Abstract
Rapidly developing approaches to acquiring and analyzing densely-sampled, single-subject fMRI data have opened new avenues for understanding the neurobiological basis of individual differences in behavior and could allow fMRI to become a more clinically useful tool. Here, we review briefly key insights from these precision functional mapping studies and a highlight significant barrier to their clinical translation. Specifically, that reliable delineation of functional brain networks in individual humans can require hours of resting-state fMRI data per-subject. We found recently that multi-echo fMRI improves the test-retest reliability of resting-state functional connectivity measurements, mitigating the need for acquiring large quantities of per -subject data. Because the benefits of multi-echo acquisitions are most pronounced in clinically important but artifact-prone brain regions, such as the subgenual cingulate and structures deep in the subcortex, this approach has the potential to increase the impact of precision functional mapping routines in both healthy and clinical populations.
Keywords: Precision functional mapping, functional brain networks, multi-echo fMRI, test-retest reliability
Introduction
Over the last two decades, resting-state fMRI has become one of the most widely used tools in cognitive neuroscience and in efforts to understand neuropsychiatric pathophysiology. Functional brain networks can be mapped non-invasively in humans from correlations in resting-state fMRI BOLD signals (Biswal et al. 1995). These correlations, commonly known as resting-state functional connectivity (FC), are thought to reflect synaptic connectivity and other properties of the tissues producing those signals, as well as processes that serve to maintain the brain’s large-scale functional organization(Power, Schlaggar, and Petersen 2014; Buckner, Krienen, and Yeo 2013).
To date, FC analyses have most often been performed at the group-level by pooling relatively small quantities of resting-state data — typically 5–15 minutes — from many persons. The pioneering MyConnectome study (Poldrack et al. 2015; Laumann et al. 2015) was a sharp departure from this approach. In this study, a single individual was scanned twice a week for a year and half—an effort that resulted in an unprecedented amount of per -subject fMRI data and a variety of physiological and psychological measurements. This proof-of-concept study was a springboard for a series of others like it that prioritized sample depth over sample size. These “dense sampling” studies have already provided remarkable new insights regarding the functional brain organization of individual human brains.
Importantly, such efforts also have the potential to become clinically useful. The efficacy of existing therapeutic interventions could in principle be improved by tailoring them to the functional neuroanatomy of individual patients. For example, patient response to brain stimulation therapies, such as repetitive transcranial magnetic stimulation or deep brain stimulation, might be related to individual idiosyncrasies in which functional networks are engaged by the stimulation target (Greene et al. 2019). Theoretically, even specific symptoms might be alleviated by selecting the appropriate functional network as a stimulation target in each patient (Horn and Fox 2020). A key barrier to realizing this level of clinical utility however is the limited reliability of single-subject fMRI data. There is a need for new approaches that can improve the reliability of FC measurements at the individual subject level. Here we review emerging insights from studies of densely-sampled individuals, as well as recent work from our group and others showing how multi-echo fMRI can be used to overcome challenges inherent to the analysis of single-subject fMRI data.
Insights gained from studying densely-sampled individual subjects
Studies of densely-sampled humans have revealed unexpected insights concerning how functional networks are organized across individuals and how they adapt to experience. Here, we highlight just a few examples. First, while many aspects of functional networks are shared across individuals, there are also focal features of an individual’s functional brain organization that deviate from central tendencies in large groups (Laumann et al. 2015; Gordon et al. 2017; Seitzman et al. 2019; Kraus et al. 2021). These deviations have been described as network variants. For example, a given anatomical area may be a node of network A in group-average data, but it may belong to network B in a given individual subject. Similarly, a brain network can appear as a single system in group-averaged data but may be fractionated into functionally distinct subnetworks in individuals (Braga and Buckner 2017; Gordon et al. 2020; DiNicola, Braga, and Buckner 2020). These findings have raised important questions regarding whether or not such individual differences are functionally relevant or epiphenomenon (D’Esposito 2019) and highlighted a significant limitation of group-average analyses. Specifically, when fMRI data from many individuals are co-registered and analyzed in a common atlas space, signals from different functional areas or networks will be artifactually mixed. In this way, individual differences in functional brain organization could obscure brain-behavior relationships traditionally measured at the group level (Bijsterbosch et al. 2018; Marek et al. 2020; Gordon and Nelson 2021).
Second, the topology (the size, shape, spatial arrangement) of functional brain networks in an individual are largely stable across time (Gratton et al. 2018) and cognitive states (Kraus et al. 2021). At the same time, there is also evidence that changes in experience and external inputs can change FC strength between network nodes (without necessarily altering topology per se). In one remarkable example, three healthy young adults casted their dominant arm for 2 weeks and underwent daily resting-state fMRI scanning (Newbold et al. 2020). The authors observed large decreases in FC between cortical regions controlling the disused arm and the rest of the somatomotor network that were restored to baseline levels after cast removal in all three individuals. Whether FC measured at the individual level can be used to track changes in functional brain networks associated with a disease trajectory, treatment response, or other more subtle forms of experimental manipulation is an open question.
There is however a major caveat concerning the stability of an individual’s functional brain organization measured using resting-state FC. Achieving highly reliable individual-specific FC measurements typically requires large amounts of data per -subject: on average, when using standard imaging approaches, 45 minutes may be required in cortex (Gordon et al. 2017) and more than 90 minutes may be required in the cerebellum and subcortex(Marek et al. 2018; Greene et al. 2019)). These requirements for large amounts of data are thought to be at least in part due to random sampling variability and the confounding influence of various image artifacts, including but not limited to head movement (Laumann et al. 2017). Collecting such large quantities of data per-subject is costly and inconvenient under normal conditions and may be an insurmountable obstacle in some clinical contexts. Thus, a major barrier to precision mapping of single subjects realizing its full potential is the limited reliability of FC measurements that can be achieved using quantities of data that can be easily obtained from a single scan.
Multi-echo fMRI
Here we briefly introduce multi-echo fMRI and review advantages and disadvantages of this approach compared to single-echo fMRI, particularly with respect to denoising and improving test-retest reliability. In a typical single-echo fMRI sequence, images are acquired once per tissue excitation after a single fixed delay (“echo time”; usually near 30 ms at 3T). Multi-echo acquisitions collect three or more images per volume at echo times spanning tens of milliseconds (ranging approximately from 10 to 90 ms at 3T). In general, having multiple echoes affords at least two advantages. First, echoes can be combined into a single time-series with improved BOLD contrast and less susceptibility artifact by weighting echoes near the estimated average rate of T2* at each voxel more heavily than those that are not(Posse et al. 1999; Poser et al. 2006). Second, how signals decay across echoes can be used during denoising to separate signals of interest (T2*-dependent or “BOLD-like”) from various forms of noise (S0-dependent or not “not-BOLD-like”). In principle, the rate of T2* decay could be modeled voxel-wise at each time point. However, this approach is sensitive to noise. Multi-echo ICA (ME-ICA) is a commonly used alternative that can classify (without training) spatially structured signals in the optimally-combined time-series as T2*-dependent and S0-dependent, primarily according to their signal decay properties (Kundu et al. 2012, 2013). Readers interested in the details of multi-echo acquisition and denoising are encouraged to refer to an excellent review by (Kundu et al. 2017).
Multiple recent investigations have demonstrated clear advantages of multi-echo acquisitions and denoising. For example,(Power et al. 2018) found that ME-ICA can remove the effects of head motion on resting-state FC, including the tendency for nearby brain regions to exhibit stronger FC than those that are further apart. This investigation also revealed however that ME-ICA is unable to remove spatially diffuse signals induced by changes in respiration (e.g., “deep breaths”), in part because they arise from alterations in pCO2 and are thus T2*-dependent(Power, Lynch, et al. 2019). Additional denoising steps, such as global signal regression or something approximating it (e.g., mean gray matter time-series regression, GODEC) may be necessary after ME-ICA is performed to remove these respiration-induced signals. Another investigation(Dipasquale et al. 2017) directly compared the performance of ME-ICA to other ICA-based algorithms commonly used to denoise single-echo data with respect to their ability to separate motion artifacts from BOLD-like signals of interest in individuals with and without Attention Deficit Hyperactivity Disorder (a clinical population that exhibits higher levels of head movement). Multi-echo data denoised using ME-ICA had better temporal signal-to-noise, exhibited less contamination from head movement, and better preserved FC between cortico-subcortical nodes of the default mode network when compared to the other denoising approaches. Similar effects have been reported in task-based fMRI studies, where task-related head movement artifacts can be difficult to disentangle from signals of interest. The simulations performed in(Lombardo et al. 2016) revealed that effect size estimates for a mentalizing fMRI task increased by an average of 24% when multi-echo data was denoised using ME-ICA, compared to other denoising approaches that do not leverage signal-decay information. Interestingly, effect size estimates increased by more than 50% in brain regions prone to signal dropout, such as the temporal pole and ventromedial prefrontal cortex, suggesting that the benefits of the multi-echo data and ME-ICA procedures can be somewhat region-specific.(Moia et al., n.d.) demonstrated that ME-ICA was the most effective way to remove motion artifacts and enhance the test-retest reliability of cerebrovascular reactivity and hemodynamic lag estimates in ten densely-sampled individuals.
Collectively, the studies reviewed above demonstrated empirically that ME-ICA can be used to separate various kinds of noise (spatially structured S0-dependent signals), including but not limited to head motion artifacts, from T2*-dependent signals of interest in fMRI data. Distinguishing variance in fMRI signals related to neurobiological and non-neurobiological factors is critical for valid and reliable inferences regarding FC measurements. This is in part what motivated us to ask in a recent study whether multi-echo fMRI could be used to improve test-retest reliability of FC measurements at the single-subject level.
Improved test-retest reliability of resting-state FC using multi-echo fMRI
In Lynch et al.(Lynch et al. 2020), we predicted that multi-echo fMRI could improve the test-retest reliability of FC measurements in at least two ways. First, we reasoned that an OC-ME time-series has better BOLD signal sensitivity and less signal dropout (compared to typical single-echo acquisition) in short T2* regions and deep subcortical structures known to exhibit especially unreliable FC in single-echo datasets(Noble et al. 2017; Noble, Scheinost, and Constable 2019). Second, as reviewed above, ME-ICA is highly effective at removing the confounding effects of head motion (while also retaining signals of interest (Dipasquale et al. 2017)) and other spatially structured S0-dependent artifacts (Kundu et al. 2012, 2013) that along with thermal noise compromise a large proportion of variance in the raw fMRI signal(Bianciardi et al. 2009) and are a major source of FC variability within a person over time(Laumann et al. 2017).
To test our hypothesis, we recruited 4 healthy adults to undergo repeated imaging (12 to 24 × 14.4 minute scans) using a multi-band multi-echo fMRI sequence. FC reliability was then quantified at each point in the brain of these individuals and compared to 14 highly-sampled individuals from three independent single-echo resting-state fMRI datasets. Time × reliability curves were used to summarize the average FC reliability value (calculated separately in cortex, subcortical structures, and cerebellum) given different amounts of data from single scans. Consistent with our prediction, these curves revealed that smaller quantities of OC-ME data were needed to achieve the same level of FC reliability in the independent single-echo fMRI datasets or in pseudo single-echo data, in which the same FC measures were derived from the second echo (TE2 = 31.11 ms) of the multi-echo scans. This effect was most pronounced when combined with ME-ICA denoising: 10 minutes of ME-ICA denoised OC-ME data yielded FC reliability values comparable to 30 minutes of single-echo fMRI. This suggests that discarding S0-dependent artifacts is important for increasing FC reliability. Interestingly, some previous studies had found that denoising procedures other than ME-ICA tend to decrease FC reliability, which has been interpreted as the removal of reliable artifacts(Noble, Scheinost, and Constable 2019). An alternative interpretation is that generic denoising methods (regression of head motion parameters or signals from nuisance compartments, frequency restricting bandpass filters) could decrease FC reliability by inadvertently removing signals of interest, given that they have no ground truth to precisely separate BOLD and non-BOLD variance in fMRI signals (Kundu et al. 2017).
Two points related to the findings of (Lynch et al. 2020) above are worth emphasizing further. First, given a relatively modest amount of data per -subject (approximately 15–30 minutes), many cortical regions (e.g., lateral prefrontal, posterior parietal, and a subset of midline cortical areas) can exhibit reliable FC in single-echo datasets. In other words, our findings should not be interpreted to mean that single-echo data, which forms the backbone of the human neuroimaging literature, is inherently unreliable. Instead, in agreement with multiple previously published reports, they indicate that some cortical and subcortical brain regions have a tendency to yield noisy, unreliable FC measurements, especially in shorter duration scans, and multi-echo fMRI can mitigate this problem. Second, obtaining reliable FC estimates that are neurobiological meaningful from short T2* brain regions where fMRI signals decay rapidly or in deep subcortical structures susceptible to certain kinds of physiological artifacts can be difficult even if large quantities of single-echo data are available for a given subject. This is an important point because the need for high quantities of data per -subject is sometimes thought of as the only barrier to achieving high quality FC measurements. Figure 1 helps illustrate this point (data shown are from (Lynch et al. 2020)). A seed (green sphere) placed in the left subgenual cingulate and substantia nigra of an individual scanned repeatedly (6 hrs. total) using both a multi-echo and single-echo multi-band sequence reveals two entirely different patterns of FC (with FC maps derived from the multi-echo dataset appearing more functionally meaningful). However, this effect is highly region specific — nearly identical FC measurements can be produced in both datasets when seeds are placed elsewhere in cortex or subcortex (see the lateral prefrontal and lateral geniculate nucleus seeds in green box). Investigators that are especially interested in the FC of these and other artifact prone brain regions at the single-subject level could consider using a multi-echo acquisition.
Figure 1.
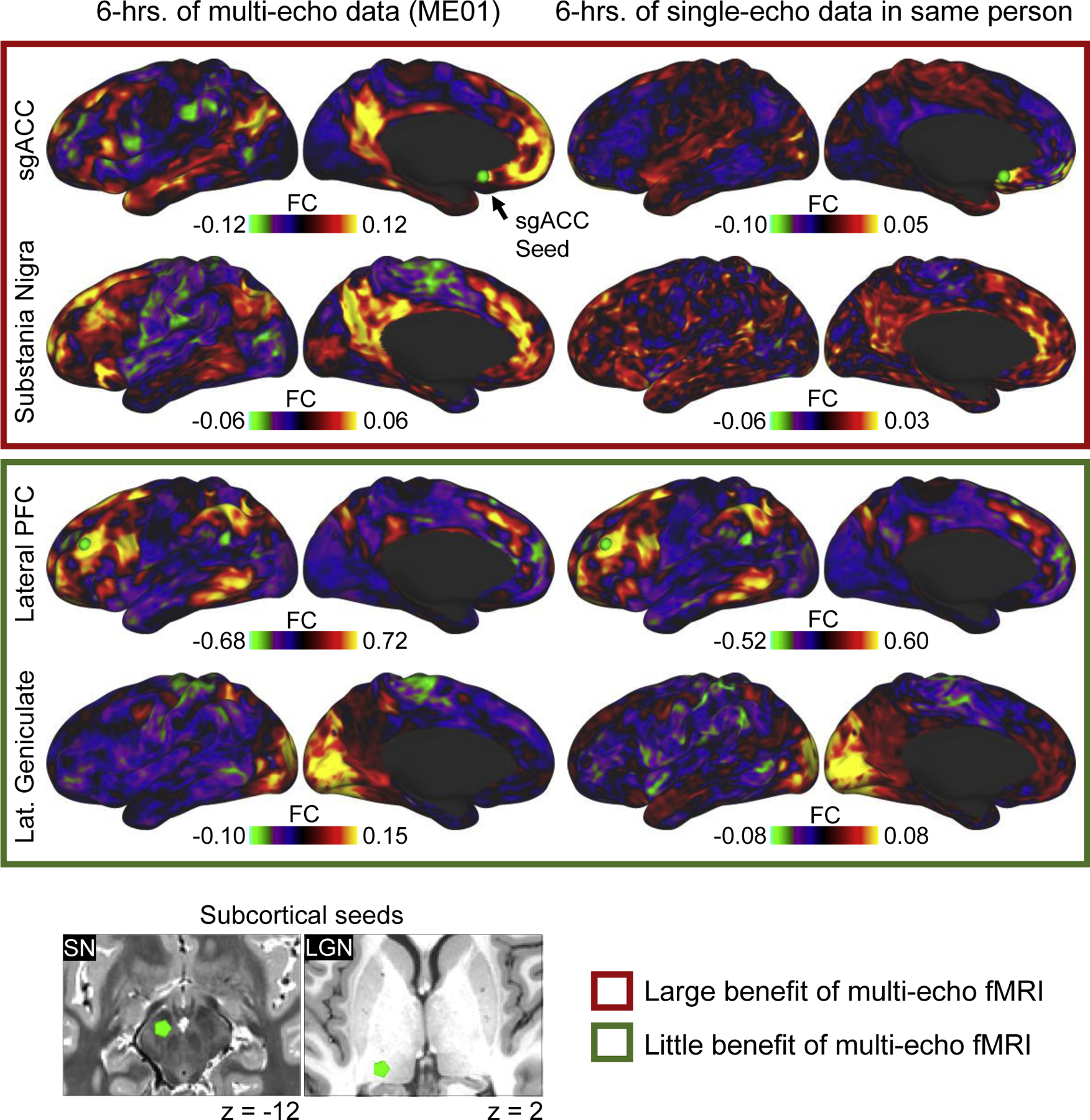
Comparing the FC of brain regions with different levels of artifact susceptibility in a highly-sampled individual. This individual underwent repeated scanning using both multi -echo and single-echo fMRI sequences for a total of six hours. The FC of four seed regions are shown using the concatenated multi-echo (left) and single-echo (right) datasets. The red box contains seeds in noise prone brain regions (subgenual cingulate cortex, brain stem nuclei) where the benefits of multi-echo fMRI appear to be more pronounced. The green box contains example seeds exhibiting FC that are similar in both multi-echo and single-echo datasets.
Future Directions: Increasing the availability and use of multi-echo fMRI
The studies reviewed above highlight the considerable advantages of multi-echo fMRI and suggest that it may be an underused technology. Less than 1% of the more than 12K resting-state studies published in the last 10 years have used a multi-echo sequence despite a number of studies reporting significant advantages associated with this technique. In this section, we describe two barriers that could have contributed to this situation and describe recent progress towards overcoming them. First, until recently there has been a relative lack of software available for preprocessing and denoising multi-echo fMRI data. To our knowledge, fMRIPrep(Esteban et al. 2019) and AFNI(Cox 1996) are the only software packages capable of preprocessing multi-echo data in an automated fashion. Multi-echo denoising is itself an active area of research and development (Caballero-Gaudes et al. 2019; DuPre et al. 2020). Tedana (TE-Dependent ANAlysis; (DuPre et al. 2020)) is a python based implementation of ME-ICA that is maintained by an open community of scientists. Second, there is a large parameter space for multi-echo data acquisition (e.g. the number and range of echo times, spatial resolution, acceleration, and repetition time, to name just a few), and there is no consensus regarding which parameter set is best. This may leave investigators that are interested in collecting multi-echo fMRI uncertain where to begin. In practice, there are trade-offs associated with any parameter set selected that need be considered in the context of the scientific goals at hand. For example, collecting more echoes could be desirable for parameter fitting but the requisite increase in repetition rate or voxel size might quickly become prohibitive for investigators that prioritize spatiotemporal resolution (Harms et al. 2018). Helpful guidelines for setting up a multi-echo acquisition can be found online (https://tedana.readthedocs.io/en/stable/acquisition.html) and in the appendix of(Dipasquale et al. 2017).
Conclusions
Precise and reliable analysis of single-subject fMRI data will open new avenues for understanding individual differences in behavior (Kong et al. 2019; Seitzman et al. 2019; Wang et al. 2020), highly-powered within-subject experimental designs (Newbold et al. 2020; Pritschet et al. 2020), and opportunities for fMRI to become more useful clinically (Gratton et al. 2019). The dependence of FC reliability on data quantity is a significant bottleneck to realizing this level of clinical utility. Considered alongside other recent investigations, the findings of (Lynch et al. 2020) indicate that multi-echo fMRI can improve the reliability of resting-state FC, in part by discarding image artifacts and increasing BOLD signal sensitivity. At the same time, it is important to be mindful that obtaining accurate and reliable descriptions of an individual’s functional brain organization is a difficult enterprise that requires a multipronged approach. For example, while multi-echo denoising is attractive because it can be highly effective at discarding the effects of head motion post hoc, investigators can also take steps to minimize head movement in the first place – for example, either through real-time motion feedback (Dosenbach et al. 2017; Greene et al. 2018) or custom headcases (Power, Silver, et al. 2019).
Highlights.
Studies of densely-sampled humans have provided new insights regarding functional brain organization at the level of individuals.
Precise delineation of functional networks in individuals has the potential to be clinically useful.
Barriers to clinical translation include the large amount of clean data per -subject that is needed for reliable measurements.
Multi-echo fMRI improves test-rest reliability and reduces the need for long or multiple scans.
The advantages of multi-echo fMRI are especially pronounced in artifact-prone brain regions that are clinically important.
Acknowledgements:
C.L. was supported by grants from NIMH, NIDA, the Rita Allen Foundation, and the Hope for Depression Research Foundation. C.J.L. was supported by an NIMH F32 National Research Service Award (F32MH120989).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: C.L. is listed as an inventor for Cornell University patent applications on neuroimaging biomarkers for depression that are pending or in preparation. The authors report no biomedical financial interests or other potential conflicts of interest.
References
- Bianciardi Marta, Fukunaga Masaki, van Gelderen Peter, Horovitz Silvina G., de Zwart Jacco A., Shmueli Karin, and Duyn Jeff H.. 2009. “Sources of Functional Magnetic Resonance Imaging Signal Fluctuations in the Human Brain at Rest: A 7 T Study.” Magnetic Resonance Imaging 27 (8): 1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch Janine Diane, Woolrich Mark W., Glasser Matthew F., Robinson Emma C., Beckmann Christian F., Van Essen David C., Harrison Samuel J., and Smith Stephen M.. 2018. “The Relationship between Spatial Configuration and Functional Connectivity of Brain Regions.” eLife. 10.7554/elife.32992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, and Hyde JS. 1995. “Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar MRI.” Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 34 (4): 537–41. [DOI] [PubMed] [Google Scholar]
- Braga Rodrigo M., and Buckner Randy L.. 2017. “Parallel Interdigitated Distributed Networks within the Individual Estimated by Intrinsic Functional Connectivity.” Neuron 95 (2): 457–71.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner Randy L., Krienen Fenna M., and Yeo B. T. Thomas. 2013. “Opportunities and Limitations of Intrinsic Functional Connectivity MRI.” Nature Neuroscience 16 (7): 832–37. [DOI] [PubMed] [Google Scholar]
- Caballero-Gaudes César, Moia Stefano, Panwar Puja, Bandettini Peter A., and Gonzalez-Castillo Javier. 2019. “A Deconvolution Algorithm for Multi-Echo Functional MRI: Multi-Echo Sparse Paradigm Free Mapping.” NeuroImage 202 (November): 116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW 1996. “AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages.” Computers and Biomedical Research, an International Journal 29 (3): 162–73. [DOI] [PubMed] [Google Scholar]
- D’Esposito Mark. 2019. “Are Individual Differences in Human Brain Organization Measured with Functional MRI Meaningful?” Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicola Lauren M., Braga Rodrigo M., and Buckner Randy L.. 2020. “Parallel Distributed Networks Dissociate Episodic and Social Functions within the Individual.” Journal of Neurophysiology 123 (3): 1144–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasquale Ottavia, Sethi Arjun, Laganà Maria Marcella, Baglio Francesca, Baselli Giuseppe, Kundu Prantik, Harrison Neil A., and Cercignani Mara. 2017. “Comparing Resting State fMRI de-Noising Approaches Using Multi- and Single-Echo Acquisitions.” PloS One 12 (3): e0173289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach Nico U. F., Koller Jonathan M., Earl Eric A., Miranda-Dominguez Oscar, Klein Rachel L., Van Andrew N., Snyder Abraham Z., et al. 2017. “Real-Time Motion Analytics during Brain MRI Improve Data Quality and Reduce Costs.” NeuroImage 161 (November): 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPre E, Salo T, Markello R, Kundu P, Whitaker K, and Handwerker D. 2020. “ME-ICA/tedana: 0.0. 9a. Zenodo” [Google Scholar]
- Esteban Oscar, Markiewicz Christopher J., Blair Ross W., Moodie Craig A., Isik A. Ilkay, Erramuzpe Asier, Kent James D., et al. 2019. “fMRIPrep: A Robust Preprocessing Pipeline for Functional MRI.” Nature Methods 16 (1): 111–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Evan M., Laumann Timothy O., Gilmore Adrian W., Newbold Dillan J., Greene Deanna J., Berg Jeffrey J., Ortega Mario, et al. 2017. “Precision Functional Mapping of Individual Human Brains.” Neuron 95 (4): 791–807.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This work found central tendencies but also remarkable variability in the topology (size, shape, spatial positioning) of functional brain networks in ten densely-sampled individuals. Individual-specific functional connectivity measurements become more reliable with additional data per-subject.
- Gordon Evan M., Laumann Timothy O., Marek Scott, Raut Ryan V., Gratton Caterina, Newbold Dillan J., Greene Deanna J., et al. 2020. “Default-Mode Network Streams for Coupling to Language and Control Systems.” Proceedings of the National Academy of Sciences of the United States of America 117 (29): 17308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon Evan M., and Nelson Steven M.. 2021. “Three Types of Individual Variation in Brain Networks Revealed by Single-Subject Functional Connectivity Analyses.” Current Opinion in Behavioral Sciences 40 (August): 79–86. [Google Scholar]
- Gratton Caterina, Kraus Brian T., Greene Deanna J., Gordon Evan M., Laumann Timothy O., Nelson Steven M., Dosenbach Nico U. F., and Petersen Steven E.. 2019. “Defining Individual-Specific Functional Neuroanatomy for Precision Psychiatry.” Biological Psychiatry. 10.1016/j.biopsych.2019.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton Caterina, Laumann Timothy O., Nielsen Ashley N., Greene Deanna J., Gordon Evan M., Gilmore Adrian W., Nelson Steven M., et al. 2018. “Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation.” Neuron 98 (2): 439–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This work demonstrated that (with sufficient data per-subject and absent extreme changes in experience or external inputs) functional brain networks are largely stable within a person over time. Changes in FC associated with task state and imaging session were found to be minimal.
- Greene Deanna J., Koller Jonathan M., Hampton Jacqueline M., Wesevich Victoria, Van Andrew N., Nguyen Annie L., Hoyt Catherine R., et al. 2018. “Behavioral Interventions for Reducing Head Motion during MRI Scans in Children.” NeuroImage 171 (May): 234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene Deanna J., Marek Scott, Gordon Evan M., Siegel Joshua S., Gratton Caterina, Laumann Timothy O., Gilmore Adrian W., et al. 2019. “Integrative and Network-Specific Connectivity of the Basal Ganglia and Thalamus Defined in Individuals.” Neuron, December. 10.1016/j.neuron.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms Michael P., Somerville Leah H., Ances Beau M., Andersson Jesper, Barch Deanna M., Bastiani Matteo, Bookheimer Susan Y., et al. 2018. “Extending the Human Connectome Project across Ages: Imaging Protocols for the Lifespan Development and Aging Projects.” NeuroImage 183 (December): 972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn Andreas, and Fox Michael D.. 2020. “Opportunities of Connectomic Neuromodulation.” NeuroImage 221 (November): 117180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Ru, Li Jingwei, Orban Csaba, Sabuncu Mert R., Liu Hesheng, Schaefer Alexander, Sun Nanbo, et al. 2019. “Spatial Topography of Individual-Specific Cortical Networks Predicts Human Cognition, Personality, and Emotion.” Cerebral Cortex 29 (6): 2533–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus Brian T., Perez Diana, Ladwig Zach, Seitzman Benjamin A., Dworetsky Ally, Petersen Steven E., and Gratton Caterina. 2021. “Network Variants Are Similar between Task and Rest States.” NeuroImage 229 (January): 117743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu Prantik, Brenowitz Noah D., Voon Valerie, Worbe Yulia, Vértes Petra E., Inati Souheil J., Saad Ziad S., Bandettini Peter A., and Bullmore Edward T.. 2013. “Integrated Strategy for Improving Functional Connectivity Mapping Using Multiecho fMRI.” Proceedings of the National Academy of Sciences of the United States of America 110 (40): 16187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu Prantik, Inati Souheil J., Evans Jennifer W., Luh Wen-Ming, and Bandettini Peter A.. 2012. “Differentiating BOLD and Non-BOLD Signals in fMRI Time Series Using Multi-Echo EPI.” NeuroImage 60 (3): 1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This investigation described a new denoising approach, called multi-echo independent component analysis (ME-ICA), capable of separating spatially structured BOLD-like (functional brain networks) and non-BOLD-like” (the effects of head movement and other image artifacts) signals in a fully automated fashion (it did not require training a classifier). ME-ICA was found to improve functional connectivity measurements at the group and single-subject level.
- Kundu Prantik, Voon Valerie, Balchandani Priti, Lombardo Michael V., Poser Benedikt A., and Bandettini Peter A.. 2017. “Multi-Echo fMRI: A Review of Applications in fMRI Denoising and Analysis of BOLD Signals.” NeuroImage 154 (July): 59–80. [DOI] [PubMed] [Google Scholar]
- Laumann Timothy O., Gordon Evan M., Adeyemo Babatunde, Snyder Abraham Z., Joo Sung Jun, Chen Mei-Yen, Gilmore Adrian W., et al. 2015. “Functional System and Areal Organization of a Highly Sampled Individual Human Brain.” Neuron 87 (3): 657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann Timothy O., Snyder Abraham Z., Mitra Anish, Gordon Evan M., Gratton Caterina, Adeyemo Babatunde, Gilmore Adrian W., et al. 2017. “On the Stability of BOLD fMRI Correlations.” Cerebral Cortex 27 (10): 4719–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo Michael V., Auyeung Bonnie, Holt Rosemary J., Waldman Jack, Ruigrok Amber N. V., Mooney Natasha, Bullmore Edward T., Baron-Cohen Simon, and Kundu Prantik. 2016. “Improving Effect Size Estimation and Statistical Power with Multi-Echo fMRI and Its Impact on Understanding the Neural Systems Supporting Mentalizing.” NeuroImage 142 (November): 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch Charles J., Power Jonathan D., Scult Matthew A., Dubin Marc, Gunning Faith M., and Liston Conor. 2020. “Rapid Precision Functional Mapping of Individuals Using Multi-Echo fMRI.” Cell Reports 33 (12): 108540. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This work found that multi-echo fMRI can enhance the test-retest reliability of functional connectivity measurements at the individual subject level. Less multi-echo data was needed to achieve the same level of FC reliability in independent single-echo datasets or in the same person scanned using a single-echo sequence. This effect was pronounced in artifact-prone brain regions when ME-ICA was used.
- Marek Scott, Siegel Joshua S., Gordon Evan M., Raut Ryan V., Gratton Caterina, Newbold Dillan J., Ortega Mario, et al. 2018. “Spatial and Temporal Organization of the Individual Human Cerebellum.” Neuron 100 (4): 977–93.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek Scott, Tervo-Clemmens Brenden, Calabro Finnegan J., Montez David F., Kay Benjamin P., Hatoum Alexander S., Rose Donohue Meghan, et al. 2020. “Towards Reproducible Brain-Wide Association Studies.” Cold Spring Harbor Laboratory. 10.1101/2020.08.21.257758. [DOI] [Google Scholar]
- Moia Stefano, Termenon Maite, Uruñuela Eneko, Chen Gang, Stickland Rachael C., Bright Molly G., and Caballero-Gaudes César. n.d. “ICA-Based Denoising Strategies in Breath-Hold Induced Cerebrovascular Reactivity Mapping with Multi Echo BOLD fMRI.” 10.1101/2020.08.18.256479. [DOI] [PMC free article] [PubMed]
- Newbold Dillan J., Laumann Timothy O., Hoyt Catherine R., Hampton Jacqueline M., Montez David F., Raut Ryan V., Ortega Mario, et al. 2020. “Plasticity and Spontaneous Activity Pulses in Disused Human Brain Circuits.” Neuron 107 (3): 580–89.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble Stephanie, Scheinost Dustin, and Todd Constable R. 2019. “A Decade of Test-Retest Reliability of Functional Connectivity: A Systematic Review and Meta-Analysis.” NeuroImage 203 (December): 116157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble Stephanie, Spann Marisa N., Tokoglu Fuyuze, Shen Xilin, Todd Constable R, and Scheinost Dustin. 2017. “Influences on the Test-Retest Reliability of Functional Connectivity MRI and Its Relationship with Behavioral Utility.” Cerebral Cortex 27 (11): 5415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack Russell A., Laumann Timothy O., Koyejo Oluwasanmi, Gregory Brenda, Hover Ashleigh, Chen Mei-Yen, Gorgolewski Krzysztof J., et al. 2015. “Long-Term Neural and Physiological Phenotyping of a Single Human.” Nature Communications 6 (December): 8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser Benedikt A., Versluis Maarten J., Hoogduin Johannes M., and Norris David G.. 2006. “BOLD Contrast Sensitivity Enhancement and Artifact Reduction with Multiecho EPI: Parallel-Acquired Inhomogeneity-Desensitized fMRI.” Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 55 (6): 1227–35. [DOI] [PubMed] [Google Scholar]
- Posse Stefan, Wiese Stefan, Gembris Daniel, Mathiak Klaus, Kessler Christoph, Grosse-Ruyken Maria-Liisa, Elghahwagi Barbara, Richards Todd, Dager Stephen R., and Kiselev Valerij G.. 1999. “Enhancement of BOLD-Contrast Sensitivity by Single-Shot Multi-Echo Functional MR Imaging.” Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 42 (1): 87–97. [DOI] [PubMed] [Google Scholar]
- Power Jonathan D., Lynch Charles J., Gilmore Adrian W., Gotts Stephen J., and Martin Alex. 2019. “Reply to Spreng et Al.: Multiecho fMRI Denoising Does Not Remove Global Motion-Associated Respiratory Signals.” Proceedings of the National Academy of Sciences of the United States of America, August. 10.1073/pnas.1909852116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power Jonathan D., Plitt Mark, Gotts Stephen J., Kundu Prantik, Voon Valerie, Bandettini Peter A., and Martin Alex. 2018. “Ridding fMRI Data of Motion-Related Influences: Removal of Signals with Distinct Spatial and Physical Bases in Multiecho Data.” Proceedings of the National Academy of Sciences of the United States of America 115 (9): E2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This work showed that ME-ICA can separate the effects of head motion from signals of interest in resting-state fMRI scans. After ME-ICA, there was little evidence of any motion artifact. Global signal changes induced by changes in respiration were not removed by ME-ICA.
- Power Jonathan D., Schlaggar Bradley L., and Petersen Steven E.. 2014. “Studying Brain Organization via Spontaneous fMRI Signal.” Neuron 84 (4): 681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power Jonathan D., Silver Benjamin M., Silverman Melanie R., Ajodan Eliana L., Bos Dienke J., and Jones Rebecca M.. 2019. “Customized Head Molds Reduce Motion during Resting State fMRI Scans.” NeuroImage 189 (April): 141–49. [DOI] [PubMed] [Google Scholar]
- Pritschet Laura, Santander Tyler, Taylor Caitlin M., Layher Evan, Yu Shuying, Miller Michael B., Grafton Scott T., and Jacobs Emily G.. 2020. “Functional Reorganization of Brain Networks across the Human Menstrual Cycle.” NeuroImage 220 (October): 117091. [DOI] [PubMed] [Google Scholar]
- Seitzman Benjamin A., Gratton Caterina, Laumann Timothy O., Gordon Evan M., Adeyemo Babatunde, Dworetsky Ally, Kraus Brian T., et al. 2019. “Trait-like Variants in Human Functional Brain Networks.” Proceedings of the National Academy of Sciences of the United States of America 116 (45): 22851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Danhong, Li Meiling, Wang Meiyun, Schoeppe Franziska, Ren Jianxun, Chen Huafu, Öngür Dost, Brady Roscoe O. Jr, Baker Justin T., and Liu Hesheng. 2020. “Individual-Specific Functional Connectivity Markers Track Dimensional and Categorical Features of Psychotic Illness.” Molecular Psychiatry 25 (9): 2119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


