Abstract
Pulmonary arterial hypertension (PAH) refers to a set of heterogeneous vascular diseases defined by elevation of pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), leading to right ventricular (RV) remodeling and often death. Early increases in pulmonary artery stiffness in PAH drive pathogenic alterations of pulmonary arterial endothelial cells (PAECs), leading to vascular remodeling. Dysregulation of microRNAs can drive PAEC dysfunction. However, the role of vascular stiffness in regulating pathogenic microRNAs in PAH is incompletely understood. Here, we demonstrated that extracellular matrix (ECM) stiffening downregulated miR-7 levels in PAECs. The RNA-binding protein quaking (QKI) has been implicated in the biogenesis of miR-7. Correspondingly, we found that ECM stiffness upregulated QKI, and QKI knockdown led to increased miR-7. Downstream of the QKI-miR-7 axis, the serine and arginine-rich splicing factor 1 (SRSF1) was identified as a direct target of miR-7. Correspondingly, SRSF1 was reciprocally upregulated in PAECs exposed to stiff ECM and was negatively correlated with miR-7. Decreased miR-7 and increased QKI and SRSF1 were observed in lungs from patients with PAH and PAH rats exposed to SU5416/hypoxia. Lastly, miR-7 upregulation inhibited human PAEC migration, whereas forced SRSF1 expression reversed this phenotype, proving that miR-7 depended upon SRSF1 to control migration. In aggregate, these results define the QKI-miR-7-SRSF1 axis as a mechanosensitive mechanism linking pulmonary arterial vascular stiffness to pathogenic endothelial function. These findings emphasize implications relevant to PAH and suggest the potential benefit of developing therapies that target this miRNA-dependent axis in PAH.
Keywords: endothelial migration, microRNA, pulmonary arterial hypertension, RNA binding protein, vascular stiffness
INTRODUCTION
Pulmonary hypertension (PH) and its particularly severe subtype pulmonary arterial hypertension (PAH) are progressive diseases driven by remodeling and matrix stiffening in the pulmonary vasculature (1). The consequences of vascular remodeling include increased pulmonary vascular resistance (PVR) and consequent right ventricular (RV) remodeling, leading to right heart failure and often death. Current therapies primarily target pulmonary vasoconstriction rather than vascular remodeling, and thus, novel molecular insights into the relationship of pulmonary vascular stiffness and PAH pathogenesis are urgently needed.
Extracellular matrix (ECM) stiffness in both the proximal and distal pulmonary arteries has been implicated as an index of PAH disease progression (2), and arterial stiffening contributes to elevated RV afterload in patients with PAH (3). The endothelium comprises as cellular monolayer that responds to physical and chemical signals to maintain vascular hemostasis (4). Endothelial cell (EC) injuries and insults have been thought to be the key to triggering PAH disease development (5, 6). Moreover, ECM stiffness can influence EC proliferation and migration (7–10). Increased migration of pulmonary arterial ECs (PAECs) is an important cause of pulmonary artery remodeling, which is a primary feature of PAH (1, 11, 12). ECM mechanics play a pivotal role in controlling EC migration (7, 13, 14); however, the molecular mechanisms by which ECs respond to the altered ECM mechanics to control migration lack definition.
MicroRNAs (miRNAs) are small, noncoding regulatory RNA molecules that regulate the level of their targets by translational repression or transcript degradation, and have profound impacts on many biological processes (15, 16). The potential therapeutic applications of miRNAs for PAH are being examined (16, 17), but a more comprehensive molecular understanding regarding their regulation has been lacking. In PAH and endothelial cells, some miRNAs have been characterized as pathogenic and induced through PH triggers such as hypoxia (18) or inflammatory cytokines (16) and acting through mechanisms such as apoptotic resistance (19) or metabolic reprogramming (7). The modulatory effects of pulmonary arterial stiffness on any specific miRNAs and their downstream mechanisms are not fully defined. In the present study, we aimed to identify mechanosensitive miRNAs in PAECs that correlated with dysregulated expression in cellular and rodent models of PAH. To identify the most functionally active miRNAs, we leveraged the principle of miRNA pleiotropy to define cohorts of long-coding mRNA transcripts in PAECs that were all differentially regulated when exposed to stiffened matrix and collectively were predicted targets of single miRNAs. Based on the differential regulation of a high number of predicted miR-7 targets, we identified microRNA-7 (miR-7) as a mechanosensitive miRNA, downregulated in PAECs when exposed to stiff matrix. The biogenesis of miR-7 is known to be negatively regulated by the RNA-binding protein quaking (QKI) (20). QKI directly interacts with the primary form of miR-7, destabilizing and reducing the production of miR-7 (21). In addition, QKI has been implicated as a regulator of endothelial barrier function (22) and smooth muscle differentiation (23, 24) during cardiovascular diseases (25). However, its activity in response to matrix stiffening and its functions in PAH have not been defined. miR-7 is also highly conserved across species and plays critical roles during embryonic development (26) and several cancers (27). In humans, miR-7 is transcribed from three genes, miR-7-1, miR-7-2, and miR-7-3, and these transcripts are processed to yield the same mature miRNA sequence, of which the 5 strand (miR-7-5p) is the most commonly studied (28). miR-7-5p (termed here as “miR-7”) has displayed tumor suppressive functions of migration and proliferation in endothelial cells (28–30), but any causative biology in PAH regulating such migration has not been described.
Of the known direct downstream targets of miR-7, the serine and arginine-rich splicing factor 1 (SRSF1, also named ASF/SF2) has been reported in nonvascular contexts (31) and holds critical functions in RNA metabolism, such as constitutive and alternative splicing (32, 33), RNA polymerase II transcription (34), and export of mature mRNA (35) and translation (36, 37). Recent studies have reported that SRSF1 has a pro-oncogenic action in cancers (38). Specifically, SRSF1 in arteries promotes injury-induced neointima formation via increased proliferation and migration of human aortic and coronary arterial smooth muscle cells, thus facilitating the intimal thickening hyperplasia (39). Whether SRSF1 holds similar functions in pulmonary vascular biology and whether miR-7 depends upon this target for such endothelial control are unknown.
Thus, via a combination of RNA sequencing and gain-of-function and loss-of-function experimentation, we investigated the hypothesis that matrix stiffening activates a QKI-miR-7-SRSF1 molecular axis, thus driving pathogenic signaling in PAECs and offering a more complete molecular explanation connecting ECM pathobiology to PAH.
MATERIALS AND METHODS
Cell Culture
Human pulmonary artery endothelial cells (PAECs, Lot No. 0000708987) were purchased from Lonza (Walkersville, MD) and were cultured in EBM-2 endothelial cell growth basic medium (Lonza) supplemented with growth factors (Lonza). Lonza primary PAECs are nontransformed human cells isolated directly from lung tissue of a single donor. PAECs are characterized by positive immunofluorescent staining for von Willebrand factor antigen and CD31/PECAM-1. Lot No. 0000708987 PAECs were derived from a 64-yr-old Caucasian male. Cell lines tested negative for human immunodeficiency virus, hepatitis B, hepatitis C, and mycoplasma. Human PAECs at passage five to ten were used for the experiments, which was begun at passage three. Human embryonic kidney HEK 293 T cells were purchased from ATCC and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Lonza) supplemented with 10% fetal bovine serum (FBS; HyClone). Our laboratory performs intermittent testing for mycoplasma and had no positive results when this work was performed. All cells were maintained in a humidified chamber at 37°C and 5% CO2.
Pulmonary Artery Endothelial Cell Culture on Matrix Stiffness
Human PAECs were grown in collagen-coated flask (Sigma) at 37°C in a humidified 5% CO2 atmosphere. Collagen-coated hydrogel preplated in culture wells (Matrigen) was generated from a mix of acrylamide and bis-acrylamide coated with collagen. Cells were seeded on top of the hydrogel, using standard cell culture techniques as we previously described (7).
Lentiviral Packaging
The human cDNA quaking QKI (CCDS5285.1) was amplified by PCR, cloned into the lentiviral vector pCDH-CMV-MCS-EF1-copGFP (System Biosciences), and confirmed by DNA sequencing. Human SRSF1 cDNA was purchased from Addgene (#99021) (40). The SRSF1 open reading frame (ORF) was then subcloned into the lentiviral vector. Viral packaging and infection were performed using pPACKH1 HIV Lentivector Packaging Kit (LV500A-1, System Biosciences) according to the standard protocol as recommend by the manufacturer.
Transfection of miRNA Mimics and miRNA Inhibitors
Human PAECs were transfected with 2 nM miR-7-5p mimic or with 20 nM miR-7-5p inhibitor (Thermo Fisher Scientific) by using Lipofectamine 2000 (Thermo Fisher Scientific) and studied 48 h later. In all experiments, an equal concentration of a nontargeting negative control mimic or an inhibitor negative control (NC) was used for nonsequence-specific effects in miRNA experiments. Verification of miR-7-5p overexpression and knockdown was performed using TaqMan quantitative PCR (qPCR), as described in MATERIALS AND METHODS.
RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
Total RNA from cells and lung tissues was isolated using QIAzol reagent (Qiagen, Valencia, CA) according to the manufacturer’s protocol. About 12 ng (for miRNA measurement) or 0.5 μg (for mRNA measurement) of total RNA was reverse transcribed into cDNA using high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific) and miRNA reverse transcription primers (Applied Biosystems, Bedford, MA). Quantitative real-time PCRs were performed on a QuantStudio Flex Real-time PCR System (Applied Biosystems, Bedford, MA) with TaqMan primers (Applied Biosystems, Bedford, MA) and miRNA qRT-PCR primers (Applied Biosystems, Bedford, MA). For mRNA control, the housekeeping gene β-actin or 18S was used. For miRNA control, the small RNA RNU48 (human) and U87 (rat) were used. The results were calculated using the 2-ΔCT method.
Immunoblotting
Immunoblotting was performed as previously described (41). Briefly, protein was prepared by lysing cells in radioimmunoprecipitation assay (RIPA) buffer (Sigma, St. Louis, MO) or SDS lysis buffer supplemented with a protease inhibitor (Thermo Fisher). Total lysates (10–20 µg) per lane were loaded on 10% or 12% SDS-PAGE, transferred onto polyvinylidene difluoride membranes (Bio-Rad), and blocked with 5% skim milk dissolved in Tween 20/Tris-buffered saline (TTBS) for 1 h at room temperature. The membranes were probed with primary antibodies against SRSF1 (Invitrogen), QKI (Abcam), or YAP (Cell Signaling) at a dilution of 1:1,000 for overnight at 4°C. Samples were normalized to vinculin (1:1,000; Cell Signaling Technologies, Danvers, MA). Protein bands were visualized, and digitized images quantified using the Image Lab software (Bio-Rad). Immunoblots are representative of at least three individual experiments. Quantitative results are an average of ≥3 individual experiments.
Cell Migration Analysis
Human PAECs were subjected to cell migration analysis in Boyden chambers. The bottom of a 12-well membrane filter (BD Biosciences) was coated with 10 μg/mL collagen I for 12 h before each experiment. Cells were transfected with miR-7 mimic or negative control for 48 h or were infected with lentivirus overexpressing SRSF1 (LV-SRSF1) or negative control (LV-GFP) for 96 h. Cells were trypsinized and washed with migration medium (EC medium containing 0.1% fatty acid-free BSA) to remove serum. Cells at a density of 1 × 105/well were then placed in the upper chamber of Transwell inserts (8-μm pore size) with migration medium. The cells were allowed to migrate toward the completed EC medium for 5 h. Nonmigrated cells from the top surface were removed by gently wiping with cotton swabs. Migrated cells were fixed with 4% paraformaldehyde for 20 min, stained with 0.5% crystal violet (Sigma-Aldrich) for 15 min, and then rinsed with PBS. The crystal violet on migrated cells was destained with 10% acetic acid, and the absorbance in individual filters was determined at Α573 nm. Cell migration ability was determined by the relative level of absorbance values versus control. Each group had three replicates, and each experiment was repeated three times.
Scratch Assay
Human PAECs cultured on collagen coated 60-mm dishes were transfected with 2 nM miR-7 mimic or negative control for 48 h. The cells were incubated until confluent and then wounded across the midline of the plate using a P200 pipette tip. PAECs were carefully washed with growth medium to remove debris and smooth the edge of the scratch. Low-serum endothelial cell media (2.5% fetal bovine serum) was used for the remainder of the assay. Wound bed closures were followed serially for 8 h, and ×10 bright-field images were taken every 4 h (EVOS XL Core Imaging System), ensuring the same plate orientation. Wound bed areas were quantified using the NIH ImageJ software (https://imagej.nih.gov/ij/).
3′-UTR Luciferase Reporter Assay
cDNA fragment corresponding to 3′UTR of the human SRSF1 gene was amplified by reverse transcription PCR from human cDNA with XhoI and NotI linkers. The PCR products were directly cloned downstream of the Renilla luciferase open reading frame in the psiCHECK2 vector (Promega), which also contains a constitutively expressed firefly luciferase gene that is used to normalize transfection efficiency. The cloned construct containing 3′UTR of SRSF1 was confirmed by DNA sequencing. HEK 293 T cells were plated into 12-well plates and cotransfected with 1 μg of indicated 3′-UTR luciferase reporter vector and miR-7-5p or negative control (NC) mimic (2 nM; Applied Biosystems), using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured using the Dual-Glo Luciferase assay kit (Promega) according to the manufacturer’s instructions. Renilla luciferase activity was normalized to the corresponding firefly luciferase activity and plotted as a percentage of the control. Each transfection was performed in triplicate at least two times.
RNA Sequencing Analysis and miRNA Target Prediction
RNA was collected from three biological replicates from each of human PAECs cultured on stiff or soft matrix for 48 h. The Illumina TruSeq RNA Sample Preparation v2 Kit was used to construct libraries for the samples, accordingly to the manufacturer’s protocol. Briefly, 150 ng of total RNA input was used. The libraries were quantified using KAPA library quantification kit. Unstranded, paired-end sequencing was then performed on Illumina HiSeq 2000 at 50 cycles/bp to generate 50 bp paired-end reads. Around 14–19 million reads per sample were generated. Postsequencing, gene expression was quantified using Salmon (42). Differential expression of stiff versus soft matrix samples was computed using DESEQ2 (43), and P values were FDR adjusted (adjusted P value) using the Benjamini–Hochberg method. miRNA target prediction was performed using TargetScan (44), which detects mRNA with conserved complementarity to nucleotides 2–7 of a given miRNA.
Immunofluorescence of Lung Sections
To process lung tissue specifically for fluorescence microscopy, before excision, lungs were flushed with PBS at constant low pressure (∼10 mmHg) via right ventricular cannulation, followed by tracheal inflation of the left lung in optimal cutting temperature medium (OCT) (Tissue-Tek, Sakura) at a pressure of ∼20 cmH2O, followed by cryopreservation in ethanol/dry ice. Tissue was then further embedded in OCT, frozen solid in cryomolds, sectioned on a Microm HM 550 at 10 μm, and stored at −80°C. Cryosections were then air-dried for 10 min at room temperature and rehydrated in 1× PBS for 15 min at room temperature. Sections were washed in 1× PBS, blocked in 1× PBS/BSA 3% supplemented with 10% donkey serum for 1 h at room temperature, and then probed with appropriate primary antibody overnight at 4°C. After incubation, slides were washed three times with 1× PBS, blocked for 1 h at room temperature, and probed with appropriate secondary antibodies in dark room, 1 h at room temperature. After washing, slides were mounted in mounting medium containing DAPI (Invitrogen).
Alexa 488-conjugated secondary antibodies (Thermo Fisher Scientific) and Cy3-conjugated α-SMA antibodies (Sigma-Aldrich, C6198, 1:300 dilution) were used for immunofluorescence as previously described (45). A primary antibody against SRSF1 (ab129108, 1/200 dilution) was purchased from Abcam, which was reported for immunofluorescence staining in rat carotid arteries as previously described (39). Pictures were captured with a Nikon A1 microscopy. Small pulmonary vessels (<100 μm diameter) present in a given tissue section (>3 vessels/section) that were not associated with bronchial airways were selected for analysis (n > 4 animals/group). Intensity of staining was quantified using ImageJ software (NIH).
Patient Samples
As previously described (46), all experimental procedures involving the use of human tissues were approved by the Partners HealthCare, Boston Children’s Hospital, and University of California, Los Angeles, Institutional Review Boards, the University of Pittsburgh, and the New England Organ Bank. Ethical approval for this study conformed to the standards of the Declaration of Helsinki.
Rodent Models of PH
Animal studies were performed as previously described (45). Male Sprague–Dawley rats (10- to 14-wk-old, Charles River) were injected with 20 mg/kg SU5416 (Sigma) followed by 3 wk of normobaric hypoxia (10% O2). Protocols involving the use of rodent materials were approved by and performed at the University of Pittsburgh School of Medicine (DLAR).
Antibody Validation
All antibodies used in this study have undergone validation. Representative full-length blots and details of antibody validation are presented in Supplemental Fig. S5 (all Supplemental figures are available at https://doi.org/10.6084/m9.figshare.12840836).
Statistical Analysis
Data analyses were conducted using Prism 8 software (GraphPad software). Data are represented as means ± SD. Normality of data distribution was determined by Shapiro–Wilk testing. For comparisons between two groups, a two-tailed unpaired Student’s t test was performed. For comparisons among three or more groups, one-way or two-way ANOVA with post hoc Tukey testing was performed. Statistical significance was considered at P < 0.05.
RESULTS
Identification of miR-7 as a Mechanosensitive miRNA downregulated in PAECs Exposed to Stiff Matrix
To leverage gene network theory for determining system-level mechanosensitive miRNAs of PAEC pathobiology, RNA sequencing of long RNA transcripts was performed on human PAECs grown on stiff matrices (50 kPa) versus soft matrices (0.5 kPa) for 48 h. We identified protein-coding genes that displayed differential expression in human PAECs exposed to stiff versus soft matrices—so-called mechanoactivated DEGs (differentially expressed genes). Then, to identify potent mechanosensitive miRNAs that may exert higher-order control over mechanoactivated DEGs, miRNAs were ranked, based on the number of their predicted total targets within this mechanoactivated DEG database. We used the well-validated TargetScan 7.2 algorithm for miRNA target prediction, given its high degree of sensitivity and specificity relative to other algorithms (44). Ultimately, by sorting the number (#) of total DEGs (the sum of upregulated and downregulated DEGs) targeted collectively by individual miRNAs, the top 10 ranked miRNAs with the largest number of mechanoactivated DEG targets were identified (Table 1; full list in Supplemental Table S1; https://doi.org/10.6084/m9.figshare.12839162). Thus, based on such inference from target gene alterations, these miRNAs were predicted to carry both dynamic and mechanoactive properties in PAECs.
Table 1.
Top ranked miRNAs with largest number of mechanoactivated DEG targets
| Ranked | miRNA | No. of Total DEGs* |
|---|---|---|
| 1 | miR-124-3p | 1,768 |
| 2 | miR-7-5p | 1,264 |
| 3 | miR-30-5p | 1,202 |
| 4 | miR-15-5p | 1,148 |
| 5 | miR-16-5p | 1,148 |
| 6 | miR-195-5p | 1,148 |
| 7 | miR-424-5p | 1,148 |
| 8 | miR-497-5p | 1,148 |
| 9 | miR-6838-5p | 1,148 |
| 10 | miR-340-5p | 1,088 |
*DEG indicates differentially expressed genes in stiff matrices.
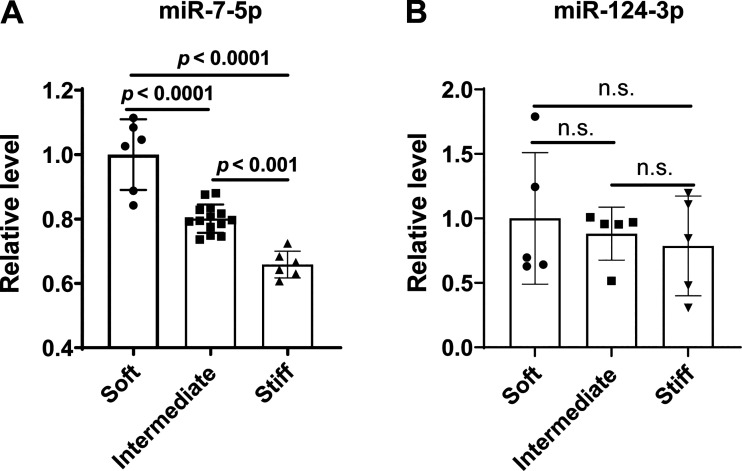
Relative miRNA activity in this context may be dictated by changes to miRNA expression level and/or independent alterations to target gene stoichiometry. To define whether the top two miRNAs, miR-124-3p and miR-7-5p, displayed alterations of expression in stiff matrix, PAECs were plated on polyacrylamide hydrogel with stiffness of 1 kPa (soft), 25 kPa (intermediate), or 50 kPa (stiff) (13). As shown in Fig. 1A, increased matrix stiffness significantly decreased miR-7-5p levels, but we found that stiff matrix stimulation did not affect the level of miR-124-3p (Fig. 1B). Two transcriptional coactivators inherent to the Hippo signaling pathway, Yes-associated protein 1 (YAP1) and TAZ [or WW Domain Containing Transcription Regulator 1 (WWRT1)], are known to be mechanoactivated by matrix stiffness (47). As positive markers of vascular stiffening, the YAP1/TAZ signaling downstream targets (Supplemental Fig. S1A) (7, 13), mRNA expression of CTGF (connective tissue growth factor), LOX (lysyl oxidase), COL3A1 (collagen type III alpha 1 chain), and COL4A1 (collagen type IV α 1 chain) were increased in human PAECs grown on a stiff matrix compared with human PAECs grown on a soft matrix (Supplemental Fig. S1, B–E). These findings demonstrate that miR-7-5p (“miR-7”) is a miRNA controlled by matrix stiffening and may contribute to PAEC pathobiology.
Figure 1.
miR-7-5p is reduced in ECM stiffening-exposed PAECs. Human PAECs were plated on polyacrylamide substrates with stiffness of 1 kPa (soft), 25 kPa (intermediate), or 50 kPa (stiff). After 48 h, total RNA was isolated and reverse transcribed to cDNA. Expression of miR-7-5p (A) and miR-124-3p (B) under stiff conditions was measured by quantitative RT-PCR with small RNA RNU48 used as an internal control. n = 5–12. Data represent the means ± SD. Statistical significance was determined by one-way ANOVA, followed by post hoc Tukey test. n.s., not significant. ECM, extracellular matrix; PAECs, pulmonary arterial endothelial cells.
ECM Stiffening Stimulates RNA-Binding Protein QKI to Attenuate miR-7 Level in Pulmonary Artery Endothelial Cells
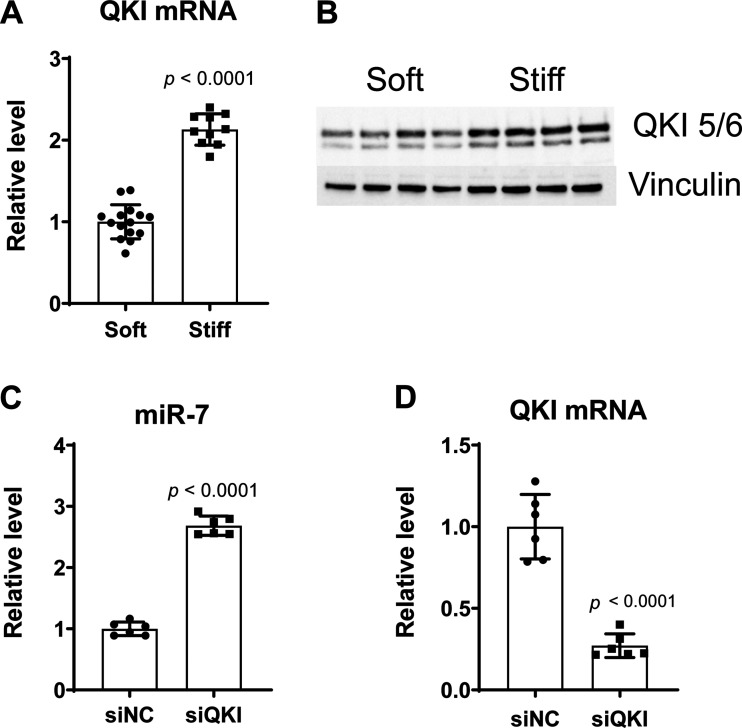
To determine whether miR-7 downregulation in matrix stiffening was correlated to RNA-binding protein quaking (QKI), QKI expression was measured in PAECs cultured on plates with stiff or soft matrices. Correspondingly, both mRNA and protein expression of QKI were increased in PAECs exposed to stiff matrix (Fig. 2, A–B). Moreover, siRNA-mediated suppression of QKI gene expression (∼80% RNA knockdown efficiency) significantly increased miR-7 levels (Fig. 2, C–D). Thus, mechanoactivation triggered by ECM stiffening induced a QKI-miR-7 regulatory axis in human PAECs.
Figure 2.
Stiffening-dependent QKI represses miR-7 in human PAECs. A: expression of QKI mRNA was increased in human PAECs cultured on stiff matrixes (50 kPa) vs. soft matrixes (1 kPa). B: immunoblot showing increased protein expression of QKI in human PAECs cultured on stiff matrixes (50 kPa). C: levels of miR-7 were upregulated in QKI siRNA knockdown PAECs. D: qRT-PCR result shows the efficiency of QKI knockdown by specific QKI-targeted siRNAs. Bar graphs show the ratios of mRNA relative to the amount of soft matrix control expression. β-Actin (mRNA) or small RNA RNU48 (miRNA) was used as an internal control. n = 6–15. Data represent the means ± SD. Statistical significance was determined by unpaired Student’s t test. siNC, negative control siRNAs; siQKI, QKI siRNAs. QKI, quaking; PAECs, pulmonary arterial endothelial cells.
Increased Matrix Stiffness Leads to Increased SRSF1 Expression
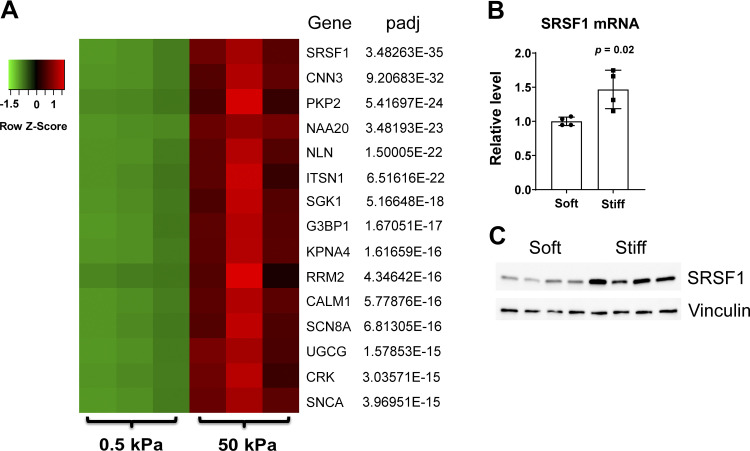
Given the computational analysis of the mechanoactivated DEG database and TargetScan miRNA target algorithm (Table 1), we further elucidated the downstream genes controlled by miR-7 upon induction by matrix stiffness. Consistent with the downregulation of miR-7 in stiff conditions, 672 putative DEG targets of miR-7 were found to be upregulated (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.12840827). Of these genes, serine and arginine-rich splicing factor 1 (SRSF1) carried the lowest adjusted P value (padj) (Fig. 3A). Furthermore, increased expression of SRSF1 mRNA and protein in stiff matrix was validated by qRT-PCR and immunoblot, respectively (Fig. 3, B–C).
Figure 3.
SRSF1 is upregulated in ECM stiffening PAECs. A: ranked by adjusted P value (padj), heat map of the top 15 genes ranked by padj in human PAECs displaying differential expression after exposure to soft (0.5 kPa) vs. stiff (50 kPa) matrices. Rows represent genes, and columns represent stiffening conditions. A color shift from green to red indicates that expression was upregulated. Color brightness represents the signal values of genes in RNA sequencing. B: expression of SRSF1 mRNA increased in human PAECs cultured on stiff (50 kPa) vs. soft (0.5 kPa) matrixes. The relative SRSF1 mRNA expression was determined by qRT-PCR. β-Actin was used as an internal control. n = 4. Data represent means ± SD. Significance was determined by unpaired Student’s t test. C: endogenous SRSF1 protein levels were determined by immunoblotting probed with anti-SRSF1 antibody using vinculin as a loading control in human PAECs cultured on stiff (50 kPa) or soft (0.5 kPa) matrixes for 48 h. SRSF1, serine and arginine-rich splicing factor 1; PAECs, pulmonary arterial endothelial cells.
miR-7 Negatively Regulates SRSF1 Expression in Human PAECs
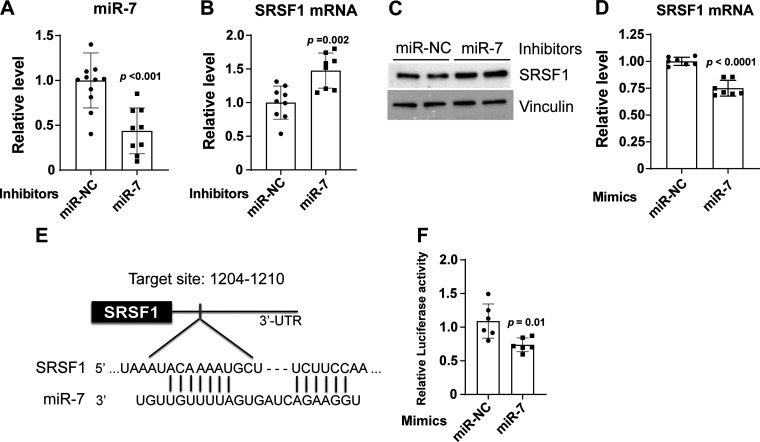
To further determine whether miR-7 regulates SRSF1 in PAECs, cells were transiently transfected with miR-7 inhibitor or mimic oligonucleotides. miR-7 knockdown in human PAECs exposed to soft matrices (Fig. 4A) significantly increased the expression of SRSF1 mRNA and protein compared with miR-NC control (Fig. 4, B–C). Conversely, SRSF1 transcript was downregulated by forced expression of miR-7 versus control (Fig. 4D). The 3′-UTR region of SRSF1 transcript contains a highly conserved binding site for miR-7, predicted using the miRNA target prediction tool TargetScan (44), and it has been reported previously (31) (Fig. 4E). To confirm, luciferase reporter vectors were constructed carrying the SRSF1 3′-UTR containing the putative miR-7 binding site. The constructs were transiently transfected into HEK293T cells along with miR-7 or negative control mimics, and the relative luciferase activity was measured after 48 h. The relative luciferase activity was decreased by 40% following miR-7 mimic transfection (Fig. 4F), thus confirming that SRSF1 is a direct target of miR-7.
Figure 4.
miR-7 directly targets SRSF1 gene. A: qRT-PCR result shows the efficiency of miR-7 knockdown in human PAECs grown on soft (1 kPa) matrices after 48-h transfection with 20 nM inhibitors of miR-7. Bar graphs show the ratios of mRNA relative to the amount of negative control inhibitor (miR-NC) expression. RNU48 was used as an internal control. B: miR-7 inhibition increased SRSF1 mRNA in human PAECs as measured by qRT-PCR. β-Actin was used as an internal control. C: miR-7 inhibition increased SRSF1 protein as measured by immunoblotting. Vinculin was used as an internal control. D: human PAECs were transfected with 2 nM miR-7 or control mimics under the same conditions as for cell migration assays (described in materials and methods). Level of SRSF1 mRNA was reduced in human PAECs transfected with 2 nM miR-7 mimics. β-Actin was used as an internal control. E: schematic representation of the putative miR-7-binding sequence in the 3′-UTR region of SRSF1 mRNA, as predicted by TargetScan (44). F: luciferase reporter assays were studied in HEK 293 T cells that cotransfected with SRSF1 3′-UTR and miR-7 or miR-NC mimics for 48 h. Renilla luciferase activity was normalized by firefly luciferase activity. n = 6–11. Data represent the means ± SD. Significance was determined by unpaired Student’s t test. SRSF1, serine and arginine-rich splicing factor 1; PAECs, pulmonary arterial endothelial cells.
QKI Regulates SRSF1 Expression in PAECs
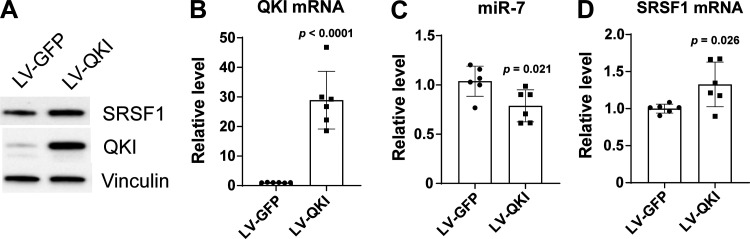
Given the mechanosensitive regulation of miR-7 by QKI (Fig. 2), we sought to determine whether, in turn, QKI regulates SRSF1 which is under the control of miR-7. To do so, we evaluated the expression of SRSF1 mRNA in PAECs overexpressing QKI using a lentivirus transduction system (Fig. 5, A–B). Compared with control lentivirus (LV-GFP), lentiviral transduction of a QKI transgene (LV-QKI) significantly decreased miR-7 level (Fig. 5C) and conversely increased SRSF1 protein and mRNA expression (Fig. 5, A and D), thus demonstrating that QKI regulates the miR-7-SRSF1 axis in PAECs.
Figure 5.
QKI overexpression increases SRSF1 level and downregulates miR-7 in human PAECs. Human PAECs were infected with lentivirus overexpressing QKI (LV-QKI) or containing empty control vector (LV-GFP). A: total proteins were extracted after 96 h of infection, and the concentrations were measured. Subsequently, immunoblotting was performed to detect the expression of SRSF1 and QKI in human PAECs. Vinculin was used as a loading control. B: total RNA was isolated from the cells after 96 h of infection. qRT-PCR analysis was performed to measure QKI mRNA expression in human PAECs. β-Actin was used as an internal control. C: levels of miR-7 were suppressed in LV-QKI PAECs compared to LV-GFP control cells. The small RNA RNU48 was used as an internal loading control. D: levels of SRSF1 mRNA were increased in LV-QKI PAECs compared to LV-GFP control cells. β-Actin was used as an internal control. n = 6. Data represent the means ± SD. Significance was determined by unpaired Student’s t test. SRSF1, serine and arginine-rich splicing factor 1; PAECs, pulmonary arterial endothelial cells.
Expression of the QKI-miR-7-SRSF1 Axis in Human and Rodent PAH Lung Tissue
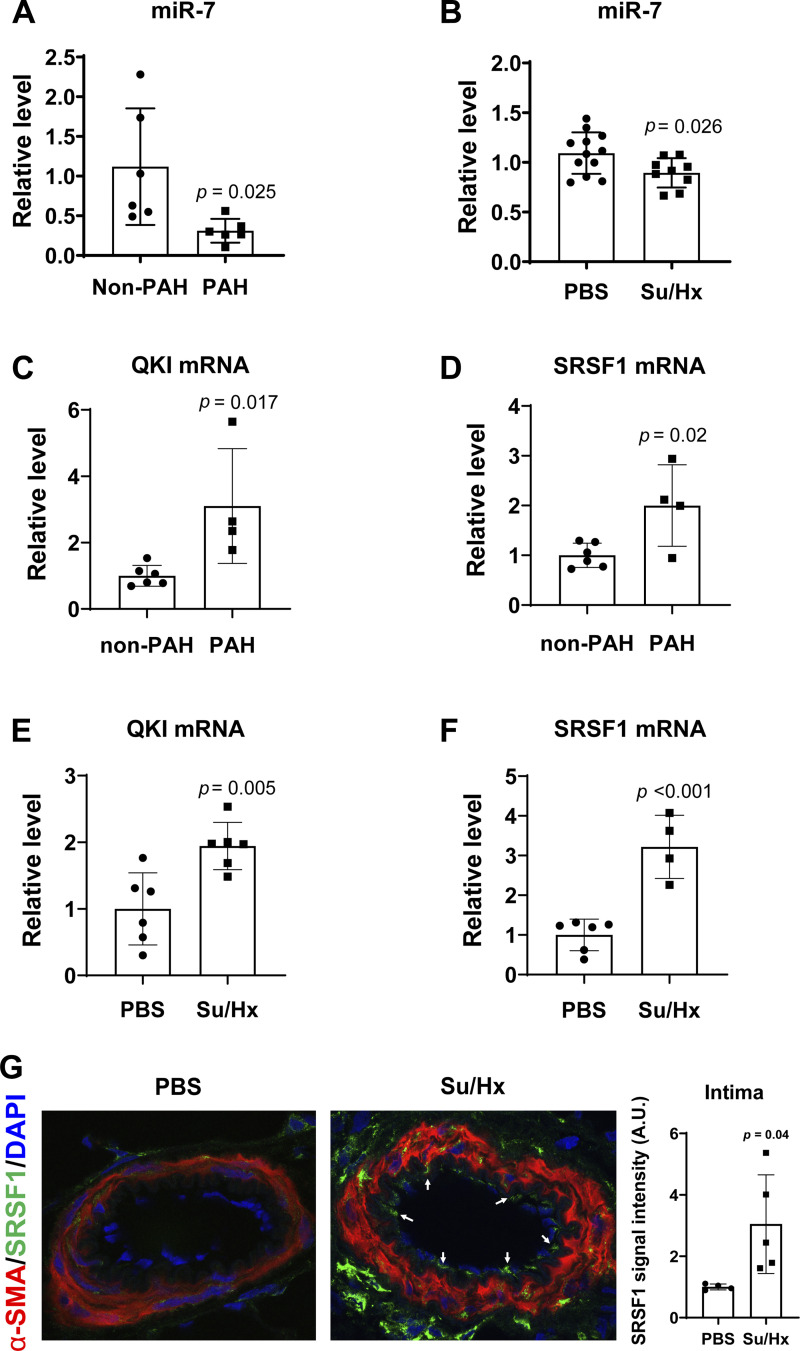
To investigate the relevance of miR-7 in PAH, we first evaluated the endogenous expression of miR-7 by qRT-PCR in human and rat PAH lungs compared with non-PAH lungs. miR-7 was downregulated in PAH tissues compared to non-PAH lungs (Fig. 6A). Similarly, miR-7 was also downregulated in lung tissues from SU5416/hypoxia (Su/Hx)-exposed rats that display distal pulmonary arterial stiffening and fibrosis (48) (Fig. 6B).
Figure 6.
The QKI-miR-7-SRSF1 axis is induced in human and rodent PAH lungs. A: miR-7 in human PAH lung tissues (n = 6) was significantly reduced versus non-PAH lung tissue (n = 6). B: miR-7 was decreased in lungs from SU5416/hypoxia (Su/Hx)-treated model of rats (n = 9) than those in PBS-treated rats (n = 12). For miRNA control, the small RNA RNU48 (human) and U87 (rat) were used. C and D: QKI and SRSF1 mRNA levels in human PAH lung tissues (n = 4) were significantly increased versus non-PAH lung tissue (n = 6) as assessed by qRT-PCR. 18S was used as an internal control. E and F: QKI and SRSF1 mRNA levels in SU5416/hypoxia (Su/Hx)-exposed rats (n = 4) were significantly increased versus PBS-treated mice (n = 6) as assessed by qRT-PCR. 18S was used as an internal control. G: SRSF1 was increased in intima of PAH pulmonary arterioles from SU5416/hypoxia (Su/Hx)-treated rat lungs (n = 5) vs. PBS-treated rat lungs (n = 4), shown by immunofluorescence. All data are shown as means ± standard deviation (SD). Statistical significance determined by unpaired Student’s t test. A.U. indicates arbitrary units. SRSF1, serine and arginine-rich splicing factor 1; PAH, pulmonary arterial hypertension.
As miR-7 was downregulated in human and rat PAH lungs (Fig. 6, A–B), we further examined whether QKI and SRSF1 mRNA levels are associated with miR-7 in PAH. The endogenous expression of QKI and SRSF1 was determined by qRT-PCR in human and rat PAH lungs compared with non-PAH lungs. Both QKI and SRSF1 were upregulated in PAH human lung tissues compared to non-PAH lung tissues (Fig. 6, C–D). Similarly, both QKI and SRSF1 were upregulated in lung tissues from SU5416/hypoxia (SU/Hx)-exposed rat models compared to normoxic control rats (Fig. 6, E–F). Of note, SRSF1 expression was increased in the intimal layers of rat PAH pulmonary arterioles (Fig. 6G). These data indicate that miR-7 downregulation may be associated with the increases of QKI and SRSF1 in PAH lungs.
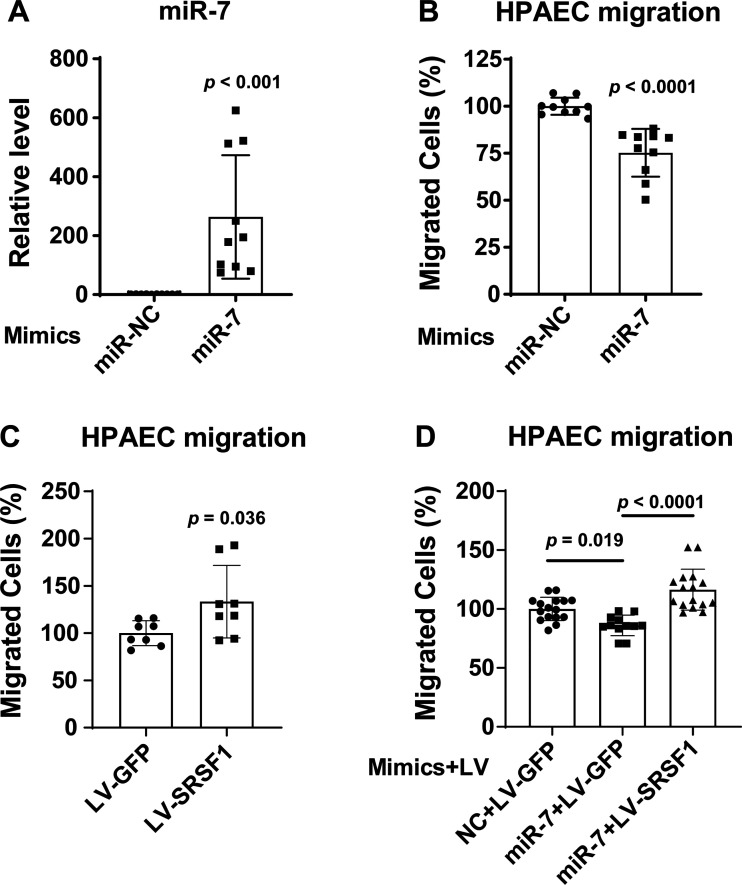
miR-7 Inhibits PAEC Migration through Targeting SRSF1
We evaluated the role of miR-7 in the migration of human PAECs by transient transfection with miR-7 mimic oligonucleotides. qRT-PCR analysis showed that this transfection increased cellular levels of mature miR-7 more than 200-fold compared with negative control (miR-NC; Fig. 7A). Boyden chamber cell migration and scratch analyses indicated that an increase in miR-7 level significantly inhibited human PAEC migration (Fig. 7B and Supplemental Fig. S2). Suppression of SRSF1 expression by miR-7 suggested that the inhibitory effect of miR-7 on PAEC migration depended upon SRSF1. To investigate, we found that forced lentiviral transduction of a SRSF1 transgene (LV-SRSF1) significantly promoted human PAEC migration (Fig. 7C) and restored cell migration even in the context of miR-7 expression in human PAECs (Fig. 7D). Together, these findings suggest that miR-7 depends upon SRSF1 in order to inhibit human PAEC migration.
Figure 7.
miR-7 inhibits human PAEC migration via SRSF1. Transwell migration assays of human PAECs transfected with miR-7 mimic and miR-NC control mimic (2 nM) were performed for 5 h. A: levels of miR-7 overexpression were determined by qRT-PCR. B: miR-7 upregulation in human PAECs decreased cell migration. Migrated cells were stained with crystal violet. Quantitation of migrated cells was performed by solubilization of crystal violet and spectrophotometric analysis at Α573 nm. The percent migrating cells in each group were compared with miR-NC migrated cells. C: transwell migration assay of human PAECs transduced with LV-SRSF1 showed increased migration compared to LV-GFP control cells. D: Concurrent SRFS1 upregulation rescued the inhibitory migration phenotype seen under miR-7 mimic treatment. n = 8–16. All data are means ± SD. Significance was determined by unpaired Student’s t test (A–C) or one-way ANOVA, followed by post hoc Tukey test (D). NC, negative control; PAECs, pulmonary arterial endothelial cells.
DISCUSSION
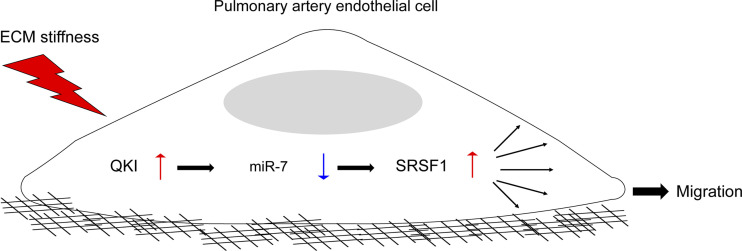
Extensive evidence supports a pathophysiological connection between pulmonary arterial stiffness and PAH development and progression (13, 48). A growing number of miRNAs have been identified as dysregulated in right ventricle and pulmonary vessels in patients with PAH (49). Yet, the molecular mechanism linking miRNAs to pulmonary arterial ECM stiffening has not previously been well defined. In this study, led by an approach to infer miRNA activity based on transcriptomic target gene sequencing, downregulation of miR-7 was shown in cultured PAECs under ECM stiffening conditions, whereas miR-7 upregulation inhibited PAEC migration accompanied by the suppression of SRSF1 expression. The endothelial miR-7-SRSF1 signal axis was negatively regulated by QKI. The biological significance of the QKI-miR-7-SRSF1 axis in pulmonary vasculature is underscored by its endogenous expression in lungs by the marked arteriolar stiffness in patients with PAH and PAH SU5416/hypoxic rats. Together, our results demonstrated a novel mechanosensitive QKI-miR-7-SRSF1 signal axis controlling endothelial migration across the pulmonary vasculature, thus offering a more comprehensive molecular perspective connecting ECM pathobiology to PAH (Fig. 8).
Figure 8.
The mechanoactivation of a QKI-miR-7-SRSF1 axis in human PAECs. Collectively, we propose that in PAECs, the QKI-miR-7-SRSF1 axis is activated by ECM stiffness to drive cellular migration. These findings not only offer fundamental insights to how endothelial miRNA pathobiology is regulated by biomechanical forces but also carry translational implications toward our understanding of PH pathogenesis. SRSF1, serine and arginine-ruch splicing factor 1; ECM, extracellular matrix; PAECs, pulmonary arterial endothelial cells.
Alterations in miRNA expression play important roles in arterial stiffening and remodeling associated with PAH disease (16, 50). We previously demonstrated mechanobiological feedback loops between ECM remodeling and YAP/TAZ in pulmonary vascular cells involving the microRNA-130/301 (miR-130/301) family and its control of collagen deposition and remodeling (13). The miR-130/301 family has been shown to regulate proliferative and migratory actions, associated with increased levels of target protein GLS (glutaminase) in pulmonary vasculature (7). The QKI-miR-7-SRSF1 axis expands upon these relationships and indicates more complex mechanoactive interactomes are active and comprise a broader role in responding to ECM stiffening in PAH. A putative function connection between miR-7 and mechanoactive Hippo signaling via YAP/TAZ may exist. For instance, two miR-7 binding sites on YAP 3′-UTR are identified via TargetScan (44) analysis, and we have observed that miR-7 mimic transfection significantly downregulates YAP protein and mRNA expression in human PAECs (Supplemental Fig. S3), suggesting YAP may be a potent downstream target of miR-7. There are several genes commonly involved in both the Hippo pathway and PAH pathogenesis, including BMPR1A (bone morphogenetic protein receptor 1 A), CCND2 (cyclinD2), and SMAD2 (mothers against decapentaplegic homolog 2), which have been studied previously in the context of PAH. This gene network overlap suggests that miR-7 may be a part of a YAP/TAZ mechanoactivated mechanism under ECM stiffness relevant to PAH. Further assessment of the link between miR-7 and these YAP1 regulatory genes will broaden our understanding of the role of miR-7 in PAH.
miR-7 has been implicated as an anti-oncogenic miRNA (anti-oncomiR) in several cancers (27), but has not extensively been studied in the context of PAH. As reported in cancer, miR-7 inhibits expression of many oncogenic regulatory factors, including EGFR (epidermal growth factor receptor), RAF-1, IGF1R (insulin-like growth factor I receptor), BCL-2 (B-cell lymphoma 2), KLF-4 (Kruppel-like factor 4), PI3KCD (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta), mTOR (mammalian target of rapamycin), and PAK1 (p21-activating kinase), through its binding to the 3′-UTR of these mRNAs (27). These direct targets may also contribute to miR-7 activity in PAECs and PAH in general, but do not fully compensate for this miRNA’s reliance on SRSF1. Recent studies demonstrated that SRSF1 is a ubiquitous RNA-binding protein and is known to regulate not only RNA splicing but also mRNA translocation and stability (51). In addition, SRSF1 has been implicated as an oncoprotein (51) that enhances cell mobility, invasion, and fibroblast transformation (38). Of note, SRSF1 has been shown to actively participate in the alternative splicing events that contribute to the phenotype modulation between proliferation and differentiated vascular smooth muscle cells (VSMCs) (52). SRSF1-deficient mice have shown protection from intimal hyperplasia in response to vascular injury (39) and reduced VSMC proliferation in injured arteries due to an inhibitory effect on apoptosis implied a prosurvival action of SRSF1 on VSMCs (39). SRSF1 promotes VSMC migration and conversely SRSF1 deletion inhibits VSMC migration (39), which is consistent to our observations of pulmonary endothelial cell migration. Future work should be geared toward greater molecular definition of how SRSF1 regulates cellular migration.
miR-7 may impact other targets and signaling mechanisms in PAH pathophysiology. For example, based on the computational analysis of the mechanoactivated DEG database and TargetScan miRNA target algorithm, we found that SGK1 (serum glucocorticoid-regulated kinase 1) may represent another mechanoregulated gene under the control of miR-7 and may be implicated in PAH pathogenesis (the 7th ranked gene candidate on Fig. 3A and Supplemental Table S2). A previous study has demonstrated that SGK1 is upregulated in hypoxia-induced PAH, and SGK1 deficiency protected PAH development induced by hypoxia and inhibited macrophage infiltration in the lung (53). Moreover, macrophages from SGK1 knockout mice produced less proinflammatory cytokines, MCP-1, IL-1β, and TNF-α (53). Chronic vascular inflammation plays a role in increased arterial stiffness (54). These findings suggest that miR-7 may be critical for the modulation of inflammatory signaling through SGK1 in association with mechanoactivated PAH pathophysiology.
Our results also offer insight into the complex evolution of PAEC phenotypes under mechanical stress. Excessive proliferation, migration, adhesion, and tube formation of endothelial cells may all be affected by matrix stiffness and have been demonstrated to promote new vessel growth and angiogenesis, leading to intimal hyperplasia and vascular abnormalities (55, 56). In PAH, the abnormality of cellular proliferative and migratory states has been displayed in plexiform lesions (12). Although the proliferation and migration of PAECs has been implicated to play a role in excessive pulmonary angiogenesis and endothelial dysfunction (57, 58), more recent study of hyperproliferative PAECs in PAH has described accompanying migratory phenotypes mediated by the YAP/TAZ-GLS signal axis on stiff matrix (7). As reported previously, miR-7 represses tubule formation and cell proliferation in vascular endothelial cells (28, 59). Interestingly, we found that miR-7 inhibits PAEC migration, but changes in miR-7 expression do not influence the cell growth of PAEC (Supplemental Fig. S4). Thus, miR-7 acts as a crucial regulator of PAEC migration independent of proliferation. Future studies will be necessary to explore how aberrant migration caused by miR-7 downregulation in stiffened matrix may contribute to other higher-order endothelial functions such as angiogenesis in PAH. In addition, miR-7 has been proposed as a potential biomarker for idiopathic inflammatory myopathies with interstitial lung disease (IIM/ILD) and esophageal squamous cell carcinoma (60). A recent report showed that miR-7 was downregulated in human artery smooth muscle cells (HASMCs) in PH associated with vascular calcification (61). Although we did not test for miR-7 expression in the extracellular space or in the plasma of patients with PAH, miR-7 expression as a circulating marker for stiffness in the PAH vasculature could have prognostic significance.
As a newly defined mechanoactive factor in PAECs, QKI has previously been demonstrated to play roles in RNA metabolism that regulate the stability and biogenesis of miRNAs (21), as well as in regulating vascular development and remodeling (25, 62). A previous study has demonstrated that QKI regulates neuronal cell proliferation by repressing the biogenesis processing of pre-miR-7 and the export of the precursor miR-7 (21). In addition to its connection to miR-7, QKI is predicted as a target of 81 conserved miRNAs by TargetScan, suggesting that other miRNAs may be involved in QKI’s role in PAEC mechanoactivation. Moreover, QKI is known to regulate not only miR-7 biogenesis but also miR-20a stability (63). BMPR2 (bone morphogenetic protein receptor type 2), mutations that have been extensively studied in PAH, is downregulated by the activity of miR-20a, as previously described during vascular remodeling of PH. Treatment with specific small antisense RNA molecules of miR-20a restores functional levels of BMPR2 and prevents vascular remodeling in hypoxia-induced pulmonary hypertension (64). These studies offer numerous possibilities to explain the ways in which QKI regulates miRNA metabolism and the exact roles of QKI in PH beyond its control of miR-7—all of which merit future investigation.
Limitations of our work should be acknowledged. Matrices of artificially defined stiffness levels are not representative of the complex pathogenic nature of PAH. Thus, PAEC culture on such matrices may result in supraphysiologic changes in gene and miRNA expression and function. Ultimately, although we provide unambiguous evidence that ECM stiffening in PAECs stimulates the QKI-miR-7-SRSF1 axis, in vivo proof of the pathogenicity of this axis in PAH is pending.
Taken together, our studies define the QKI-miR-7-SRSF1 axis as a mechanoactivated pathway in PAECs stimulated by ECM stiffening and with relevance to human and animal forms of PAH. Our data underscore the link of biomechanical forces with post-transcriptional miRNA regulation in endothelial cells, with potential implications for future diagnostic and therapeutic strategies aimed to preserve endothelial homeostasis in PAH.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S5: https://doi.org/10.6084/m9.figshare.12840836.
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.12839162.
Supplemental Table S2: https://doi.org/10.6084/m9.figshare.12840827.
GRANTS
This work was supported by the National Institutes of Health (R01 HL124021, HL 122596, HL 138437, and UH2 TR002073) and American Heart Association (18EIA33900027; to S.Y.C.).
DISCLOSURES
S.Y.C. has served as a consultant for Zogenix, Aerpio, and United Therapeutics; S.Y.C. is a director, officer, and shareholder in Numa Therapeutics; S.Y.C. holds research grants from Actelion and Pfizer. S.Y.C has filed patent applications regarding the targeting of metabolism in pulmonary hypertension. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
C.-S.C.W. and S.Y.C. conceived and designed research; C.-S.C.W., N.H., Y.T., L.D.H., L.E.E., and T.B. performed experiments; C.-S.C.W., S.Y.C., N.H., A.H., L.D.H., G.S., and S.K. analyzed data; C.-S.C.W., S.Y.C., N.H., A.H., Y.T., L.D.H., G.S., S.K., and T.B. interpreted results of experiments; C.-S.C.W., S.Y.C., and N.H. prepared figures; C.-S.C.W., and S.Y.C. drafted manuscript; C.-S.C.W., S.Y.C., N.H., L.D.H., and S.K. edited and revised manuscript; C.-S.C.W., S.Y.C., N.H., A.H., Y.T., L.D.H., L.E.E., G.S., S.K., and T.B. approved final version of manuscript.
REFERENCES
- 1.Good RB, Gilbane AJ, Trinder SL, Denton CP, Coghlan G, Abraham DJ, Holmes AM. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 185: 1850–1858, 2015. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Gan CT-J, Lankhaar J-W, Westerhof N, Marcus JT, Becker A, Twisk JWR, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132: 1906–1912, 2007. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 1: 212–223, 2011. doi: 10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krüger-Genge A, Blocki A, Franke R-P, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci 20: 4411, 2019. doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44: 14–30, 2008. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranchoux B, Harvey LD, Ayon RJ, Babicheva A, Bonnet S, Chan SY, Yuan JXJ, Perez VJ. Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape. Pulm Circ 8: 2045893217752912, 2018. doi: 10.1177/2045893217752912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sughara M, Qi Z, Gorcsan J 3rd, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, Marco TD, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science 276: 1425–1428, 1997. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan J-L, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA 99: 3546–3551, 2002. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Huang NF, Hsu S. Mechanotransduction in endothelial cell migration. J Cell Biochem 96: 1110–1126, 2005. doi: 10.1002/jcb.20614. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Hwangbo C, Hu X, Kang Y, Papangeli I, Mehrotra D, Park H, Ju H, McLean DL, Comhair SA, Erzurum SC, Chun HJ. Restoration of impaired endothelial myocyte enhancer factor 2 function rescues pulmonary arterial hypertension. Circulation 131: 190–199, 2015. doi: 10.1161/CIRCULATIONAHA.114.013339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrell NW, Adnot S, Archer SL, Dupuis J, Lloyd Jones P, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JXJ, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang Y-Y, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, Vargas SO, Haley KJ, Waxman AB, Chau BN, Fredenburgh LE, Chan SY. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep 13: 1016–1032, 2015. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thenappan T, Chan SY, Weir EK. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 315: H1322–H1331, 2018. doi: 10.1152/ajpheart.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis 35: 3–11, 2015. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negi V, Chan SY. Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2: e91327, 2017. doi: 10.1172/jci.insight.91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodcock C-SC, Chan SY. The search for disease-modifying therapies in pulmonary hypertension. J Cardiovasc Pharmacol Ther 24: 334–354, 2019. doi: 10.1177/1074248419829172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu C-G, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2007. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White K, Dempsie Y, Caruso P, Wallace E, McDonald RA, Stevens H, Hatley ME, Rooij EV, Morrell NW, MacLean MR, Baker AH. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension 64: 185–194, 2014. doi: 10.1161/HYPERTENSIONAHA.113.03037. [DOI] [PubMed] [Google Scholar]
- 20.Vernet C, Artzt K. STAR, a gene family involved in signal transduction and activation of RNA. Trends Genet 13: 479–484, 1997. doi: 10.1016/s0168-9525(97)01269-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Vogel G, Yu Z, Richard S. The QKI-5 and QKI-6 RNA binding proteins regulate the expression of microRNA 7 in glial cells. Mol Cell Biol 33: 1233–1243, 2013. doi: 10.1128/MCB.01604-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bruin RG, van der Veer EP, Prins J, Lee DH, Dane MJC, Zhang H, Roeten MK, Bijkerk R, de Boer HC, Rabelink TJ, van Zonneveld AJ, van Gils JM. The RNA-binding protein quaking maintains endothelial barrier function and affects VE-cadherin and β-catenin protein expression. Sci Rep 6: 21643, 2016. doi: 10.1038/srep21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Takakura N, Oike Y, Imanaka T, Araki K, Suda T, Kaname T, Kondo T, Abe K, Yamamura K-I. Defective smooth muscle development in qkI-deficient mice. Dev Growth Differ 45: 449–462, 2003. doi: 10.1111/j.1440-169x.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 24.van der Veer EP, de Bruin RG , Kraaijeveld AO, Vries MRd, Bot I, Pera T, , et al. Quaking, an RNA-binding protein, is a critical regulator of vascular smooth muscle cell phenotype. Circ Res 113: 1065–1075, 2013. doi: 10.1161/CIRCRESAHA.113.301302. [DOI] [PubMed] [Google Scholar]
- 25.de Bruin RG, Rabelink TJ, van Zonneveld AJ, van der Veer EP. Emerging roles for RNA-binding proteins as effectors and regulators of cardiovascular disease. Eur Heart J 38: 1380–1388, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Horsham JL, Ganda C, Kalinowski FC, Brown RAM, Epis MR, Leedman PJ. MicroRNA-7: A miRNA with expanding roles in development and disease. Int J Biochem Cell Biol 69: 215–224, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi T, Todaka H, Sugiyama Y, Ono M, Tamaki N, Hatano E, Takezaki Y, Hanazaki K, Miwa T, Lai S, Morisawa K, Tsuda M, Taniguchi T, Sakamoto S. Suppression of microRNA-7 (miR-7) biogenesis by nuclear factor 90-nuclear factor 45 complex (NF90-NF45) controls cell proliferation in hepatocellular carcinoma. J Biol Chem 291: 21074–21084, 2016. doi: 10.1074/jbc.M116.748210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Liu Y, Li L, Xu Z, Bi B, Wang Y, Li JY. MiR-7-5p is frequently downregulated in glioblastoma microvasculature and inhibits vascular endothelial cell proliferation by targeting RAF1. Tumour Biol 35: 10177–10184, 2014. doi: 10.1007/s13277-014-2318-x. [DOI] [PubMed] [Google Scholar]
- 29.Babae N, Bourajjaj M, Liu Y, Van Beijnum JR, Cerisoli F, Scaria PV, Verheul M, Van Berkel MP, Pieters EHE, Van Haastert RJ, Yousefi A, Mastrobattista E, Storm G, Berezikov E, Cuppen E, Woodle M, Schaapveld RQJ, Prevost GP, Griffioen AW, Van Noort PI, Schiffelers RM. Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma. Oncotarget 5: 6687–6700, 2014. doi: 10.18632/oncotarget.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Y-X, Bradbury R, Flamini V, Wu B, Jordan N, Jiang WG. MicroRNA-7 suppresses the homing and migration potential of human endothelial cells to highly metastatic human breast cancer cells. Br J Cancer 117: 89–101, 2017. doi: 10.1038/bjc.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Sun S, Tu K, Gao Y, Xie B, Krainer AR, Zhu J. A splicing-independent function of SF2/ASF in microRNA processing. Mol Cell 38: 67–77, 2010. doi: 10.1016/j.molcel.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krainer AR, Conway GC, Kozak D. Purification and characterization of pre-mRNA splicing factor SF2 from HeLa cells. Genes Dev 4: 1158–1171, 1990. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- 33.Mayeda A, Krainer AR. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell 68: 365–375, 1992. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- 34.Zhong X-Y, Wang P, Han J, Rosenfeld MG, Fu X-D. SR proteins in vertical integration of gene expression from transcription to RNA processing to translation. Mol Cell 35: 1–10, 2009. doi: 10.1016/j.molcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Gattoni R, Stévenin J, Steitz JA. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol Cell 11: 837–843, 2003. doi: 10.1016/s1097-2765(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 36.Karni R, Hippo Y, Lowe SW, Krainer AR. The splicing-factor oncoprotein SF2/ASF activates mTORC1. Proc Natl Acad Sci USA 105: 15323–15327, 2008. doi: 10.1073/pnas.0801376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michlewski G, Sanford JR, Cáceres JF. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell 30: 179–189, 2008. doi: 10.1016/j.molcel.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol 14: 185–193, 2007. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie N, Chen M, Dai R, Zhang Y, Zhao H, Song Z, Zhang L, Li Z, Feng Y, Gao H, Wang L, Zhang T, Xiao R-P, Wu J, Cao C-M. SRSF1 promotes vascular smooth muscle cell proliferation through a Δ133p53/EGR1/KLF5 pathway. Nat Commun 8: 16016, 2017. doi: 10.1038/ncomms16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang X, Zheng M, Wang P, Mok BW-Y, Liu S, Lau S-Y, Chen P, Liu Y-C, Liu H, Chen Y, Song W, Yuen K-Y, Chen H. An NS-segment exonic splicing enhancer regulates influenza A virus replication in mammalian cells. Nat Commun 8: 14751, 2017. doi: 10.1038/ncomms14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodcock C-SC, Huang Y, Woodcock SR, Salvatore SR, Singh B, Golin-Bisello F, Davidson NE, Neumann CA, Freeman BA, Wendell SG. Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion, and tumor growth. J Biol Chem 293: 1120–1137, 2018. doi: 10.1074/jbc.M117.814368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14: 417–419, 2017. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife 4: e05005, 2015. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Q, Tai Y-Y, Tang Y, Zhao J, Negi V, Culley MK, , et al. BOLA (BolA family member 3) deficiency controls endothelial metabolism and glycine homeostasis in pulmonary hypertension. Circulation 139: 2238–2255, 2019. doi: 10.1161/CIRCULATIONAHA.118.035889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, Graham BB, Kumar R, Black SM, Fratz S, Fineman JR, West JD, Haley KJ, Waxman AB, Chau BN, Cottrill KA, Chan SY. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest 124: 3514–3528, 2014. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 48.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AMF, Velandia MMS, Vitali S, Colas RA, Norris PC, Marinković A, Liu X, Ma J, Rose CD, Lee S-J, Comhair SAA, Erzurum SC, McDonald JD, Serhan CN, Walsh SR, Tschumperlin DJ, Fredenburgh LE. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 1: e86987, 2016. doi: 10.1172/jci.insight.86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chun HJ, Bonnet S, Chan SY. Translational advances in the field of pulmonary hypertension. Translating microRNA biology in pulmonary hypertension. It will take more than “miR” words. Am J Respir Crit Care Med 195: 167–178, 2017. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun W, Chan SY. Pulmonary arterial stiffness: an early and pervasive driver of pulmonary arterial hypertension. Front Med (Lausanne) 5: 204, 2018. doi: 10.3389/fmed.2018.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das S, Krainer AR. Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer. Mol Cancer Res 12: 1195–1204, 2014. doi: 10.1158/1541-7786.MCR-14-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llorian M, Gooding C, Bellora N, Hallegger M, Buckroyd A, Wang X, Rajgor D, Kayikci M, Feltham J, Ule J, Eyras E, Smith CWJ. The alternative splicing program of differentiated smooth muscle cells involves concerted non-productive splicing of post-transcriptional regulators. Nucleic Acids Res 44: 8933–8950, 2016. doi: 10.1093/nar/gkw560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xi X, Zhang J, Wang J, Chen Y, Zhang W, Zhang X, Du J, Zhu G. SGK1 mediates hypoxic pulmonary hypertension through promoting macrophage infiltration and activation. Anal Cell Pathol (Amst) 2019: 3013765, 2019. doi: 10.1155/2019/3013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 46: 1118–1122, 2005. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 55.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 109: 159–165, 2004. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 56.Masri FA, Anand-Apte B, Vasanji A, Xu W, Goggans T, Drazba J, Erzurum SC. Definitive evidence of fundamental and inherent alteration in the phenotype of primary pulmonary hypertension endothelial cells in angiogenesis. Chest 128: 571S, 2005. doi: 10.1378/chest.128.6_suppl.571S. [DOI] [PubMed] [Google Scholar]
- 57.Liang S, Yu H, Chen X, Shen T, Cui Z, Si G, Zhang J, Cheng Y, Jia S, Song S, Zhang X, Yu X. PDGF-BB/KLF4/VEGF signaling axis in pulmonary artery endothelial cell angiogenesis. Cell Physiol Biochem 41: 2333–2349, 2017. doi: 10.1159/000475652. [DOI] [PubMed] [Google Scholar]
- 58.Ma C, Wang Y, Shen T, Zhang C, Ma J, Zhang L, Liu F, Zhu D. Placenta growth factor mediates angiogenesis in hypoxic pulmonary hypertension. Prostaglandins Leukot Essent Fatty Acids 89: 159–168, 2013. doi: 10.1016/j.plefa.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Wen L, Wei X, Wang Q, Xu L, Zhang H, Liu W. Inhibition of miR-7 promotes angiogenesis in human umbilical vein endothelial cells by upregulating VEGF via KLF4. Oncol Rep 36: 1569–1575, 2016. doi: 10.3892/or.2016.4912. [DOI] [PubMed] [Google Scholar]
- 60.Dong W, Li B, Wang J, Song Y, Zhang Z, Fu C, Zhang P. Diagnostic and predictive significance of serum microRNA-7 in esophageal squamous cell carcinoma. Oncol Rep 35: 1449–1456, 2016. doi: 10.3892/or.2015.4499. [DOI] [PubMed] [Google Scholar]
- 61.Ma C, Gu R, Wang X, He S, Bai J, Zhang L, Zhang J, Li Q, Qu L, Xin W, Jiang Y, Li F, Zhao X, Zhu D. circRNA CDR1as promotes pulmonary artery smooth muscle cell calcification by upregulating CAMK2D and CNN3 via sponging miR-7-5p. Mol Ther Nucleic Acids 22: 530–541, 2020. doi: 10.1016/j.omtn.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bohnsack BL, Lai L, Northrop JL, Justice MJ, Hirschi KK. Visceral endoderm function is regulated by quaking and required for vascular development. Genesis 44: 93–104, 2006. doi: 10.1002/gene.20189. [DOI] [PubMed] [Google Scholar]
- 63.Chen A-J, Paik J-H, Zhang H, Shukla SA, Mortensen R, Hu J, Ying H, Hu B, Hurt J, Farny N, Dong C, Xiao Y, Wang YA, Silver PA, Chin L, Vasudevan S, DePinho RA. STAR RNA-binding protein Quaking suppresses cancer via stabilization of specific miRNA. Genes Dev 26: 1459–1472, 2012. doi: 10.1101/gad.189001.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, Huber LC. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J 35: 3203–3211, 2014. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]