Abstract
BACKGROUND AND PURPOSE: Although sonography is the primary imaging technique for evaluating the developing fetus, significant limitations exist in the sonographic prenatal diagnosis of many brain disorders. Fast MR imaging is increasingly being used to determine the underlying cause of nonspecific fetal CNS abnormalities detected sonographically and to confirm or provide further support for such anomalies. Our goal was to determine the value of MR imaging in establishing the diagnosis of fetal CNS anomalies, to ascertain how this information might be used for patient counseling, and to assess its impact on pregnancy management.
METHODS: We prospectively performed MR examinations of 73 fetuses (66 pregnancies) with suspected CNS abnormalities and compared these with available fetal sonograms, postnatal images, and clinical examinations. Retrospectively, the impact on patient counseling and pregnancy management was analyzed.
RESULTS: Images of diagnostic quality were routinely obtained with in utero MR imaging, which was particularly valuable in detecting heterotopia, callosal anomalies, and posterior fossa malformations, and for providing excellent anatomic information. We believe that 24 (46%) of 52 clinical cases were managed differently from the way they would have been on the basis of sonographic findings alone. In every case, the referring physicians thought that MR imaging provided a measure of confidence that was not previously available and that was valuable for counseling patients and for making more informed decisions.
CONCLUSION: Sonography is the leading technique for fetal assessment and provides reliable, inexpensive diagnostic images. Fast MR imaging is an important adjunctive tool for prenatal imaging in those instances in which a complex anomaly is suspected by sonography, when fetal surgery is contemplated, or when a definitive diagnosis cannot be determined.
Advances in technology and the need for accurate prenatal diagnoses have spurred vast improvements in fetal diagnosis and therapy. The advances being made in the arena of in utero surgery, particularly the repair of myelomeningoceles and the ex utero intrapartum treatment of prenatal airway obstruction (1–5) have necessitated the acquisition of high-anatomic-resolution fetal images. Although sonography remains the primary imaging technique for evaluating the developing fetus, significant limitations still exist in the sonographic prenatal diagnosis of many brain disorders. These limitations are largely due to obscuration of portions of fetal intracranial anatomy caused by reverberative artifacts of the bony calvarium and to the low sensitivity of fetal sonography to malformations of cerebral cortical development as well as to subtle destructive lesions of the cerebrum and cerebellum. Sonographic diagnoses can also be hampered by engagement of the fetal brain deep in the maternal pelvis, oligohydramnios, and maternal body habitus.
MR imaging does not suffer from these restraints, as it is not hindered by the presence of bone and may be facilitated by oligohydramnios, which sometimes results in decreased fetal motion. Further, MR imaging has a higher intrinsic sensitivity than sonography to contrast between various cerebral tissues. Moreover, the superior spatial and contrast resolution of MR imaging has the potential 1) to provide anatomic information not previously available for patient counseling and decision making, 2) to help in identifying those who may potentially benefit from prenatal intervention, and 3) to aid in fetal surgical planning.
Recent work has proved that fetal MR imaging can depict subtle brain anomalies not currently detectable by fetal sonography. MR imaging is increasingly being used to determine the underlying cause of nonspecific sonographically identified CNS abnormalities in the fetus and to confirm or provide further evidence of anomalies depicted by sonography (6–26). In this study, we sought to determine the diagnostic utility of fetal MR imaging and to discover what value the additional information about brain abnormalities may have for counseling parents in decisions regarding the management of the remainder of the pregnancy.
Methods
Since November 1996, when our institution first gained the ability to perform the single-shot fast spin-echo (ssFSE) sequences that facilitate fetal MR imaging, more than 90 fetal MR imaging examinations have been performed. Of these, the 66 that were performed to assess abnormalities of the fetal CNS constitute the basis of this report; the remainder were referred for non-CNS anomalies and are being reported separately. Some of these patients have already been reported previously (27, 28).
The 66 patients, carrying a total of 73 fetuses, were referred to our university-based center for further evaluation of fetal anomalies detected by screening sonography performed in community centers. Most subjects had confirmatory sonography at our institution, typically within 24 to 96 hours of the MR examination. Although we have been using MR imaging to examine fetuses since November 1996, 62 (94%) of the 66 studies were performed within the last 24 months of the study period.
MR imaging was performed on a high-performance 1.5-T superconducting magnet. A surface coil was used to maximize image quality, either a dedicated two-element pelvic coil or a four-element torso phased-array coil, depending on patient size and gestational age.
A coronal localizer fast multiplanar spoiled gradient refocused echo (FMPSPGR) sequence (90–120/4.2/1 [TR/TE/excitations], 70° flip angle, 8–10-mm slice thickness with a 1–2-mm gap, and 32–40-cm field of view) was acquired to determine fetal position. This was used to guide the initial imaging plane, which was selected to be anatomic to the fetal section in question. Subsequent sequences were prescribed in planes axial, coronal, and sagittal to the fetal brain and/or spine.
Modern ultrafast sequences were used to minimize image degradation by fetal motion and to allow images to be obtained during maternal breath-holding. T2-weighted images were the mainstay of the examination and were acquired with an ssFSE sequence (∞/100/0.5 [TR/TEeff/excitations], flip angle = 90°), which generally provided 14 to 17 slices in 20 to 25 seconds. Other imaging parameters included a field of view of 24 to 30 cm, a matrix size of 256 × 192, a bandwidth of 15.6 kHz for FMPSPGR and 31.5 to 62.5 kHz for ssFSE, and a slice thickness of 3.0 to 5.0 mm. The typical examination time was 45 minutes.
Thin-section T1-weighted images with the FMPSPGR sequence were obtained through the brain in 42 patients, but only provided satisfactory contrast resolution in older (beyond 26 weeks) fetuses. FMPSPGR images of younger fetuses (with smaller heads) were hindered by poor signal-to-noise ratio and poor gray/white matter contrast. All patients had ssFSE T2-weighted imaging; seven patients were studied with only T2-weighted sequences. Excessive fetal motion was rarely a problem, with only four of the 66 examinations significantly degraded by motion artifacts. However, it was not unusual for one or two sequences to be rendered nondiagnostic because of motion, requiring that sequences be repeated.
MR examinations were interpreted prospectively by at least two, and often three, radiologists, who were fully aware of the sonographic findings. Most of these studies were clinically indicated and, therefore, the interpreters felt strongly that knowledge of the indications for the examination and of the regions of suspected sonographic abnormalities heightened the sensitivity and accuracy of the MR interpretations. The emphasis of this study was to establish a diagnosis with MR imaging rather than to compare the two techniques; thus, all available information was used.
Gestational ages of the fetuses studied ranged from 18 to 39 weeks by conventional menstrual dating. No MR examination was performed during the period of organogenesis or at any time before 18 weeks' gestation. No contrast agent or sedative (maternal or fetal) was used. MR imaging was not performed as a screening tool; thus, although a review of the literature indicated no apparent danger to the developing fetus in tissue culture, animals, or humans (29–35), only fetuses with a sonographic abnormality were referred for MR imaging. In this setting, any small potential risk from the procedure was outweighed by the benefit of the additional diagnostic information that MR imaging could provide. All patients were counseled about the experimental nature of fetal MR imaging before the examination, and all studies were performed with the approval of our university's Committee on Human Research (investigational review board).
The impact of the MR imaging information on management of the pregnancy was analyzed retrospectively at the completion of the study period. An experienced maternal-fetal medicine specialist reviewed the clinical and imaging data and determined whether there had been a change in management on the basis of the MR data and, subjectively, whether the confidence level in decision making was improved, even if there was no change in the management of the pregnancy.
Results
Imaging Findings
The 73 fetuses showed a spectrum of CNS disorders (see Table). A subset of these patients is enrolled in an ongoing prospective assessment of isolated fetal ventriculomegaly, which was the most common finding (22 patients) (Fig 1). In eight fetuses, sonographic findings were suggestive of Dandy-Walker spectrum or other posterior fossa abnormalities (Fig 2). One of these fetuses was found to have a mega-cisterna magna associated with multiple subependymal heterotopia, which was confirmed by postnatal MR imaging. Another fetus had a Dandy-Walker malformation and a marked supratentorial cortical abnormality (Fig 3), which were ultimately revealed to be part of the hydrolethalus syndrome. This unusual autosomal recessive disorder is characterized by multisystem anomalies with hydrocephalus and parenchymal malformations (36). A small cerebellar hemisphere was diagnosed in a patient with a congenital diaphragmatic hernia.
Findings in 66 fetal MR examinations

fig 1.

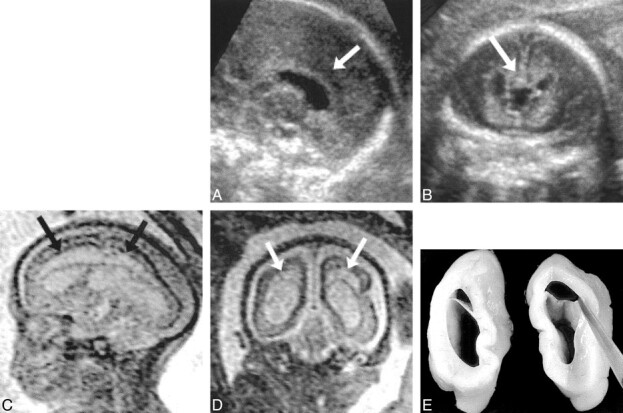
Patient 13: isolated ventriculomegaly (23 weeks' gestation).
A and B, ssFSE T2-weighted images (∞/97/0.5) in the axial plane (A) show ventriculomegaly with 11-mm diameter at the atrium (arrow) and normal signal in the adjacent parenchyma. Coronal plane (B) reveals the normal hypointense signal of the germinal matrix (arrowheads).
fig 2.
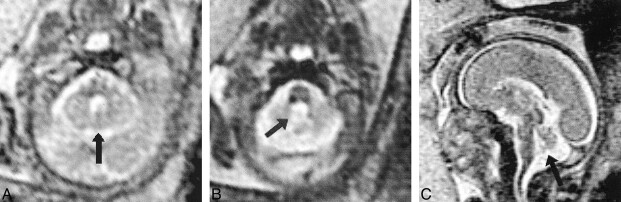
Patient 20: inferior vermian hypogenesis (23 weeks' gestation).
A, Axial ssFSE image (∞/98/0.5) shows the normal superior vermis (arrow).
B, Subjacent section shows CSF communication with the fourth ventricle (arrow).
C, Midline sagittal image shows hypogenetic inferior vermis with normal superior and hypoplastic inferior lobules (arrow).
fig 3.
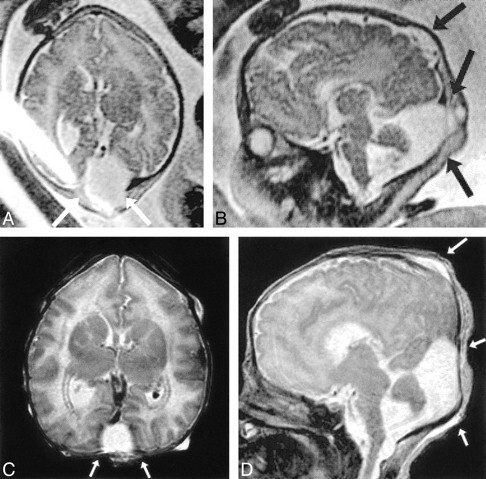
Patient 23: hydrolethalus syndrome with postnatal correlation (39 weeks' gestation).
A, Prenatal axial ssFSE image (∞/97/0.5) at the level of the third ventricle shows calvarial defect and meningocele (arrows).
B, Prenatal sagittal ssFSE image shows vermian hypogenesis, large fourth ventricle, elevated tentorium (Dandy-Walker malformation), and calvarial defects (arrows).
C and D, Postnatal axial (C) and sagittal (D) T2-weighted sequences (3000/120/1 and 3000/102/2, respectively) confirm the prenatal findings, although the meningoceles (arrows) are less apparent due to positional flattening.
Seventeen fetuses were referred for suspected periventricular leukomalacia (PVL), including one with multifocal parenchymal destruction. Twelve of these referrals were in the setting of twin-twin transfusion syndrome. This category also includes one fetus in whom we found destruction of a previously documented corpus callosum and concurrent development of marked bilateral cystic PVL (Fig 4). Another highly instructive case was a fetus referred for isolated ventriculomegaly, who was noted to have hypogenesis of the corpus callosum (Fig 5). In retrospect, this finding could be inferred but could not be diagnosed definitively on the basis of sonographic findings.
fig 4.
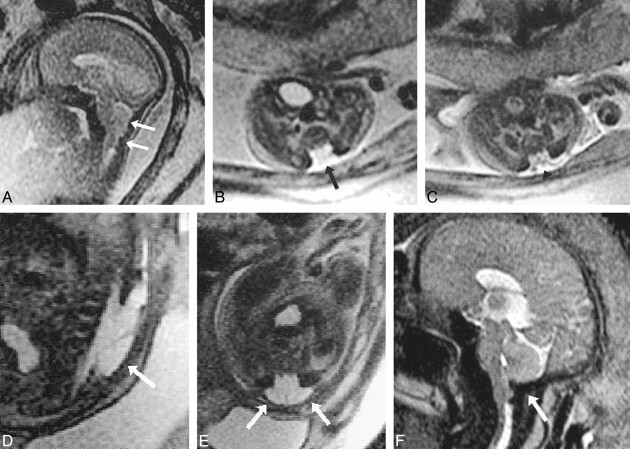
Patient 37: cystic PVL and secondary absence of the corpus callosum (25 weeks' gestation).
A and B, Sagittal (A) and coronal (B) views from sonograms obtained at 22 weeks' gestation show normal corpus callosum (arrows).
C and D, Sagittal (C) and coronal (D) ssFSE images (∞/98/0.5) reveal development of cystic PVL (arrows) and absence of the corpus callosum.
E, Postmortem coronal section confirms the MR findings.
fig 5.

Patient 39: hypogenesis of the corpus callosum (22 weeks' gestation).
A, Sagittal ssFSE image (∞/98/0.5) shows the genu and anterior body of the corpus callosum (arrow). The posterior body and splenium are absent.
B, Axial ssFSE image confirms the presence of the genu (white arrow). The cavum septi pellucidi (black arrows) is only seen when the genu and anterior callosal body are present. The absent splenium is apparent on this image, as the posterior interhemispheric fissure is continuous with the cavum.
Four fetuses were found to have Chiari II malformation and myelomeningocele at MR imaging both before and after in utero surgical repair (Fig 6). A Gibbus spinal deformity was confirmed in a twin gestation, and a spinal anomaly was a clinical consideration in a fetus with abnormally hyperextended lower extremities. Other findings were isolated hemorrhage, which was intraventricular (IVH) or subependymal (SEH) (n = 8), hydrocephalus (n = 3), and corpus callosal anomalies (n = 2).
fig 6.
Patient 17: Chiari II malformation and myelomeningocele (23 weeks' gestation).
A, Sagittal ssFSE image (∞/96/0.5) shows the poorly formed posterior fossa floor and downward cerebellar herniation (arrows).
B and C, Axial ssFSE images at the level of the lumbosacral region show absent posterior elements (arrow, B) and exposed neural elements (myelomeningocele, arrowhead, C).
D and E, Sagittal (D) and axial (E) ssFSE images (∞/98/0.5) 13 days after in utero repair show hypointense dural patch (arrows) over defect.
F, Sagittal ssFSE image (∞/97/0.5) approximately 10 weeks after repair suggests improved development of the floor of the posterior fossa (suboccipital bone, arrow) and reduced hindbrain herniation.
A single patient was confirmed to have a vein of Galen malformation (Fig 7), as seen on prenatal sonograms. We also detected neck abnormalities in two fetuses; the first with congenital high-airway-obstruction syndrome and the second with a large precervical teratoma.
fig 7.
Patient 30: vein of Galen malformation (33 weeks' gestation).
A, Sagittal ssFSE image (∞/98/0.5) shows large signal void caused by rapid flow in the vein of Galen varix and dilated straight sinus (arrows).
B, Axial FMPSPGR image (100/4.2/1) shows hyperintensity caused by flow-related enhancement in the varix (arrow).
Two patients were referred for anomalies suggested by sonographic findings (suspected tuberous sclerosis, prompted by the presence of an intracardiac mass, and a subcutaneous fluid collection, which was thought to be a posterior cephalocele), but brain MR imaging detected no abnormality. Another patient was referred for decreased fetal movement in the setting of multiple systemic anomalies; however, at MR imaging, the brain was normal.
Follow-up
Confirmation of fetal MR findings was established in 47 (64%) of the 73 fetuses at the time of this writing. Confirmation was achieved by follow-up imaging in 22 patients, including postnatal MR imaging in 10, postnatal clinical examination in 11, and surgical and/or pathologic examination in 14. Nine fetuses died either spontaneously or because the pregnancy was terminated, and, as is often the case in this situation, no pathologic data were obtained. Of these, the characteristic MR and sonographic findings were thought to be unequivocally diagnostic in five (co-twin with a spinal anomaly, hydrocephalus with hypoplastic cerebellar hemisphere, focal PVL, agenesis of the corpus callosum, and IVH with ventriculomegaly). Seventeen pregnancies are ongoing as of this writing.
Overall, there was one false-negative MR finding, as the patient with suspected tuberous sclerosis was ultimately found to have evidence of subependymal nodules at postnatal MR imaging performed at 7 months of age. No false-positive MR studies were revealed by the follow-up.
Although the effects of the information obtained by fetal MR imaging on pregnancy management were not studied prospectively in consultation with our fetal treatment team, we believe that 24 (46%) of 52 pregnancies were managed differently from the way they would have been on the basis of sonographic diagnosis alone. Fourteen patients are enrolled in the prospective study of isolated ventriculomegaly, and were imaged for research purposes. In the remaining 28 pregnancies, the referring physicians felt strongly that the confirmatory technique provided a measure of confidence that was not previously available and that was extremely valuable for counseling patients and for making more informed decisions. Four patients were imaged late in their pregnancies (after 30 weeks' gestation) and opportunities for fetal surgical intervention or significant changes in management were significantly reduced.
Discussion
Since the early descriptions of normal fetal brain morphology and maturation with MR imaging (18, 22, 24, 25, 37–39), the clinical utility of fetal MR imaging as an adjunct to screening sonography has been well documented (2, 6, 7, 9–12, 14, 40, 41). Our series lends further support to previous MR studies establishing the superb diagnostic capabilities of MR imaging, particularly in assessing disorders of neuronal migration and in defining the structures of the corpus callosum and posterior fossa. The value of an additional noninvasive technique to confirm sonographically suspected CNS abnormalities cannot be overstated, especially when this information aids in making pregnancy management decisions. Indeed, our impressive increase in the number of requested examinations, from five in 1996–1997, to 13 in 1998, to 48 in 1999 and through March 2000, reflects the referring physician's perception of the value of MR imaging.
The crucial decisions made with the additional information documented in this series underscore the importance of having these studies performed and interpreted by physicians with training and experience in imaging the developing brain. As experts in the imaging assessment of CNS development and maldevelopment, the modern neuroradiologist is highly qualified to acquire the optimal images and to recognize injuries and structural malformations. One of the most valuable applications of this technology is in the detection of heterotopia and other malformations of cortical development. Developmental abnormalities, such as ventriculomegaly, agenesis of the corpus callosum, and Dandy-Walker malformation, are associated with significantly better prognoses when they are not accompanied by cortical malformations (42–44). The sensitivity of sonography to these often subtle parenchymal abnormalities is low. Although the sensitivity of fetal MR imaging in detecting these entities is unknown and may be difficult to determine prospectively, the ability of MR imaging to detect waves of migrating cells in the fetal brain (45) and to clearly differentiate gray matter from white matter in the developing brain suggests an important role for this technique in the assessment of fetal malformations or suspected brain malformations identified by sonography.
Fetal MR imaging is a unique tool for learning more about the developing brain in vivo. It is known that insults to the fetal brain occurring before 20 to 21 weeks' gestation result in parenchymal necrosis without astrogliosis, whereas insults after approximately 26 weeks' gestation will leave behind some telltale scarring and, likely, some residual tissue (46–48). A striking example of this phenomenon in our study was the fetus with callosal destruction due to extensive white matter necrosis. Without prenatal imaging, which clearly showed an intact corpus callosum, our neuropathologist would not have been able to differentiate this acquired callosal absence from a primary agenesis. This observation suggests that infants with marked white matter hypoplasia and absence of the corpus callosum may have an acquired, in utero injury and not a genetic/developmental malformation. Additionally, our neuropathologists believed that the periventricular cystic changes in the fetal brain would most likely have been interpreted as marked ventriculomegaly had the prenatal MR studies not been available for review. As fetal brains are notoriously difficult to remove in toto and to formalin-fix, fetal imaging has the potential to impact the interpretation of fetal and neonatal autopsies as well as to teach us lessons about normal and abnormal brain development.
Ten (14%) of the 73 fetuses in our study had grade I SEH on MR images. The rate of frequency is generally believed to be less than 1% (49). This high prevalence is most likely the result of improvements in the ability to detect SEH by sonography, the introduction of MR imaging, and a referral bias in our patient population rather than to an actual increase in frequency. The clinical impact of a small SEH in the fetal brain is under investigation by our colleagues in pediatrics but is generally considered to be low (49). The relative paucity of neurologic symptoms from these lesions makes it uncommon for these patients to have clinically indicated postnatal imaging, and many of the lesions have been found to resolve on serial images. It is even possible that the MR detection of a small (grade I) SEH is superior to pathologic examination, given the relatively thick brain sections the neuropathologist is often forced to cut.
Accurate assessment of the posterior fossa is difficult by fetal sonography. As the fetal skull becomes progressively ossified, accurate assessment of the cerebellum is made more problematic. Occasionally, a prominent vallecula may be mistaken for inferior vermian agenesis on antenatal sonograms. In addition, distinguishing the more severe forms of the Dandy-Walker complex (true vermian agenesis and cerebellar hypoplasia) from the incidental finding of a mega-cisterna magna cannot always be done unerringly, even by the most skilled observer (50–52). The diagnosis of cerebellar hemispheric hypoplasia (found in one of our patients) may also be more difficult as the skull base matures. The normal development of the cerebellar vermis progresses into the second trimester and, as a result, the prenatal diagnosis of Dandy-Walker malformation should not be considered before a well-documented gestational age of 18 weeks. Before then, the normal appearance of the incompletely developed cerebellum should not be overinterpreted as anomalous (51–53). Our observations confirm previous reports that the cerebellar vermis can be reliably visualized by fetal MR imaging (7, 9–11, 14, 15).
Recent reports have also called into question the accuracy of measuring the atrium of the lateral ventricle by sonography (49, 54). This is an important issue, as fetuses with ventriculomegaly have a substantially higher rate of neurodevelopmental problems in childhood than do those with normal ventricular size (44, 55–58). Three patients referred to our institution with the diagnosis of isolated ventriculomegaly were found on MR imaging to have ventricles that measured within the range of normal (≤ 10 mm atrial diameter). The brain parenchyma was unremarkable by MR imaging. All three patients have had normal postnatal clinical courses; one is now 11 months old, one is 13 months old (and had normal postnatal brain MR findings), and the third was normal at birth and lost to follow-up. Since fetal karyotyping is often recommended for fetuses with ventriculomegaly at our institution, the confirmation of normal ventricular size can considerably reduce parental anxiety and potentially prevent an invasive diagnostic procedure.
Detailed prenatal diagnoses are mandatory for planning and follow-up of fetal surgery, such as myelomeningocele repair, if further advances are to be made in these promising areas. Assessment of the degree of hindbrain herniation and posterior fossa development is one of the necessary tools for determining the efficacy of this procedure (3, 4); these measurements are accurate and easily reproduced by MR imaging. The frequent occurrence of postsurgical oligohydramnios also favors MR imaging in the postsurgical radiologic assessment.
Early planning of postnatal interventions is another advantage of high-resolution prenatal imaging. For example, in our patient with a vein of Galen malformation, well before delivery the neurointerventional radiologists had ample opportunity to review the imaging, be in contact with the referring physicians, and discuss treatment options with the family. In this way, they were able to expedite postnatal clinical assessment and minimize delay in treatment after delivery. Other investigators (6) have postulated that late-term prenatal MR imaging could eliminate the need for postnatal MR imaging, which typically requires patient sedation. Current technology does not accord fetal MR imaging the same diagnostic quality and variety of pulse sequences as are available in the sedated postnatal examination; however, in a critically ill neonate, treatment can readily be based on the information available from prenatal imaging, thus potentially eliminating delays.
The most important criterion by which new techniques are judged is their effect on patient management. Although not prospectively studied, it is our view that the results of the MR examination changed patient management in 24 of our 52 clinical cases and increased confidence of the referring physicians in making management decisions in the remaining 28. Thus, in properly selected patients, fetal MR imaging can have an enormous impact on patient management.
Conclusion
Sonography remains the mainstay of fetal assessment. However, MR imaging of the fetal CNS is evolving as a powerful tool for obtaining additional information with which selected patients and their health-care professionals can make important pregnancy management decisions. The confirmation of an abnormality suspected by sonography provides an extra measure of certainty for the patient and provider, whereas the refutation of a suspected anomaly may prevent needless patient anxiety and potentially obviate invasive procedures. Although not prospectively studied, nearly half of our clinical cases had a significant management change based on the MR imaging data, and, in all pregnancies, the referring physicians believed that a confirmatory technique provided a measure of confidence for them and the patient that had not previously been available.
The information obtained by high-resolution in utero imaging can add to our understanding of brain development and maldevelopment. We believe that it is essential for physicians with training and experience in MR imaging of developmental anomalies of the brain to supervise the image acquisition and interpretation of these examinations. In this way, the highest possible standards of MR interpretation can be provided for these patients.
TABLE 1:
Continued

Footnotes
Presented in part at the annual meeting of the American Society of Neuroradiology, Atlanta, April 2000.
Address reprint requests to Erin M. Simon, MD, OTR Department of Radiology, Division of Neuroradiology, L 358, University of California, San Francisco, 505 Parnassus Ave, Box 0628, San Francisco, CA, 94143.
References
- 1.Hecher K, Plath H, Bregenzer T, et al. Endoscopic laser surgery versus serial amniocenteses in the treatment of severe twin-twin transfusion syndrome. Am J Obstet Gynecol 1999;180:717-724 [DOI] [PubMed] [Google Scholar]
- 2.Hubbard AM, Harty P. Prenatal magnetic resonance imaging of fetal anomalies. Semin Roentgenol 1999;34:41-47 [DOI] [PubMed] [Google Scholar]
- 3.Tulipan N, Bruner JP. Myelomeningocele repair in utero: a report of three cases. Pediatr Neurosurg 1998;28:177-180 [DOI] [PubMed] [Google Scholar]
- 4.Tulipan N, Hernanz-Schulman M, Bruner JP. Reduced hindbrain herniation after intrauterine myelomeningocele repair: a report of four cases. Pediatr Neurosurg 1998;29:274-278 [DOI] [PubMed] [Google Scholar]
- 5.Mychaliska G, Bealer J, Graf J, et al. Operating on placental support: the ex utero intrapartum treatment (EXIT) procedure. J Pediatr Surg 1997;32:227-231 [DOI] [PubMed] [Google Scholar]
- 6.Levine D, Barnes PD, Edelman RR. Obstetric MR imaging. Radiology 1999;211:609-617 [DOI] [PubMed] [Google Scholar]
- 7.Bilaniuk LT. Magnetic resonance imaging of the fetal brain. Semin Roentgenol 1999;34:48-61 [DOI] [PubMed] [Google Scholar]
- 8.Levine D, Barnes PD. Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology 1999;210:751-758 [DOI] [PubMed] [Google Scholar]
- 9.Levine D, Barnes PD, Madsen JR, et al. Fetal CNS anomalies revealed on ultrafast MR imaging. AJR Am J Roentgenol 1999;172:813-818 [DOI] [PubMed] [Google Scholar]
- 10.Resta M, Burdi N, Medicamento N. Magnetic resonance imaging of normal and pathologic fetal brain. Childs Nerv Syst 1998;14:151-154 [DOI] [PubMed] [Google Scholar]
- 11.Sonigo PC, Rypens FF, Carteret M, et al. MR imaging of fetal cerebral anomalies. Pediatr Radiol 1998;28:212-222 [DOI] [PubMed] [Google Scholar]
- 12.Garel C, Brisse H, Sebag G, et al. Magnetic resonance imaging of the fetus. Pediatr Radiol 1998;28:201-211 [DOI] [PubMed] [Google Scholar]
- 13.Kirkinen P, Partanen K, Vainio P, Ryynanen M. MRI in obstetrics: a supplementary method for ultrasonography. Ann Med 1996;28:131-136 [DOI] [PubMed] [Google Scholar]
- 14.Levine D, Barnes PD, Madsen JR, et al. Fetal CNS anomalies: MR imaging augments sonographic diagnosis. Radiology 1997;204:635-642 [DOI] [PubMed] [Google Scholar]
- 15.Garel C, Sebag G, Brisse H, et al. Magnetic resonance imaging of the fetus: contribution to antenatal diagnosis. Presse Med 1996;25:452-456 [PubMed] [Google Scholar]
- 16.Levine D, Hatabu H, Gaa J, et al. Fetal anatomy revealed with fast MR sequences. AJR Am J Roentgenol 1996;167:905-908 [DOI] [PubMed] [Google Scholar]
- 17.Colletti PM. Computer-assisted imaging of the fetus with magnetic resonance imaging. Comput Med Imaging Graph 1996;20:491-496 [DOI] [PubMed] [Google Scholar]
- 18.Revel MP, Pons JC, Lelaidier C, et al. Magnetic resonance imaging of the fetus: a study of 20 cases performed without curarization. Prenat Diagn 1993;13:775-799 [DOI] [PubMed] [Google Scholar]
- 19.D'Ercole C, Girard N, Boubli L, et al. Prenatal diagnosis of fetal cerebral abnormalities by ultrasonography and magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol 1993;50:177-184 [DOI] [PubMed] [Google Scholar]
- 20.Girard NJ, Raybaud CA. In vivo MRI of fetal brain cellular migration. J Comput Assist Tomogr 1992;16:265-267 [DOI] [PubMed] [Google Scholar]
- 21.Garden AS, Weindling AM, Griffiths RD, Martin PA. Fast-scan magnetic resonance imaging of fetal anomalies. Br J Obstet Gynaecol 1991;98:1217-1222 [DOI] [PubMed] [Google Scholar]
- 22.Garden AS, Griffiths RD, Weindling AM, Martin PA. Fast-scan magnetic resonance imaging in fetal visualization. Am J Obstet Gynecol 1991;164:1190-1196 [DOI] [PubMed] [Google Scholar]
- 23.Dinh DH, Wright RM, Hanigan WC. The use of magnetic resonance imaging for the diagnosis of fetal intracranial anomalies. Childs Nerv Syst 1990;6:212-215 [DOI] [PubMed] [Google Scholar]
- 24.Hata T, Makihara K, Aoki S, et al. Magnetic resonance imaging of the fetus: initial experience. Gynecol Obstet Invest 1990;29:255-258 [DOI] [PubMed] [Google Scholar]
- 25.Powell MC, Worthington BS, Buckley JM, Symonds EM. Magnetic resonance imaging in obstetrics, II: fetal anatomy. Br J Obstet Gynaecol 1988;95:38-46 [DOI] [PubMed] [Google Scholar]
- 26.McCarthy SM, Filly RA, Stark DD, et al. Magnetic resonance imaging of fetal anomalies in utero: early experience. AJR Am J Roentgenol 1985;145:677-682 [DOI] [PubMed] [Google Scholar]
- 27.Coakley FV, Hricak H, Filly RA, Barkovich AJ, Harrison MR. Complex fetal disorders: effect of MR imaging on management: preliminary clinical experience. Radiology 1999;213:691-696 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell LA, Simon EM, Filly RA, Barkovich AJ. Antenatal diagnosis of subependymal heterotopia. AJNR Am J Neuroradiol 2000;21:296-300 [PMC free article] [PubMed] [Google Scholar]
- 29.Baker PN, Johnson IR, Harvey PR, et al. A three-year follow-up of children imaged in utero with echo-planar magnetic resonance. Am J Obstet Gynecol 1994;170:32-33 [DOI] [PubMed] [Google Scholar]
- 30.Mevissen M, Buntenkotter S, Loscher W. Effects of static and time-varying (50-Hz) magnetic fields on reproduction and fetal development in rats. Teratology 1994;50:229-237 [DOI] [PubMed] [Google Scholar]
- 31.Kanal E, Gillen J, Evans JA, et al. Survey of reproductive health among female MR workers. Radiology 1993;187:395-399 [DOI] [PubMed] [Google Scholar]
- 32.Tyndall DA, Sulik KK. Effects of magnetic resonance imaging on eye development in the C57BL/6J mouse. Teratology 1991;43:263-275 [DOI] [PubMed] [Google Scholar]
- 33.Beers GJ. Biological effects of weak electromagnetic fields from 0 Hz to 200 MHz: a survey of the literature with special emphasis on possible magnetic resonance effects. Magn Reson Imaging 1989;7:309-331 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JL, Crooks LE. NMR imaging produces no observable mutations or cytotoxicity in mammalian cells. AJR Am J Roentgenol 1982;139:583-585 [DOI] [PubMed] [Google Scholar]
- 35.Wolff S, Crooks LE, Brown P, et al. Test for DNA and chromosomal damage induced by nuclear magnetic resonance imaging. Radiology 1980;136:707-710 [DOI] [PubMed] [Google Scholar]
- 36.de Ravel TJ, van der Griendt MC, Evan P, Wright CA. Hydrolethalus syndrome in a non-Finnish family: confirmation of the entity and early prenatal diagnosis. Prenat Diagn 1999;19:279-281 [DOI] [PubMed] [Google Scholar]
- 37.Chong BW, Babcock CJ, Salamat MS, et al. A magnetic resonance template for normal neuronal migration in the fetus. Neurosurgery 1996;39:110-116 [DOI] [PubMed] [Google Scholar]
- 38.Girard NJ, Raybaud CA, Poncet M. In vivo MR study of brain maturation in normal fetuses. AJNR Am J Neuroradiol 1995;16:407-413 [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong E, Schleicher A, Omran H, et al. Ontogeny of human gyrification. Cereb Cortex 1995;1:56-63 [DOI] [PubMed] [Google Scholar]
- 40.Levine D, Barnes PD, Sher S, et al. Fetal fast MR imaging: reproducibility, technical quality, and conspicuity of anatomy. Radiology 1998;206:549-554 [DOI] [PubMed] [Google Scholar]
- 41.Yamashita Y, Namimoto T, Abe Y, et al. MR imaging of the fetus by a HASTE sequence. AJR Am J Roentgenol 1997;168:513-519 [DOI] [PubMed] [Google Scholar]
- 42.Kalidasan V, Carroll T, Allcutt D, Fitzgerald RJ. The Dandy-Walker syndrome: a 10-year experience of its management and outcome. Eur J Pediatr Surg 1995;5::16-18 [DOI] [PubMed] [Google Scholar]
- 43.Gupta JK, Lilford RJ. Assessment and management of fetal agenesis of the corpus callosum. Prenat Diagn 1995;15:301-312 [DOI] [PubMed] [Google Scholar]
- 44.Gupta JK, Bryce FC, Lilford RJ. Management of apparently isolated fetal ventriculomegaly. Obstet Gynecol Surv 1994;49:716-721 [DOI] [PubMed] [Google Scholar]
- 45.Volpe JJ. Neuronal proliferation, migration, organization, and myelination. In: Neurology of the Newborn. 3rd ed. Philadelphia: Saunders 1995;79-82
- 46.Barkovich AJ, Truit CL. Brain damage from perinatal asphyxia: correlation of MR findings with gestational age. AJNR Am J Neuroradiol 1990;11:1087-1096 [PMC free article] [PubMed] [Google Scholar]
- 47.Barkovich AJ, Rowley HA, Bollen A. Correlation of prenatal events with the development of polymicrogyria. AJNR Am J Neuroradiol 1995;16:822-827 [PMC free article] [PubMed] [Google Scholar]
- 48.Toti P, De Felice C, Palmeri ML, et al. Inflammatory pathogenesis of cortical polymicrogyria: an autopsy study. Pediatr Res 1998;44:291-296 [DOI] [PubMed] [Google Scholar]
- 49.Behnke M, Eyler FD, Garvan CW, et al. Cranial ultrasound abnormalities identified at birth: their relationship to perinatal risk and neurobehavioral outcome. Pediatrics 1999;103:41-46 [DOI] [PubMed] [Google Scholar]
- 50.Hertzberg BS, Kliewer MA, Bowie JD. Sonographic evaluation of fetal CNS: technical and interpretive pitfalls. AJR Am J Roentgenol 1998;172:523-527 [DOI] [PubMed] [Google Scholar]
- 51.Bromley B, Nadel AS, Pauker S, et al. Closure of the cerebellar vermis: evaluation with second trimester US. Radiology 1994;193:761-763 [DOI] [PubMed] [Google Scholar]
- 52.Laing FC, Frates MC, Brown DL, et al. Sonography of the fetal posterior fossa: false appearance of mega-cisterna magna and Dandy-Walker variant. Radiology 1994;192:247-251 [DOI] [PubMed] [Google Scholar]
- 53.Babcock CJ, Chong BW, Salamat MS, et al. Sonographic anatomy of the developing cerebellum: normal embryology can resemble pathology. AJR Am J Roentgenol 1996;166:427-433 [DOI] [PubMed] [Google Scholar]
- 54.Hertzberg BS, Kliewer MA, Bowie JD. Fetal cerebral ventriculomegaly: misidentification of the true medial boundary of the ventricle at US. Radiology 1997;205:813-816 [DOI] [PubMed] [Google Scholar]
- 55.Bloom SL, Bloom DD, Dellanebbia C, et al. The developmental outcome of children with antenatal mild isolated ventriculomegaly. Obstet Gynecol 1997;90:93-97 [DOI] [PubMed] [Google Scholar]
- 56.Patel MD, Filly AL, Hersh DR, Goldstein RB. Isolated mild fetal cerebral ventriculomegaly: clinical course and outcome. Radiology 1994;192:759-764 [DOI] [PubMed] [Google Scholar]
- 57.Achiron R, Schimmel M, Achiron A, Mashiach S. Fetal mild idiopathic lateral ventriculomegaly: is there a correlation with fetal trisomy? Ultrasound Obstet Gynecol 1993;3:89-92 [DOI] [PubMed] [Google Scholar]
- 58.Bromley B, Frigoletto FD, Benacerraf BR. Mild fetal lateral cerebral ventriculomegaly: clinical course and outcome. Am J Obstet Gynecol 1991;164:863-867 [DOI] [PubMed] [Google Scholar]