Keywords: fluorescence microscopy, intravital microscopy, kidney, O'Brien Center
Abstract
The Indiana O’Brien Center for Advanced Microscopic Analysis is a National Institutes of Health (NIH) P30-funded research center dedicated to the development and dissemination of advanced methods of optical microscopy to support renal researchers throughout the world. The Indiana O’Brien Center was founded in 2002 as an NIH P-50 project with the original goal of helping researchers realize the potential of intravital multiphoton microscopy as a tool for understanding renal physiology and pathophysiology. The center has since expanded into the development and implementation of large-scale, high-content tissue cytometry. The advanced imaging capabilities of the center are made available to renal researchers worldwide via collaborations and a unique fellowship program. Center outreach is accomplished through an enrichment core that oversees a seminar series, an informational website, and a biennial workshop featuring hands-on training from members of the Indiana O’Brien Center and imaging experts from around the world.
ORIGINS OF THE INDIANA O’BRIEN CENTER
The origins of the Indiana O’Brien Center can be traced back to 1994, when Bruce Molitoris took the position as Director of the Nephrology Division of the Indiana School of Medicine. Bruce Molitoris came to Indiana with a vision of establishing a research program focused on the cell biology of renal disease, a program in which microscopy would play a primary role. In 1996, he established a core microscopy facility, the Indiana Center for Biological Microscopy, based on a Bio-Rad MRC1024 confocal microscope. In 1999, the Bio-Rad microscope was upgraded to perform multiphoton microscopy, a technique whose unique mechanism of fluorescence excitation gives it the capability to image biological tissues at depths that are inaccessible to confocal microscopy. The extended reach of multiphoton microscopy was initially exploited to characterize the ornate three-dimensional structure of the kidney in development and disease (Fig. 1A) (1, 2). In 2001, the new system was used to collect the first multiphoton fluorescence images of the living kidney in situ (Fig. 1B) (3).
Figure 1.
Early multiphoton microscopy of the rodent kidney. A: projected image volume collected from the kidney of an inv/inv mouse model of polycystic kidney disease. B: three-color image collected from the kidney of a living rat following intravenous injection of Hoechst 33342 (blue nuclei), 500-kDa fluorescein dextran (green peritubular capillaries), and 10-kDa rhodamine dextran (red endosomes in proximal tubules and bright red concentration in distal lumens). [Image in B was adapted from Dunn et al. (3).] Scale bars = 40 µm in length.
By providing a high-resolution window deep into biological tissues, multiphoton microscopy gave biologists their first opportunities to visualize and quantify the dynamic and interactive behavior of individual cells and even subcellular organelles in the relevant context of the intact, living animal. The Indiana O’Brien Center was founded in 2002 to support the growth and development of multiphoton microscopy as an effective tool for understanding the physiology and pathophysiology of the kidney. Consistent with the overall mission of the National Institute of Diabetes and Digestive and Kidney Diseases O’Brien Centers to support research and facilitate collaborations in renal research (4), the goals of the original Indiana O’Brien Center were to 1) provide renal researchers with access to the unique experimental capabilities of the team of investigators at Indiana University, 2) develop methods to mitigate the technical challenges of multiphoton microscopy of the kidney, and 3) broaden the understanding and appreciation of new microscopy techniques in the renal research community.
SUPPORT OF RENAL RESEARCH BY THE INDIANA O’BRIEN CENTER
The success of the Indiana O’Brien Center has depended, in part, on the growth of the Indiana Center for Biological Microscopy (ICBM), the microscopy facility that provides its home. Over the past 18 years, investments from the Indiana University, the Lilly Foundation, and the National Institutes of Health (NIH) have supported the acquisition of a series of increasingly advanced microscope systems and the construction of a purpose-designed microscopy suite, with dedicated space for individual microscopy systems, digital image analysis workstations, and small animal surgery. Currently supporting more than 160 NIH-funded projects, the ICBM has been used by the investigators of the Indiana University to generate data for more than 500 publications. The growth of the ICBM has significantly expanded the capabilities of the Indiana O’Brien Center, currently providing it with access to two Leica SP8 confocal/multiphoton microscopes, an Olympus FV1000 confocal/multiphoton microscope, a spinning disk confocal microscope, a Keyence BZ-X800 fluorescence microscope, an Akoya CODEX multiplex fluorescence imaging system, and multiple image processing workstations running software for two- and three-dimensional image analysis (described in detail in Supplemental Table S1; all Supplemental material is available at 10.6084/m9.figshare.13818251).
Implementation of Intravital Microscopy As A Tool in Renal Research
The success of the Indiana O’Brien Center can be sourced to the creative efforts of a team of experienced animal surgeons, microscopists, clinicians, and cell biologists (see acknowledgements). Novel methods of surgery and tissue presentation had to be developed to immobilize the kidney to submicron levels. Methods of anesthesia and monitoring had to be developed to maintain the animal in a physiological condition for imaging studies lasting hours. Methods for administering fluorescent probes and drugs had to be developed to ensure appropriate delivery to target cells within the constraints of the animal’s blood volume and without introducing motion artifacts to the preparation. Finally, several methods had to be developed to optimize intravital microscopy for renal research including minimizing motion artifacts, increasing reach and resolution, and validating fluorescent probes. The Indiana O’Brien Center has also developed and applied new techniques to extend the capabilities of intravital microscopy, such as fluorescence lifetime imaging and fluorescence resonance energy transfer (FRET). The use of these novel approaches by internal and external users led to paradigm-shifting observations that have been critical to increasing our understanding of basic mechanisms of renal physiology and to uncovering novel pathways of injury and repair in animal models of acute and chronic renal disease. Examples, too numerous to describe in detail, include studies of albumin handling by proximal tubules (5–9), mechanisms of sepsis (10–13), nephrotoxic and ischemic injury (14–21), nanoparticle therapeutics (22), vascular function (23, 24), mitochondrial function (13, 25), cell death, (3, 26, 27), immune responses (16, 19, 27), diabetic nephropathy (28), bacterial infection (29–32), and renal transport (17, 33–37). A few examples of these studies are shown in Fig. 2.
Figure 2.
Intravital microscopy studies of the Indiana O’Brien Center. A: proximal tubule endocytosis of filtered Alexa 568-albumin in the rat. Multiphoton fluorescence image of the kidney of a living rat after intravenous injection of Hoechst 33342 (blue nuclei) before (A) and 24 min after (B) intravenous injection of Alexa 568-albumin. Alexa 568-albumin can be seen in the glomerular and intertubular capillaries and in endosomes of proximal tubule cells. Scale bar = 30 µm. [Figure adapted from Russo et al. (6).] C and D: oxidative stress in S2 segments of the mouse proximal tubule 2 h after intravenous injection of bacterial endotoxin. Endotoxin (red) binds to S1 segments, but oxidative stress can be observed in downstream S2 segments (green, 2′,7′-dichlorofluorescin diacetate). Scale bar = 40 µm in length. [Figure adapted from Hato et al. (11).] E: colonization of the rat proximal tubule by Escherichia coli following tubular microperfusion. At time 0, green fluorescent protein-expressing bacteria were injected into the lumens of tubules outlined with blue dextran. A fraction of the injected bacteria bound to the tubules, subsequently increasing in number and inducing tubular and endothelial injury. Scale bar = 30 µm in length. [Figure adapted from Melican et al. (32).] G, glomerulus; H2DCFDA, 2′,7′-dichlorodihydrofluorescein diacetate; LPS, lipopolysaccharide; PT, proximal tubule.
Development of Image-Analysis Software
A secondary focus of the Indiana O’Brien Center is the development of image-analysis software, a critical component of modern microscopy. Examples of software developed by the center include the following:
Voxx scientific volume-rendering software, which puts real-time volume rendering into the hands of nearly anyone with a personal computer (38). Voxx continues to be a mainstay tool of the Indiana O’Brien Center, has been cited in over 100 papers, and has been downloaded from the O’Brien website more than 6,000 times.
Image Motion Artifact Reduction Tool (IMART) software (Fig. 3, A–F), which digitally corrects motion artifacts in sequences of images collected over time or in three-dimensions (39, 40), has been downloaded by nearly 100 investigators. IMART software was used to remove motion artifacts from a series of images collected from the kidney of a living mouse treated with endotoxin, enabling a quantitative analysis of immune cell motility (41).
Spatial Temporal Analysis of Fieldwise Flow (STAFF), an ImageJ-based software tool that uses kymographs generated from images collected by high-speed intravital microscopy to provide essentially continuous measurements of microvascular flow across entire two-dimensional images (Fig. 3, G–I) (42, 43).
DeepSynth, a software tool that uses machine learning to accomplish highly accurate segmentation of nuclei in three-dimensional image volumes (44–47).
Volumetric Tool for Exploration and Analysis (VTEA), a unique ImageJ-based software tool that provides a complete integrated workflow from segmentation, through quantitation to data analysis via a simple, interactive user interface (Fig. 4) (48). VTEA-based tissue cytometry has made critical contributions to studies of the processes underlying kidney stone formation conducted as part of an NIH-funded program project (49, 50) and represents a cornerstone technology of the Indiana University contributions to the Kidney Precision Medicine Project (51–54).
Figure 3.
Image-analysis software developed by the Indiana O’Brien Center. A − F: Image Motion Artifact Reduction Tool (IMART) digital correction of a time series of images of 500-kDa molecular weight red dextran and 3-kDa molecular weight green dextran collected from the kidney of a living rat following intravenous injection. A: single frame. B and C: results of rigid and nonrigid registration depicted as YT and XT images collected along the lines in A. D: the original and registered time series depicted as three-dimensional volumes. IMART registration enabled measurement of microvascular leakage, as shown in E and F, which show measurements of interstitial (open circles) and capillary fluorescence (closed symbols) over time. [Figure adapted from Dunn et al. (39).] G−I: Spatial Temporal Analysis of Fieldwise Flow (STAFF) image-analysis software for continuous measurement of microvascular flow in two-dimensional networks. G: time series of images collected from the liver of a living mouse depicted as a three-dimensional volume. H: velocity map derived from STAFF measurements obtained at one time point. I: time series of continuously measured velocities obtained from sinusoidal segments with high and low/intermittent flow. The field was 663 µm across. [Figure adapted from Clendenon et al. (43).] AU, arbitrary units; PT, proximal tubule; RBC, red blood cell.
Figure 4.
Volumetric Tool for Exploration and Analysis (VTEA)-based tissue cytometry of the human kidney. A: maximum projection of combined fluorescence images of DAPI (gray), phalloidin (green), and antibodies to Tamm–Horsfall protein (THP; cyan), aquaporin-1 (AQP1; magenta), myeloperoxidase (MPO; red), CD68 (yellow), and CD3 (white). Arrows indicate two regions of immune cell infiltrates. B: corresponding ×4 magnification images of the regions indicated in the two boxes indicated in A. C: scatterplots of the fluorescence intensity of THP vs. AQP1 and MPO vs. CD68, respectively, with gates used to identify specific cell types. Scale bars = 1 mm in A and 250 µm in B. [Figure adapted from Ferkowicz et al. (52).]
All of these software tools are available online, either through the O’Brien Center (Voxx, IMART, and DeepSynth) or as ImageJ plugins (STAFF and VTEA).
Developing and Implementing Large-Scale Tissue Cytometry for Renal Research
Recent developments in automated optical sectioning microscope systems have enabled biomedical researchers to conduct high-resolution, three-dimensional microscopy at the scale of millimeters, if not centimeters, in biological tissues. With the implementation of multiple laser lines and methods of spectral deconvolution, these systems allow renal researchers to characterize four to eight different parameters in tissue slices spanning the entire kidney. Microscopy at this scale offers several advantages over conventional approaches to tissue histology. First, large-scale volumes support interrogation of hundreds of thousands of cells, minimizing sample bias and enhancing the ability to detect rare events. Second, large-scale volumes capture mesoscale tissue structures (e.g., the vasculature, glomeruli, and tubular segments) necessary to interpret cell distribution and function. Third, the characteristics of morphologically complex cells, such as immune cells, and the interactions between cells can only be properly captured in three-dimensional volumes.
Large-scale cytometry has proven to be a powerful new technique for high-content analysis of the kidney (as discussed in the description of the VTEA software above) and a variety of other tissues (55–57). However, tissue cytometry presents several challenges that include image acquisition, processing, and data analysis and visualization. The Indiana O’Brien Center is ideally positioned to address these challenges to help renal investigators realize the potential of large-scale tissue cytometry for their research. In addition to the extensive expertise in three-dimensional cytometry of the kidney, the center is hosted by the ICBM, a microscopy resource equipped with multiple automated confocal/multiphoton microscope systems and an established infrastructure-guaranteeing reliable and efficient system performance. Indiana University has provided the Indiana O’Brien Center with free access to the computer and network hardware necessary to address the computational and data management requirements of large-scale microscopy. Based on the success and promise of tissue cytometry as a powerful tool for interrogating renal physiology, we have incorporated quantitative, three-dimensional tissue cytometry as a new core offering of the Indiana O’Brien Center. The overarching goal of the three-dimensional tissue imaging core is to make large-scale, quantitative three-dimensional microscopy accessible and productive for renal researchers, regardless of their institutional infrastructure or their expertise in imaging. Services of the core consist of two components. First, the core provides imaging services, which can include the preparation of samples, collection of high-resolution image volumes by confocal microscopy, and combination of these image volumes into single, multi-parameter hypervolumes. Second, the core will provide access, training, and support to investigators in the use of a high-performance image-analysis system that can be used by remote investigators in a client-server format.
COLLATERAL BENEFITS OF THE INDIANA O’BRIEN CENTER
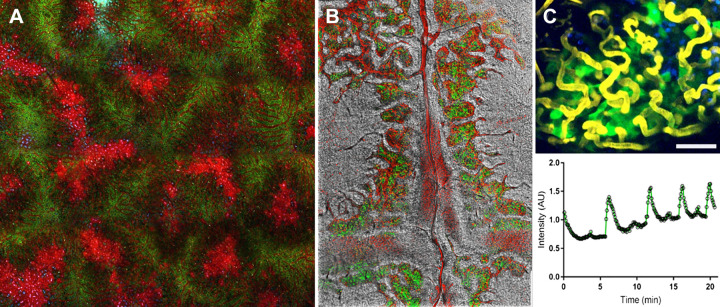
The development and ongoing funding of the Indiana O’Brien have yielded enormous collateral benefits beyond the O’Brien Center itself. The development of techniques and support infrastructure for intravital microscopy have led to the development of methods of intravital microscopy of additional organ systems (Fig. 5), which has subsequently developed into a centerpiece technology of several project grants. Methods of intravital microscopy of the liver were developed to analyze the effects of drugs on hepatic transport (58–60), leading to funding of a U01 project collaboration with investigators from the Indiana Biocomplexity Institute to develop predictive models of drug hepatotoxicity (61–63). Methods of intravital microscopy of the bone marrow niche were developed (64, 65) and incorporated into a unique intravital microscopy core of the P30 Indiana Comprehensive Center of Excellence in Hematology. Methods of intravital microscopy, fluorescent protein biosensor analysis, and in vivo gene expression developed by the O’Brien Center were applied for in vivo imaging of the mouse pancreas and mouse and human islets transplanted into the rodent kidney (66). These techniques were subsequently incorporated into a unique core of the Indiana Diabetes Research Center, a core that was a strong asset in the successful application for NIH P30 funding. The techniques of three-dimensional tissue cytometry recently developed by the O’Brien group have become critical components of an NIH-funded program project addressing the processes underlying kidney stone formation and the NIH Kidney Precision Medicine Project.
Figure 5.
Dissemination of intravital microscopy at the Indiana University. A: intravital microscopy image of the liver of a living mouse following treatment with acetaminophen. The red fluorescence of Texas red-dextran was found in the sinusoidal capillaries and the cytosol of necrotic hepatocytes. The green fluorescence derives from rhodamine 123, which accumulates only in mitochondria of healthy cells. The image is a projection of a mosaic of 16 image volumes. Scale bar = 200 µm. B: mosaic image volume collected from the calvarium of a living green fluorescent protein (GFP)-Lys mouse (provided by Malgorzata Kamocka and Nadia Carlesso). The red fluorescence of Texas red-dextran was found in the capillaries. The green fluorescence derives from GFP expressed in myelomonocytic cells. Scale bar = 1 mm. C: intravital microscopy image collected from the pancreas of a mouse transduced with adeno-associated virus vector serotype 8 expressing the calcium biosensor GCaMP6s (green islet cells) following injection with Texas red-dextran (yellow vasculature) Scale bar = 50 µm. The graph at the bottom shows calcium responses of the islet over time after intraperitoneal injection of glucose. [Image in C adapted from Reissaus et al. (66).] AU, arbitrary units.
Funding of these center grants has been key to significant institutional investment into the ICBM and to successful applications to NIH for shared instrumentation grants, which have funded acquisition of two multiphoton microscope systems, further enhancing the technical capabilities of the ICBM.
OUTREACH TO THE RENAL RESEARCH COMMUNITY
The primary goals of the Indiana O’Brien Center are not only to support the research of renal investigators but also to broaden the understanding of light microscopy as a tool in renal research and to encourage its dissemination to additional laboratories. Accordingly, the services of the Indiana O’Brien Center are presented each year at the annual meeting of the American Society of Nephrology, in some cases via dedicated symposia. The Indiana O’Brien Center also actively supports education through tours and workshops presented in a variety of programs at the Indiana University for local researchers, postdoctoral fellows, graduate and undergraduate students, and visiting high-school students. Education and dissemination are also accomplished through the ongoing publication of detailed protocols as they are developed and a biennial workshop, as described in the following two sections.
Publications and Videos of Detailed Protocols
In addition to publications describing novel imaging techniques developed by the center, or the biological results obtained using these techniques, the center has also published numerous protocols written to a level of technical detail that allows their accurate reproduction by most laboratories.
These protocols are particularly important for the dissemination of intravital microscopy as they describe many of the esoteric “tricks” necessary for its success. Detailed protocols have been produced describing intravital techniques developed to image the kidney (67) and additional organ systems, such as the lung (68), liver (58, 59), and pancreas (66). Other protocols describe the development of specialized techniques, such as intravital imaging of thrombus formation (69) and intravital imaging of FRET biosensors (70). In some cases, protocols are presented in the form of videos, for example, describing methods of measuring glomerular permeability (71), hematopoietic cell engraftment (65), and microvascular flow (42).
Many of these procedures are also detailed in a recent volume of the journal Methods, edited by Ken Dunn and Richard Day of the Indiana O’Brien Center. This volume presents detailed descriptions of surgical procedures (72), techniques of in vivo fluorescence lifetime imaging (73), the specialized techniques developed with the Molitoris and Dagher laboratories (41, 74), and techniques of intravital microscopy of the liver (59). The volume also includes articles from experts describing intravital microscopy of additional organ systems (75, 76), different imaging modalities (77–79), and specialized window preparations for long-term intravital studies (80, 81).
Indiana O’Brien Microscopy Workshops
One of the most significant and successful components of the education and dissemination activities of the Indiana O’Brien Center has been a workshop presented every 2 years in Indianapolis, IN. Consisting of 4–5 days of lectures and hands-on laboratory exercises, the workshop has been presented 7 times since 2006, providing hands-on training in intravital and other forms of microscopy to over 120 attendees, including faculty, postdoctoral fellows, graduate students, and directors of microscopy facilities.
Over the years, students attending the workshop have benefited from the generous participation of world-class experts in light microscopy, such as Joerg Bewersdorf, JiXin Chen, Richard Day, Iain Drummond, Enrico Gratton, George Patterson, Claire Walczak, and Simon Watkins; some of the original developers of multiphoton microscopy, including Jeff Squier, Sam Wells, Chris Xu, and Warren Zipfel; leading experts of intravital microscopy, such as John Lemasters, Charles Lin, Vladislav Verkhusha, and Roberto Weigert; leaders in the development of intravital microscopy of the kidney, such as Andrew Hall, Fadi Lakkis, and Janos Peti-Peterdi; and alternative methods of in vivo kidney imaging, such as those developed by Kevin Bennett, Yu Chen, and Xingde Li (details about each workshop can be found at http://medicine.iupui.edu/NEPH/OBRIEN/NEWWORKSHOP).
In many cases, training at the Indiana O’Brien Center has provided the springboard for investigators to develop intravital microscopy as a primary tool in their own research laboratories. For example, building on their training at the Indiana O’Brien Center, Andrew Hall has implemented intravital microscopy for his studies of renal physiology, Fadi Lakkis has adapted methods of intravital microscopy for his studies of the transplanted kidney, and Agneta Richter-Dahlfors has implemented intravital microscopy of pyelonephritis and other bacterial infections to extend the studies conducted with the Molitoris laboratory. Roberto Weigert, a student at the first O’Brien workshop, has since developed intravital microscopy of a variety of different organ systems into an active research program and core resource at the National Cancer Institute.
SERVICES AND RESOURCES OF THE CURRENT INDIANA O’BRIEN CENTER
As described above in Support of Renal Research by the Indiana O’Brien Center, the services of the Indiana O’Brien Center are provided through the imaging and image analysis equipment of the ICBM (Supplemental Table S1). As delineated below and shown in Fig. 6, the center is organized around two service cores, the Intravital Microscopy and Three-Dimensional Microscopy cores, whose services are made available through a fellowship program. The service cores are themselves supported by technical development cores dedicated to fluorescent protein biosensor techniques and digital image analysis. The outreach mission of the O’Brien Center is accomplished by an enrichment program core.
Figure 6.
Schematic diagram of the services and resources of the Indiana O’Brien Center. Research services are mediated through the Fellowship Program, which provides funds to support microscopy of living animals through the Intravital Microscopy Core and large-scale microscopy and tissue cytometry through the three-dimensional (3-D) Tissue Imaging Core. The Biosensor Development and Expression Core validates and provides fluorescent protein biosensors and transgenic animals to support studies conducted by the Intravital Microscopy Core. The Digital Analysis and Development Core develops software solutions to support quantitative analysis of studies conducted by the Intravital and 3-D Tissue Imaging Cores, including the development and support of the DINAVID online image-analysis system. The developments of the Indiana O’Brien Center are disseminated through the O’Brien Enrichment program, which also organizes and presents a biennial workshop on cutting-edge techniques in microscopy.
The Indiana O’Brien Fellowship Program
The Indiana O’Brien Fellowship Program is designed to provide a bridge for investigators interested in conducting feasibility studies using the techniques developed by the center. As described above, most of these techniques depend on a confluence of costly equipment, extensive infrastructure, and specialized expertise that is found only at few institutions. The fellowship program provides funds that allow investigators to utilize the resources of the Indiana O’Brien Center, including use of microscope equipment, technical support, and expert consultation. As necessary, project funding may also include funds for laboratory personnel or trainees to travel to Indianapolis and spend up to a month conducting studies. This opportunity, we believe, is especially valuable, exposing the investigator to the procedural nuances that may be critical to a technique and providing them with unique insights into the potential and limitations of the approach. Project funding is based on review of an application available online (http://medicine.iupui.edu/neph/obrien/fellow). The fellowship program (previously known as the Pilot and Feasibility Program) has been highly successful, having supported more than 47 projects since its inception in 2007.
The Indiana O’Brien Intravital Microscopy Core
The Indiana O’Brien Intravital Microscopy Core is designed to help renal researchers capitalize on the potential of intravital microscopy as a tool for renal research. This is accomplished via three major goals. The first goal is to work with O’Brien Fellows to develop and conduct intravital microscopy studies customized to the specific needs of their hypotheses and experimental system. Services include study design, procurement of animal models, conduct of microscopy, and image and data analysis, conducted in collaboration with the Digital Image Analysis and Development Core. The Core supports intravital studies of glomerular function by maintaining a colony of Munich-Wistar Fromter rats, which were obtained from Roland Blantz (University of California-San Diego O’Brien Center). The Munich-Wistar Fromter rat is, to our knowledge, the only strain of rats with significant numbers of glomeruli within the reach of intravital multiphoton microscopy. To date, the Indiana O’Brien Center has provided these rats to six investigative teams including the Department of Physiology at the Medical College of Wisconsin (Aron Geurts) and the University of Michigan (Roger Wiggins) for the purpose of creating transgenic rat models to enhance the understanding of glomerular disease processes. The second goal is to develop solutions to some of the current challenges to intravital microscopy of the kidney, including working with the Biosensor Development and Expression Core, to develop transgenic animals and methods of in vivo gene transfer and imaging to support the use of fluorescent protein biosensors in intravital studies of the kidney (70, 82). The third goal is to develop techniques that capitalize on new technical developments such as in vivo two-photon fluorescence lifetime microscopy and multiphoton imaging at excitation wavelengths longer than 1 mm.
The Indiana O’Brien Three-Dimensional Microscopy Core
The Indiana O’Brien Three-Dimensional Microscopy Core was designed to help renal researchers capitalize on the potential of high-content, large-scale tissue cytometry as a tool for renal researchers. Most renal researchers now have access to microscope systems capable of collecting multiplexed, high-resolution three-dimensional images of biological tissues at the scale of millimeters, if not centimeters. Image volumes of this size and complexity are enormously rich in potential information. However, microscopy at this scale introduces new sets of challenges with respect to visualization, quantitative analysis, and data handling. Few investigators have the technical expertise, institutional infrastructure, and computational resources necessary to capitalize on the enormous potential of high-resolution, high-content image volumes collected at this scale. The goal of the Indiana O’Brien Three-dimensional Microscopy Core is to make large-scale tissue cytometry accessible and productive for renal researchers, regardless of their institutional infrastructure or their expertise in imaging. This goal is accomplished via a service in which O’Brien Center personnel will use ICBM microscope systems to collect high-resolution, high-content three-dimensional image volumes from samples provided by renal investigators. The component image volumes will then be combined into large (millimeter-scale) image volumes, preprocessed (spectral deconvolution and segmentation), and posted on a powerful image processing computer (DINAVID) developed by the Digital Analysis and Development Core by Edward Delp’s laboratory at Purdue. Investigators will then be given access to this system, through which they can interactively visualize, explore, and quantitatively analyze their image volumes using custom-designed software. A parallel goal of the Digital Analysis and Development Core is to develop methods of image analysis to facilitate, extend, and enhance three-dimensional tissue cytometry (e.g., segmentation).
The Indiana O’Brien Enrichment Core
The Indiana O’Brien Enrichment Core was designed to disseminate the techniques developed by the center and to broaden the appreciation of optical microscopy as a tool for renal research. As previously discussed, this is partially accomplished through publications. However, dissemination is also directly supported by The Indiana O’Brien Enrichment Core, which maintains an online website portal for information and software, orchestrates a journal club, tours and appearances at national meetings, and, every 2 years, presents the Indiana O’Brien Microscopy Workshop. In past years, the workshop has typically been attended by 20−25 students. Information about the upcoming 2021 workshop and past workshops can be found at http://medicine.iupui.edu/NEPH/OBRIEN/NEWWORKSHOP.
FUTURE DIRECTIONS
The past 20 years have seen an explosion in biomedical imaging technology. New techniques are now capable of resolving subcellular structures at the scale of macromolecular complexes, entire centimeter-scale organisms in three dimensions, three-dimensional processes occurring in fractions of a second, and the simultaneous distribution of tens of proteins and thousands of RNAs. Crucially, each of these techniques has been implemented in commercially available instruments, putting these powerful capabilities into the hands of biomedical researchers.
We are currently exploring incorporation of some of these powerful new techniques into the services of the Indiana O’Brien Center. In particular, we have recently implemented highly multiplexed imaging of proteins, based upon CODEX technology and spatial transcriptomics, based upon 10× Genomics technology. We believe that these techniques can be combined and implemented using the quantitative three-dimensional tissue cytometry framework that we have developed to provide researchers with a unique integrated approach to tissue analysis. When combined with our large-scale imaging capabilities, these novel technologies will open a new window for understanding the complex behavior of the kidney in health and disease.
We are also exploring “superresolution” microscopy as a tool for understanding the intimate cross talk between organelles and other cellular structures that is at the heart of the normal functioning of cells and their responses to injury. The current infrastructure of the Indiana O’Brien Center is ideal to host and implement superresolution microscopy as a tool to extend our window of the kidney from the entire organ down to nanoscale events and structures.
The Indiana O’Brien Center has strived over the past 20 years to help renal researchers avail themselves of the latest techniques in biomedical microscopy. The incorporation of novel technologies such as CODEX, spatial transcriptomics, and superresolution microscopy will ensure our growth into the future and support our continued efforts to provide the renal community with information and state-of-the-art tools to image the kidney and understand its structure and function in health and disease.
SUPPLEMENTAL DATA
Supplemental Material is available at https://doi.org/10.6084/m9.figshare.13818251.v3.
GRANTS
This work was supported by National Institutes of Health O’Brien Center Grant P30DK079312 (to P.C.D.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.W.D. prepared figures; K.W.D. and P.C.D. drafted manuscript; K.W.D., B.A.M., and P.C.D. edited and revised manuscript; K.W.D., B.A.M., and P.C.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The success of the Indiana O’Brien Center reflects the combined efforts of a team of investigators that has evolved since its beginnings in 2002. The center has benefited from generous contributions from all of the faculty of the Division of Nephrology of Indiana University but particularly from Tarek Ashkar, Simon Atkinson, Robert Bacallao, Takashi Hato, Katherine Kelly, Carrie Phillips, Tim Sutton, and Mark Wagner. The center has also been strengthened by the participation of investigators outside the Indiana University Nephrology group, whose complementary expertise has furthered the development of methods of image analysis (Edward Delp of Purdue University and Paul Salama of Indiana University-Purdue University Indianapolis) and in vivo imaging of fluorescent biosensors (Rudolph Juliano and Xiao Xiao of the University of North Carolina). Techniques of animal surgery and preparation critical to intravital microscopy of the kidney were pioneered by Ruben Sandoval, Sylvia Campos-Bilderback, Henry Mang, and George Rhodes and subsequently adapted by Michelle Martinez, Barbara Sturonas-Brown, and Cliff Babbey. Other research associates in the Division of Nephrology have played an enormous role in the development of quantitative techniques that are critical to the ongoing mission of the Indiana O’Brien Center, including Jason Byars, Jeff Clendenon, Sherry Clendenon, Exing Wang, and Seth Winfree. The success of the workshops has depended on the participation of researchers from around the world, who have generously taken time out of their busy schedules to travel to Indianapolis to work one on one with our students. Finally, we would also like to acknowledge the tireless contributions of Malgorzata Kamocka, who has so successfully overseen the administration of the center webpage, workshop, and fellowship program.
REFERENCES
- 1.Phillips CL, Arend LJ, Filson AJ, Kojetin DJ, Clendenon JL, Fang S, Dunn KW. Three-dimensional imaging of embryonic mouse kidney by two-photon microscopy. Am J Pathol 158: 49–55, 2001. doi: 10.1016/S0002-9440(10)63943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips CL, Miller KJ, Filson AJ, Nurnberger J, Clendenon JL, Cook GW, Dunn KW, Overbeek PA, Gattone VH 2nd, Bacallao RL. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J Am Soc Nephrol 15: 1744–1755, 2004. doi: 10.1097/01.asn.0000131520.07008.b3. [DOI] [PubMed] [Google Scholar]
- 3.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905–C916, 2002. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 4.Staruschenko A, Brooks HL. O'Brien kidney research centers. Am J Physiol Renal Physiol 319: F1042, 2020. doi: 10.1152/ajprenal.00580.2020. [DOI] [PubMed] [Google Scholar]
- 5.Endres BT, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Kamocka MM, McDermott-Roe C, Staruschenko A, Molitoris BA, Geurts AM, Palygin O. Intravital imaging of the kidney in a rat model of salt-sensitive hypertension. Am J Physiol Renal Physiol 313: F163–F173, 2017. doi: 10.1152/ajprenal.00466.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20: 489–494, 2009. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 8.Saleh MA, Sandoval RM, Rhodes GJ, Campos-Bilderback SB, Molitoris BA, Pollock DM. Chronic endothelin-1 infusion elevates glomerular sieving coefficient and proximal tubular albumin reuptake in the rat. Life Sci 91: 634–637, 2012. doi: 10.1016/j.lfs.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner MC, Myslinski J, Pratap S, Flores B, Rhodes G, Campos-Bilderback SB, Sandoval RM, Kumar S, Patel M, Ashish Molitoris BA. Mechanism of increased clearance of glycated albumin by proximal tubule cells. Am J Physiol Renal Physiol 310: F1089–F1102, 2016. doi: 10.1152/ajprenal.00605.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290: F1034–F1043, 2006. doi: 10.1152/ajprenal.00414.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hato T, Winfree S, Day R, Sandoval RM, Molitoris BA, Yoder MC, Wiggins RC, Zheng Y, Dunn KW, Dagher PC. Two-photon intravital fluorescence lifetime imaging of the kidney reveals cell-type specific metabolic signatures. J Am Soc Nephrol 28: 2420–2430, 2017. doi: 10.1681/ASN.2016101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hato T, Winfree S, Kalakeche R, Dube S, Kumar R, Yoshimoto M, Plotkin Z, Dagher PC. The macrophage mediates the renoprotective effects of endotoxin preconditioning. J Am Soc Nephrol 26: 1347–1362, 2015. doi: 10.1681/ASN.2014060561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, Dagher PC. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22: 1505–1516, 2011. doi: 10.1681/ASN.2011020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn KW, Sandoval RM, Molitoris BA. Intravital imaging of the kidney using multiparameter multiphoton microscopy. Nephron Exp Nephrol 94: e7–e11, 2003. doi: 10.1159/000070813. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW. Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am J Physiol Renal Physiol 293: F245–F254, 2007. doi: 10.1152/ajprenal.00477.2006. [DOI] [PubMed] [Google Scholar]
- 17.Imamura R, Isaka Y, Sandoval RM, Ori A, Adamsky S, Feinstein E, Molitoris BA, Takahara S. Intravital two-photon microscopy assessment of renal protection efficacy of siRNA for p53 in experimental rat kidney transplantation models. Cell Transplant 19: 1659–1670, 2010. doi: 10.3727/096368910X516619. [DOI] [PubMed] [Google Scholar]
- 18.Liu XF, Wei J, Zhou Q, Molitoris BA, Sandoval R, Kobayashi H, Okada R, Nagaya T, Karim B, Butcher D, Pastan I. Immunotoxin SS1P is rapidly removed by proximal tubule cells of kidney, whose damage contributes to albumin loss in urine. Proc Natl Acad Sci USA 117: 6086–6091, 2020. doi: 10.1073/pnas.1919038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharfuddin AA, Sandoval RM, Berg DT, McDougal GE, Campos SB, Phillips CL, Jones BE, Gupta A, Grinnell BW, Molitoris BA. Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol 20: 524–534, 2009. doi: 10.1681/ASN.2008060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton TA, Kelly KJ, Mang HE, Plotkin Z, Sandoval RM, Dagher PC. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 288: F91–F97, 2005. doi: 10.1152/ajprenal.00051.2004. [DOI] [PubMed] [Google Scholar]
- 21.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 285: F191–F198, 2003. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 22.Uchida M, Maier B, Waghwani HK, Selivanovitch E, Pay SL, Avera J, Yun E, Sandoval RM, Molitoris BA, Zollman A, Douglas T, Hato T. The archaeal Dps nanocage targets kidney proximal tubules via glomerular filtration. J Clin Invest 129: 3941–3951, 2019. doi: 10.1172/JCI127511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrell N, Sandoval RM, Bian A, Campos-Bilderback SB, Molitoris BA, Fissell WH. Shear stress is normalized in glomerular capillaries following (5/6) nephrectomy. Am J Physiol Renal Physiol 308: F588–F593, 2015. doi: 10.1152/ajprenal.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCurley A, Alimperti S, Campos-Bilderback SB, Sandoval RM, Calvino JE, Reynolds TL, Quigley C, Mugford JW, Polacheck WJ, Gomez IG, Dovey J, Marsh G, Huang A, Qian F, Weinreb PH, Dolinski BM, Moore S, Duffield JS, Chen CS, Molitoris BA, Violette SM, Crackower MA. Inhibition of alphavbeta5 integrin attenuates vascular permeability and protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 28: 1741–1752, 2017. doi: 10.1681/ASN.2016020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall AM, Rhodes GJ, Sandoval RM, Corridon PR, Molitoris BA. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int 83: 72–83, 2013. doi: 10.1038/ki.2012.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly KJ, Sandoval RM, Dunn KW, Molitoris BA, Dagher PC. A novel method to determine specificity and sensitivity of the TUNEL reaction in the quantitation of apoptosis. Am J Physiol Cell Physiol 284: C1309–C1318, 2003. doi: 10.1152/ajpcell.00353.2002. [DOI] [PubMed] [Google Scholar]
- 27.Kelly KJ, Sutton TA, Weathered N, Ray N, Caldwell EJ, Plotkin Z, Dagher PC. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 287: F760–F766, 2004. doi: 10.1152/ajprenal.00050.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol 297: F923–F931, 2009. doi: 10.1152/ajprenal.00205.2009. [DOI] [PubMed] [Google Scholar]
- 29.Choong FX, Regberg J, Udekwu KI, Richter-Dahlfors A. Intravital models of infection lay the foundation for tissue microbiology. Future Microbiol 7: 519–533, 2012. doi: 10.2217/fmb.12.18. [DOI] [PubMed] [Google Scholar]
- 30.Mansson LE, Melican K, Boekel J, Sandoval RM, Hautefort I, Tanner GA, Molitoris BA, Richter-Dahlfors A. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell Microbiol 9: 413–424, 2007. doi: 10.1111/j.1462-5822.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 31.Melican K, Boekel J, Mansson LE, Sandoval RM, Tanner GA, Kallskog O, Palm F, Molitoris BA, Richter-Dahlfors A. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol 10: 1987–1998, 2008. doi: 10.1111/j.1462-5822.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 32.Melican K, Sandoval RM, Kader A, Josefsson L, Tanner GA, Molitoris BA, Richter-Dahlfors A. Uropathogenic Escherichia coli P and Type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog 7: e1001298, 2011. doi: 10.1371/journal.ppat.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hato T, Friedman AN, Mang H, Plotkin Z, Dube S, Hutchins GD, Territo PR, McCarthy BP, Riley AA, Pichumani K, Malloy CR, Harris RA, Dagher PC, Sutton TA. Novel application of complementary imaging techniques to examine in vivo glucose metabolism in the kidney. Am J Physiol Renal Physiol 310: F717–F725, 2016. doi: 10.1152/ajprenal.00535.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horbelt M, Wotzlaw C, Sutton TA, Molitoris BA, Philipp T, Kribben A, Fandrey J, Pietruck F. Organic cation transport in the rat kidney in vivo visualized by time-resolved two-photon microscopy. Kidney Int 72: 422–429, 2007. doi: 10.1038/sj.ki.5002317. [DOI] [PubMed] [Google Scholar]
- 35.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20: 1754–1764, 2009. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandoval RM, Kennedy MD, Low PS, Molitoris BA. Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol 287: C517–C526, 2004. doi: 10.1152/ajpcell.00006.2004. [DOI] [PubMed] [Google Scholar]
- 37.Tanner GA, Sandoval RM, Dunn KW. Two-photon in vivo microscopy of sulfonefluorescein secretion in normal and cystic rat kidneys. Am J Physiol Renal Physiol 286: F152–F160, 2004. doi: 10.1152/ajprenal.00264.2003. [DOI] [PubMed] [Google Scholar]
- 38.Clendenon JL, Phillips CL, Sandoval RM, Fang S, Dunn KW. Voxx: a PC-based, near real-time volume rendering system for biological microscopy. Am J Physiol Cell Physiol 282: C213–C218, 2002. doi: 10.1152/ajpcell.2002.282.1.C213. [DOI] [PubMed] [Google Scholar]
- 39.Dunn KW, Lorenz KS, Salama P, Delp EJ. IMART software for correction of motion artifacts in images collected in intravital microscopy. Intravital 3: e28210, 2014. doi: 10.4161/intv.28210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenz KS, Salama P, Dunn KW, Delp EJ. Digital correction of motion artefacts in microscopy image sequences collected from living animals using rigid and nonrigid registration. J Microsc 245: 148–160, 2012. doi: 10.1111/j.1365-2818.2011.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hato T, Winfree S, Dagher PC. Intravital imaging of the kidney. Methods 128: 33–39, 2017. doi: 10.1016/j.ymeth.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clendenon SG, Fu X, Von HR, Clendenon JL, Sluka JP, Winfree S, Mang H, Martinez M, Filson A, Klaunig JE, Glazier JA, Dunn KW. Spatial temporal analysis of fieldwise flow in microvasculature. J Vis Exp , 2019. [31789313] doi: 10.3791/60493. [DOI] [PubMed] [Google Scholar]
- 43.Clendenon SG, Fu X, Von Hoene RA, Clendenon JL, Sluka JP, Winfree S, Mang H, Martinez M, Filson AJ, Klaunig JE, Glazier JA, Dunn KW. A simple automated method for continuous fieldwise measurement of microvascular hemodynamics. Microvasc Res 123: 7–13, 2019. doi: 10.1016/j.mvr.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn KW, Fu C, Ho DJ, Lee S, Han S, Salama P, Delp EJ. DeepSynth: Three-dimensional nuclear segmentation of biological images using neural networks trained with synthetic data. Sci Rep 9: 18295, 2019. doi: 10.1038/s41598-019-54244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu CC, Ho DJ, Han S, Salama P, Dunn KW, Delp EJ. Nuclei Segmentation of Fluorescence Microscopy Images Using Convolutional Neural Networks. 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017). Melbourne, Australia, 2017, 704–708. [Google Scholar]
- 46.Ho DJ, Fu CC, Salama P, Dunn KW, Delp EJ. Nuclei detection and segmentation of fluorescence microscopy images using three dimensional convolutional neural networks. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 2018, pp. 418–422, doi: 10.1109/ISBI.2018.8363606. [DOI] [Google Scholar]
- 47.Ho DJ, Fu CC, Salama P, Dunn KW, Delp EJ. Nuclei Segmentation of Fluorescence Microscopy Images Using Three Dimensional Convolutional Neural Networks. 2017 IEEE Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), 2017, 834–842.doi: 10.1109/CVPRW.2017.116. [DOI] [Google Scholar]
- 48.Winfree S, Khan S, Micanovic R, Eadon MT, Kelly KJ, Sutton TA, Phillips CL, Dunn KW, El-Achkar TM. Quantitative three-dimensional tissue cytometry to study kidney tissue and resident immune cells. J Am Soc Nephrol 28: 2108–2118, 2017. doi: 10.1681/ASN.2016091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makki MS, Winfree S, Lingeman JE, Witzmann FA, Worcester EM, Krambeck AE, Coe FL, Evan AP, Bledsoe S, Bergsland KJ, Khochare S, Barwinska D, Williams JC Jr, El-Achkar TM. A precision medicine approach uncovers a unique signature of neutrophils in patients with brushite kidney stones. Kidney Int Rep 5: 663–677, 2020. doi: 10.1016/j.ekir.2020.02.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winfree S, Weiler C, Bledsoe SB, Gardner T, Sommer AJ, Evan AP, Lingeman JE, Krambeck AE, Worcester EM, El-Achkar TM, Williams JC Jr.. Multimodal imaging reveals a unique autofluorescence signature of Randall's plaque. Urolithiasis 49: 123–135, 2021. doi: 10.1007/s00240-020-01216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Achkar TM, Eadon MT, Menon R, Lake BB, Sigdel TK, Alexandrov T, , et al. A multimodal and integrated approach to interrogate human kidney biopsies with rigor and reproducibility: guidelines from the kidney precision medicine project. Physiol Genomics 53: 1–11, 2020. doi: 10.1152/physiolgenomics.00104.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferkowicz MJ, Winfree S, Sabo AR, Kamocka MM, Khochare S, Barwinska D, Eadon MT, Cheng YH, Phillips CL, Sutton TA, Kelly KJ, Dagher PC, El-Achkar TM, Dunn KW; Kidney Precision Medicine Project. Large-scale, three-dimensional tissue cytometry of the human kidney: a complete and accessible pipeline. Lab Invest , 2021. doi: 10.1038/s41374-020-00518-w. [33408350] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winfree S, Dagher PC, Dunn KW, Eadon MT, Ferkowicz M, Barwinska D, Kelly KJ, Sutton TA, El-Achkar TM. Quantitative large-scale three-dimensional imaging of human kidney biopsies: a bridge to precision medicine in kidney disease. Nephron 140: 134–139, 2018. doi: 10.1159/000490006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winfree S, Ferkowicz MJ, Dagher PC, Kelly KJ, Eadon MT, Sutton TA, Markel TA, Yoder MC, Dunn KW, El-Achkar TM. Large-scale 3-dimensional quantitative imaging of tissues: state-of-the-art and translational implications. Transl Res 189: 1–12, 2017. doi: 10.1016/j.trsl.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coutu DL, Kokkaliaris KD, Kunz L, Schroeder T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat Biotechnol 35: 1202–1210, 2017. doi: 10.1038/nbt.4006. [DOI] [PubMed] [Google Scholar]
- 56.Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37: 364–376, 2012. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halse H, Colebatch AJ, Petrone P, Henderson MA, Mills JK, Snow H, Westwood JA, Sandhu S, Raleigh JM, Behren A, Cebon J, Darcy PK, Kershaw MH, McArthur GA, Gyorki DE, Neeson PJ. Multiplex immunohistochemistry accurately defines the immune context of metastatic melanoma. Sci Rep 8: 11158, 2018. doi: 10.1038/s41598-018-28944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babbey CM, Ryan JC, Gill EM, Ghabril MS, Burch CR, Paulman A, Dunn KW. Quantitative intravital microscopy of hepatic transport. Intravital 1: 44–53, 2012. doi: 10.4161/intv.21296. [DOI] [Google Scholar]
- 59.Dunn KW, Ryan JC. Using quantitative intravital multiphoton microscopy to dissect hepatic transport in rats. Methods 128: 40–51, 2017. doi: 10.1016/j.ymeth.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Ryan J, Morgan RE, Chen Y, Volak LP, Dunn RT 2nd, Dunn KW. Intravital multiphoton microscopy with fluorescent bile salts in rats as an in vivo biomarker for hepatobiliary transport inhibition. Drug Metab Dispos 46: 704–718, 2018. doi: 10.1124/dmd.117.079277. [DOI] [PubMed] [Google Scholar]
- 61.Adhyapok P, Fu X, Sluka JP, Clendenon SG, Sluka VD, Wang Z, Dunn K, Klaunig JE, Glazier JA. A computational model of liver tissue damage and repair. PLoS One 15: e0243451, 2020. doi: 10.1371/journal.pone.0243451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunn KW, Martinez MM, Wang Z, Mang HE, Clendenon SG, Sluka JP, Glazier JA, Klaunig JE. Mitochondrial depolarization and repolarization in the early stages of acetaminophen hepatotoxicity in mice. Toxicology 439: 152464, 2020. doi: 10.1016/j.tox.2020.152464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu X, Sluka JP, Clendenon SG, Dunn KW, Wang Z, Klaunig JE, Glazier JA. Modeling of xenobiotic transport and metabolism in virtual hepatic lobule models. PLoS One 13: e0198060, 2018. doi: 10.1371/journal.pone.0198060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chitteti BR, Kobayashi M, Cheng Y, Zhang H, Poteat BA, Broxmeyer HE, Pelus LM, Hanenberg H, Zollman A, Kamocka MM, Carlesso N, Cardoso AA, Kacena MA, Srour EF. CD166 regulates human and murine hematopoietic stem cells and the hematopoietic niche. Blood 124: 519–529, 2014. doi: 10.1182/blood-2014-03-565721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Kamocka MM, Zollman A, Carlesso N. Combining intravital fluorescent microscopy (IVFM) with genetic models to study engraftment dynamics of hematopoietic cells to bone marrow niches. J Vis Exp 121: 5423, 2017. doi: 10.3791/54253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reissaus CA, Pineros AR, Twigg AN, Orr KS, Conteh AM, Martinez MM, Kamocka MM, Day RN, Tersey SA, Mirmira RG, Dunn KW, Linnemann AK. A versatile, portable intravital microscopy platform for studying beta-cell biology in vivo. Sci Rep 9: 8449, 2019. doi: 10.1038/s41598-019-44777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunn KW, Sutton TA, Sandoval RM. Live-animal imaging of renal function by multiphoton microscopy. Curr Protoc Cytom 83: 12.9.1–12.9.25, 2018. [29345326] doi: 10.1002/cpcy.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Presson RG Jr, Brown MB, Fisher AJ, Sandoval RM, Dunn KW, Lorenz KS, Delp EJ, Salama P, Molitoris BA, Petrache I. Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact. Am J Pathol 179: 75–82, 2011. doi: 10.1016/j.ajpath.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamocka MM, Mu J, Liu X, Chen N, Zollman A, Sturonas-Brown B, Dunn K, Xu Z, Chen DZ, Alber MS, Rosen ED. Two-photon intravital imaging of thrombus development. J Biomed Opt 15: 016020, 2010. doi: 10.1117/1.3322676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Day RN, Tao W, Dunn KW. A simple approach for measuring FRET in fluorescent biosensors using two-photon microscopy. Nat Protoc 11: 2066–2080, 2016. doi: 10.1038/nprot.2016.121. [DOI] [PubMed] [Google Scholar]
- 71.Sandoval RM, Molitoris BA. Quantifying glomerular permeability of fluorescent macromolecules using 2-photon microscopy in Munich Wistar rats. J Vis Exp 74: 50052, 2013. doi: 10.3791/50052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhodes GJ. Surgical preparation of rats and mice for intravital microscopic imaging of abdominal organs. Methods 128: 129–138, 2017. doi: 10.1016/j.ymeth.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winfree S, Hato T, Day RN. Intravital microscopy of biosensor activities and intrinsic metabolic states. Methods 128: 95–104, 2017. doi: 10.1016/j.ymeth.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandoval RM, Molitoris BA. Intravital multiphoton microscopy as a tool for studying renal physiology and pathophysiology. Methods 128: 20–32, 2017. doi: 10.1016/j.ymeth.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amornphimoltham P, Thompson J, Melis N, Weigert R. Non-invasive intravital imaging of head and neck squamous cell carcinomas in live mice. Methods 128: 3–11, 2017. doi: 10.1016/j.ymeth.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Surewaard BGJ, Kubes P. Measurement of bacterial capture and phagosome maturation of Kupffer cells by intravital microscopy. Methods 128: 12–19, 2017. doi: 10.1016/j.ymeth.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Conway JRW, Warren SC, Timpson P. Context-dependent intravital imaging of therapeutic response using intramolecular FRET biosensors. Methods 128: 78–94, 2017. doi: 10.1016/j.ymeth.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 78.Lee HJ, Cheng JX. Imaging chemistry inside living cells by stimulated Raman scattering microscopy. Methods 128: 119–128, 2017. doi: 10.1016/j.ymeth.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 79.Mazumder N, Deka G, Wu WW, Gogoi A, Zhuo GY, Kao FJ. Polarization resolved second harmonic microscopy. Methods 128: 105–118, 2017. doi: 10.1016/j.ymeth.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 80.Entenberg D, Pastoriza JM, Oktay MH, Voiculescu S, Wang Y, Sosa MS, Aguirre-Ghiso J, Condeelis J. Time-lapsed, large-volume, high-resolution intravital imaging for tissue-wide analysis of single cell dynamics. Methods 128: 65–77, 2017. doi: 10.1016/j.ymeth.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prunier C, Chen N, Ritsma L, Vrisekoop N. Procedures and applications of long-term intravital microscopy. Methods 128: 52–64, 2017. doi: 10.1016/j.ymeth.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 82.Tao W, Rubart M, Ryan J, Xiao X, Qiao C, Hato T, Davidson MW, Dunn KW, Day RN. A practical method for monitoring FRET-based biosensors in living animals using two-photon microscopy. Am J Physiol Cell Physiol 309: C724–C735, 2015. doi: 10.1152/ajpcell.00182.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]