Abstract
Right ventricular (RV) function determines outcome in pulmonary arterial hypertension (PAH). RV pressure-volume loops, the gold standard for measuring RV function, are difficult to analyze. Our aim was to investigate whether simple assessments of RV pressure-volume loop morphology and RV systolic pressure differential reflect PAH severity and RV function. We analyzed multibeat RV pressure-volume loops (obtained by conductance catheterization with preload reduction) in 77 patients with PAH and 15 patients without pulmonary hypertension in two centers. Patients were categorized according to their pressure-volume loop shape (triangular, quadratic, trapezoid, or notched). RV systolic pressure differential was defined as end-systolic minus beginning-systolic pressure (ESP − BSP), augmentation index as ESP − BSP/pulse pressure, pulmonary arterial capacitance (PAC) as stroke volume/pulse pressure, and RV-arterial coupling as end-systolic/arterial elastance (Ees/Ea). Trapezoid and notched pressure-volume loops were associated with the highest afterload (Ea), augmentation index, pulmonary vascular resistance (PVR), mean pulmonary arterial pressure, stroke work, B-type natriuretic peptide, and the lowest Ees/Ea and PAC. Multivariate linear regression identified Ea, PVR, and stroke work as the main determinants of ESP − BSP. ESP − BSP also significantly correlated with multibeat Ees/Ea (Spearman’s ρ: −0.518, P < 0.001). A separate retrospective analysis of 113 patients with PAH showed that ESP − BSP obtained by routine right heart catheterization significantly correlated with a noninvasive surrogate of RV-arterial coupling (tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure ratio; ρ: −0.376, P < 0.001). In conclusion, pressure-volume loop shape and RV systolic pressure differential predominately depend on afterload and PAH severity and reflect RV-arterial coupling in PAH.
Keywords: pressure-volume relationship, pulmonary arterial hypertension, right ventricle, right ventricle-pulmonary arterial, systolic function coupling
INTRODUCTION
Right ventricular (RV) function is the main determinant of symptomatology and outcome in pulmonary arterial hypertension (PAH) (1). The gold-standard method to evaluate RV function in PAH relies on invasive measurement of RV-pulmonary arterial (PA) coupling defined as the ratio of end-systolic to arterial elastances (Ees/Ea) (2, 3). The method relies on single-beat or multibeat measurements of instantaneous pressures and volumes throughout the cardiac cycle with results plotted as pressure-volume (PV) loops (3). The normal RV PV loop has a rounded shape with early systolic peaking of pressure (4), whereas the normal left ventricular PV loop has a square shape with end-systolic pressure (ESP) easily identified at its upper left corner (5). With progression of pulmonary hypertension (PH), the shape of the RV PV loop changes, with late systolic peaking of RV pressure and increased ESP eventually resulting in a square shape resembling that of the left ventricular PV loop (6, 7). Along with these morphological changes, the difference between beginning-systolic pressure (BSP) and ESP changes from negative to positive (6), and the gradient between ESP and mean pulmonary arterial pressure (mPAP) increases (8). How all these changes relate to the metrics of RV afterload and RV-PA coupling in PAH is incompletely understood. Furthermore, although RV PV loops provide a wealth of information on RV function, their analysis is demanding and requires experience and time; single-beat methods are a valuable alternative to the multibeat method but their reliance on complex mathematical extrapolation and careful selection of data points during isovolumetric phases means they are vulnerable to interrater variability (9). It is therefore difficult to apply the methods to clinical care. An improved understanding of RV PV loop morphology and RV systolic pressure trends may help to broaden the utility and clinical applicability of RV PV loop data.
We therefore investigated the RV systolic pressure differential and morphology of PV loops and assessed their relationships to Ees/Ea ratios and key hemodynamic parameters and biomarkers in patients with PAH and patients without PH.
METHODS
The raw data that underpin this study are available from the corresponding authors on reasonable request.
Study Design and Patients
The study included patients with PAH and patients without PH from two centers: the Universities of Giessen and Marburg Lung Center [n = 50, Giessen, Germany; patients enrolled in the Right Heart I study (ClinicalTrials.gov identifier: NCT03403868) between February 2017 and March 2020] and the Johns Hopkins University School of Medicine (n = 42, Baltimore, MD; patients who underwent multibeat PV loop catheterization between 2013 and 2016). All participating patients gave written informed consent, and the studies were approved by the local ethics committees. Some of the patients were included in previous reports (10–13) and were reanalyzed for the present study. The patients were diagnosed according to current guidelines (14) and all patients with PAH received targeted therapies based on clinical evaluation and best standard of care. Patients without PH were disease controls referred for the evaluation of dyspnea or with clinical suspicion of PH.
Right heart catheterization was performed before PV catheterization according to current recommendations (15). Pulmonary arterial capacitance (PAC) was calculated as stroke volume (SV)/pulse pressure (16). Augmentation index was defined as ESP − BSP/pulse pressure (17).
PV Catheterization
PV catheterization was performed as described previously in each center (10, 11, 18). Preload reduction was achieved by balloon occlusion of the inferior vena cava in the Giessen center (12, 19) and by a gentle Valsalva maneuver (with concomitant manual external compression applied to the abdominal right upper quadrant to induce partial external inferior vena cava occlusion, if needed) in the Johns Hopkins center (10, 11).
Multibeat Ees was determined by a tangent fitted on the end-systolic portions of the PV loops at decreasing venous return. Ea was calculated as ESP/SV (18). The single-beat method for the calculation of Ees was also applied as previously described (9, 20). Briefly, to measure single-beat Ees, an RV pressure curve was analyzed to obtain the first derivative of pressure over time (dP/dt) during isovolumetric contraction and isovolumetric relaxation. Sine wave extrapolation was then used to calculate a theoretical isovolumetric maximum pressure (Pmax). Single-beat Ees was calculated as (Pmax − ESP)/SV.
RV-arterial coupling was calculated as Ees/Ea (21). Stroke work was calculated as SV × pressure and was defined by an intracardiac analyzer [Inca (CD Leycom, Zoetermeer, The Netherlands) or custom WinPVAN software].
BSP was defined as the pressure at the onset of ventricular ejection following maximal isovolumic contraction (dP/dt max), identified during offline analysis. ESP was automatically identified in real time by the intracardiac analyzer at the maximum pressure/volume ratio toward the end of ventricular ejection and rechecked by the authors. The RV systolic pressure differential was defined as ESP − BSP. Investigators who were blinded to the clinical data categorized patients according to the shape of their PV loops [triangular (ESP − BSP ≤ −4 mmHg), quadratic (ESP − BSP > −4 to 6 mmHg), trapezoid, or notched (both ESP − BSP > 6 mmHg; trapezoid vs. notched distinguished by visual adjudication)] (Fig. 1, A–D).
Figure 1.
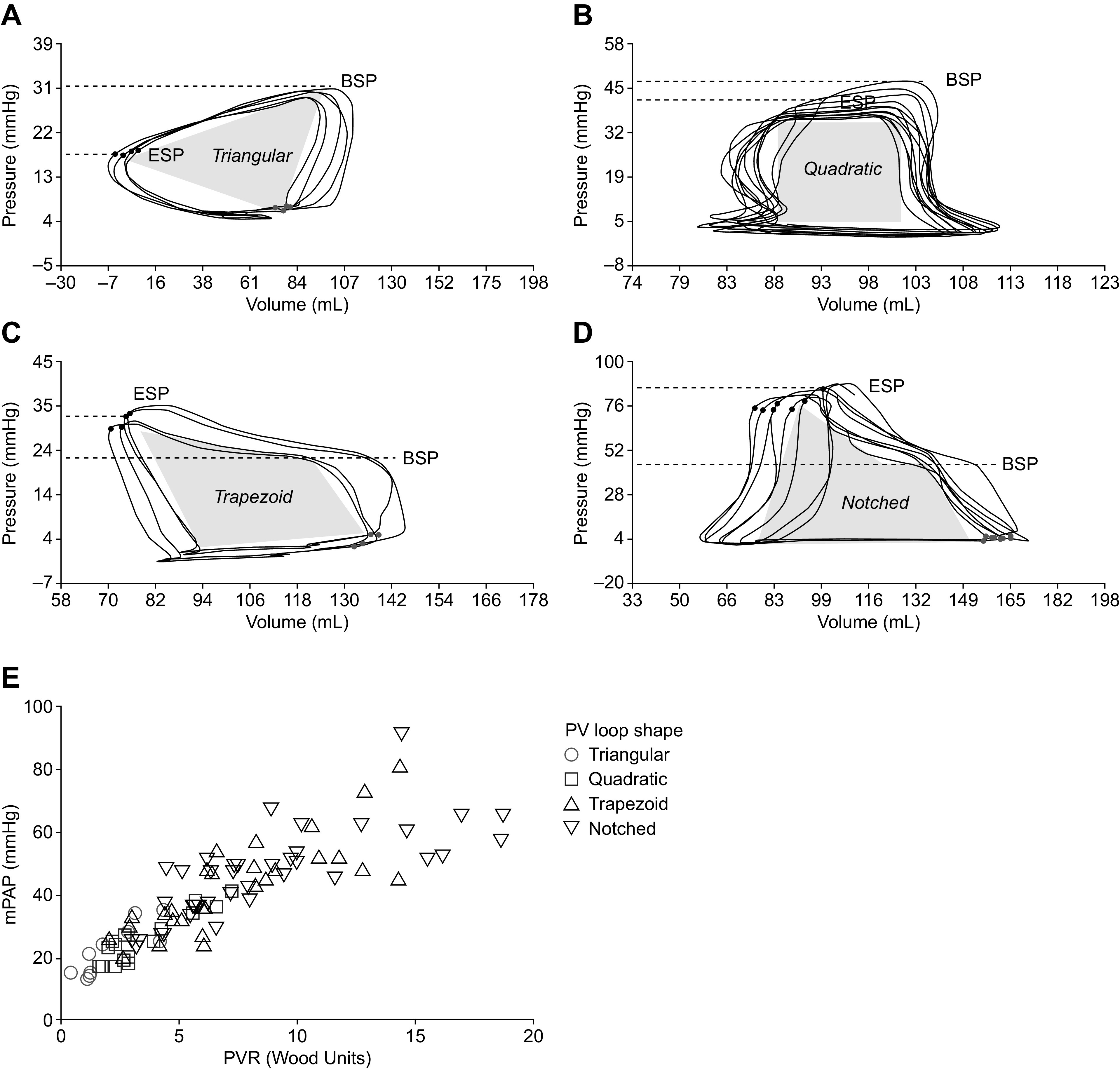
Characteristic RV PV loop shapes and RV systolic pressure differentials. Representative multibeat pressure-volume loops from a patient with triangular RV PV loops and a negative RV systolic pressure differential (A), a patient with quadratic RV PV loops and a low RV systolic pressure differential (B), a patient with trapezoid RV PV loops and a high RV systolic pressure differential (C), and a patient with notched RV PV loops and a high RV systolic pressure differential (D). E: the relationship between mPAP, PVR, and PV loop shape in all patients. BSP, beginning-systolic pressure; ESP, end-systolic pressure; mPAP, mean pulmonary arterial pressure; PV, pressure-volume; PVR, pulmonary vascular resistance; RV, right ventricular.
Additional Retrospective Analyses
We performed a retrospective analysis of the RV outflow tract (RVOT) systolic flow profile of a subgroup of previously published patients with PAH who underwent Doppler echocardiography as well as PV catheterization (22) and had available RVOT measurements (n = 24). The RVOT systolic flow profile was analyzed as described in detail previously (23) and the flow velocity at notching (Vnotch) and the peak velocity of the postnotching acceleration flow (Vpost) were obtained.
A second retrospective cohort was included to evaluate the association between RV-PA coupling and the RV systolic pressure differential without using PV catheterization. The retrospective cohort comprised 113 patients with PAH who underwent routine Swan–Ganz right heart catheterization (RHC) and echocardiography and were included in the Giessen PH Registry (24) between October 2018 and November 2020. Pressure values were continuously assessed during routine RHC as previously described (25). ESP and BSP were directly assessed from RV pressure curves, defining BSP as the pressure directly after dP/dt max and ESP as the pressure directly before dP/dt min. To assess RV-PA coupling in the retrospective cohort, we used the echocardiographic tricuspid annular plane systolic excursion (TAPSE)/pulmonary arterial systolic pressure (PASP) ratio which has been previously validated against multibeat (12) and single-beat Ees/Ea (22). Echocardiographic acquisition of TAPSE and PASP was adherent to current recommendations (26).
Statistical Analyses
Adherence to a Gaussian distribution was determined using the Kolmogorov–Smirnov test and visual assessment of histograms. Correlations were measured with Spearman’s rank correlation coefficient. Differences between groups were analyzed with the independent-samples Kruskal–Wallis test, Pearson χ2 test, Mann–Whitney U test, independent t test, or one-way analysis of variance, as appropriate. We used univariate and multivariate linear regression analysis with ESP − BSP as the dependent variable to determine the association with PV loop and key hemodynamic parameters. Variables with P < 0.001 in univariate analyses were added to the multivariate model. Multicollinearity was assessed using the variance inflation factor. Non-normally distributed parameters were natural log transformed for linear regression analysis and rechecked by visual assessment of histograms.
For analysis, SPSS, v. 23.0 and 27.0 (IBM, Armonk, NY) and Prism 8 (GraphPad Software, San Diego, CA) were used. P < 0.05 was considered statistically significant.
RESULTS
Patients
We included 77 patients with PAH and 15 patients without PH who had undergone multibeat PV loop analysis. The characteristics of the two cohorts are displayed in Table 1. The majority of the PAH cohort presented with idiopathic PAH. About half (50.6%) of the patients with PAH were on specific dual or triple combination therapy, and most were in World Health Organization functional class III. Right heart catheterization showed precapillary PH with substantial elevation of pulmonary vascular resistance (PVR) in the PAH cohort. The cohort without PH showed preserved RV-PA coupling with low pressure values overall.
Table 1.
Characteristics of the study cohort
| Patients With PAH, n = 77 | Patients With Exclusion of PH, n = 15 | |
|---|---|---|
| Male/female | 24/53 | 14/1 |
| Age, yr | 56 ± 13 | 59 ± 17 |
| BNP, pg/mL | 121 [53 to 371]a | 34 [18 to 34]b |
| NT-proBNP, pg/mL | 200 [100 to 861]c | 132 [71 to 269]d |
| PH subtype | ||
| Idiopathic PAH | 51 (66.2) | |
| Heritable PAH | 1 (1.3) | |
| PAH associated with | ||
| Portal hypertension | 4 (5.2) | |
| Connective tissue disease | 20 (26.0) | |
| Pulmonary veno-occlusive disease | 1 (1.3) | |
| Concomitant disease | ||
| Lupus erythematous | 1 (6.7) | |
| Mixed connective tissue disease | 1 (6.7) | |
| Scleroderma | 10 (66.7) | |
| Left heart disease | 1 (6.7) | |
| Dyspnea other causes | 2 (13.3) | |
| Specific combination therapy | ||
| Dual therapy | 21 (22.8) | |
| Triple therapy | 16 (20.8) | |
| World Health Organization functional class | ||
| I | 4 (5.2) | |
| II | 27 (35.1) | 11 (73.3)e |
| III | 43 (55.8) | 3 (20.0)e |
| IV | 3 (3.9) | |
| Right heart catheterization | ||
| mPAP, mmHg | 43 ± 14 | 19 ± 4 |
| Right atrial pressure, mmHg | 7 [4 to 10] | 3 [2 to 6] |
| PVR, Wood units | 6.4 [4.4 to 9.9] | 1.8 [1.2 to 2.9] |
| Cardiac index, L/min/m2 | 2.6 ± 0.6 | 2.9 ± 0.6 |
| PAWP, mmHg | 10 ± 3 | 9 ± 3 |
| PAC, mL/mmHg | 1.7 [1.3 to 2.7] | 3.0 [2.2 to 5.2] |
| RV pressure-volume loop measurements | ||
| Ea, mmHg/mL | 0.7 [0.5 to 1.1] | 0.4 [0.3 to 0.5] |
| Ees, mmHg/mL | 0.6 [0.4 to 0.8] | 0.7 [0.6 to 0.9] |
| τ, ms | 45 ± 15 | 46 ± 27d |
| ESP, mmHg | 59 [43 to 80] | 27 [23 to 31] |
| BSP, mmHg | 42 [32 to 55] | 28 [24 to 34] |
| End-diastolic pressure, mmHg | 8 [5 to 11] | 4 [3 to 5] |
| Stroke work, mL·mmHg | 2,505 [1,805 to 3,925] | 1,669 [1,364 to 1,946]d |
| Augmentation index | 0.44 [0.26 to 0.62] | −0.05[−0.40 to 1.77] |
Values represent n/n, n (%), means ± standard deviation, or median [interquartile range]. BNP, B-type natriuretic peptide; BSP, beginning-systolic pressure; Ea, arterial elastance; Ees, end-systolic elastance; ESP, end-systolic pressure; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAC, pulmonary arterial capacitance; PAH, pulmonary arterial hypertension; PAWP, pulmonary arterial wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular.
n = 45.
n = 3.
n = 30.
n = 12.
n = 14.
In the PAH cohort, the PV loop measurements showed an increase in Ea but not Ees compared with the cohort without PH, resulting in a median Ees/Ea ratio of 0.8 (Table 2), which is close to the threshold for RV-PA uncoupling (Ees/Ea < 0.7) (11, 12). ESP and end-diastolic pressure were elevated approximately twofold in the PAH cohort compared with the cohort with exclusion of PH, whereas BSP showed a smaller increase. This translated into a high RV systolic pressure differential overall in the PAH cohort (Table 2), although individual differentials showed a wide range of negative to positive values.
Table 2.
RV systolic pressure differential and Ees/Ea stratified by cohort and PV loop shape
| Multibeat Ees/Ea |
RV Systolic Pressure Differential, mmHg |
Single-Beat Ees/Ea |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median [IQR] | P | n | Median [IQR] | P | n | Median [IQR] | P | |
| Cohort | <0.001* | <0.001* | 0.080* | ||||||
| PAH | 77 | 0.8 [0.5 to 1.0] | 77 | 17 [9 to 32] | 76 | 0.8 [0.5 to 1.1] | |||
| PH excluded | 15 | 1.7 [1.1 to 2.3] | 15 | −1 [−7.6 to 3.7] | 14 | 1.0 [0.6 to 1.1] | |||
| PV loop shape | 0.006† | <0.001† | 0.051† | ||||||
| Triangular | 10 | 1.9 [1.2 to 2.4] | 10 | −11 [−15 to −7] | 10 | 1.0 [0.7 to 1.2] | |||
| Quadratic | 16 | 1.1 [0.8 to 1.7] | 16 | 0.2 [−0.8 to 3] | 16 | 1.0 [0.7 to 1.2] | |||
| Trapezoid | 28 | 0.7 [0.5 to 0.9] | 28 | 17 [10 to 29] | 27 | 0.8 [0.5 to 1.0] | |||
| Notched | 38 | 0.8 [0.4 to 1.1] | 38 | 25 [16 to 35] | 37 | 0.7 [0.4 to 1.1] | |||
Ea, arterial elastance; Ees, end-systolic elastance; IQR, interquartile range; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PV, pressure-volume; RV, right ventricular.
MannWhitney U test (PAH vs PH excluded).
KruskalWallis test across PV loop shapes.
In the overall study population, four distinct morphologies of PV loops were identified: triangular with an early systolic pressure peak with BSP > ESP (Fig. 1A), rectangular with ESP close to BSP (Fig. 1B), trapezoid with late systolic peaking of pressure and ESP > BSP (Fig. 1C), and the same but with identifiable midsystolic notching (Fig. 1D). Patients with a systolic RV notch had the highest mPAP and PVR, whereas patients with quadratic or triangular PV loops had substantially lower pressure and resistance (Fig. 1E). A high proportion of patients receiving dual combination therapy for PAH had trapezoid (38%) or notched (48%) PV loop shapes; a similar distribution was observed in patients receiving triple combination therapy (trapezoid, 50%; notched, 44%) (Supplemental Fig. S1; all Supplemental materials are available at https://dx.doi.org/10.17504/protocols.io.br4em8te). Patients with idiopathic PAH and those with connective tissue disease-associated PAH showed no significant difference in the distribution of PV loop shapes (P = 0.63; Supplemental Fig. S2) or the RV systolic pressure differential (P = 0.85).
Within the PAH cohort, a direct comparison between patients from the Giessen center and those from the Johns Hopkins center showed significant differences in sex, etiology, mPAP, τ, and BSP (Supplemental Table S1).
Association of RV PV Loop Morphology with Hemodynamics, B-Type Natriuretic Peptide, Ea, and Ees/Ea
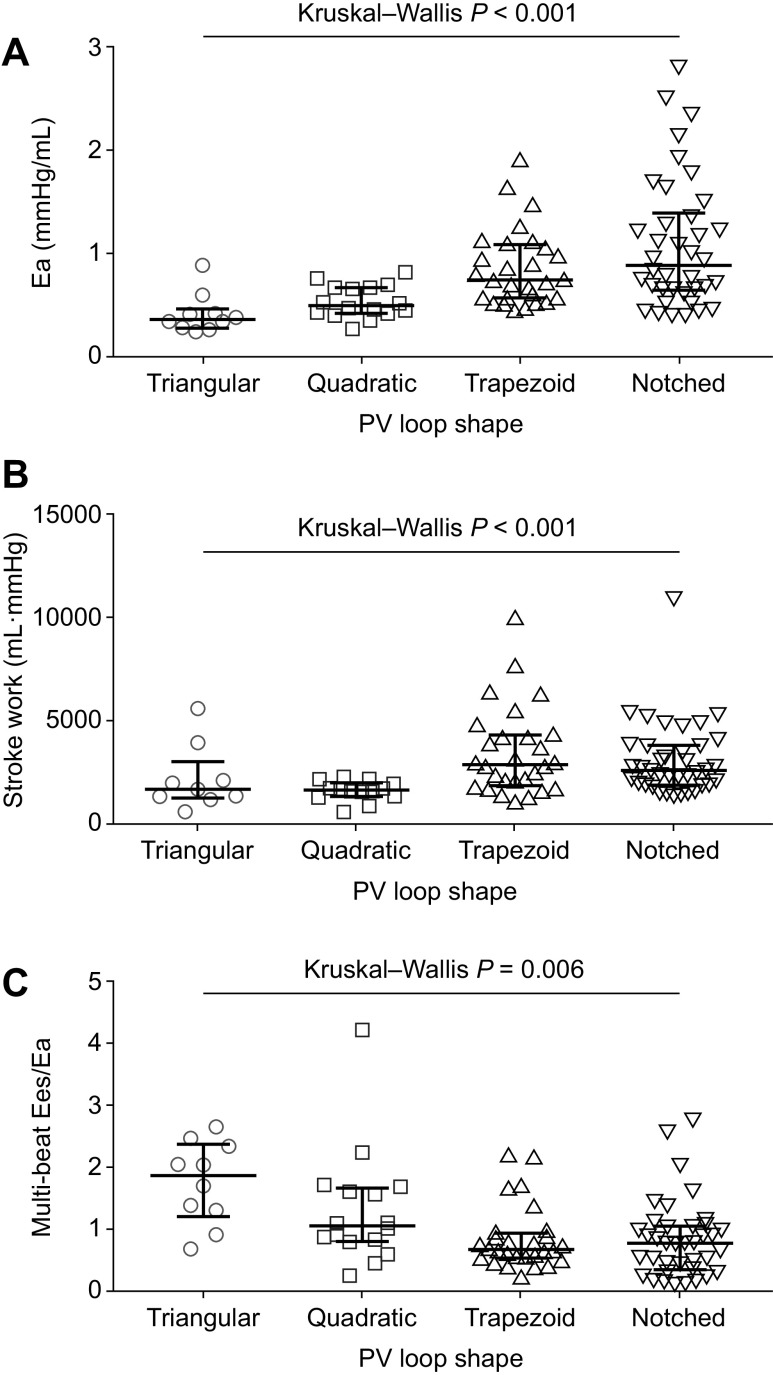
In all patients, we stratified key hemodynamic measurements, B-type natriuretic peptide (BNP) levels, and PV loop-derived parameters by the four characteristic PV loop shapes. The trapezoid and notched PV loop shapes were associated with the highest mPAP and PVR (Fig. 2, A and B), the lowest PAC (Fig. 2C), the highest augmentation index (Fig. 2D) and BNP levels (Fig. 2E), the highest Ea and stroke work (Fig. 3, A and B), and lowest multibeat Ees/Ea (Fig. 3C). Of note, multibeat Ees was not significantly different across the PV loop shapes (P = 0.890). Single-beat Ees/Ea stratified by PV loop shape showed a comparable pattern to multibeat Ees/Ea, albeit without statistical significance (Table 2).
Figure 2.
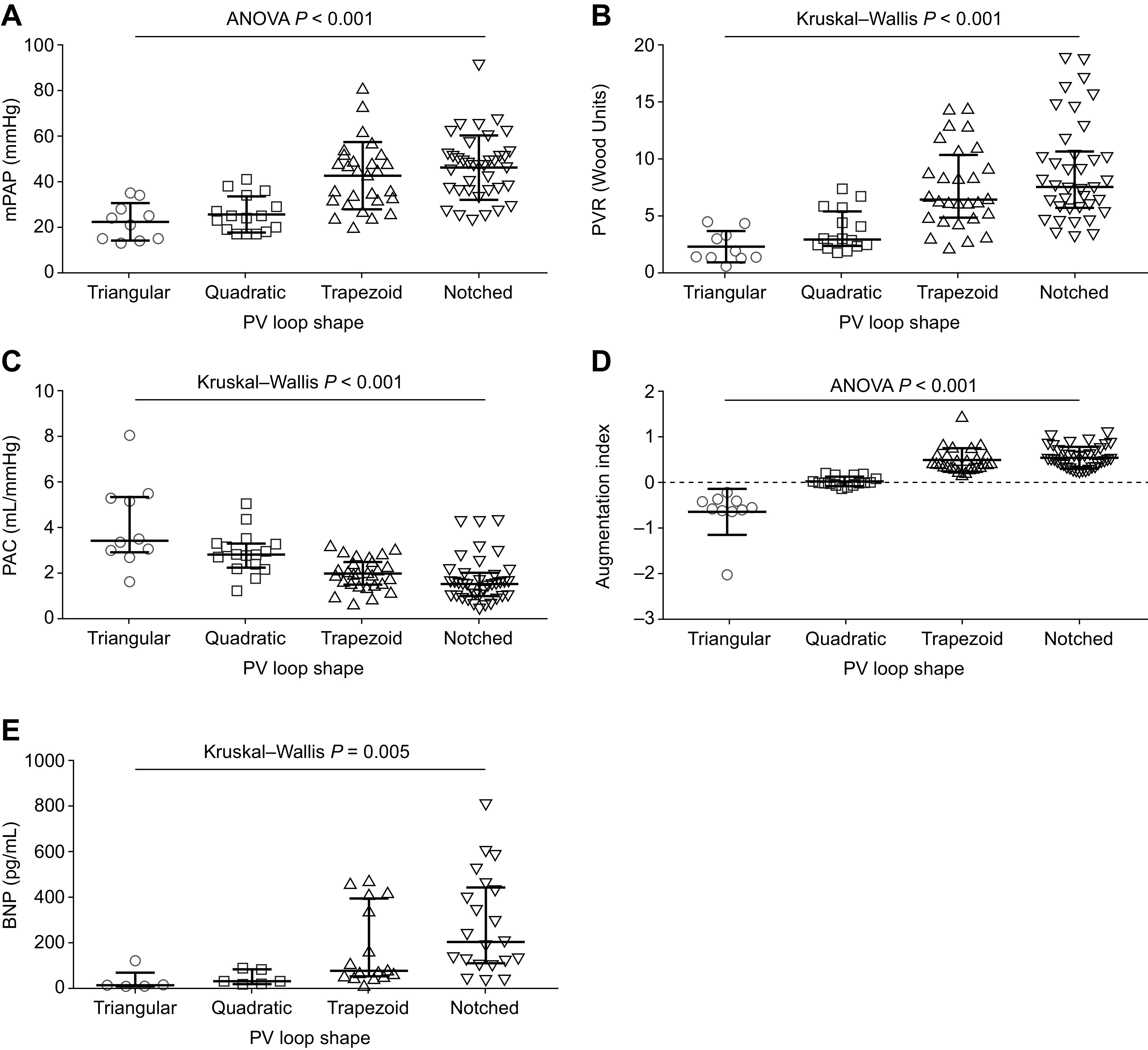
Association of right ventricular PV loop shape with right heart catheter measurements, augmentation index, and BNP levels. Right heart catheter measurements (PAH, n = 77; non-PH, n = 15) (A–C), augmentation index (PAH, n = 77; non-PH, n = 15) (D), and BNP levels (PAH, n = 45; non-PH, n = 3) (E) were stratified by PV loop shape. Means and standard deviations are shown for mPAP (A) and augmentation index (D). Medians and interquartile ranges are shown for PVR (B), PAC (C), and BNP (E). BNP, B-type natriuretic peptide; mPAP, mean pulmonary arterial pressure; PAC, pulmonary arterial capacitance; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PV, pressure-volume; PVR, pulmonary vascular resistance.
Figure 3.
Association of right ventricular PV loop shape with PV loop parameters. Ea (PAH, n = 77; non-PH, n = 15) (A), stroke work (PAH, n = 77; non-PH, n = 12) (B), and multibeat Ees/Ea (PAH, n = 77; non-PH, n = 15) (C) were stratified by PV loop shape. Medians and interquartile ranges are shown in each graph. Ea, arterial elastance; Ees, end-systolic elastance; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PV, pressure-volume.
As shown in Table 2, the trapezoid and notched PV loop shapes indicate RV-PA uncoupling defined as Ees/Ea < 0.7 (11, 12) measured using the single-beat or multibeat method. The characteristic and corresponding pattern of the RV systolic pressure differential across PV loop shapes is also shown in Table 2; the RV systolic pressure differential adjusted for SV [(ESP − BSP)/SV] showed a similar pattern (Supplemental Table S2).
Association of the RV Systolic Pressure Differential with Hemodynamics, BNP, Ea, and Ees/Ea
In correlation analyses of the overall study population, the RV systolic pressure differential was significantly associated with Ea and multibeat Ees/Ea (Fig. 4) as well as single-beat Ees/Ea (Supplemental Fig. S3); patients who had negative or low RV systolic pressure differentials showed lower Ea and better RV-arterial coupling than those with high differentials. Moreover, the RV systolic pressure differential was significantly associated with mPAP, PVR, PAC, stroke work, and BNP and N-terminal proBNP levels (Fig. 5). The association of the RV systolic pressure differential with Ea, RV-arterial coupling, and key hemodynamic parameters remained consistent when assessed in each participating center separately (Supplemental Table S3). The RV systolic pressure differential adjusted for SV [(ESP − BSP)/SV] was also significantly associated with PAC and multibeat Ees/Ea (Supplemental Fig. S4).
Figure 4.
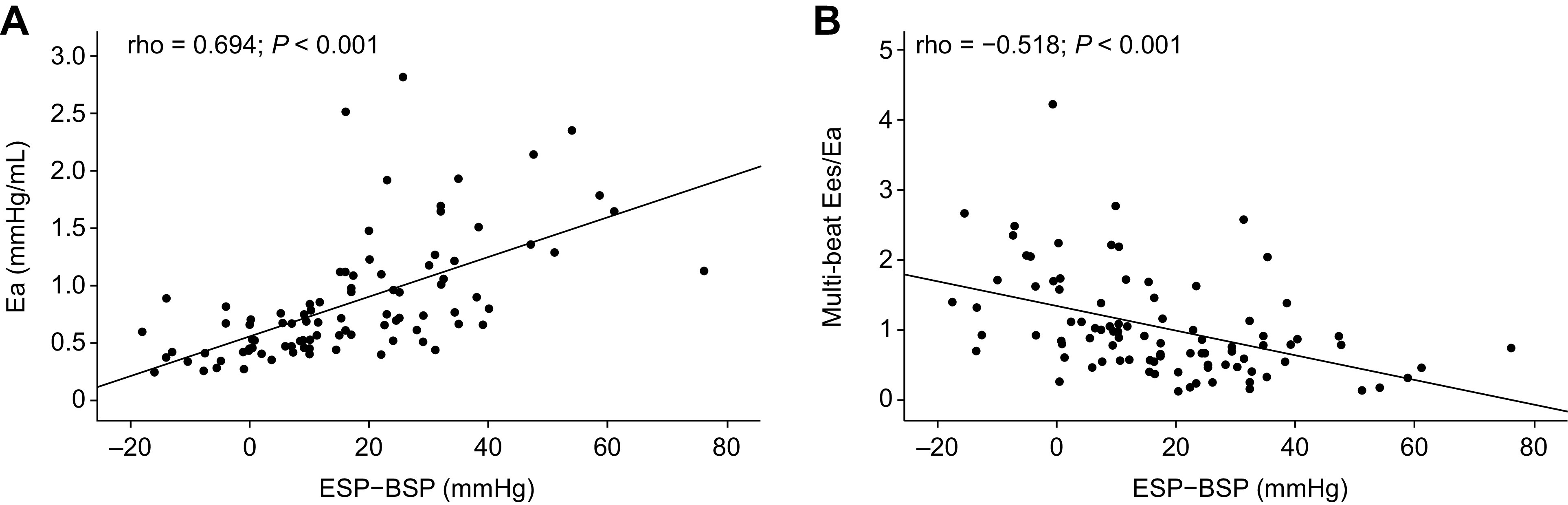
Association of the RV systolic pressure differential with afterload and RV-pulmonary arterial coupling. Spearman correlations showed significant associations of the RV systolic pressure differential (ESP − BSP) with afterload (Ea; PAH, n = 77; non-PH, n = 15) (A) and RV-pulmonary arterial coupling (multibeat Ees/Ea; PAH, n = 77; non-PH, n = 15) (B). BSP, beginning-systolic pressure; Ea, arterial elastance; Ees, end-systolic elastance; ESP, end-systolic pressure; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; RV, right ventricular.
Figure 5.
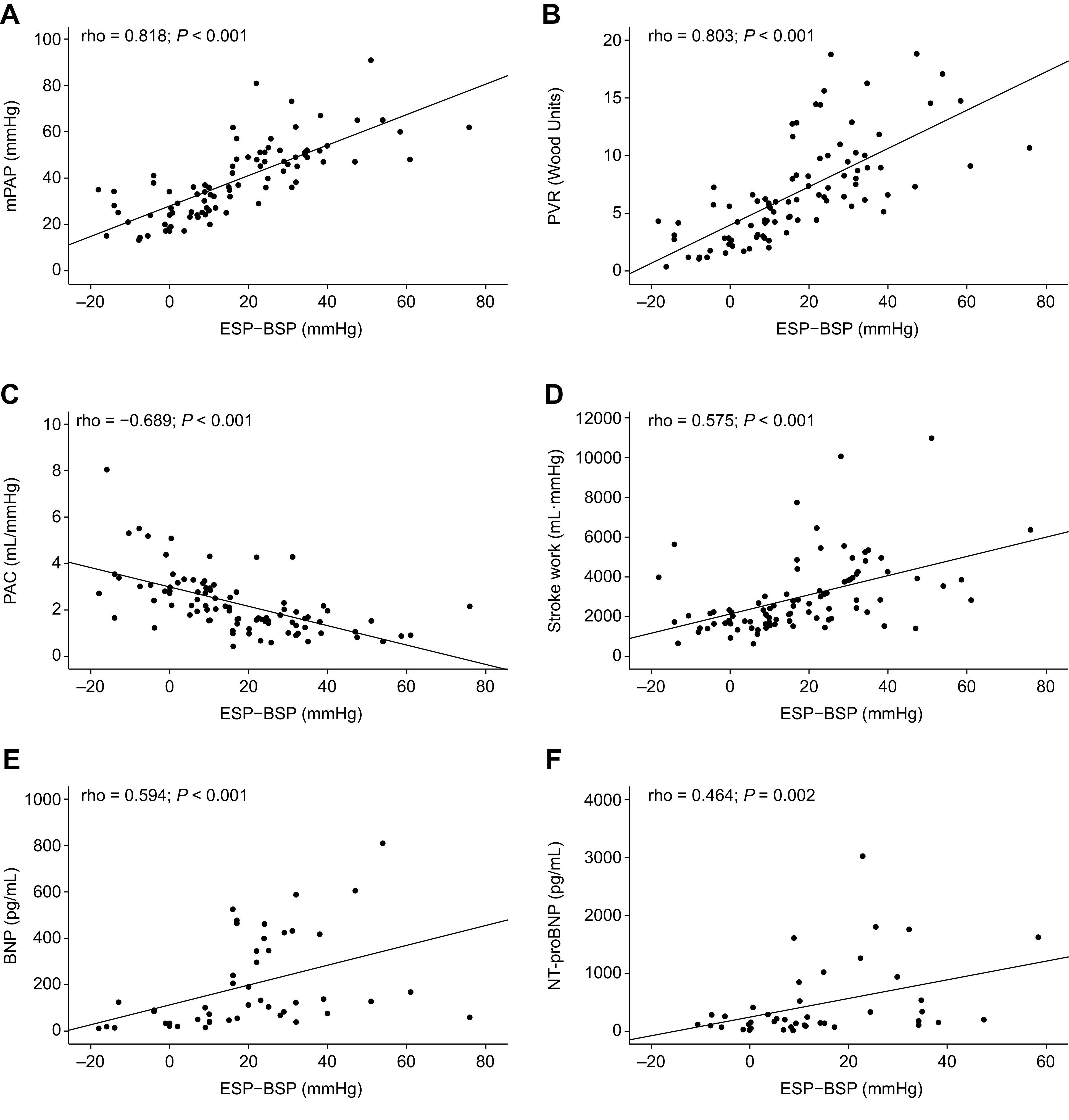
Association of the RV systolic pressure differential with key hemodynamic parameters and BNP. Spearman correlations showed significant associations of the RV systolic pressure differential (ESP − BSP) with mPAP (PAH, n = 77; non-PH, n = 15) (A), PVR (PAH, n = 77; non-PH, n = 15) (B), PAC (PAH, n = 77; non-PH, n = 15) (C), stroke work (PAH, n = 77; non-PH, n = 12) (D), BNP (PAH, n = 45; non-PH, n = 3) (E), and NT-proBNP (PAH, n = 30; non-PH, n = 12) (F). BNP, B-type natriuretic peptide; BSP, beginning-systolic pressure; ESP, end-systolic pressure; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PAC, pulmonary arterial capacitance; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RV, right ventricular.
In univariate linear regression analysis, the afterload-dependent indices Ea, PVR, and PAC as well as stroke work and multibeat Ees/Ea were associated with the RV systolic pressure differential. Using multivariate analysis (adjusting for all variables with P < 0.001 in univariate analysis), Ea, PVR, and stroke work remained significant determinants of the RV systolic pressure differential (Table 3).
Table 3.
Determinants of RV systolic pressure differential in univariate and multivariate linear regression analysis
| B Coefficient (95% CI)* | P | |
|---|---|---|
| Univariate | ||
| Ees/Ea | −11.99 (−16.62 to −0.7.37) | <0.001 |
| Ea, mmHg/mL | 22.26 (17.13 to 27.39) | <0.001 |
| Ees, mmHg/mL | 1.35 (−5.25 to 7.96) | 0.69 |
| τ, ms | 0.072 (−0.16 to 0.31) | 0.55 |
| Stroke work, mL·mmHg | 17.22 (11.34 to 23.12) | <0.001 |
| Pulmonary arterial capacitance, mL/mmHg | −20.20 (−25.65 to −14.76) | <0.001 |
| Pulmonary vascular resistance, Wood units | 18.23 (14.64 to 21.80) | <0.001 |
| Multivariate† | ||
| Ea, mmHg/mL | 8.16 (0.42 to 15.91) | 0.04 |
| Stroke work, mL·mmHg | 8.26 (3.10 to 13.42) | <0.001 |
| Pulmonary vascular resistance, Wood units | 10.91 (4.34 to 17.48) | 0.001 |
CI, confidence interval; Ea, arterial elastance; Ees, end-systolic elastance; RV, right ventricular.
Unstandardized coefficients.
Adjusted with backward stepwise selection for all variables with P < 0.001 in univariate analysis.
Additional Retrospective Analyses
In the retrospective cohort of patients with PAH who underwent Doppler echocardiography as well as PV catheterization (n = 24), we were able to show a significant association of Vnotch and Vpost with the RV systolic pressure differential (Supplemental Fig. S5).
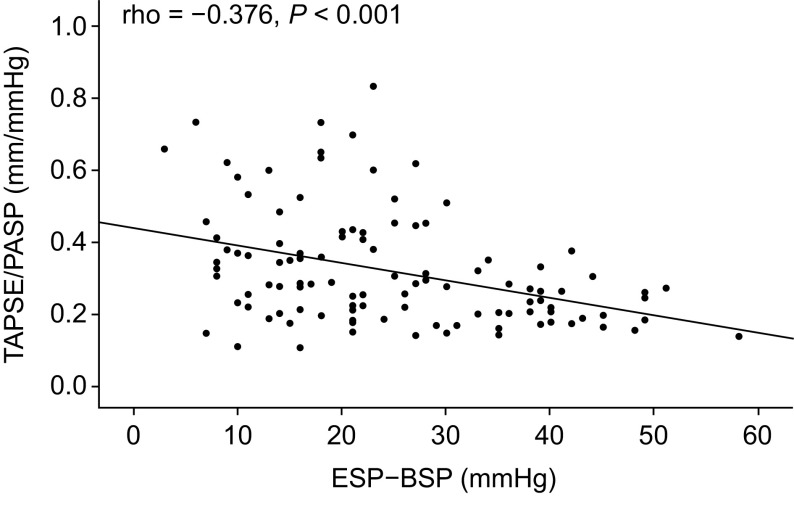
In the second retrospective cohort of patients with PAH (n = 113), the RV systolic pressure differential obtained from routine RHC showed a significant correlation with TAPSE/PASP (Fig. 6). Key hemodynamic and echocardiographic parameters in this cohort were as follows: mPAP, 44 ± 12 mmHg; PAWP, 11 ± 4 mmHg; cardiac output, 4.5 ± 1.1 L/min; PVR, 7.4 ± 3.7 Wood units; ESP, 58 ± 19 mmHg; BSP, 34 ± 13 mmHg; ESP − BSP, 25 ± 12 mmHg; and TAPSE/PASP, 0.32 ± 0.16 mm/mmHg.
Figure 6.
Association of the RV systolic pressure differential obtained during routine RHC with a non-invasive surrogate of RV-pulmonary arterial coupling (TAPSE/PASP) in a retrospective pulmonary arterial hypertension cohort from the Giessen Pulmonary Hypertension Registry (n = 113; Spearman correlation). BSP, beginning-systolic pressure; ESP, end-systolic pressure; PASP, pulmonary arterial systolic pressure; RHC, right heart catheterization; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion.
DISCUSSION
The present results show that RV PV loop morphology and systolic pressure differential are predominantly related to RV afterload and allow for a stratification of the adequacy of RV functional adaptation to increased afterload in patients with PAH. Moreover, understanding RV PV loop morphology and RV systolic pressure differential alone is sufficient to gain an in-depth appreciation of the patient’s pulmonary vascular disease and RV-PA coupling. Even routine measurement of the RV systolic pressure differential by RHC associates with a simple, bed-side ready surrogate of RV-PA coupling (the TAPSE/PASP ratio), which itself has proven its prognostic relevance across various disease entities (27).
Ventricular function is defined by the PV relationship (1–4). Major morphological differences between normal left ventricular PV loops [which are rectangular with a negligible ESP − BSP gradient (5)] and normal RV PV loops [which are rounded with a negative ESP − BSP gradient (4, 6)] are explained by differences in aortic and pulmonary arterial impedance (4). Previous studies have shown that RV PV loops in PH have triangular or rectangular aspects with late systolic peaking of pressure and inversion of the ESP − BSP gradient (7, 28). The systemic right ventricle (e.g., after a Mustard operation for transposition of the great arteries) is also characterized by rectangular PV loops (7). The present results confirm the relationship between RV PV loop morphology and the severity of PH as assessed by mPAP, PVR, and PAC. However, RV PV loop morphology was also tightly correlated to Ees/Ea and BNP. Therefore, RV PV loop morphology appears to be an indicator of both the severity of PH and RV systolic functional adaptation to afterload.
The shape of RV PV loops appears to be determined by PA pressure and flow wave morphology. Increased pulse pressure and late systolic peaking of pressure, which were noted in the earliest right heart catheterizations in patients with severe PH (29), are explained by a combination of low PAC (30) and return of the first reflected pressure wave on the forward wave associated with ventricular systole (31, 32). The contribution of pressure wave reflection can be quantified by the augmentation index (the difference between the inflection point on the ascending pressure stroke and peak systolic pressure, divided by pulse pressure) (17, 31). We considered quantifying wave reflection by calculation of the augmentation index (17) in the pulmonary artery as well as in the right ventricle, but could not identify the inflection point on the ascending limb of RV and PA pressure in a sufficient number of patients. However, this was easily appreciated on the RV PV loop when pressure rose during ejection. Decreased PAC and/or proximal reflection (e.g., in chronic thromboembolic PH) cause a late-systolic or midsystolic deceleration of flow (33–35), resulting from a returning reflected wave during ventricular systole (36, 37). Time to notching on PA flow curves is decreased in the presence of proximal PA obstruction (36) (as occurs in chronic thromboembolic PH) but is also proportional to PH severity (35), as higher PVR decreases PAC and increases wave speed (37). In addition, our data indicate that a higher RV systolic pressure differential translates into higher notching of the RVOT systolic Doppler flow velocity envelope; this association might indirectly underscore the impact of wave reflection on the PV loop shape. A combination of these alterations in the morphology of PA pressure and flow curves explains the triangular, square, and eventually notched trapezoid shape of PV loops found in the present study.
As measuring multibeat PV loops with high-fidelity technology is demanding, there has been interest in assessing RV-PA coupling solely from single RV pressure curves (38, 39). It has been shown that the Ees/Ea ratio can be simplified for volumes as Pmax/ESP − 1, where Pmax is calculated from early- and late-systolic portions of the RV pressure curve and represents the peak isovolumetric pressure of a nonejecting beat (40). This pressure-only derived estimate of RV-PA coupling has been shown to be correlated to multibeat Ees/Ea (12). In the present study, we reasoned that the progressive increase in ESP with increasing ESP − BSP gradients (the RV systolic pressure differential) (7, 28) would reflect the Ees/Ea ratio. Our results showed significant associations of ESP − BSP with Ees/Ea in correlation and univariate linear regression analyses but not in the multivariate analysis. Ea remained significantly associated with ESP − BSP in the multivariate analysis, suggesting that the association of ESP − BSP with RV-arterial coupling is driven by elevated load in patients with lower coupling values. Currently, it is a matter of debate, whether ESP in PH is closer to mPAP or to right ventricular systolic pressure (41, 42). Our data show that in the setting of PH, ESP is more closely related to RV systolic pressure than mPAP.
Limitations
The study is limited by the small sample of only 77 patients with PAH and only 15 patients without PH. This may have influenced the findings of subgroup analyses, such as the lack of difference in PV loop shape distribution between treatment groups (dual vs. triple combination therapy) and between PAH subtypes (idiopathic PAH vs. connective tissue disease-associated PAH). However, PAH is a rare disease and the methods of evaluation used in our study are very invasive. It is therefore difficult to repeat the study in much larger populations of patients. Nevertheless, the impact of systemic prostacyclin and different PH subtypes on the RV PV loop shape merits further investigation. The study is also limited by differences between the two participating centers in patient characteristics and methods for preload reduction [although the Valsalva method has been previously validated against invasive preload reduction by balloon occlusion (43)]. These differences must be considered when interpreting the data. In addition, it remains unknown if the RV PV loop shape changes over time in response to increased or decreased afterload. This merits further investigation. The acute effects of specific pulmonary vasoactive therapy on the RV PV loop shape and on load-independent RV function should also be explored in future studies. As recently highlighted by the American Thoracic Society (2), the relevance of RV diastolic function should be further assessed.
During the recruiting period of the study, updated recommendations for the hemodynamic definition of PH were published (44). According to the new definition, 5 of the 15 controls would be diagnosed as having PH with mPAP between 21 mmHg and 24 mmHg. However, only two of those controls (both with connective tissue disease) had a PVR above 3 Wood units and would therefore be classed as having PAH according to the new definition. As we did not separately analyze PH and controls in the main figures, key correlations, or linear regression analysis, we believe that the impact of the new definition on our findings is minimal.
CONCLUSIONS
We have presented a comprehensive analysis of RV PV loop shape in relation to load and coupling. The data clearly indicate that increasing load is associated with increasing ESP − BSP differential and with alterations in PV loop shape (from triangular to quadratic, trapezoid, and finally notched). Both the shape of PV loops and the RV systolic pressure differential allow for the assessment of RV adaptation to afterload in PH. Trapezoid and notched PV loop shapes indicate patients with RV-PA uncoupling in a direct visual assessment.
GRANTS
This work was supported by the Excellence Cluster Cardio-Pulmonary System (ECCPS) and the Collaborative Research Center (SFB) 1213 – Pulmonary Hypertension and Cor Pulmonale, Grant number SFB1213/1, project B08 (German Research Foundation, Bonn, Germany). This work was also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health Grants K23‐HL146889 (to S. Hsu) and R01‐HL114910 (to P. M. Hassoun, R. S. Tedford, D. A. Kass), and the Jerome Greene Scholarship (to S. Hsu).
DISCLOSURES
M. J. Richter has received support from United Therapeutics and Bayer; speaker fees from Bayer, Actelion, Mundipharma, Roche, and OMT; and consultancy fees from Bayer. H. A. Ghofrani has received consultancy fees from Bayer, Actelion, Pfizer, Merck, GSK, and Novartis; fees for participation in advisory boards from Bayer, Pfizer, GSK, Actelion, and Takeda; lecture fees from Bayer HealthCare, GSK, Actelion, and Encysive/Pfizer; industry-sponsored grants from Bayer HealthCare, Aires, Encysive/Pfizer, and Novartis; and sponsored grants from the German Research Foundation, Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research. R. Naeije has relationships with drug companies including AOPOrphan Pharmaceuticals, Actelion, Bayer, Reata, Lung Biotechnology Corporation, and United Therapeutics. In addition to being an investigator in trials involving these companies, relationships include consultancy service, research grants, and membership of scientific advisory boards. W. Seeger has received speaker/consultancy fees from Pfizer and Bayer Pharma AG. H. Gall has received fees from Actelion, AstraZeneca, Bayer, BMS, GSK, Janssen-Cilag, Lilly, MSD, Novartis, OMT, Pfizer, and United Therapeutics. R. J. Tedford reports no direct conflicts pertinent to the development of this manuscript. Other general conflicts include consulting relationships with Medtronic, Arena Pharmaceuticals, Aria CV, and United Therapeutics. R. J. Tedford is on a steering committee for Medtronic and the research advisory board for Abiomed. He also does hemodynamic core laboratory work for Actelion and Merck. K. Tello has received speaking fees from Actelion and Bayer. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
M.J.R., S.H., H.A.G., F.G., W.S., H.G., and K.T. conceived and designed research; M.J.R., S.H., H.A.G., F.G., W.S., H.G., R.J.T., and K.T. performed experiments; M.J.R., H.G., and K.T. analyzed data; M.J.R., H.A.G., W.S., H.G., and K.T. interpreted results of experiments; M.J.R., H.A.G., W.S., H.G., R.J.T., and K.T. prepared figures; M.J.R., S.H., A.Y., I.V., H.A.G., R.N., S.H., F.G., W.S., H.G., R.J.T., and K.T. drafted manuscript; M.J.R., S.H., A.Y., F.H.-S., I.V., H.A.G., R.N., S.H., F.G., W.S., H.G., R.J.T., and K.T. edited and revised manuscript; M.J.R., S.H., A.Y., F.H.-S., I.V., H.A.G., R.N., S.H., F.G., W.S., H.G., R.J.T., and K.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Claire Mulligan, PhD (Beacon Medical Communications Ltd, Brighton, UK) for editorial support, funded by the University of Giessen. We also thank Prof. David A. Kass and Prof. Paul M. Hassoun (Johns Hopkins University School of Medicine) for funding and patient enrollment.
REFERENCES
- 1.Ren X, Johns RA, Gao WD. EXPRESS: right heart in pulmonary hypertension: from adaptation to failure. Pulm Circ 9: 204589401984561, 2019. doi: 10.1177/2045894019845611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, Mathai SC, Mercier O, Michelakis ED, Naeije R, Tuder RM, Ventetuolo CE, Vieillard-Baron A, Voelkel NF, Vonk-Noordegraaf A, Hassoun PM; American Thoracic Society Assembly on Pulmonary Circulation. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An official American Thoracic Society Research Statement. Am J Respir Crit Care Med 198: e15–e43, 2018. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanz J, Sanchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol 73: 1463–1482, 2019. doi: 10.1016/j.jacc.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 4.Maughan WL, Shoukas AA, Sagawa K, Weisfeldt ML. Instantaneous pressure-volume relationship of the canine right ventricle. Circ Res 44: 309–315, 1979. doi: 10.1161/01.res.44.3.309. [DOI] [PubMed] [Google Scholar]
- 5.Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res 32: 314–322, 1973. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- 6.McCabe C, White PA, Hoole SP, Axell RG, Priest AN, Gopalan D, Taboada D, MacKenzie Ross R, Morrell NW, Shapiro LM, Pepke-Zaba J. Right ventricular dysfunction in chronic thromboembolic obstruction of the pulmonary artery: a pressure-volume study using the conductance catheter. J Appl Physiol (1985) 116: 355–363, 2014. doi: 10.1152/japplphysiol.01123.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redington AN, Gray HH, Hodson ME, Rigby ML, Oldershaw PJ. Characterisation of the normal right ventricular pressure-volume relation by biplane angiography and simultaneous micromanometer pressure measurements. Br Heart J 59: 23–30, 1988. doi: 10.1136/hrt.59.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tello K, Richter MJ, Axmann J, Buhmann M, Seeger W, Naeije R, Ghofrani HA, Gall H. More on single-beat estimation of right ventriculoarterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med 198: 816–818, 2018. doi: 10.1164/rccm.201802-0283LE. [DOI] [PubMed] [Google Scholar]
- 9.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 284: H1625–H1630, 2003. doi: 10.1152/ajpheart.01023.2002. [DOI] [PubMed] [Google Scholar]
- 10.Hsu S, Houston BA, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, Damico RL, Kolb TM, Hummers LK, Shah AA, McMahan Z, Corona-Villalobos CP, Zimmerman SL, Wigley FM, Hassoun PM, Kass DA, Tedford RJ. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation 133: 2413–2422, 2016. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu S, Simpson CE, Houston BA, Wand A, Sato T, Kolb TM, Mathai SC, Kass DA, Hassoun PM, Damico RL, Tedford RJ. Multi-beat right ventricular-arterial coupling predicts clinical worsening in pulmonary arterial hypertension. J Am Heart Assoc 9: e016031, 2020. doi: 10.1161/JAHA.119.016031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter MJ, Peters D, Ghofrani HA, Naeije R, Roller F, Sommer N, Gall H, Grimminger F, Seeger W, Tello K. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med 201: 116–119, 2020. doi: 10.1164/rccm.201906-1195LE. [DOI] [PubMed] [Google Scholar]
- 13.Tello K, Dalmer A, Husain-Syed F, Seeger W, Naeije R, Ghofrani HA, Gall H, Richter MJ. Multibeat right ventricular-arterial coupling during a positive acute vasoreactivity test. Am J Respir Crit Care Med 199: e41–e42, 2019. doi: 10.1164/rccm.201809-1787IM. [DOI] [PubMed] [Google Scholar]
- 14.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 46: 903–975, 2015. [Erratum in Eur Respir J 46: 1855-1856, 2015]. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 15.Frost A, Badesch D, Gibbs JSR, Gopalan D, Khanna D, Manes A, Oudiz R, Satoh T, Torres F, Torbicki A. Diagnosis of pulmonary hypertension. Eur Respir J 53: 1801904, 2019. doi: 10.1183/13993003.01904-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French S, Amsallem M, Ouazani N, Li S, Kudelko K, Zamanian RT, Haddad F, Chung L. Non-invasive right ventricular load adaptability indices in patients with scleroderma-associated pulmonary arterial hypertension. Pulm Circ 8: 204589401878826, 2018. doi: 10.1177/2045894018788268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation 62: 105–116, 1980. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 18.Tello K, Dalmer A, Axmann J, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Seeger W, Sommer N, Wilhelm J, Gall H, Richter MJ. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail 12: e005512, 2019. doi: 10.1161/CIRCHEARTFAILURE.118.005512. [DOI] [PubMed] [Google Scholar]
- 19.Tello K, Richter MJ, Yogeswaran A, Ghofrani HA, Naeije R, Vanderpool R, Gall H, Tedford RJ, Seeger W, Lahm T. Sex differences in right ventricular-pulmonary arterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med 202: 1042–1046, 2020. doi: 10.1164/rccm.202003-0807LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter MJ, Badagliacca R, Wan J, Vanderpool R, Dalmer A, Ghofrani HA, Harth S, Seeger W, Gall H, Naeije R, Tello K. Right ventricular dyssynchrony: from load-independent right ventricular function to wall stress in severe pulmonary arterial hypertension. Pulm Circ 10: 2045894020925759, 2020. doi: 10.1177/2045894020925759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tello K, Seeger W, Naeije R, Vanderpool R, Ghofrani HA, Richter M, Tedford RJ, Bogaard HJ. Right heart failure in pulmonary hypertension: diagnosis and new perspectives on vascular and direct right ventricular treatment. Br J Pharmacol 178: 90–107, 2019. doi: 10.1111/bph.14866. [DOI] [PubMed] [Google Scholar]
- 22.Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Mohajerani E, Seeger W, Herberg U, Sommer N, Gall H, Richter MJ. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging 12: e009047, 2019. doi: 10.1161/CIRCIMAGING.119.009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahama H, McCully RB, Frantz RP, Kane GC. Unraveling the RV ejection doppler envelope: insight into pulmonary artery hemodynamics and disease severity. JACC Cardiovasc Imaging 10: 1268–1277, 2017. doi: 10.1016/j.jcmg.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA. The Giessen pulmonary Hypertension registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant 36: 957–967, 2017. doi: 10.1016/j.healun.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Yogeswaran A, Richter MJ, Sommer N, Ghofrani HA, Seeger W, Gall H, Tello K. Evaluation of pulmonary hypertension by right heart catheterisation: does timing matter? Eur Respir J 56: 1901892, 2020. doi: 10.1183/13993003.01892-2019. [DOI] [PubMed] [Google Scholar]
- 26.Kiely DG, Levin D, Hassoun P, Ivy DD, Jone PN, Bwika J, Kawut SM, Lordan J, Lungu A, Mazurek J, Moledina S, Olschewski H, Peacock A, Puri GD, Rahaghi F, Schafer M, Schiebler M, Screaton N, Tawhai M, Van Beek EJ, Vonk-Noordegraaf A, Vanderpool RR, Wort J, Zhao L, Wild J, Vogel-Claussen J, Swift AJ. EXPRESS: Statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ 9: 204589401984199, 2019.doi: 10.1177/2045894019841990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guazzi M. Use of TAPSE/PASP ratio in pulmonary arterial hypertension: an easy shortcut in a congested road. Int J Cardiol 266: 242–244, 2018. doi: 10.1016/j.ijcard.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 28.McCabe C, White PA, Rana BS, Gopalan D, Agrawal B, Pepke-Zaba J, Hoole SP. Right ventricle functional assessment: have new techniques supplanted the old faithful conductance catheter? Cardiol Rev 22: 233–240, 2014. doi: 10.1097/CRD.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 29.Cournand A, Bloomfield RA, Lauson HD. Double lumen catheter for intravenous and intracardiac blood sampling and pressure recording. Proc Soc Exp Biol Med 60: 73–75, 1945. doi: 10.3181/00379727-60-15095. [DOI] [PubMed] [Google Scholar]
- 30.Elzinga G, Piene H, de Jong JP. Left and right ventricular pump function and consequences of having two pumps in one heart. A study on the isolated cat heart. Circ Res 46: 564–574, 1980. doi: 10.1161/01.res.46.4.564. [DOI] [PubMed] [Google Scholar]
- 31.Castelain V, Herve P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol 37: 1085–1092, 2001. doi: 10.1016/S0735-1097(00)01212-2. [DOI] [PubMed] [Google Scholar]
- 32.Laskey WK, Ferrari VA, Palevsky HI, Kussmaul WG. Pulmonary artery hemodynamics in primary pulmonary hypertension. J Am Coll Cardiol 21: 406–412, 1993. doi: 10.1016/0735-1097(93)90682-Q. [DOI] [PubMed] [Google Scholar]
- 33.Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, Mishima M, Uematsu M, Shimazu T, Hori M, Abe H. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 68: 302–309, 1983. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 34.Torbicki A, Kurzyna M, Ciurzynski M, Pruszczyk P, Pacho R, Kuch-Wocial A, Szulc M. Proximal pulmonary emboli modify right ventricular ejection pattern. Eur Respir J 13: 616–621, 1999. doi: 10.1183/09031936.99.13361699. [DOI] [PubMed] [Google Scholar]
- 35.Turkevich D, Groves BM, Micco A, Trapp JA, Reeves JT. Early partial systolic closure of the pulmonic valve relates to severity of pulmonary hypertension. Am Heart J 115: 409–418, 1988. doi: 10.1016/0002-8703(88)90489-9. [DOI] [PubMed] [Google Scholar]
- 36.Furuno Y, Nagamoto Y, Fujita M, Kaku T, Sakurai S, Kuroiwa A. Reflection as a cause of mid-systolic deceleration of pulmonary flow wave in dogs with acute pulmonary hypertension: comparison of pulmonary artery constriction with pulmonary embolisation. Cardiovasc Res 25: 118–124, 1991. doi: 10.1093/cvr/25.2.118. [DOI] [PubMed] [Google Scholar]
- 37.Naeije R, Huez S. Reflections on wave reflections in chronic thromboembolic pulmonary hypertension. Eur Heart J 28: 785–787, 2007. doi: 10.1093/eurheartj/ehm040. [DOI] [PubMed] [Google Scholar]
- 38.Naeije R, Richter MJ, Vanderpool R, Tello K. When it all comes down to pressure: right ventricular ejection fraction at cardiac catheterisation. Eur Respir J 55: 1902341, 2020. doi: 10.1183/13993003.02341-2019. [DOI] [PubMed] [Google Scholar]
- 39.Trip P, Kind T, van de Veerdonk MC, Marcus JT, de Man FS, Westerhof N, Vonk-Noordegraaf A. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant 32: 50–55, 2013. doi: 10.1016/j.healun.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 40.Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, Mathier MA, Lacomis J, Champion HC, Simon MA. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 101: 37–43, 2015. doi: 10.1136/heartjnl-2014-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brener MI, Burkhoff D, Sunagawa K. Effective arterial elastance in the pulmonary arterial circulation: derivation, assumptions, and clinical applications. Circ Heart Fail 13: e006591, 2020. doi: 10.1161/CIRCHEARTFAILURE.119.006591. [DOI] [PubMed] [Google Scholar]
- 42.Tedford RJ, Hsu S, Kass DA. Letter by Tedford et al regarding article, "Effective Arterial Elastance in the Pulmonary Arterial Circulation: Derivation, Assumptions, and Clinical Applications". Circ Heart Fail 13: e007081, 2020. doi: 10.1161/CIRCHEARTFAILURE.120.007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, Boyce D, Kelemen BW, Bacher AC, Shah AA, Hummers LK, Wigley FM, Russell SD, Saggar R, Saggar R, Maughan WL, Hassoun PM, Kass DA. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 6: 953–963, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 53: 1801913, 2019. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]