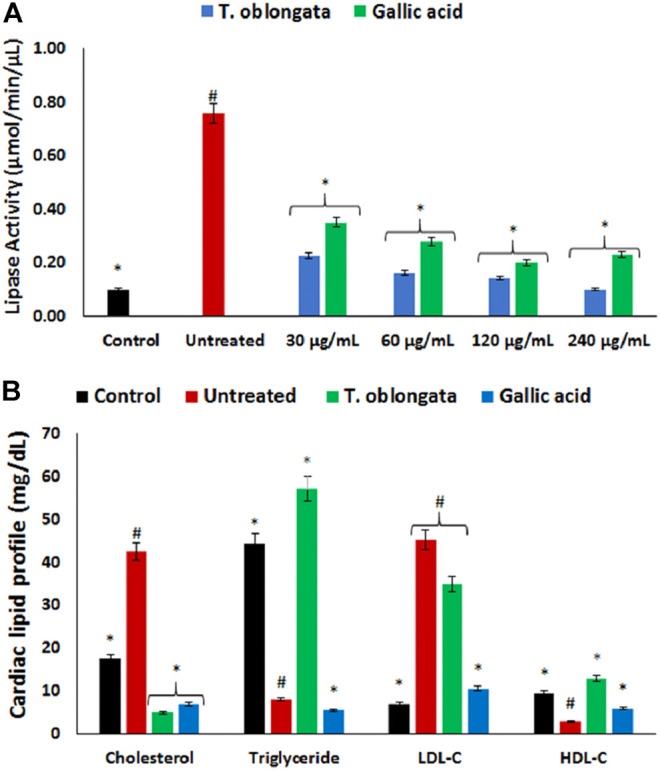
Effect of T. oblongata on (A) lipase activity and (B) lipid profile in oxidative cardiomyopathy. Data = mean ± SD; n = 3. *Statistically significant compared to untreated cardiac tissues; #statistically significant compared to the control (normal cardiac tissues).
Induction of oxidative cardiac injury led to significant (p < 0.05) elevation of cardiac levels of cholesterol and LDL-C, with concomitant decreased levels of triglycerides and HDL-C as shown in Figure 4B. Following treatment with the DCM extract, there was a significant depletion in cardiac level of cholesterol with concomitant elevation of triglyceride and HDL-C levels. Although the level of LDL-C was reduced in the treated tissues, it was however not statistically significant.
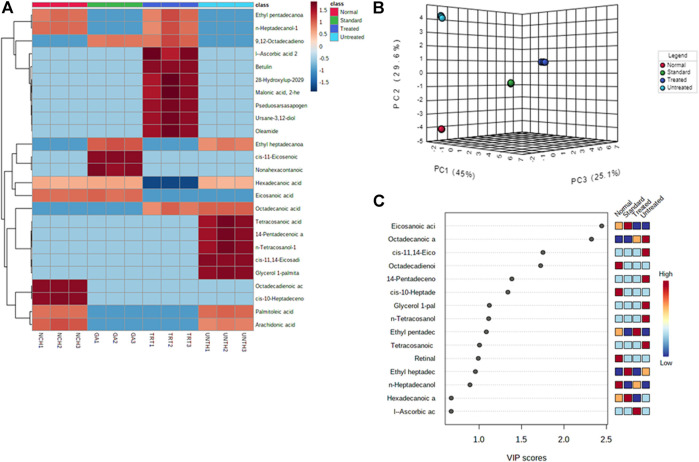
GC-MS analysis of the extracted lipid metabolites revealed the presence of unsaturated fatty acid, saturated fatty acid, fatty ester, fatty alcohol, fatty amide, glycerol and steroids in the cardiac tissue as shown in Table 1. Induction of cardiac tissue led to the complete depletion of octadecadienoic acid (Z,Z)-; cis-10-heptadecenoic acid; eicosanoic acid; n-heptadecanol-1 and retinal; with concomitant generation of 14-pentadecenoic acid; octadecanoic acid; tetracosanoic acid; cis-11,14-eicosadienoic acid, methyl ester; n-tetracosanol-1 and glycerol 1-palmitate. Treatment with the DCM extract led to the depletion of oxidative generated 14-pentadecenoic acid; tetracosanoic acid; cis-11,14-eicosadienoic acid, methyl ester; n-tetracosanol-1 and glycerol 1-palmitate, with concomitant generation of 9,12-Octadecadienoic acid (Z,Z)-; cis-11-eicosenoic acid; malonic acid, 2-hexyl tetradecyl ester; l-(+)-Ascorbic acid 2,6-dihexadecanoate; pentanoic acid, heptadecyl ester; 13-tetradecen-1-ol acetate; oleamide and pseduosarsasapogenin-5,20-dien methyl ether. Non lipid metabolites consisting of ursane-3,12-diol; betulin and 28-hydroxylup-20(29)-ene-3,21-dione were also identified in cardiac tissues treated with the DCM extract.
Distinct changes and distribution of the cardiac metabolites were observed for all the experimental groups as portrayed by the negative values and heat intensity of the heat map following clustering analysis (Figure 5A). This is also supported by the distinctive changes between the pair wise score plots between the selected principal components (PCs) of metabolites from the treated tissues and that of the untreated (Figure 5B). This is further corroborated by the distribution of the important identified features (metabolites) and their respective concentrations in each experimental group by Partial Least Squares - Discriminant Analysis (PLS-DA) as shown in Figure 5C.
FIGURE 5.
(A) Heat map; (B) PC and (C) VIP scores of identified cardiac lipid metabolites. NCH = normal cardiac tissues; UNTH = untreated cardiac tissues; TRT: T. oblongata–treated cardiac tissues; and GA: gallic acid–treated cardiac tissues.
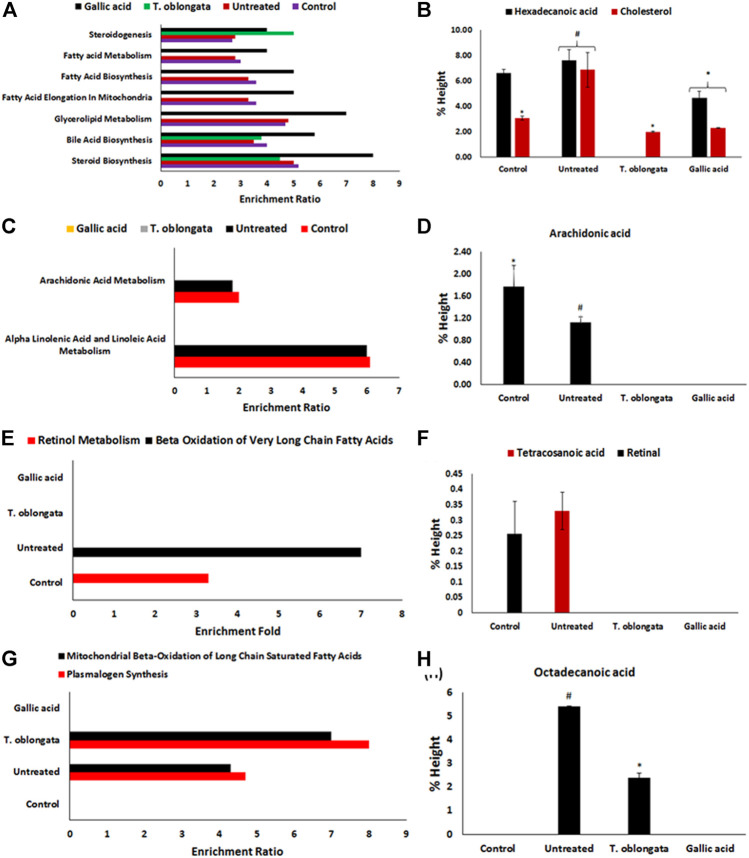
Pathway enrichment of the identified cardiac lipid metabolites revealed an inactivation of retinol metabolism pathway, with concomitant activation of beta oxidation of very long chain fatty acids, plasmalogen synthesis and mitochondrial beta-oxidation of long chain saturated fatty acids pathways as depicted in Figure 6 and Table 2. Treatment with the DCM extract did not reactivate the retinol metabolism pathway but inactivated oxidative-activated beta oxidation of very long chain fatty acids. It further inactivated arachidonic acid metabolism, glycerolipid metabolism, fatty acid elongation in mitochondria, fatty acid biosynthesis, fatty acid metabolism and steroidogenesis pathways. The oxidative-activated pathways were inactivated in cardiac tissues treated with gallic acid.
FIGURE 6.
(A) Fold enrichment for steroid biosynthesis, bile acid biosynthesis, glycerolipid metabolism, fatty acid elongation in mitochondria, fatty acid biosynthesis, fatty acid metabolism and steroidogenesis pathways and their (B) metabolites concentrations; (C) fold enrichment for alpha linolenic acid and linoleic acid metabolism, and arachidonic acid metabolism pathways and their (D) metabolites; (E) fold enrichment for beta oxidation of very long chain fatty acids and retinol metabolism pathways and their (F) metabolites; (G) fold enrichment for plasmalogen synthesis and mitochondrial beta-oxidation of long chain saturated fatty acids and their (H) metabolites.
TABLE 2.
Identified pathways in experimental cardiac tissues.
| Pathways | Normal | Untreated | T. Oblongata | Gallic acid |
|---|---|---|---|---|
| Steroid biosynthesis | X | X | X | X |
| Bile acid biosynthesis | X | X | X | X |
| Alpha linolenic acid and linoleic acid metabolism | X | X | – | – |
| Glycerolipid metabolism | X | X | – | X |
| Fatty acid elongation in mitochondria | X | X | – | X |
| Fatty acid biosynthesis | X | X | – | X |
| Retinol metabolism | X | – | – | – |
| Fatty acid metabolism | X | X | – | X |
| Steroidogenesis | X | X | X | X |
| Arachidonic acid metabolism | X | X | – | – |
| Beta oxidation of very long chain fatty acids | – | X | – | – |
| Plasmalogen synthesis | – | X | X | – |
| Mitochondrial beta-oxidation of long chain saturated fatty acids | – | X | X | – |
X = detected; – = not detected.
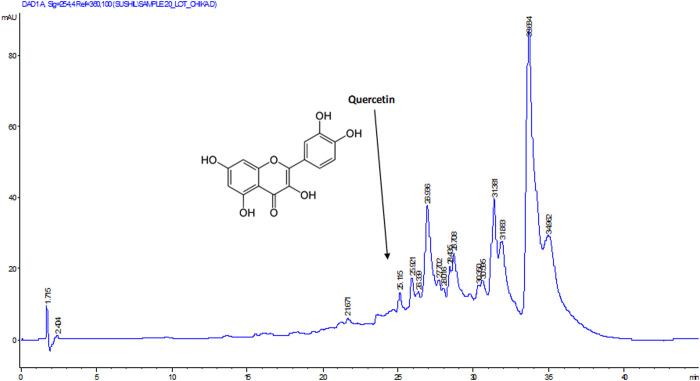
To determine the bioactive compound that may be responsible for the studied biological activity of the DCM extract, the extract was subjected to HPLC analysis. As depicted in Figure 7, quercetin was identified as the active compound in the extract.
FIGURE 7.
HPLC chromatogram of DCM extract of T. oblongata.
Discussion
Cardiotoxicity is a major global health issue as it has been implicated in the pathogenesis of cardiovascular dysfunction which may lead to cardiac failure if not well treated and managed (Poulter, 2003; Wold et al., 2005; Li et al., 2019). Several factors including lipotoxicity, oxidative imbalance, exacerbated ACE activity and altered bioenergetics have been implicated in the pathophysiology of cardiovascular diseases. The high cost associated with its treatment coupled with the side effect of synthesized drugs are of major concerns to health practitioners. This have led to an increased paradigm shift to natural products for cheap and affordable drugs with little or no side effects. The present study reports the cardioprotective effect of T. oblongata and the possible mechanism and pathways by which it brings about its effect.
Oxidative stress has been implicated as a major mechanism in the pathogenesis and progression of cardiotoxicity and other cardiological dysfunctions (Fearon and Faux, 2009; Angsutararux et al., 2015). This has been attributed to suppressed endogenous antioxidant activity owing to increased free radical productions in the cardiac tissues. In the present study, the depleted GSH level, SOD and catalase activities (Figures 1A–C) depicts an onset of oxidative stress following induction of oxidative cardiac injury. This is further evidenced by the elevated cardiac MDA level (Figure 1D) which indicates a peroxidative effect on the cardiac lipids following the induction of oxidative cardiac injury. These altered activities and levels correspond with previous reports on the occurrence of oxidative stress after induction of oxidative injury in isolated hearts (Erukainure et al., 2020a; Salau et al., 2020b). The onset of oxidative stress can be attributed to iron-catalyzed Fenton’s and Haber-Weiss reactions following the incubation of heart tissues with Fe2+. The exacerbated GSH level, SOD and catalase activities, with concomitant suppressed MDA level in cardiac tissues treated with the DCM extract of T. oblongata indicate an antioxidative and anti-peroxidative effect of the extract. This corroborates previous reports on the antioxidant cardioprotective effect of plants (Wattanapitayakul et al., 2005; Adegbola et al., 2017). The antioxidative activity of the extract may be attributed to the identified flavonoid, quercetin (Figure 7). Quercetin is among the common flavonoids found in most fruits, herbs and vegetables, and has been reported for its potent antioxidant and cardioprotective properties (Zhang et al., 2011; Chen et al., 2013).
Increased ACE activity has been implicated in the pathophysiology of cardiotoxicity and other cardiovascular dysfunctions (Ferrario and Strawn, 2006; Salau et al., 2020b). The enzyme catalyzes the hydrolysis of angiotensin I to angiotensin II, with the latter possessing a vasoconstrictive effect leading to hypertension, myocyte death and hypertrophy (Fiordaliso et al., 2000; Agunloye et al., 2019). The increased ACE activity following the induction of oxidative cardiac injury (Figure 2A), therefore suggests an exacerbated cardiac level angiotensin II. The depleted ACE activity following treatment with the DCM extract of T. oblongata indicates an ACE-inhibitory effect of the extract, thus depicting reduced cardiac levels of angiotensin II. This corroborates the ability of the extract to inhibit ACE activity in vitro (Table 1 and Supplementary Figure S1). ACE-inhibitors have been reported as potent therapies in the treatment and management of cardiovascular diseases, which may be part of the possible mechanism by which the extract protect against oxidative-mediated cardiotoxicity. This corroborates previous reports on the use medicinal plants as ACE inhibitors (Khan and Kumar, 2019; Le et al., 2020). The ACE inhibitory activity of the extract may be attributed to the presence of quercetin, which has also been reported for its ACE inhibitory activity (Huang et al., 2017; Luo et al., 2017).
The elevated acetylcholinesterase activity following the induction of oxidative cardiac injury (Figure 2B) suggests a decreased cardiac level of acetylcholine, thus depicting a cholinergic dysfunction. This can be attributed to the fact the enzyme catalyzes the hydrolysis of acetylcholine to acetate and choline. Acetylcholine is important in muscular contraction and also facilitates vasodilation via the muscarinic receptors (Kellogg et al., 2005; Agunloye et al., 2019), thus indicating that decreased concentrations could have detrimental effect on the normal physiological function of the heart. Several studies have reported acetylcholinesterase inhibition as a therapeutic mechanism in the treatment and management of cardiotoxicity and other cardiovascular dysfunctions (Gavioli et al., 2014; Roy et al., 2014; Wu et al., 2017). The decreased acetylcholinesterase in the treated cardiac tissues therefore suggests a therapeutic effect against oxidative-mediated cardiotoxicity. This corresponds with several reports on the ability of medicinal plants to inhibit cardiac acetylcholinesterase activities (Agunloye et al., 2019; Salau et al., 2020b).
Purinergic enzyme activities have been reported to play an important role in the normal physiological activities of the heart. These enzymes phospho-hydrolyze adenosine triphosphate (ATP) and adenosine monophosphate (AMP) to produce the endogenous signaling nucleotide, adenosine which has been reported as a potent dilator and an important factor in bioenergetics (Das and Maulik, 1996; Burnstock, 2017). The increased ATPase and decreased ENTPDase activities following the induction of oxidative cardiac injury (Figures 3A,B) indicate a decreased cardiac level of ATP and adenosine. These levels in the untreated cardiac tissues corresponds with previous reports on altered purinergic activities in oxidative injuries (Salau et al., 2020a; Erukainure et al., 2020b). The increased ATPase activity suggests a diminished cardiac level of ATP and depicts an altered bioenergetic activity as ATP is an important energy-signaling molecule. Altered cardiac bioenergetics have been implicated in the pathophysiology of oxidative stress, cardiotoxicity and other cardiovascular dysfunctions (Das and Maulik, 1996). Both ATP and adenosine have been reported for their vasodilatory activities in coronary vessels (Kirby et al., 2010). The decreased ATPase and elevated ENTPDase activities following treatment with T. oblongata DCM extract therefore indicates an improved purinergic activity which may suggest improved bioenergetics.
The normal heart function depends on lipids as its energy fuel for its physiological functions via hydrolysis of cardiac triglyceride by lipase to fatty acids (Ritterhoff and Tian, 2017). The heart does not produce triglyceride and thus depends on its supply from the liver. The elevated lipase activity following the induction of oxidative cardiac injury (Figure 4A) therefore suggests an incessant breakdown of cardiac triglyceride, which corroborates with the depleted cardiac level of triglyceride (Figure 4B). The elevated lipase activity and reduced triglyceride level in the untreated cardiac tissues suggests abnormality in FA supply and utilization. This may further indicate a suppressed ATP level which also corroborates the increased ATPase activity (Figure 3A). This may therefore portray a metabolic dependence on aerobic glycolysis for cardiac energy (ATP) supply (Stanley, 2001; Salau et al., 2020b). The depleted lipase activity and elevated triglyceride level in cardiac tissues treated with T. oblongata extract therefore indicate an arrest of incessant lipolysis of triglyceride and improved lipid utilization, which also suggests an improved cardiac bioenergetic.
The elevated cholesterol and LDL-C, with concomitant depleted HDL-C following the induction of oxidative cardiac injury (Figure 4B) indicates a disrupted cardiac lipid spectrum and insinuate a cardiac lipotoxic effect which has been linked to the pathophysiology of cardiotoxicity (Poulter, 2003). High concentrations of cholesterol and LDL-C have also been implicated in vasoconstriction and linked to most cardiovascular dysfunctions (Lamping et al., 1994; Pfister and Campbell, 1996; Kopkan et al., 2009). The decreased cholesterol and LDL-C levels as well as elevated HDL-C level on treatment with T. oblongata therefore indicates an antilipemic effect of the extract against oxidative-disrupted cardiac lipid profile. This correlates with previous reports on the ability of medicinal plants to maintain cardiac lipid spectrum in oxidative-mediated cardiotoxicity (Erukainure et al., 2020a).
An occurrence of cardiac lipotoxicity on induction of oxidative cardiac injury was further depicted by dysregulated lipid metabolic pathways and altered lipid metabolites (Tables 2, 3 and Figures 5, 6). Dysregulated lipid metabolism in the heart leading to free fatty acids (FFAs) have been implicated among the pathophysiology of cardiotoxicity and other cardiovascular dysfunctions (Antozzi and Zeviani, 1997; Ventura-Clapier et al., 2004). The elevated cardiac level of cholesterol in the untreated tissues corroborates that of the cardiac lipid spectrum (Figure 4A) and the major metabolites for steroid biosynthesis, bile acid biosynthesis and steroidogenesis pathways (Figures 6A,B). The inactivation of retinol metabolism in the untreated cardiac tissue can be attributed to the complete depletion of retinal (Figures 6E,F) and corroborates previous reports linking the downregulation of the pathway to impaired cardiac lipid metabolism and heart failure (Osorio et al., 2002; Lee et al., 2014). The activation of beta oxidation of very long chain fatty acids and mitochondrial beta-oxidation of long chain saturated fatty acids pathways can be attributed to the metabolites octadecanoic acid and tetracosanoic acid (Figures 6E–H). The incessant activation of these pathways has been implicated in increased production of NADH, FADH2 and acetyl-CoA (Marín-García and Goldenthal, 2002). Acetyl-CoA is further broken down in the tricarboxylic acid (TCA) cycle to generate NADH, FADH2 and ATP. High concentrations of these electron carriers have been reported to cause a high mitochondrial membrane potential leading to the inhibition of the electron transport (Brownlee, 2001; Du et al., 2001). This automatically reduces oxygen (O2) to superoxide (O2 −). Thus, it can be insinuated that the continuous production of O2 − and concomitant low SOD activity (Figure 1B) in the untreated tissues may be an oxidative mechanism on induction of oxidative cardiac injury.
TABLE 3.
GC-MS identified lipid metabolites in experimental cardiac tissues.
| Classes | Compounds | Control | Untreated | T. oblongata | Gallic acid |
|---|---|---|---|---|---|
| Unsaturated fatty acid | Palmitoleic acid | 1.84 ± 0.18 | 2.62 ± 0.16 | ND | ND |
| Octadecadienoic acid (Z,Z)- | 3.56 ± 0.12 | ND | ND | ND | |
| cis-10-Heptadecenoic acid | 0.87 ± 0.10 | ND | ND | ND | |
| Eicosanoic acid | 5.19 ± 0.10 | ND | ND | 3.49 ± 0.29 | |
| Arachidonic acid | 1.78 ± 0.38 | 1.12 ± 0.10 | ND | ND | |
| 14-Pentadecenoic acid | ND | 1.34 ± 0.29 | ND | ND | |
| Octadecanoic acid | 5.42 ± 0.01 | 2.38 ± 0.21 | ND | ||
| 9,12-Octadecadienoic acid (Z,Z)– | ND | ND | 1.48 ± 0.15 | 0.73 ± 0.11 | |
| cis-11-Eicosenoic acid | ND | ND | ND | 1.01 ± 0.10 | |
| Saturated fatty acid | Hexadecanoic acid | 6.60 ± 0.33 | 7.60 ± 087 | ND | 4.68 ± 0.50 |
| Tetracosanoic acid | ND | 0.33 ± 0.06 | ND | ND | |
| Fatty ester | Ethyl hexadecanoate | 1.28 ± 0.24 | 1.11 ± 0.21 | 1.39 ± 0.27 | 1.68 ± 0.27 |
| Ethyl pentadecanoate | 2.78 ± 0.61 | 2.44 ± 0.50 | 2.84 ± 0.57 | 2.72 ± 0.52 | |
| cis-11,14-Eicosadienoic acid, methyl ester | ND | 5.06 ± 0.40 | ND | ND | |
| Ethyl heptadecanoate | ND | ND | ND | ND | |
| Malonic acid, 2-hexyl tetradecyl ester | ND | ND | 0.15 ± 0.01 | ND | |
| l–(+)–Ascorbic acid 2,6-dihexadecanoate | ND | ND | 11.96 ± 9.16 | ND | |
| Pentanoic acid, heptadecyl ester | ND | ND | 0.17 ± 0.01 | ND | |
| Fatty alcohol | Pentadecanol | 0.37 ± 0.03 | 1.59 ± 0.30 | 0.32 ± 0.02 | 0.85 ± 0.10 |
| n-Heptadecanol-1 | 0.86 ± 0.07 | ND | 0.72 ± 0.09 | ND | |
| n-Tetracosanol-1 | ND | 0.49 ± 0.09 | ND | ND | |
| 13-Tetradecen-1-ol acetate | ND | ND | 0.20 ± 0.03 | ND | |
| Fatty amide | Oleamide | ND | ND | 0.26 ± 0.03 | ND |
| Glycerol | Glycerol 1-palmitate | ND | 0.50 ± 0.03 | ND | ND |
| Steroids | Retinal | 0.26 ± 0.11 | ND | ND | ND |
| Cholesterol | 3.08 ± 0.18 | 6.87 ± 1.36 | 1.99 ± 0.04 | 2.29 ± 0.02 | |
| Pseduosarsasapogenin-5,20-dien methyl ether | ND | ND | 0.34 ± 0.06 | ||
| Non-lipid | Ursane-3,12-diol | ND | ND | 0.35 ± 0.04 | ND |
| Betulin | ND | ND | 0.38 ± 0.13 | ND | |
| 28-Hydroxylup-20(29)-ene-3,21-dione | ND | ND | 0.40 ± 0.01 | ND | |
| Nonahexacontanoic acid | ND | ND | ND | 0.32 ± 0.02 |
Values = mean ± SD; n = 3. ND = not detected.
Inactivation of beta oxidation of very long chain fatty acids in tissues treated with T. oblongata DCM extract therefore suggests a decreased production of the electron carriers which depicts a suppressed production of O2 −. This corroborates the absence of tetracosanoic acid and the high SOD activity. The inactivation of alpha linolenic acid and linoleic acid metabolism, glycerolipid metabolism, fatty acid elongation in mitochondria, fatty acid biosynthesis, and fatty acid metabolism suggests a possible hypolipidemic mechanism by which the extract mitigates oxidative-mediated lipid dysmetabolism in oxidative-mediated cardiotoxicity.
As depicted in Figure 7, quercetin was identified as an active component of the DCM fraction of T. oblongata. Quercetin ranks among the most popular phenolics as it is commonly found in fruits, vegetables, and herbs (Zhang et al., 2011). Its potent antioxidant properties have been reported and has been exploited in the treatment and management of various oxidative-mediated ailments such as diabetes, cardiovascular diseases, and cancer (Zhang et al., 2011; Georgiev et al., 2014; Patel et al., 2018). The studied activities of T. oblongata in the present study may therefore be attributed to the presence of quercetin, which may also work in synergy with other unidentified phytochemicals.
Conclusion
These results indicate the cardioprotective effect of T. oblongata against oxidative-mediated cardiotoxicity. This is evidenced by the ability of its DCM extract to mitigate lipotoxicity and modulate dysregulated cardiometabolic activities as shown by its antioxidative activity and suppressive effects on ACE, acetylcholinesterase and lipase activities, while modulating cardiac lipid dysmetabolism. The presence of quercetin in the extract may contribute to these activities. However, further in vivo studies are recommended to identify the molecular mechanism that maybe involved in the cardioprotective effect of the extract and its bioactive compounds.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the animal ethical committee of the University of KwaZulu-Natal, Durban, South Africa.
Author Contributions
Conceptualization: MM, MJ, CC and OLE; Plant material and Indigenous Usage: MJ and MM; Experiments: OE; CC; VS; and MI. Metabolomics: OE and NK; Original manuscript draft: OE; CC and VS; Manuscript editing: all authors; Funding and Supervision: MM.
Funding
This work was supported by research funding from the National Research Foundation; and the Department of Science and Innovation (DSI) through the IKS-Based Technology Innovations. OE is thankful to the University of the Free State, Bloemfontein, South Africa for Incentives for Rated Researchers (2019060769); and the National Research Foundation (NRF) for Scarce Skills Postdoctoral Research Grant (UID: 132822).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.610835/full#supplementary-material
References
- Adegbola P., Aderibigbe I., Hammed W., Omotayo T. (2017). Antioxidant and Anti-inflammatory Medicinal Plants Have Potential Role in the Treatment of Cardiovascular Disease: a Review. Am. J. Cardiovasc. Dis. 7 (2), 19–32. [PMC free article] [PubMed] [Google Scholar]
- Adewoye O. E., Bolarinwa A. F., Olorunsogo O. O. (2000). Ca++, Mg++-ATPase Activity in Insulin-dependent and Non-insulin Dependent Diabetic Nigerians. Afr. J. Med. Med. Sci. 29 (3–4), 195–199. [PubMed] [Google Scholar]
- Aebi H. (1984). “[13] Catalase In Vitro,” in Meth. Enzymol (Elsevier; ), 105, 121–126. 10.1016/s0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- Agunloye O. M., Oboh G., Ademiluyi A. O., Ademosun A. O., Akindahunsi A. A., Oyagbemi A. A., et al. (2019). Cardio-protective and Antioxidant Properties of Caffeic Acid and Chlorogenic Acid: Mechanistic Role of Angiotensin Converting Enzyme, Cholinesterase and Arginase Activities in Cyclosporine Induced Hypertensive Rats. Biomed. Pharmacother. 109, 450–458. 10.1016/j.biopha.2018.10.044 [DOI] [PubMed] [Google Scholar]
- Akomolafe S. F., Akinyemi A. J., Ogunsuyi O. B., Oyeleye S. I., Oboh G., Adeoyo O. O., et al. (2017). Effect of Caffeine, Caffeic Acid and Their Various Combinations on Enzymes of Cholinergic, Monoaminergic and Purinergic Systems Critical to Neurodegeneration in Rat Brain-In Vitro. NeuroToxicology 62, 6–13. 10.1016/j.neuro.2017.04.008 [DOI] [PubMed] [Google Scholar]
- Angsutararux P., Luanpitpong S., Issaragrisil S. (2015). Chemotherapy-induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid. Med. Cel. Longev. 2015, 795602. 10.1155/2015/795602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antozzi C., Zeviani M. (1997). Cardiomyopathies in Disorders of Oxidative Metabolism. Cardiovasc. Res. 35, 184–199. 10.1016/s0008-6363(97)00141-7 [DOI] [PubMed] [Google Scholar]
- Brownlee M. (2001). Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 414 (6865), 813–820. 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2017). Purinergic Signaling in the Cardiovascular System. Circ. Res. 120 (1), 207–228. 10.1161/circresaha.116.309726 [DOI] [PubMed] [Google Scholar]
- Chen J-Y., Hu R-Y., Chou H-C. (2013). Quercetin-induced Cardioprotection against Doxorubicin Cytotoxicity. J. Biomed. Sci. 20 (1), 95. 10.1186/1423-0127-20-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J., Soufan O., Li C., Caraus I., Li S., Bourque G., et al. (2018). MetaboAnalyst 4.0: towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 46 (W1), W486–W494. 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury P., Soulsby M. (2002). Lipid Peroxidation in Rat Brain Is Increased by Simulated Weightlessness and Decreased by a Soy-Protein Diet. Ann. Clin. Lab. Sci. 32, 188–192. [PubMed] [Google Scholar]
- Das D., Maulik N. (1996). “Bioenergetics, Ischemic Contracture and Reperfusion Injury,” in Myocardial Ischemia: Mechanisms, Reperfusion, Protection (Springer; ), 76, 155–173. 10.1007/978-3-0348-8988-9_10 [DOI] [PubMed] [Google Scholar]
- Drosatos K., Schulze P. C. (2013). Cardiac Lipotoxicity: Molecular Pathways and Therapeutic Implications. Curr. Heart Fail. Rep. 10 (2), 109–121. 10.1007/s11897-013-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X. L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. (2001). Hyperglycemia Inhibits Endothelial Nitric Oxide Synthase Activity by Posttranslational Modification at the Akt Site. J. Clin. Invest. 108 (9), 1341–1348. 10.1172/jci11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G. L., Courtney K. D., Andres V., Jr, Featherstone R. M. (1961). A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 7, 88–95. 10.1016/0006-2952(61)90145-9 [DOI] [PubMed] [Google Scholar]
- Ellman G. L. (1959). Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 82, 70–77. 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- Erukainure O. L., Mopuri R., Oyebode O. A., Koorbanally N. A., Islam M. S. (2017). Dacryodes Edulis Enhances Antioxidant Activities, Suppresses DNA Fragmentation in Oxidative Pancreatic and Hepatic Injuries; and Inhibits Carbohydrate Digestive Enzymes Linked to Type 2 Diabetes. Biomed. Pharmacother. 96, 37–47. 10.1016/j.biopha.2017.09.106 [DOI] [PubMed] [Google Scholar]
- Erukainure O. L., Chukwuma C. I., Matsabisa M. G., Salau V. F., Koorbanally N. A., Islam M. S. (2020a). Buddleja Saligna Willd (Loganiaceae) Inhibits Angiotensin-Converting Enzyme Activity in Oxidative Cardiopathy with Concomitant Modulation of Nucleotide Hydrolyzing Enzymatic Activities and Dysregulated Lipid Metabolic Pathways. J. Ethnopharmacology 248, 112358. 10.1016/j.jep.2019.112358 [DOI] [PubMed] [Google Scholar]
- Erukainure O. L., Salau V. F., Oyenihi A. B., Mshicileli N., Islam M. S. (2020b). Strawberry Fruit (Fragaria X Ananassa Cv. Romina) Extenuates Iron-Induced Cardiac Oxidative Injury via Effects on Redox Balance, Angiotensin-Converting Enzyme, Purinergic Activities, and Metabolic Pathways. J. Food Biochem. 44 (8), e13315. 10.1111/jfbc.13315 [DOI] [PubMed] [Google Scholar]
- Fearon I. M., Faux S. P. (2009). Oxidative Stress and Cardiovascular Disease: Novel Tools Give (Free) Radical Insight. J. Mol. Cell Cardiol. 47, 372–381. 10.1016/j.yjmcc.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Ferrario C. M., Strawn W. B. (2006). Role of the Renin-Angiotensin-Aldosterone System and Proinflammatory Mediators in Cardiovascular Disease. Am. J. Cardiol. 98, 121–128. 10.1016/j.amjcard.2006.01.059 [DOI] [PubMed] [Google Scholar]
- Fiordaliso F., Li B., Latini R., Sonnenblick E. H., Anversa P., Leri A., et al. (2000). Myocyte Death in Streptozotocin-Induced Diabetes in Rats Is Angiotensin II- Dependent. Lab. Invest. 80, 513–527. 10.1038/labinvest.3780057 [DOI] [PubMed] [Google Scholar]
- Foden W., Potter L. (2005). Ipomoea Oblongata E.Mey. Ex Choisy. National Assessment. Red List of South African plants version 2020.1. [Online]. Available: http://redlist.sanbi.org/species.php?species=1625-89 (Accessed August 13, 2020).
- Gavioli M., Lara A., Almeida P. W. M., Lima A. M., Damasceno D. D., Rocha-Resende C., et al. (2014). Cholinergic Signaling Exerts Protective Effects in Models of Sympathetic Hyperactivity-Induced Cardiac Dysfunction. PLoS One 9, e100179. 10.1371/journal.pone.0100179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B., Bünning P., Riordan J. F. (1979). A Continuous Spectrophotometric Assay for Angiotensin Converting Enzyme. Anal. Biochem. 95, 540–548. 10.1016/0003-2697(79)90769-3 [DOI] [PubMed] [Google Scholar]
- Huang W-Y., Fu L., Li C-Y., Xu L-P., Zhang L-X., Zhang W-M. (2017). Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects. J. Food Sci. 82, 1239–1246. 10.1111/1750-3841.13706 [DOI] [PubMed] [Google Scholar]
- Hutchings A., Scott A., Lewis G., Cunningham A. (1996). Zulu Medicinal Plants. Pietermaritzburg. South Africa: University of Natal Press. [Google Scholar]
- Jiménez-González S., Marín-Royo G., Jurado-López R., Bartolomé M. V., Romero-Miranda A., Luaces M., et al. (2020). The Crosstalk between Cardiac Lipotoxicity and Mitochondrial Oxidative Stress in the Cardiac Alterations in Diet-Induced Obesity in Rats. Cells 9, 451. 10.3390/cells9020451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar P., Das B., Viswanathan P. (1984). A Modified Spectrophotometric Assay of Superoxide Dismutase. Indian J. Biochem. Biophys. 21 (2), 130–132. [PubMed] [Google Scholar]
- Kellogg D. L., Jr, Zhao J. L., Coey U., Green J. V. (2005). Acetylcholine-induced Vasodilation Is Mediated by Nitric Oxide and Prostaglandins in Human Skin. J. Appl. Physiol. 98, 629–632. 10.1152/japplphysiol.00728.2004 [DOI] [PubMed] [Google Scholar]
- Khan M. Y., Kumar V. (2019). Mechanism & Inhibition Kinetics of Bioassay-Guided Fractions of Indian Medicinal Plants and Foods as ACE Inhibitors. J. Traditional Complement. Med. 9, 73–84. 10.1016/j.jtcme.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Lee Y. M., Kim H., Kim J., Jang D. S., Kim J. H., et al. (2010). Anti-obesity Effect of Morus Bombycis Root Extract: Anti-lipase Activity and Lipolytic Effect. J. Ethnopharmacology 130, 621–624. 10.1016/j.jep.2010.05.053 [DOI] [PubMed] [Google Scholar]
- Kirby B. S., Crecelius A. R., Voyles W. F., Dinenno F. A. (2010). Vasodilatory Responsiveness to Adenosine Triphosphate in Ageing Humans. J. Physiol. 588, 4017–4027. 10.1113/jphysiol.2010.197814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopkan L., Khan M. A. H., Lis A., Awayda M. S., Majid D. S. A. (2009). Cholesterol Induces Renal Vasoconstriction and Anti-natriuresis by Inhibiting Nitric Oxide Production in Anesthetized Rats. Am. J. Physiology-Renal Physiol. 297, F1606–F1613. 10.1152/ajprenal.90743.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose L. S., Moteetee A., Van Vuuren S. (2015). Ethnobotanical Survey of Medicinal Plants Used in the Maseru District of Lesotho. J. Ethnopharmacol. 170, 184–200. 10.1016/j.jep.2015.04.047 [DOI] [PubMed] [Google Scholar]
- Lamping K. G., Piegors D. J., Benzuly K. H., Armstrong M. L., Heistad D. D. (1994). Enhanced Coronary Vasoconstrictive Response to Serotonin Subsides after Removal of Dietary Cholesterol in Atherosclerotic Monkeys. Arterioscler Thromb. 14, 951–957. 10.1161/01.atv.14.6.951 [DOI] [PubMed] [Google Scholar]
- Le X. T., Phi X. T., Phi X. T., Nguyen D. T. T., Pham H. T. N., Nguyen Van T., et al. (2020). Angiotensin-converting Enzyme Inhibitory Activity of Some Vietnamese Medicinal Plants. Vjste 62, 77–82. 10.31276/vjste.62(2).77-82 [DOI] [Google Scholar]
- Lee S-A., Jiang H., Trent C. M., Yuen J. J., Narayanasamy S., Curley R. W., Jr, et al. (2014). Cardiac Dysfunction in β-carotene-15,15′-dioxygenase-deficient Mice Is Associated with Altered Retinoid and Lipid Metabolism. Am. J. Physiology-Heart Circulatory Physiol. 307, H1675–H1684. 10.1152/ajpheart.00548.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Chen L., Yu Y., Yang B., Li P., Tan X. Q. (2019). Resveratrol Alleviates Hypoxia/reoxygenation Injury induced Mitochondrial Oxidative Stress in Cardiomyocytes. Mol. Med. Rep. 19, 2774–2780. 10.3892/mmr.2019.9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Zhang C., Liu Q., Ou S., Zhang L., Peng X. (2017). Combinative Effect of Sardine Peptides and Quercetin Alleviates Hypertension through Inhibition of Angiotensin I Converting Enzyme Activity and Inflammation. Food Res. Int. 100, 579–585. 10.1016/j.foodres.2017.07.019 [DOI] [PubMed] [Google Scholar]
- Marín-García J., Goldenthal M. J. (2002). Fatty Acid Metabolism in Cardiac Failure: Biochemical, Genetic and Cellular Analysis. Cardiovasc. Res. 54, 516–527. 10.1016/s0008-6363(01)00552-1 [DOI] [PubMed] [Google Scholar]
- Osorio J. C., Stanley W. C., Linke A., Castellari M., Diep Q. N., Panchal A. R., et al. (2002). Impaired Myocardial Fatty Acid Oxidation and Reduced Protein Expression of Retinoid X Receptor-α in Pacing-Induced Heart Failure. Circulation 106, 606–612. 10.1161/01.cir.0000023531.22727.c1 [DOI] [PubMed] [Google Scholar]
- Pfister S. L., Campbell W. B. (1996). Reduced Pulmonary Artery Vasoconstriction to Methacholine in Cholesterol-Fed Rabbits. Hypertension 27, 804–810. 10.1161/01.hyp.27.3.804 [DOI] [PubMed] [Google Scholar]
- Poulter N. (2003). Global Risk of Cardiovascular Disease. Heart 89, ii2–ii5. 10.1136/heart.89.suppl_2.ii2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston-Hooper K., Jannasch A., Adamec J., Sepúlveda M. (2011). “The Use of Two-Dimensional Gas Chromatography–Time-Of-Flight Mass Spectrometry (Gc× Gc–Tof-Ms) for Metabolomic Analysis of Polar Metabolites,” in Metabolic Profiling. Editor Metz T. O. (Springer; ), 708, 205–211. 10.1007/978-1-61737-985-7_12 [DOI] [PubMed] [Google Scholar]
- Ritterhoff J., Tian R. (2017). Metabolism in Cardiomyopathy: Every Substrate Matters. Cardiovasc. Res. 113, 411–421. 10.1093/cvr/cvx017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Guatimosim S., Prado V. F., Gros R., Prado M. A. M. (2014). Cholinergic Activity as a New Target in Diseases of the Heart. Mol. Med. 20, 527–537. 10.2119/molmed.2014.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salau V. F., Erukainure O. L., Ibeji C. U., Olasehinde T. A., Koorbanally N. A., Islam M. S. (2020a). Vanillin and Vanillic Acid Modulate Antioxidant Defense System via Amelioration of Metabolic Complications Linked to Fe 2+-induced Brain Tissues Damage. Metab. Brain Dis. 35 (5), 727–738. 10.1007/s11011-020-00545-y [DOI] [PubMed] [Google Scholar]
- Salau V. F., Erukainure O. L., Islam M. S. (2020b). Caffeic Acid Protects against Iron-Induced Cardiotoxicity by Suppressing Angiotensin-Converting Enzyme Activity and Modulating Lipid Spectrum, Gluconeogenesis and Nucleotide Hydrolyzing Enzyme Activities. Biol. Trace Elem. Res. 199 (3), 1052–1061. 10.1007/s12011-020-02227-3 [DOI] [PubMed] [Google Scholar]
- Semenya S. S., Madamombe-Manduna I., Mashele S. S., Polori K. L. (2018). Ethno-medical Botany and Some Biological Activities of Ipomoea Oblongata Collected in the Free State Province, South Africa. J. Biol. Sci. 18, 441–449. 10.4314/ahs.v18i4.29 [DOI] [Google Scholar]
- Shalaby S. M., Zakora M., Otte J. (2006). Performance of Two Commonly Used Angiotensin-Converting Enzyme Inhibition Assays Using FA-PGG and HHL as Substrates. J. Dairy Res. 73, 178–186. 10.1017/s0022029905001639 [DOI] [PubMed] [Google Scholar]
- Srivastava D., Rauniyar N. (2020). Medicinal Plants of Genus Ipomoea. Beau Bassin. Mauritius: LAP LAMBERT Academic Publishing. [Google Scholar]
- Stanley W. C. (2001). Cardiac Energetics during Ischaemia and the Rationale for Metabolic Interventions. Coron. Artery Dis. 12 (Suppl. 1), S3–S7. [PubMed] [Google Scholar]
- Udenigwe C. C., Lin Y-S., Hou W-C., Aluko R. E. (2009). Kinetics of the Inhibition of Renin and Angiotensin I-Converting Enzyme by Flaxseed Protein Hydrolysate Fractions. J. Funct. Foods 1, 199–207. 10.1016/j.jff.2009.01.009 [DOI] [Google Scholar]
- Ventura-Clapier R., Garnier A., Veksler V. (2004). Energy Metabolism in Heart Failure. J. Physiol. 555, 1–13. 10.1113/jphysiol.2003.055095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanapitayakul S. K., Chularojmontri L., Herunsalee A., Charuchongkolwongse S., Niumsakul S., Bauer J. A. (2005). Screening of Antioxidants from Medicinal Plants for Cardioprotective Effect against Doxorubicin Toxicity. Basic Clin. Pharmacol. Toxicol. 96, 80–87. 10.1111/j.1742-7843.2005.pto960112.x [DOI] [PubMed] [Google Scholar]
- Wold L. E., Ceylan-Isik A. F., Ren J. (2005). Oxidative Stress and Stress Signaling: Menace of Diabetic Cardiomyopathy. Acta Pharmacologica Sinica 26, 908–917. 10.1111/j.1745-7254.2005.00146.x [DOI] [PubMed] [Google Scholar]
- Wu S. J., Li Y. C., Shi Z. W., Lin Z. H., Rao Z. H., Tai S. C., et al. (2017). Alteration of Cholinergic Anti-inflammatory Pathway in Rat with Ischemic Cardiomyopathy–Modified Electrophysiological Function of Heart. J. Am. Heart Assoc. 6 (9), e006510. 10.1161/JAHA.117.006510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Swarts S. G., Yin L., Liu C., Tian Y., Cao Y., et al. (2011). Antioxidant Properties of Quercetin. Boston, United States: Springer, 283–289. 10.1007/978-1-4419-7756-4_38 10.1007/978-1-4419-7756-4_38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.