Abstract
BACKGROUND AND PURPOSE: The use of liquid embolic agents for embolization of cerebral aneurysms has been reported in the neurosurgical literature. The most important limitation of this technique is the relatively poor control of migration of the liquid embolic agent into the parent artery. We performed an experimental aneurysm study using a liquid embolic agent and different protective devices to evaluate the safety and technical feasibility of this endovascular technique.
METHODS: Forty lateral aneurysms were surgically constructed on 20 common carotid arteries of swine. Onyx alone was used to obliterate eight aneurysms. Onyx was also used in combination with microcoils (n = 11), microstents (n = 6), balloons inflated proximally to the neck of the aneurysm (n = 6), and across the neck of the aneurysm (n = 7). One control aneurysm was embolized with Guglielmi detachable coils (GDCs) alone.
RESULTS: The use of a microballoon across the neck of the aneurysm, a microstent deployed across the neck of the aneurysm, or the deposit of GDCs into the aneurysm allowed faster and more complete filling of the aneurysm with Onyx. However, these protection devices did not totally preclude intractable migration of Onyx into the parent artery (migration rate, 9–33%).
CONCLUSION: Although complete occlusion of experimental aneurysms with Onyx is feasible using protective devices, migration of the liquid embolic agent into the parent artery or intracranially remains a difficult challenge. Further experimental studies need to be performed to master this technique and to select those aneurysms that can be safely treated in clinical practice.
The use of liquid embolic agents for occlusion of intracranial aneurysms has not been universally accepted as a standard therapy, despite the appearance of numerous articles describing their experimental and clinical use (1–12). An important limitation of this technique is the difficulty in controlling the liquid embolic agent when it reaches the neck of the aneurysm. Previous reports have suggested that gravity and use of protective devices, such as balloons, coils, or stents, could play a positive role in preventing migration of the liquid embolic agent into the parent artery or cranium (1–12). The aim of this study was to evaluate the technique of injecting Onyx (a liquid embolic agent) into an aneurysm, alone or in combination with protective devices. Particular attention was paid to obtaining complete occlusion of the aneurysm, depicting intractable migration of the liquid embolic agent distal to the neck of the aneurysm, and collecting angiographic and histopathologic findings.
Methods
Liquid Embolic Agent
Onyx (formerly called Embolyx; Micro Therapeutics Inc [MTI], Irvine, CA) is a biocompatible polymer (ethylene–vinyl alcohol copolymer; EVOH) dissolved in its organic solvent, dimethyl sulfoxide (DMSO). The EVOH is composed of a random mixture of two subunits: ethylene (hydrophobic) and vinyl alcohol (hydrophilic). Micronized tantalum powder was added to the polymer/solvent mixture to obtain appropriate radiopacity. When this mixture contacts a liquid agent, such as blood, DMSO rapidly diffuses away from the mixture, causing in situ precipitation and solidification of the polymer. The liquid agent becomes spongy and occlusive without adhering to the vascular wall.
Both 6% and 8% Onyx (6–8% EVOH and 92–94% DMSO) have been used for the embolization of cerebral arteriovenous malformations and vascular tumors (13, 14). In this study, 12% Onyx was chosen for the treatment of aneurysms. This solution, with a higher concentration of EVOH, increases the viscosity and tensile strength of the liquid embolic agent.
Endovascular Delivery System
A newly designed double-lumen microcatheter system was used to deliver the Onyx into the aneurysm. This double-lumen coaxial microcatheter included a 1.7F inner and a 2.7F outer microcatheter (Fig 1). The inner microcatheter was designed for the injection of Onyx into the aneurysm and the outer microcatheter was used to infuse saline to wash out the DMSO from the aneurysm. Stagnation of DMSO within the aneurysm delays solidification of the Onyx. The fast solidification of the outer surface of Onyx was a key feature in decreasing its migration into the parent artery (even when the inside mass was still liquid). The 2.7F outer microcatheter was connected to a manual pulsatile saline flushing system. Continuous or intermittent flushing of saline was performed during the injection of Onyx into the aneurysm.
fig 1.

Double-lumen microcatheter placed in the silicone-made aneurysm. A small 1.7F microcatheter system was used for the injection of Onyx (black arrow indicates direction of flow), and a large 2.7F microcatheter system was used for the injection of saline to flush the stagnant DMSO in the aneurysmal sac (white arrows indicate direction of flow)
Construction and Embolization of Aneurysms
All animal experiments were conducted in accordance with policies set by the UCLA Chancellor's Animal Research Committee and National Institutes of Health guidelines. The animals were 3 to 4 months old, weighed 30 to 40 kg, were of mixed sex, and were maintained on a standard laboratory diet. After an overnight fast, each swine was premedicated with intramuscular ketamine (20 mg/kg) and xylazine (2 mg/kg). General anesthesia was maintained with mechanical ventilation and inhalation of 0.5% to 1.5% halothane after endotracheal intubation.
Forty lateral-wall aneurysms were surgically constructed by a single neurosurgeon using both common carotid arteries in 20 swine. This technique of aneurysm construction has been reported previously (15). The two aneurysms constructed in each animal were similar in diameter (8–10 mm) and neck size (6 mm). The right external jugular vein was isolated and divided into two equal segments to make two aneurysms of equal size. A 6-mm-long arteriotomy was performed using a premade 6-mm paper slit that could be inserted into the arterial incision. The end-to-side anastomosis was made using 7–0 Prolene. This technique permitted us to manufacture aneurysms with a neck size of 6 mm. The height of the aneurysm was determined by the closure of the open end of the venous graft using 3–0 silk (8 mm). The width of the aneurysm was determined by the degree of removal of adventitia of the venous graft.
Five subgroups of animals were identified. The percentage of occlusion of the aneurysm, the injection time, and the frequency of migration of embolic material into the parent artery were evaluated in all five subgroups. Aneurysms in group 1 were embolized using 12% Onyx alone (n = 8). Aneurysms in group 2 were embolized using Onyx in combination with Guglielmi detachable coils (GDCs) (n = 11). Aneurysms in group 3 were embolized using Onyx in combination with microstents (GFX II; Arterial Vascular Engineering, Santa Rosa, CA) deposited across the neck of the aneurysm (n = 6). Aneurysms in group 4 were embolized using Onyx in combination with balloon occlusion of the parent artery (Zeppelin balloon guiding catheter; Medtronic Micro Interventional Systems [MIS], Sunnyvale, CA) proximal to the location of the aneurysm (n = 6). And aneurysms in group 5 were treated with Onyx in combination with balloon occlusion across the neck of the aneurysm (Grapevine balloons; MIS) (n = 7). One aneurysm was embolized with GDCs alone (size: 3 × 8 to 8 × 20). Histologic findings in this aneurysm were compared with results observed in aneurysms embolized with Onyx. One aneurysm was excluded from the study owing to inadvertent surgical dissection of the parent artery. All endovascular treatment of aneurysms was performed immediately after their construction by a single investigator.
Group 1: Onyx Alone (n = 8)
A 6F sheath was placed in the right femoral artery after standard Seldinger puncture and catheterization. Selective common carotid arteriography was performed using a 6F Envoy guiding catheter (Cordis Corp, Miami Lakes, FL). The aneurysms were shown in angiographic orthogonal projections. An intravenous bolus of 3000 U of heparin was injected to prevent thromboembolic complications. The 1.7/2.7F double-lumen microcatheter (MTI) and a Silver speed microguidewire (MTI) were advanced through the guiding catheter, and the tip of the 1.7F microcatheter was positioned in the center of the aneurysm. A slow injection of Onyx under fluoroscopic control was performed with the goal of filling the aneurysm and minimizing the migration of embolic material into the carotid artery. Postembolization angiography was performed in all cases and the injection time and amount of Onyx delivered into the aneurysm were recorded.
The delivery technique for Onyx was as follows: 0.25 mL of DMSO was injected into the 1.7F microcatheter to fill its dead space; 1 mL of Onyx was then aspirated into a 1-mL syringe, and the first 0.25 mL was then slowly injected over 40 seconds to displace the DMSO from the microcatheter; Onyx continued to be injected slowly under digital fluoroscopy. Intermittent saline injections through the 2.7F microcatheter were performed to wash out the DMSO from the aneurysm to accelerate solidification of the Onyx mass. Special efforts were made to prevent the Onyx from acquiring a tail shape (mass becoming threadlike) as it flowed into the aneurysm. The presence of this tail shape increased the possibility of Onyx migrating into the parent artery. If the Onyx mass became tail-shaped, the tip of the microcatheter was repositioned in a different area of the aneurysm and the injection of Onyx continued.
Group 2: Onyx with GDCs (n = 11)
The 1.7/2.7F double-lumen microcatheter was located in a similar position as in group 1. One GDC-10 coil (6 × 20 mm) was placed in the aneurysm through the inner 1.7F microcatheter. Deployment of the GDC into the aneurysm changed its flow dynamics and decreased the possibility of Onyx migrating into the parent artery. Subsequently, 12% Onyx was slowly injected through the 1.7F microcatheter into the mesh of the coil under fluoroscopic supervision. Again, intermittent flushing of normal saline was done through the 2.7F microcatheter located in the neck of the aneurysm.
Group 3: Onyx with Microstents (n = 6)
A GFX II balloon expandable microstent (length, 12 mm; maximum expanded diameter, 4.0 mm) was used in this study. The stent was premounted on a 3.0F microballoon system. The dead space of the balloon catheter was filled with 50% contrast material (Omnipaque) to visualize the balloon's inflation and deflation. The stent was coaxially delivered through a 6F guiding catheter across the neck of the aneurysm. After stent deployment, a carotid angiogram was obtained to document flow changes in the aneurysm. The 1.7/2.7F double-lumen microcatheter was then positioned into the aneurysm through the metallic mesh of the stent. Twelve percent Onyx was then slowly delivered into the aneurysm until it reached the stent surface. If a residual lumen of the aneurysm was identified, the double-lumen microcatheter was repositioned through the mesh of the stent and the embolization continued.
Group 4: Onyx Delivered with Balloon Occlusion of the Parent Artery Proximal to the Aneurysm (n = 6)
A 7F sheath was placed in the left femoral artery and a 6.4F balloon catheter (Zeppelin; MIS) was placed in the common carotid artery. The balloon was positioned proximal to the neck of the aneurysm. The double-lumen microcatheter was advanced through the balloon tip, and the tip of the 1.7F microcatheter was placed near the dome of the aneurysm. During the injection of Onyx, the balloon temporarily occluded the carotid artery proximal to the location of the neck of the aneurysm. The injection of Onyx was terminated when complete or nearly complete occlusion of the aneurysm was achieved or when intractable migration of Onyx was detected.
Group 5: Onyx with Balloon Occlusion of Aneurysmal Neck (n = 7)
Intraaneurysmal pressure (IAP) measurements were taken during embolization of an aneurysm with balloon-assisted technology (before embolization in one case). We documented changes in IAP when Onyx was injected into an occluded or semioccluded aneurysm through an inflated balloon across its neck. The technicalities of this method of IAP measurement have been described elsewhere (16). A Tracker 18 microcatheter (Target Therapeutics, Fremont, CA) was surgically placed in the dome of the aneurysm and was connected to a pressure transducer. A double-lumen microcatheter was then placed coaxially into the aneurysm through a 6F guiding catheter. Measurements of intraaneurysmal systolic, diastolic, and mean arterial pressures were taken while changing the position of an inflated balloon across the neck of the aneurysm or in the parent artery.
Six French sheaths were placed in the left and right femoral arteries, and 6F guiding catheters (Envoy; Cordis) were placed in the carotid artery harboring the aneurysm. One guiding catheter was at the origin of the carotid artery and the other was positioned close to the neck of the aneurysm. A microcatheter and a microballoon catheter (Grapevine; MIS) were advanced through the guiding catheters. The tip of the microcatheter was placed in the center of the aneurysm. The balloon was then placed across the neck of the aneurysm and inflated to occlude approximately 90% of its orifice. The inflow zone of the aneurysm remained open to avoid stagnation of DMSO within the aneurysm and to decrease IAP during embolization.
Onyx was then slowly injected into the aneurysm until satisfactory embolization was achieved or intractable Onyx migration was depicted. If the risk of intractable migration was suspected, the balloon was completely inflated, sealing the neck of the aneurysm for 2 to 4 minutes.
Angiographic Evaluation
Immediate pre- and postembolization angiograms were obtained in all cases to evaluate anatomic results and migration/protrusion of Onyx into the parent artery. Follow-up angiograms were obtained at 14 days in groups 1, 2, 3, and 5. Periodic follow-up studies were not obtained in group 4 because we expected findings similar to those in group 1. These angiographic evaluations were all performed by the same investigator.
Histopathologic Results
After 14 days, the swine were sacrificed using standard approved procedures. Both carotid arteries were opened following their vertical axis, and the parent artery and the neck of the aneurysm were analyzed macroscopically.
The harvested aneurysms were fixed with 10% formaldehyde and sent to an outside independent institute (Pathology Associates Inc, Frederick, MD). They were embedded in methylmethacrylate, and histologic sections were cut using a diamond band saw. Four longitudinal sections of the aneurysm were obtained through the neck, approximately 50 μm thick, polished and surface-stained with hematoxylin-eosin, Goldner's trichrome, and Paragon stains.
The degree of aneurysmal thrombosis, inflammation, neointimal hyperplasia, and neoendothelial coverage was graded as follows: grade 0 = no inflammation, grade 1 = minimal, grade 2 = mild, grade 3 = moderate, grade 4 = marked, and grade 5 = severe. These histologic findings were graded by an independent pathologist at the outside institution.
Results
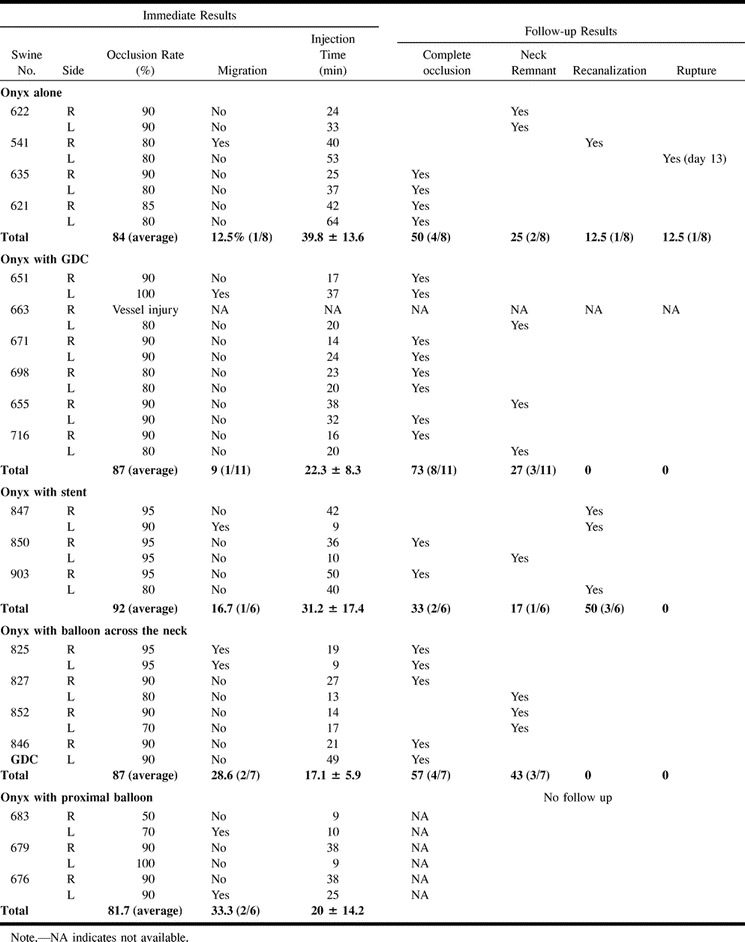
The overall angiographic results are summarized in the Table.
Angiographic findings in 20 swine with 40 experimental lateral-wall aneurysms
Group 1: Aneurysms Embolized with Onyx Alone
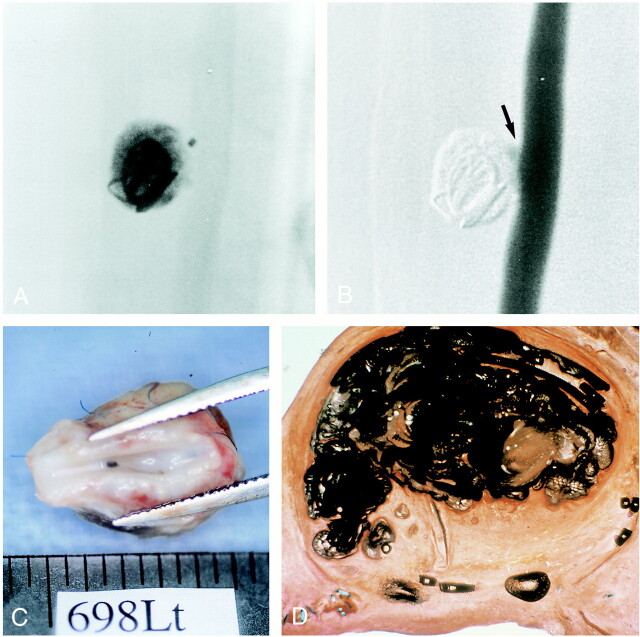
A very slow injection of Onyx was required to decrease its migration into the parent artery. The average injection time was 40 minutes for each aneurysm. With this slow injection technique, 70% to 90% (average, 84%) occlusion of the aneurysm was achieved. A neck remnant was seen in all cases. Migration of Onyx into the parent artery was observed in one of eight aneurysms (12.5%). Angiographic follow-up at 14 days showed complete aneurysmal occlusion in four cases (50%) and a small neck remnant in two cases (25%). One aneurysm (12.5%) showed recanalization and regrowth (Fig 2A). One aneurysm (12.5%) ruptured 13 days after embolization. Migration of Onyx into the parent artery was observed in one case (Fig 2B). There was no parent artery occlusion in this group. On macroscopic analysis, three of four completely occluded aneurysms had their necks completely covered with neointima and neoendothelium, and one aneurysm showed partial neoendothelial coverage.
fig 2.
A, Sagittal view of aneurysm embolized with Onyx. Note incomplete packing of the aneurysm, multiple fragmentation of the embolic mass (open arrow), and recanalization of the aneurysm inflow zone (solid arrow) (hematoxylin-eosin, ×4).
B, Macroscopic appearance of the neck of the aneurysm with Onyx migration into the parent artery (arrow). No significant thrombus formation was observed.
Group 2: Aneurysms Embolized with Onyx and GDCs
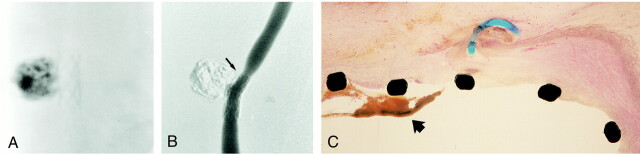
The placement of GDCs into an aneurysm allowed faster injection of Onyx, as the GDC loops acted as a protective mesh to reduce Onyx migration into the parent artery (Fig 3A and B). Sometimes it was difficult to see the Onyx through the coil mesh, because GDCs are more radiopaque than Onyx. This problem was especially important when GDCs created a dense three-dimensional basket in the aneurysm. When a single GDC could be anchored in the aneurysm, the injection of Onyx was denser and faster. The average injection time was 22 minutes, and the average occlusion rate was 87%. There was one case of minimal intractable migration of Onyx into the carotid artery.
fig 3.
A, Nonsubtraction angiogram shows dense packing of the aneurysmal sac with the combination of a GDC and Onyx. The coil was placed in the aneurysm.
B, Digital subtraction view shows small neck remnant at the inflow zone (arrow).
C, Macroscopic appearance of the neck of the aneurysm with complete neoendothelial lining.
D, Sagittal view of aneurysm embolized with Onyx and a GDC. Note denser packing with Onyx and endothelial lining across the neck of the aneurysm.
Fourteen days after embolization, angiograms showed complete occlusion in eight (73%) of 11 aneurysms. A small neck remnant was observed in three aneurysms (27%). There were no cases of aneurysmal recanalization or rupture. Macroscopic and histologic examinations showed that five of eight totally occluded aneurysms had their necks completely covered with neointima and neoendothelium (Fig 3C and D), and three aneurysms showed nearly complete coverage of their necks. Two of three aneurysms with small neck remnants showed partial neoendothelial coverage, and one aneurysm lacked neointimal reaction.
Group 3: Aneurysms Embolized with Onyx and Stents
Metallic microstents were successfully deployed across the neck of the aneurysms in all cases. After stent deployment there was some flow stagnation in the aneurysm. The degree of flow stagnation achieved with the GFX stent was less than that with the Wallstents (unpublished data). Onyx could be injected in all cases through the 1.7F microcatheter positioned in the aneurysm through the metallic mesh of both types of stents (Fig 4A). Immediate postembolization angiography showed that six aneurysms had a small neck remnant and two aneurysms showed 70% occlusion. The average occlusion rate was 92%. The average time of injection of Onyx was 31 minutes. Migration of Onyx into the parent artery was noticed in one case.
fig 4.
A, Plain radiograph shows immediate poststenting and Onyx embolization of the aneurysm.
B, Follow-up angiogram at 14 days shows moderate degree of stenosis of the parent artery due to intimal hyperplasia (note stent deformity, arrow). The aneurysm was completely occluded with Onyx.
C, Light microscopic findings of aneurysmal neck 14 days after embolization with Onyx and microstent show thrombus deposition on stent wire (arrow) (hematoxylin-eosin, ×10).
Follow-up angiograms at 14 days showed that two aneurysms (33%) were completely occluded and one aneurysm (17%) had an unchanged small neck remnant. Three aneurysms (50%) showed recanalization and regrowth. Although the average occlusion rate in this subgroup was the highest, the GFX II stent did not prevent aneurysmal recanalization or intractable migration of Onyx into the parent artery. Angiographic and histopathologic evaluation showed some intimal hyperplasia and artery stenosis in all cases, owing to expansion of the stent (Fig 4B). Macroscopically, two cases showed complete coverage of the neck with neointima and four aneurysms had no endothelial coverage across their necks. Macro- or microscopic evidence of thrombotic attachments was observed on the surface of the stents in all cases (Fig 4C).
Group 4: Aneurysms Embolized with Onyx and Concomitant Proximal Balloon Occlusion of the Parent Artery
Complete balloon occlusion of the common carotid artery proximal to the aneurysm produced marked blood stagnation in the aneurysm in all cases. However, complete flow arrest into the sac of the aneurysm was never achieved because of the retrograde blood flow from the distal portion of the parent artery. Although the mean injection time was 20 minutes (range, 9–38 minutes), the behavior of the Onyx in the aneurysm was unpredictable and uncontrollable.
Complete or nearly complete occlusion (more than 90%) was observed in four aneurysms (67%) and incomplete occlusion (less than 90%) was shown in two aneurysms (33%). The average occlusion rate was 82%. Intractable migration of Onyx occurred in two aneurysms (33%).
Group 5: Embolization of the Aneurysm with Onyx and Concomitant Balloon Occlusion of Its Neck
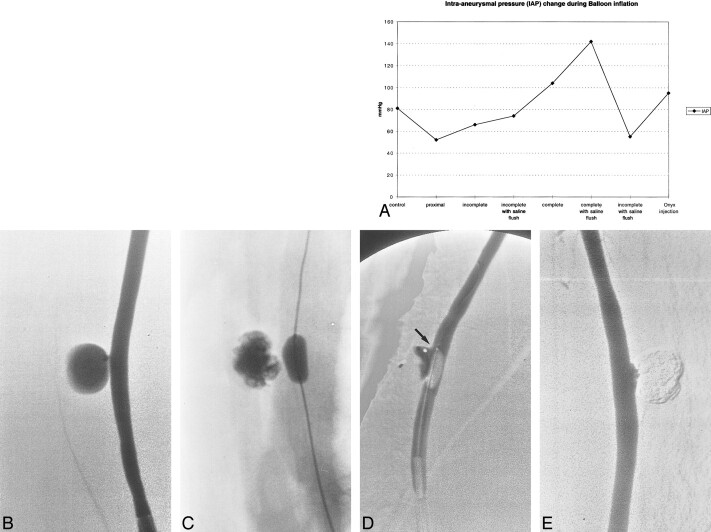
IAP Measurements (Fig 5A)
fig 5.
A, Graph shows IAP change during balloon inflation. Note significant increase of IAP with complete balloon inflation with or without saline flush.
B, Angiogram of experimental aneurysm created on the common carotid artery of a swine.
C, Approximately 80% occlusion of the aneurysm was achieved with combination of Onyx and a microballoon.
D, Embolization of the remnant at the inflow zone using nearly complete occlusion technique. Note small space between balloon and distal portion of the aneurysmal neck (arrow).
E, Example of successful occlusion of the aneurysm with Onyx and balloon technique. Note small concave shape of the Onyx along with balloon shape.
A slight reduction in IAP was observed when the balloon was inflated in the parent artery, proximal to the aneurysm, or when it incompletely occluded the neck of the aneurysm. Similar findings were observed with or without delivery of pulsatile saline flush. However, when the balloon was completely inflated across the neck of the aneurysm, IAP increased significantly. This increase in IAP was more dramatic when the saline pulsatile flow was used. On the basis of these findings, we used the incomplete balloon occlusion technique in the chronic study.
Embolization of Aneurysm with Onyx
After obtaining a diagnostic angiogram (Fig 5B), the microballoon catheter and the double-lumen microcatheter were advanced at the neck of the aneurysm. The microballoon was not inflated until the double-lumen microcatheter was almost totally filled with Onyx (0.25 mL). Then the balloon was inflated, sparing the distal portion of the aneurysmal neck. Using this balloon technique, 80% to 90% of the aneurysmal sac was occluded easily, without intractable migration of Onyx (Fig 5C). However, occlusion of the last small space closest to the balloon was always technically challenging (Fig 5D). The microballoon had to be inflated completely to avoid migration of Onyx into the parent artery. Such a small neck remnant was almost always observed at the inflow zone of the aneurysm. Five (71%) of seven aneurysms were embolized completely or nearly completely (Fig 5E), with an average injection time of 17 minutes (range, 9–27 minutes). The average immediate occlusion rate was 87% and the migration rate was 29% (two of seven cases). Follow-up angiography at 14 days showed complete occlusion in four aneurysms (57%) (Fig 5D) and a small neck remnant in three aneurysms (43%). There were no cases of aneurysmal recanalization or rupture. In cases complicated by migration of Onyx into the parent artery, the embolic agent was covered with a neointima and there was no parent artery occlusion.
Histopathologic Findings
Overall Findings
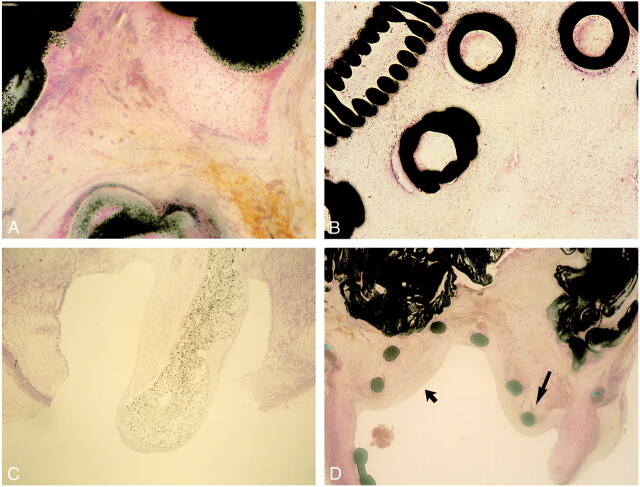
The sac of the aneurysm was partially occupied with Onyx and the remainder of the sac contained a mature, well-vascularized fibrous tissue matrix with interlacing bundles of fibroblasts. There was a mild to moderate inflammatory response (grade 2–4), which concentrated along the surface of the Onyx and decreased in intensity away from the polymer (Fig 6A). The control histopathologic specimen (aneurysm embolized only with GDCs) showed minimal to mild inflammatory reaction (grade 1–2) (Fig 6B). The inflammatory infiltrate was composed mostly of lymphocytes and macrophages, with rare neutrophils, and a moderate number of multinucleated foreign body giant cells. There was no evidence of angionecrosis of the wall of the aneurysm or in the parent artery.
fig 6.
A, Light microscopic findings in region of aneurysmal dome 14 day after embolization with Onyx (hematoxylin-eosin, ×25). Dense connective tissue containing moderate number of mixed inflammatory cells is observed along with polymer surfaces. No angionecrosis is present.
B, Light microscopic findings in region of aneurysmal dome 14 days after embolization with GDCs (hematoxylin-eosin, ×25). Minimum to mild inflammatory reaction is observed along with coils.
C, Light microscopic findings of aneurysmal neck 14 days after embolization with Onyx. Note tonguelike migration of Onyx into the parent artery and complete coverage with neointima (hematoxylin-eosin, ×10).
D, Light microscopic findings of aneurysmal neck 14 days after embolization with Onyx and microstent. Note significant intimal hyperplasia (wide arrow) and stent deformity due to tissue reaction (thin arrow) (hematoxylin-eosin, ×5).
Onyx Alone, with Balloon Protection and GDCs
Completely embolized aneurysm: The neck of the aneurysm contained relatively mature fibrous connective tissue with a surface of neoendothelium and fibromuscular neointima.
Incompletely embolized aneurysm: When aneurysms were incompletely occluded, the sacs were filled with a mixture of fibrin and unorganized thrombus. There was minimal organized fibrous tissue along the surface of the Onyx. At the neck of the aneurysm, there was a layer of fibrin on the polymer surface facing the arterial lumen.
Migration of Onyx into the parent artery: In the neck of the aneurysm, there was a tonguelike section of polymer that extended from the aneurysmal sac into the lumen of the parent artery. The polymer in the parent artery was covered by a layer of endothelium-lined, fibromuscular neointima (Fig 6C).
Onyx with Stent
The stent wires were surrounded by granulation tissue that was covered by a surface of endothelialized fibromuscular neointima (Fig 6D). There were small focal areas of hemorrhage and scattered lymphocytes and histiocytes throughout the granulation tissue surrounding the stent wires.
Discussion
Endovascular treatment of cerebral aneurysms has become a valid alternative to conventional neurosurgical clipping. The advent of GDCs provided safe and effective treatment for a large population of cerebral aneurysms (17–24). Some anatomic limitations associated with the use of GDCs in aneurysms have been identified. GDCs are less effective in wide-necked or large/giant aneurysms (anatomic limitation) (19, 20, 23). In this type of aneurysm, it is difficult to achieve complete packing and control of the aneurysm inflow with GDC technology.
GDCs produce a mild reactive inflammatory response in the aneurysm, promoting a limited and delayed scar formation (biological limitation) (15). This mild inflammatory response offers weak anchoring tissue for the coils and limited protection from coil compaction and recanalization in wide-necked aneurysms.
Use of liquid embolic agents is a possible alternative for the treatment of some cerebral aneurysms (1–12). However, this therapeutic technique has not been universally accepted owing to intrinsic technical limitations. One major limitation is the delivery of liquid embolic agent into the sac of the aneurysm without any possibility of repositioning or retrieval. Some experimental and clinical methods have been tested to decrease distal migration of the liquid embolic agent, such as intraaneurysmal flow control with proximal balloon protection, balloon inflation across the neck of the aneurysm, and use of metallic stents.
Kinugasa and coworkers (2–5, 10) have described the use of cellulose acetate polymer for embolization of intracranial aneurysms. They used the effect of gravity and flow control by balloon inflation. Because cellulose acetate polymer is heavier than blood, the risk of distal migration seemed less likely when the aneurysm pointed downward (gravity effect). Our in vitro study showed that gravity alone caused a concomitant delayed solidification of Onyx owing to delayed washout of the DMSO from the sac of the aneurysm (25).
A potential way to decrease migration is to accelerate the solidification of the outer surface of the liquid embolic agent in the aneurysmal sac. We believe that a quicker solidification time creates a more stable polymer morphology around the tip of the microcatheter. The 1.7/2.7F double-lumen microcatheter system allowed the injection of Onyx with concomitant flushing of normal saline into the aneurysm. The saline flush accelerated the washout of DMSO from the aneurysm and allowed a faster and more homogeneous solidification of Onyx in the aneurysm.
Onyx without Flow Control
A very slow injection of Onyx with concomitant intermittent flushing saline technique was performed to keep Onyx from assuming a tail shape. The speed of injection of the polymer was approximately 1.0 mL/h. When we detected a tail-shape appearance of Onyx migrating to the neck of the aneurysm, we discontinued the injection of Onyx and continued the saline flushing. This very slow and discontinuous injection technique extended the time of the procedure. Without flow control in the parent artery, 70% to 80% occlusion was achieved in all cases. However, it was extremely difficult to achieve higher occlusion rates owing to the potential risk of Onyx migrating to the parent artery.
Onyx with GDCs
In contrast to using Onyx alone, deployment of a GDC in the aneurysm allowed faster and denser injection of Onyx and a higher rate of occlusion. The GDC changed the blood flow in the aneurysm, decreasing turbulence. Appropriate coil placement was confirmed by angiography. One limitation of this technique was the poor visualization of the Onyx caused by the higher radiopacity of the GDC. This technical difficulty resulted in intractable migration of Onyx in one case. The presence of a GDC mesh in the outflow zone of the aneurysm enabled faster and more controlled injection of Onyx.
Onyx with Stents
The use of microstents for intracranial aneurysms has recently been reported (26–28). The use of stents for aneurysms is based on the premise that stents located across the neck of an aneurysm may produce progressive aneurysmal thrombosis due to flow stagnation and may prevent migration of embolic material into the parent artery. In this study, the quality of blood flow in aneurysms (turbulent versus laminar) was not significantly changed by deployment of GFX II stents. The GFX II is one of the most flexible stents on the market. It can potentially be placed in serpiginous arteries, such as intracranial carotid or vertebral arteries. However, the metallic mesh of the coil was not dense enough to alter flow patterns in these experimental, wide-necked aneurysms. The other limitation observed with this technique was the development of chronic stenosis of the parent artery and potential thrombus formation on the surface of the stent facing the blood flow. These technical limitations may be solved in the future with the development of more flexible microstents manufactured with biocompatible materials.
Onyx with Balloons
The combined use of liquid embolic material with balloon protection has been described by several investigators (1–7, 9–12). In this study, we showed that although the proximal balloon occlusion of the parent artery decreased the blood flow in an aneurysm, there was always an unpredictable and uncontrollable back flow from the carotid artery distal to the aneurysm via the rete mirabile (anastomosis of bilateral ascending pharyngeal artery).
Balloon inflation across the neck of the aneurysm was a more promising technique than proximal balloon occlusion of the parent artery. It allowed a faster and denser injection of Onyx into the aneurysm, but had the potential limitation of increasing IAP. Although the IAP measurement in this study was preliminary, the trend of pressure changes was consistent. One way to decrease this sudden increase of IAP was to perform a subtotal occlusion of the neck of the aneurysm, sparing part of the aneurysmal inflow. With this technique, 80% to 90% occlusion was safely achieved. Complete aneurysmal occlusion with Onyx required complete balloon occlusion of the neck of the aneurysm. Two migrations occurred during injection of Onyx into the small residual space closest to the neck of the aneurysm. We believe that full inflation of the balloon does not always seal the aneurysm completely. Some lateral slits in the aneurysmal neck may remain unprotected, and it is through them that the Onyx can migrate into the parent artery. Pressure is another possible mechanism for migration. Injection of Onyx into the small, closed, residual space may increase IAP. High IAP may open the small space between the elastic wall of the experimental aneurysm and the microballoon. The balloons used in this study were originally designed for intracranial angioplasty, not for the temporary occlusion of intracranial aneurysms. Technical innovations on these balloons and the creation of new protective devices to be positioned in the neck of the aneurysm while delivering a liquid embolic agent may offer better anatomic outcomes in the immediate future.
Conclusion
The embolization of experimental, lateral-wall, wide-necked aneurysms with liquid embolic agents combined with protective devices, such as GDCs, microstents, and microballoons, is feasible. The protection devices allow faster and denser injection of liquid embolic agent into the aneurysm; however, the rate of migration of liquid embolic agent into the parent artery is still too high if this technique is to be used in clinical practice. In general, embolization is more controlled and predictable in experimental aneurysms than in clinical practice. Additional technical innovations are necessary to decrease potential complications.
Acknowledgments
We gratefully acknowledge the technical assistance of John Robert and Shawn McGill in the laboratories of Leo G. Rigler Radiological Research Center at University of California, Los Angeles. We thank Joan R. Wicks, of Pathology Associates Inc, for the histopathologic evaluation, and Brian Strauss and Tom Whalen, of Micro Therapeutics Inc, for technical support.
References
- 1.Debrun GM, Varsos V, Liszczak Davis KR, Heros RS, Zervas NT. Obliteration of experimental aneurysms in dogs with isobutyl-cyanoacrylate. J Neurosurg 1984;61:37-43 [DOI] [PubMed] [Google Scholar]
- 2.Kinugasa K, Mandai S, Kamata I, et al. Prophylactic thrombosis to prevent new bleeding and to delay aneurysm surgery. Neurosurgery 1995;36:661-667 [DOI] [PubMed] [Google Scholar]
- 3.Kinugasa K, Mandai S, Terai Y, et al. Direct thrombosis of aneurysm with cellulose acetate polymer (CAP), II: preliminary clinical experience. J Neurosurg 1992;77:501-507 [DOI] [PubMed] [Google Scholar]
- 4.Kinugasa K, Mandai S, Tsuchida K, et al. Cellulose acetate polymer thrombosis for the emergency treatment of aneurysms: angiographic findings, clinical experience and histopathological study. Neurosurgery 1994;34:694-701 [DOI] [PubMed] [Google Scholar]
- 5.Kinugasa K, Mandai S, Tsuchida K, Kamata I, Ohmoto T. Direct thrombosis of a pseudoaneurysm after obliteration of a carotid-cavernous fistula with cellulose acetate polymer: technical case report. Neurosurgery 1994;35:755-759 [DOI] [PubMed] [Google Scholar]
- 6.Macdonald RL, Mojtahedi S, Johns L, Kowakczuk A. Randomized comparison of Guglielmi detachable coils and cellulose acetate polymer for treatment of aneurysms in dogs. Stroke 1998;29:478-486 [DOI] [PubMed] [Google Scholar]
- 7.Mandai S, Kinugasa K, Ohmoto T. Direct thrombosis of aneurysms with cellulose acetate polymer, I: results of thrombosis in experimental aneurysms. J Neurosurg 1992;77:497-500 [DOI] [PubMed] [Google Scholar]
- 8.Mandai S, Rüfenacht DA, Kinugasa K, Ohmoto T. An in vitro study to define the term “dense packing” of aneurysms with detachable coils, liquid embolic material and their combination. Intervent Neuroradiol 1998;4 (Suppl 1):173-177 [DOI] [PubMed] [Google Scholar]
- 9.Nishi S, Taki W, Nakahara I, et al. Embolization of cerebral aneurysms with a liquid embolus, EVAL mixture: report of three cases. Acta Neurochir (Wien) 1996;138:294-300 [DOI] [PubMed] [Google Scholar]
- 10.Sugiu K, Kinugasa K, Mandai S, Tokunaga K, Ohmoto T. Direct thrombosis of experimental aneurysms with cellulose acetate polymer (CAP): technical aspects, angiographic follow up, and histological study. J Neurosurg 1995;83:531-538 [DOI] [PubMed] [Google Scholar]
- 11.Szikora I, Guterman LR, Standard SC, Walhloo AK, Hopkins LN. Endovascular treatment of experimental aneurysms with liquid polymers: the protective potential of stents. Neurosurgery 1996;38:339-347 [DOI] [PubMed] [Google Scholar]
- 12.Taki W, Nishi S, Yamashita K, et al. Selection and combination of various endovascular techniques in the treatment of giant aneurysms. J Neurosurg 1992;77:37-42 [DOI] [PubMed] [Google Scholar]
- 13.Murayama Y, Viñuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery 1998;43:1164-1175 [DOI] [PubMed] [Google Scholar]
- 14.Murayama Y, Viñuela F, Zenteno M, et al. Nonadhesive liquid embolic agent for neurovascular applications: experimental studies and preliminary clinical utilization in brain arteriovenous malformations. Intervent Neuroradiol 1997;3S-101
- 15.Murayama Y, Viñuela F, Suzuki, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997;40:1233-1244 [DOI] [PubMed] [Google Scholar]
- 16.Akiba Y, Murayama Y, Viñuela F, Lefkowitz MA, Duckwiler GR, Gobin YP. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysms, 1: experimental evaluation. Neurosurgery 1999;45:519-530 [DOI] [PubMed] [Google Scholar]
- 17.Byrne JV, Sohn MJ, Molyneux AJ. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656-663 [DOI] [PubMed] [Google Scholar]
- 18.Guglielmi G, Viñuela F, Dion J, et al. Electrothrombosis of saccular aneurysms via endovascular approach, 2: preliminary clinical experience. J Neurosurg 1991;75:8-14 [DOI] [PubMed] [Google Scholar]
- 19.Guglielmi G, Viñuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach, 1: electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1-7 [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa M, Murayama Y, Viñuela F, Duckwiler GR, Gobin YP, Guglielmi G. Long-term angiographic follow up of neck remnants in cerebral aneurysms treated with Guglielmi detachable coils. J Neruosurg (in press)
- 21.Murayama Y, Viñuela F, Duckwiler GR, Gobin YP, Guglielmi G. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg 1999;90:207-214 [DOI] [PubMed] [Google Scholar]
- 22.Vanninen R, Koivisto T, Saari T, Hernesniemi J, Vapalahti M. Ruptured intracranial aneurysms: acute endovascular treatment with electrolytically detachable coils—a prospective randomized study. Radiology 1999;211:325-336 [DOI] [PubMed] [Google Scholar]
- 23.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475-482 [DOI] [PubMed] [Google Scholar]
- 24.Zubillaga AF, Guglielmi G, Viñuela F, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol 1994;15:815-820 [PMC free article] [PubMed] [Google Scholar]
- 25.Murayama Y, Akiba Y, Walker B, Werneth R, Pecor R. Endovascular treatment of experimental aneurysms with liquid embolic agents: in vitro hemodynamic evaluation. Proceedings of the 36th Annual Meeting of the ASNR, Philadelphia, PA, May 1998
- 26.Lylyk P, Ceratto R, Hurviz D, Basso A. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery 1998;43:385-388 [DOI] [PubMed] [Google Scholar]
- 27.Mericle RA, Lanzino G, Wakhloo AK, Guterman LR, Hopkins LN. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery 1998;43:1229-1234 [DOI] [PubMed] [Google Scholar]
- 28.Sekhon LHS, Morgan MK, Sorby W, Grinnel V. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery 1998;43:380-384 [DOI] [PubMed] [Google Scholar]