Abstract
Noninvasive sampling of the distal airspace in patients with acute respiratory distress syndrome (ARDS) has long eluded clinical and translational researchers. We recently reported that fluid collected from heat moisture exchange (HME) filters closely mirrors fluid directly aspirated from the distal airspace. In the current study, we sought to determine fluid yield from different HME types, optimal HME circuit dwell time, and reliability of HME fluid in reflecting the distal airspace. We studied fluid yield from four different filter types by loading increasing volumes of saline and measuring volumes of fluid recovered. We collected filters after 1, 2, and 4 h of dwell time for measurement of fluid volume and total protein from 13 subjects. After identifying 4 h as the optimal dwell time, we measured total protein and IgM in HME fluid from 42 subjects with ARDS and nine with hydrostatic pulmonary edema (HYDRO). We found that the fluid yield varies greatly by filter type. With timed sample collection, fluid recovery increased with increasing circuit dwell time with a median volume of 2.0 mL [interquartile range (IQR) 1.2–2.7] after 4 h. Total protein was higher in the 42 subjects with ARDS compared with nine with HYDRO [median 708 µg/mL (IQR 244–2017) vs. 364 µg/mL (IQR 136–578), P = 0.047], confirming that total protein concentration in HME is higher in ARDS compared with hydrostatic edema. These studies establish a standardized HME fluid collection protocol and confirm that HME fluid analysis is a novel noninvasive tool for the study of the distal airspace in ARDS.
Keywords: acute respiratory distress syndrome, ARDS, biomarkers, exhaled breath, pulmonary edema
INTRODUCTION
Despite decades of research in acute respiratory distress syndrome (ARDS), challenges still remain in prevention, diagnosis, prognosis, and treatment. These persistent challenges are driven, in part, by the relative inaccessibility of the airspace of patients, leading to a dearth of biological samples from the lungs of patients with ARDS. Our group recently reported (1) that fluid (HMEF) collected from heat moisture exchange (HME) filters routinely used in the mechanical ventilator circuit is similar in protein composition to pulmonary edema fluid collected by direct aspiration in patients with acute pulmonary edema due to ARDS or hydrostatic (cardiogenic) pulmonary edema (HYDRO). Further, HMEF from patients with ARDS had higher levels of inflammatory and lung injury markers compared with HMEF from HYDRO patients. In our original publication, there was significant variability in the length of time the HME filter was in place (ranging from 1 to 12 h) in the ventilator circuit before filter collection. For analysis of HMEF to be used to rigorously answer research questions, standardized methods of patient enrollment and filter collection are needed.
Our study had three goals. The first goal was to determine whether HMEF yield differed according to HME filter type as filters from different manufacturers vary by size, shape, and materials. The second goal was to identify the optimal HME filter circuit dwell time to balance the need to collect sufficient fluid volumes for study on the majority of patients enrolled with the need to sample over a short period of time given the dynamic physiology of critically ill patients. The third goal was to test whether measurement of total protein in HMEF collected with a standardized collection protocol reflects the known differences in total protein found in the airspace in ARDS versus hydrostatic edema. We identify that a 4-h circuit dwell time using an AirLife HME filter was optimal for the collection of sufficient sample volume and demonstrate that total protein can be measured in HMEF collected according to this standard protocol.
METHODS
Patient Selection
Patients in the Vanderbilt University Medical Center adult intensive care units were screened daily by trained study nurses for mechanical ventilation status and bilateral radiographic infiltrates consistent with pulmonary edema. Patients who met these inclusion criteria were immediately enrolled. A waiver of informed consent was granted by the Vanderbilt University Medical Center Institutional Review Board as HME filter exchange is part of usual care and presents minimal risk to the study participants. Patients were enrolled within the first 24 h after intubation, when possible; a few patients transferred from other facilities or intubated on a weekend day were enrolled later than 24 h after intubation. All patients who were enrolled were classified post hoc as HYDRO, ARDS, or mixed edema (2). ARDS was diagnosed using the Berlin definition (3) by two physician investigators with a third providing adjudication in cases of disagreement as described previously (4). Those assessed to have a cardiac cause of pulmonary edema were classified as HYDRO. Subjects were classified as having mixed edema if they met the Berlin criteria for ARDS but also had concomitant fluid overload or cardiac dysfunction. For the timed filter study, we also included three mechanically ventilated patients without ARDS to determine whether fluid could be collected from patients who did not have overt radiographic pulmonary edema. Patients were excluded from analysis if they had suffered a cardiac arrest before HMEF collection or if they had a bronchoscopy with bronchoalveolar lavage in the 6 h before HMEF collection.
Filter Yield by Type
To test whether the type of HME filter used influences the amount of fluid that can be recovered by centrifugation of the filter, we compared four commonly used filter types in a series of benchtop experiments: The AirLife Adult HME Filter (no. 003003) and the AirLife Neonatal HME Filter (no. 003011) (Carefusion, San Diego, CA) used at our institution, the Apifil from Advanced Life Sciences (#AFHMEA) (Woodbridge, IL) used routinely at another institution and the No Port Straight Adult Filter (#DYNJAAHME12) from Medline (Northfield, IL), which was briefly used in our institution as a substitute for the AirLife filter. Filters were preloaded with increasing volumes of normal saline (0.025–3.0 mL) based on the volume of HMEF recovered in our prior study (1) and processed as described in Sample Collection and Processing.
Timed Sample Collection
After determining that AirLife HME filters had the highest fluid yield, the Adult version of this filter was used for the subsequent studies. At the time of patient enrollment, the existing HME filter was removed from the ventilator circuit and replaced with a fresh AirLife HME filter unit. Trained research personnel collected three successive HME units on the day of enrollment after 1, 2, and 4 h of dwell time on the first 13 study subjects. Based on the yield of HMEF, subsequent patients (n = 51) had HMEF collected on enrollment after a 4-h circuit dwell time.
Sample Collection and Processing
Following fluid loading (for yield studies) or sample collection (for patient studies), each sample was transported immediately at room temperature to the laboratory. HME filters were placed in modified 50-mL conical tubes (or unmodified 15-mL conical tubes for neonatal HME filters) and spun at 4°C for 10 min at 2,100 g in a swinging bucket centrifuge to collect HMEF. The 50-mL conical tubes were cut down so that they could fit in the centrifuge bucket then modified using four machined screws inserted just below the cut top to hold the HME filter in place during centrifugation (Fig. 1, A and B). The modified conical tubes can be washed, sterilized, and reused. Total volume of recovered HMEF was measured, then aliquoted, and stored at −80°C for later analysis.
Figure 1.
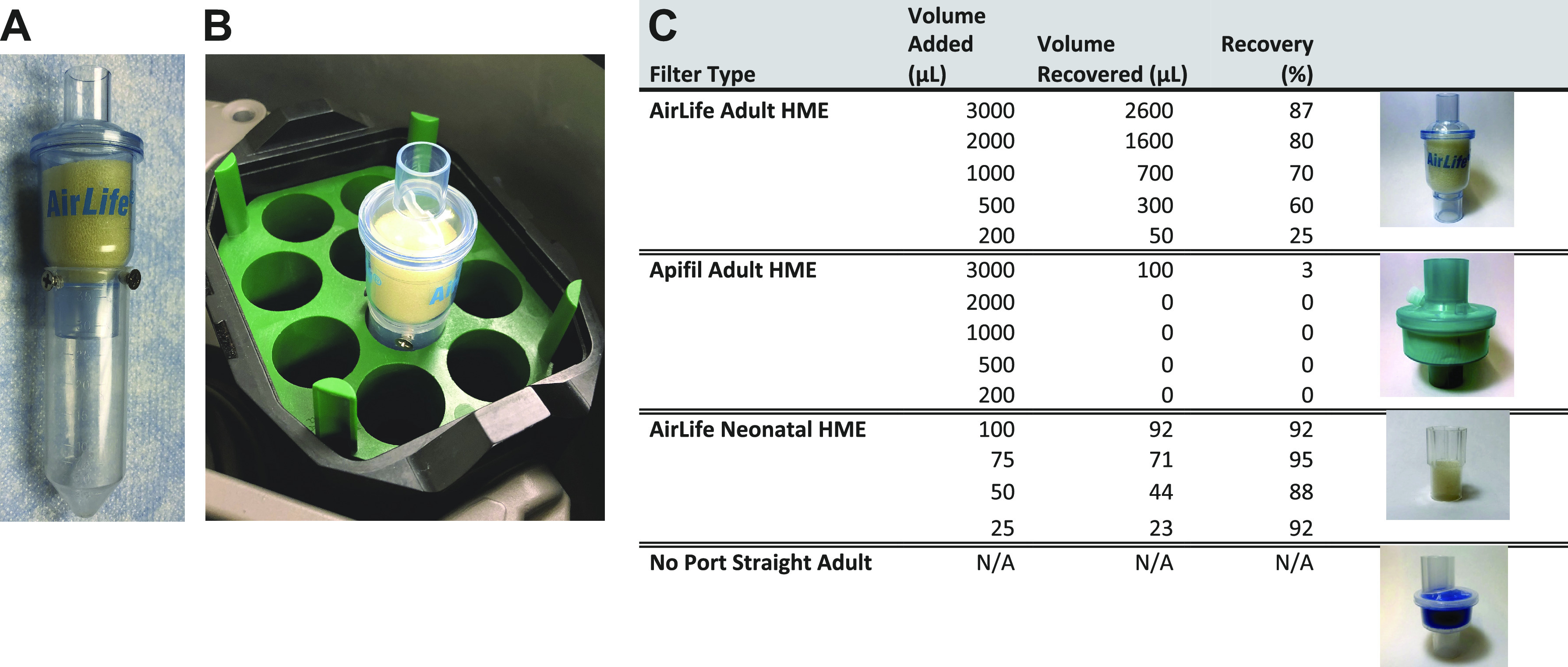
Fluid recovery of pre-loaded volume by filter type. 50-mL conical tubes were modified using four screws to firmly hold the HME filter in place prior to placing the tube in the centrifuge (A and B). Increasing volumes of saline were added to four different HME filters (three adult and one neonatal). Filters were placed in customized tubes and fluid was recovered by centrifugation. C: percent recovery was calculated by volume recovered/volume loaded. HME, heat moisture exchange.
Measurement of Protein and IgM
Total protein was measured in duplicate by bicinchoninic acid assay (Pierce, Rockford, IL). IgM was measured in duplicate by sandwich ELISA; ab137982 (Abcam, Cambridge, MA).
Statistical Analysis
Continuous data are expressed as median, interquartile range. All data were analyzed using nonparametric statistical comparisons. The Kruskal–Wallis test was used to compare two or more groups. A P value less than 0.05 was considered to be statistically significant.
RESULTS
Comparison of Fluid Recovery from Different Types of HMEF
Fluid recovery is shown in Fig. 1C. The Adult AirLife demonstrated good yield at all loading volumes with a range of 25%–87% recovery as loading volumes increased (percent recovery of loaded volume of 25%, 60%, 70%, 80%, and 87% at 0.2, 0.5, 1, 2, and 3 mL, respectively). Likewise, the neonatal AirLife had the excellent yield (88%–92%) even at very small volumes with a saturation volume of 0.5 mL. By contrast, the Apifil HME, which has a very different shape and material composition than the AirLife, had essentially no fluid recovered. After loading 3-mL saline, only 0.1 mL was recovered, with the other loading volumes having no fluid recovered. Finally, the Medline filter has an offset alignment of tubing connectors causing centrifugation to generate sufficient torque to damage the holder device and release the filter unit mid-spin. Following centrifugation of a study filter of this type, the customized holder device was cracked and no fluid was recovered. Additional yield studies using this filter were aborted. Based on these studies, subsequent patient studies utilized the adult AirLife filter.
Patient Characteristics
There were 13 subjects in the timed sample collection, eight with ARDS, one with HYDRO, one with mixed edema, and three who were mechanically ventilated without pulmonary edema (as an additional control). After establishing an optimal dwell time of 4 h, 51 additional subjects were enrolled, 42 with ARDS and nine with HYDRO. Patient characteristics for these subjects are in Table 1. The median time from endotracheal intubation to 4-h HMEF collection was 30.0 h (IQR 22.5–66.5 h).
Table 1.
Subject characteristics for 4-h filter collection
| ARDS (n = 42) | HYDRO (n = 9) | |
|---|---|---|
| Age, yr; median (IQR) | 53 (41–63) | 72 (38–85) |
| Male sex, % | 16 (38%) | 6 (67%) |
| Race | ||
| White | 33 (79%) | 6 (66%) |
| Black | 7 (17%) | 3 (33%) |
| Other/unknown | 2 (4%) | |
| Time from intubation to HMEF collection, h; median (IQR) | 29 (20–70) | 31 (19–65) |
| Past or current smoker, % | 22 (52%) | 7 (78%) |
| / | 109 (87–169) | 233 (182–395) |
| / | 118 (92–147) | 225 (121–257) |
| Tidal volume, mL | 375 (335–420) | 420 (400–500) |
| Positive end-expiratory pressure (PEEP, cmH2O) | 8 (3, 5–9) | 5 (5–8) |
| Minute ventilation, L/min | 8.9 (6.6–10.4) | 6.8 (6.0–9.0) |
| 0.50 (0.45–0.72) | 0.40 (0.30–0.51) | |
| RALE score, median (IQR) | 20 (16–27) | 14 (11–22) |
| APACHE II, median (IQR) | 20 (13–24) | 25 (17–29) |
| Cause of ARDS, n (%) | ||
| Nonpulmonary sepsis | 6 (14%) | |
| Pulmonary sepsis | 31 (74%) | |
| Other | 5 (12%) | |
| Ventilator-free days, median (IQR) | 7 (2–16) | 8 (2–12) |
| Survived to hospital discharge, % | 57% | 56% |
ARDS, acute respiratory distress syndrome; IQR, interquartile range; RALE, Radiographic Assessment of Lung Edema.
Timed Sample Collection
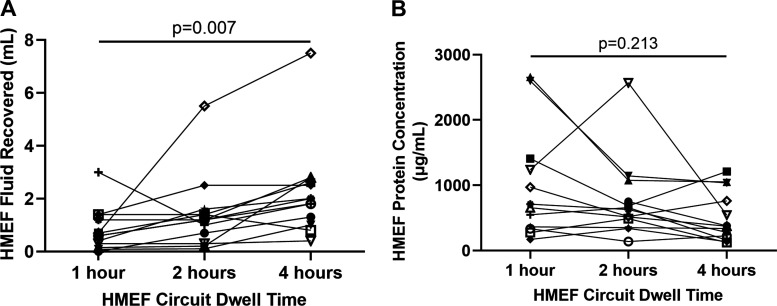
In the 13 subjects who had samples collected after 1, 2, or 4 h of HMEF circuit dwell time on the same study day, we found that HMEF volume increased significantly (P = 0.007) as dwell time increased (Fig. 2A) with a median volume of 0.6 mL (IQR 0.23–1.3), 1.2 mL (IQR 0.5–1.5), and 2.0 mL (IQR 1.15–2.7) after 1, 2, and 4 h, respectively. We also assessed what percent of samples at each of the dwell times had a low yield (defined as less than 0.5 mL) and found that to be 31% at 1 h, 23% at 2 h, and 8% at 4 h. This further confirms that the majority of patients have sufficient samples for multiple studies at the 4-h dwell time. While fluid volume increased steadily over time, total protein concentration at the three time points did not differ significantly (Fig. 2B). There was a similar trend of increasing volume with increasing dwell time when restricted to the eight patients with ARDS (P = 0.182). Interestingly, three subjects in this cohort did not meet the criteria for ARDS or HYDRO and still had similar fluid volumes collected over time. Given these results, we selected the 4-h filter dwell time as the optimal timing for collection (see Supplemental Data for Standard Operating Procedure (SOP); https://doi.org/10.6084/m9.figshare.13640774.v1), providing a balance between adequate volume of fluid collected while maintaining stable total protein concentrations.
Figure 2.
Timed HMEF sample collection. HME filters were collected after 1, 2, and 4 h of circuit dwell time from 13 mechanically ventilated patients with ARDS, HYDRO, mixed, or another cause of respiratory failure. HMEF volume was measured at each time point and recovery increased as dwell time increased (A). Protein concentrations were measured in the same samples and did not change significantly as HMEF dwell time increased (B). ARDS, acute respiratory distress syndrome; HME, heat moisture exchange.
HMEF Protein Concentrations Are Higher in ARDS versus HYDRO
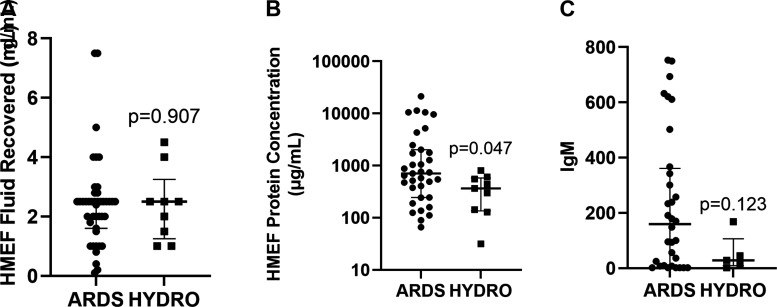
After optimizing dwell time at 4 h, we enrolled 51 additional subjects, 42 with ARDS and nine with HYDRO. The total volume of HMEF recovered did not differ between ARDS and HYDRO (Fig. 3A), but subjects with ARDS had significantly higher HMEF total protein when compared with HYDRO (Fig. 3B). To confirm that the high total protein levels in ARDS HMEF represented transit of large proteins across a damaged alveolar-capillary barrier (10), we measured IgM and found that IgM levels tended to be higher in patients with ARDS although this trend did not reach statistical significance (Fig. 3C). It is important to note that power for the IgM analysis was limited as only five of the nine HYDRO patients had sufficient HMEF fluid available for IgM measurements.
Figure 3.
HMEF comparison between ARDS and HYDRO. HMEF was collected after 4 h of HME circuit dwell time within the first 2 days of endotracheal intubation from ARDS (n = 42) and HYDRO (n = 9) patients. There was no difference in total volume of fluid collected (A), but protein concentration was significantly higher in fluid from ARDS (n = 35) patients versus HYDRO (n = 9) (B) and there was a trend toward higher IgM in patients with ARDS (n = 35) compared with HYDRO (n = 5) patients (C). ARDS, acute respiratory distress syndrome; HME, heat moisture exchange.
DISCUSSION
The goal of this study was to optimize a standardized patient enrollment and collection method for HMEF as a tool to study the distal airspace in patients with ARDS. We found that the type of filter used is critically important; some HME filters are not suitable for HMEF collection by our centrifugation methods. After systematically studying a range of dwell times, we selected 4 h as the optimal HME filter circuit dwell time in patients with ARDS and HYDRO. Four hours allow for sufficient fluid to be recovered (range 0.5–7.5 mL) without a significant change in protein concentration compared with 1 and 2 h of HME filter circuit dwell time. These findings suggest that the feasibility of collecting HMEF differs substantially by HME filter type. Investigators who wish to study HMEF should test the particular filter used at their institution to ensure that adequate fluid recovery is feasible, realizing that different assays may require different volumes of fluid for analysis. Importantly, these studies validate our earlier report that HMEF protein levels are higher in ARDS versus HYDRO and lend supporting evidence that HMEF may contain clinically and biologically relevant information.
Our study supports the idea that HMEF may be a better tool to sample the distal airspace than other existing methods. The heterogeneous patient cohort enrolled in this study suggests that HMEF can be easily and noninvasively collected after 4 h of dwell time in patients with ARDS or HYDRO, a clear advantage over other methods. Direct aspiration of undiluted pulmonary edema fluid (EF) is another method to directly sample the distal airspace and has been used in many studies to understand the biology of the injured lung (5, 7, 11–16). However, direct aspiration of the undiluted pulmonary edema fluid from the distal airspace only yields edema fluid in a minority of patients in whom sampling is attempted, resulting in significant sampling bias. Exhaled breath condensate has also been studied as a method of sampling the airspace in ARDS (6, 9, 17) but requires a specialized collection apparatus. By contrast, collection of HMEF utilizes equipment that is routinely in place in the ventilator circuit. Further, this study validates our prior findings that ARDS patients have higher HMEF protein concentrations, both total protein and a trend toward higher IgM, compared with HYDRO, consistent with increased permeability of the alveolar-capillary barrier in ARDS. This finding is consistent with similar observations in undiluted edema fluid (2, 8, 18), further validating HMEF as a window to the airspace in patients with acute pulmonary edema.
Our study has several strengths. First, we performed systematic characterization of different HME filters, illustrating that filter choice is an important variable. We also found that, despite its small size, the AirLife Neonatal HME filter has excellent fluid yield in the laboratory. Thus, the potential for extending HMEF collection to the neonatal population exists. Second, we developed a standardized protocol to optimize filter dwell time by comparing different dwell times in the same patients for HMEF volume yield. With this rigorous method, we established a 4-h dwell time as the optimal balance between sufficient fluid volume recovery in the majority of subjects and a reasonable duration of sampling. Third, we established that HMEF can be collected from patients who do not have overt pulmonary edema, potentially paving the way for studies of mechanically ventilated patients with chronic obstructive pulmonary disease, asthma, or other respiratory illnesses. Finally, we have created a cohort of subjects with HMEF that is carefully phenotyped for ARDS, HYDRO, or mixed etiology that can be used to probe the biological underpinnings of ARDS.
This study also has some limitations. This is a single-center study; whether these results will replicate across other institutions remains to be seen. Second, we did not do an exhaustive comparison of all HME filters that are available on the market; instead, we focused on filters used in our institution and at colleagues’ institutions. Thus, the findings cannot be extrapolated to other filter types. Third, our measurements were restricted to total protein and IgM (10). Because we were primarily interested in establishing standardized collections, we did not measure any biomarkers of biologically relevant pathways such as inflammation or lung epithelial injury in subjects without pulmonary edema so we do not yet know whether HMEF will be a robust biological sample for the study of mechanically ventilated patients with respiratory illnesses other than acute pulmonary edema due to ARDS or HYDRO. Here, we showed significantly different protein concentrations between ARDS and HYDRO, but we do not yet know if this is true for other biomarkers of acute lung injury which will need to be tested in future studies. Finally, although we did test fluid recovery from the AirLife Neonatal HME filter, we have not yet collected samples from mechanically ventilated neonates. Whether neonatal HMEF will be reflective of the neonatal airspace during mechanical ventilation remains to be tested.
In conclusion, this study established both an optimal filter type (the AirLife adult and neonatal HME filters) and circuit dwell time (4 h) allowing for standardization of collection methods for studies of HMEF. Further, using these standardized methods, we confirmed that total protein concentration is higher in HMEF from patients with ARDS compared with hydrostatic pulmonary edema, consistent with prior studies showing higher airspace protein in ARDS. This finding, in particular, suggests that HMEF has the potential to be an excellent tool to study the biologic mechanisms of human ARDS.
GRANTS
This work was supported in part by Department of Defense Grant W81XWH-18-1-0683 and NIH National Heart, Lung, and Blood Institute Grant R35HL150783.
DISCLOSURES
J.A.B. receives support from Omniox for work unrelated to the current manuscript. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.A.B., E.J.P., J.S.S., V.E.K., C.M.S., and L.B.W. conceived and designed research; J.B.M., V.E.K., L.E.H., E.W., N.E.W., and B.S. performed experiments; J.A.B., L.E.H., J.E.M., N.E.W., C.M.S., and L.B.W. analyzed data; J.A.B., V.E.K., C.M.S., and L.B.W. interpreted results of experiments; J.A.B., J.B.M., and J.E.M. prepared figures; J.A.B. drafted manuscript; J.A.B., J.B.M., E.J.P., J.S.S., V.E.K., L.E.H., E.W., B.S., J.E.M., N.E.W., C.M.S., and L.B.W. edited and revised manuscript; J.A.B., J.B.M., E.J.P., J.S.S., V.E.K., L.E.H., E.W., B.S., J.E.M., N.E.W., C.M.S., and L.B.W. approved final version of manuscript.
REFERENCES
- 1.McNeil JB, Shaver CM, Kerchberger VE, Russell DW, Grove BS, Warren MA, Wickersham NE, Ware LB, McDonald WH, Bastarache JA. Novel method for non-invasive sampling of the distal airspace in acute respiratory distress syndrome. Am J Respir Crit Care Med 197: 1027–1035, 2018. doi: 10.1164/rccm.201707-1474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the etiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J 35: 331–337, 2010. doi: 10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 307: 2526–2533, 2012. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Murphy LS, Wickersham N, McNeil JB, Shaver CM, May AK, Bastarache JA, Ware LB. Endothelial glycocalyx degradation is more severe in patients with non-pulmonary sepsis compared to pulmonary sepsis and associates with risk of ARDS and other organ dysfunction. Ann Intensive Care 7: 102, 2017. doi: 10.1186/s13613-017-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calfee CS, Eisner MD, Parsons PE, Thompson BT, Conner ER Jr, Matthay MA, Ware LB; NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med 35: 248–257, 2009. doi: 10.1007/s00134-008-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter CT, Price PV, Christman BW. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 114: 1653–1659, 1998. doi: 10.1378/chest.114.6.1653. [DOI] [PubMed] [Google Scholar]
- 7.Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med 31: 20–27, 2003. doi: 10.1097/00003246-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Fein A, Grossman RF, Jones JG, Overland E, Pitts L, Murray JF, Staub NC. The value of edema protein measurements in patients with pulmonary edema. Am J Med 67: 32–38, 1979. doi: 10.1016/0002-9343(79)90066-4. [DOI] [PubMed] [Google Scholar]
- 9.Gessner C, Dihazi H, Brettschneider S, Hammerschmidt S, Kuhn H, Eschrich K, Keller T, Engelmann L, Sack U, Wirtz H. Presence of cytokeratins in exhaled breath condensate of mechanical ventilated patients. Respir Med 102: 299–306, 2008. doi: 10.1016/j.rmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Hendrickson CM, Abbott J, Zhuo H, Liu KD, Calfee CS, Matthay MA, Network NA, NHLBI ARDS Network. Higher mini-BAL total protein concentration in early ARDS predicts faster resolution of lung injury measured by more ventilator-free days. Am J Physiol Lung Cell Mol Physiol 312: L579–L585, 2017. doi: 10.1152/ajplung.00381.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol 161: 1783–1796, 2002. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 297: L1035–L1041, 2009. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastarache JA, Ong T, Matthay MA, Ware LB. Alveolar fluid clearance is faster in women with acute lung injury compared to men. J Crit Care 26: 249–256, 2010. doi: 10.1016/j.jcrc.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowler RP, Duda B, Chan ED, Enghild JJ, Ware LB, Matthay MA, Duncan MW. Proteomic analysis of pulmonary edema fluid and plasma in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 286: L1095–L1104, 2004. doi: 10.1152/ajplung.00304.2003. [DOI] [PubMed] [Google Scholar]
- 15.Olman MA, White KE, Ware LB, Simmons WL, Benveniste EN, Zhu S, Pugin J, Matthay MA. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 β-induced IL-6 expression. J Immunol 172: 2668–2677, 2004. doi: 10.4049/jimmunol.172.4.2668. [DOI] [PubMed] [Google Scholar]
- 16.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 17.Montuschi P, Collins JV, Ciabattoni G, Lazzeri N, Corradi M, Kharitonov SA, Barnes PJ. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am J Respir Crit Care Med 162: 1175–1177, 2000. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- 18.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]