Abstract
Summary: We report two patients with symptomatic high-grade stenosis of the basilar artery refractory to appropriate maximal medical therapy in whom endovascular stenting was performed successfully without preliminary balloon angioplasty. Excellent angiographic results were achieved and there were no procedural or periprocedural complications. The patients were asymptomatic and neurologically intact at a mean clinical follow-up of 6.5 months. Primary stenting of basilar artery stenosis may be an alternative to balloon angioplasty for patients with symptomatic lesions refractory to medical therapy or in whom anticoagulation is contraindicated.
Patients with symptomatic stenosis of the basilar artery are usually initially treated medically with antiplatelet agents and/or systemic anticoagulation. Despite this, the risk of stroke remains high. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study reported the annual risk of stroke in the basilar artery territory as 20% for patients with symptomatic basilar artery stenosis treated with aspirin and 12% for those treated with warfarin (1). For the subset of patients for whom medical therapy fails, the therapeutic options are limited. Surgical bypass-grafting for basilar artery stenosis is technically demanding and the results are often disappointing (2). Successful balloon angioplasty of the basilar artery was first reported by Sundt et al in 1980; however, intraprocedural vessel rupture, elastic recoil, and flow-limiting intimal dissection remain potential problems that have restricted its use (2).
The advent of new-generation, flexible stents has enabled reliable and atraumatic percutaneous access of the intracranial vasculature. We report two cases in which elective primary stenting without preceding balloon angioplasty of symptomatic, high-grade, basilar artery stenosis was performed without complications and with excellent angiographic results (no residual stenosis). The possible indications for primary stenting of basilar artery occlusive disease and the technical aspects of this procedure are discussed.
Case Reports
Case 1
A 77-year-old man presented with an 18-month history of recurrent drop attacks. He was symptomatic despite having taken oral clopidogrel, 75 mg/day, for the preceding 6 months. An MR angiogram suggested the diagnosis of basilar artery stenosis, which was subsequently confirmed by a diagnostic cerebral angiogram. In view of the patient's drop attacks, systemic anticoagulation with warfarin was considered contraindicated because of the risk of cerebral hemorrhage occurring with potential head trauma. At presentation, the patient was neurologically intact, and a baseline MR image of the brain revealed no definite evidence of posterior fossa infarction. The patient was examined by a neurovascular neurologist at our institution who agreed the symptoms were related to vertebrobasilar ischemia. In view of this, endovascular therapy of the basilar lesion was performed.
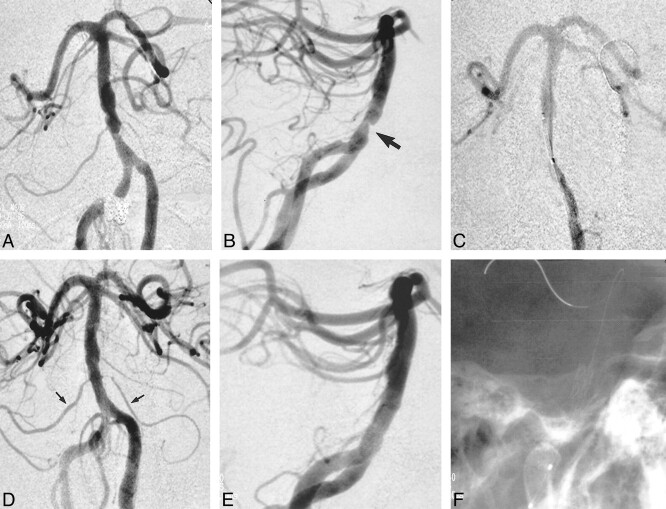
Diagnostic cerebral angiography revealed a high-grade, eccentric, atherosclerotic stenosis (>75%) of the lower basilar artery above the level of the right anterior inferior cerebellar artery (AICA). The left AICA arose from the ipsilateral posterior inferior cerebellar artery (PICA). Additionally, there was severe focal stenosis of both posterior cerebral arteries at the P1/P2 junctions, and a moderately severe stenosis of the left vertebrobasilar junction. The right vertebral artery was dominant. The posterior communicating arteries (PCoAs) were absent bilaterally (Fig 1).
fig 1.
Anteroposterior oblique views of the basilar artery.
A, Eccentric high-grade (>75%) stenosis of the lower basilar artery above the origin of the right AICA. The left AICA arises from the left PICA. Note bilateral stenosis of the posterior cerebral arteries (arrows) and a stenosis involving the distal left vertebral artery at the vertebrobasilar junction.
B, Stent is positioned across the stenosis without preliminary balloon angioplasty.
C, After stent deployment, normal vessel lumen is restored. Although not obvious on this image, the inferior margin of the stent covers the right AICA origin; however, the vessel continues to opacify normally (arrow).
D, Nonsubtracted lateral oblique view of the posterior fossa shows the basilar artery metallic stent posterior to the clivus (arrow).
Endovascular treatment was performed using an AVE GFX-2 stent (Advanced Vascular Engineering, Santa Rosa, CA) with a nominal diameter of 4 mm and a length of 12 mm. The stent-deployment balloon was inflated to its nominal pressure of 8 atm (unconstrained stent diameter of 4 mm). The normal midbasilar artery diameter was 3.9 mm. An excellent angiographic result was achieved with complete normalization of the vessel diameter. There were no procedural complications, and the patient was asymptomatic and neurologically intact at the 7-month clinical follow-up examination.
Case 2
A 57-year-old obese man with a history of hypertension, diabetes mellitus, hypercholesterolemia, and coronary artery disease presented with recurrent episodes of vertigo associated with nausea and vomiting and severe gait ataxia. MR imaging and MR angiography of the brain revealed a small area of infarction within the left cerebellar hemisphere associated with a high-grade stenosis of the basilar artery. The patient was started on oral clopidogrel, 75 mg/day; however, the episodes of gait ataxia persisted over the next 4 months. Systemic anticoagulation was considered contraindicated because of his episodes of severe gait ataxia. Neurologic examination performed at presentation to our institution was unremarkable apart from a mild right upper limb pronator drift and difficulty performing tandem gait. The patient was examined by a neurovascular neurologist, who agreed his symptoms were related to vertebrobasilar ischemia.
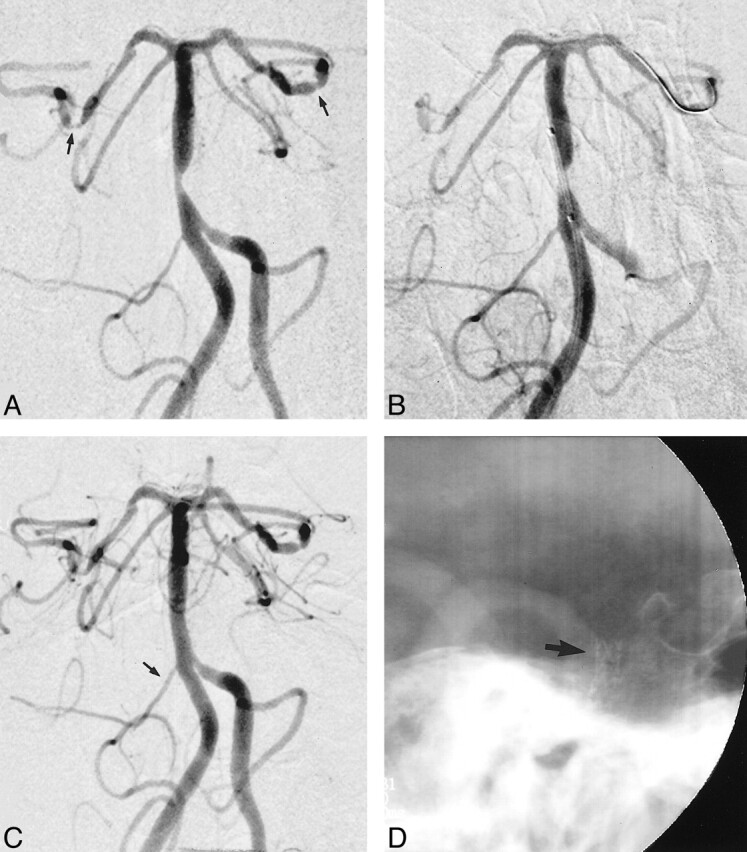
Diagnostic cerebral angiography revealed a high-grade, eccentric, atherosclerotic stenosis (>70%) of the lower basilar artery immediately below the origins of the AICAs. The right PCoA was absent and of normal size on the left. The vertebral arteries were codominant (Fig 2).
fig 2.
A and B, Anteroposterior (A) and lateral (B) views of the basilar artery show an eccentric high-grade (>70%) stenosis of the lower basilar artery projecting from the anterior wall (arrow, B). The AICAs originate immediately above the lesion.
C, Anteroposterior view of the basilar artery shows positioning of the stent across the lesion without preliminary balloon angioplasty. Note that positioning of the stent delivery catheter across the stenosis results in significant flow compromise, confirming the high-grade nature of this lesion.
D and E, Anteroposterior (D) and lateral (E) views of the basilar artery after endovascular stent deployment show restoration of normal vessel lumen. Although not obvious on these images, the superior margin of the stent covers the origins of both AICAs (arrows, D), which, however, continue to opacify normally.
F, Nonsubtracted lateral oblique view of the posterior fossa shows the basilar artery metallic stent. The tip of the stent delivery catheter has been retracted inferiorly with the 0.014-inch exchange guidewire still in place.
Endovascular treatment was performed using an AVE GFX-2 stent with a nominal diameter of 3.5 mm and a length of 8 mm. The stent-deployment balloon was inflated to a pressure of 10 atm (unconstrained diameter of 3.6 mm). The normal midbasilar artery diameter was 3.7 mm. An excellent angiographic result was achieved with complete normalization of the vessel diameter. There were no procedural complications, and the patient was asymptomatic and neurologically intact at the 6-month clinical follow-up examination.
Description of the Technique
In both our cases, oral aspirin, 325 mg/day, was started 3 days before the procedure in addition to the oral clopidogrel, 75 mg/day, the patients were already taking. With the patients under general anesthesia, a 7F groin sheath was inserted into the right common femoral artery, and a full diagnostic cerebral angiogram was obtained, which revealed high-grade, eccentric, atherosclerotic stenosis (>70%) of the lower basilar artery in both cases.
A loading dose of 5000 U heparin was administered intravenously, resulting in an activated clotting time of more than 250 seconds. After this, the patients were maintained on a continuous heparin infusion of 1200 U/h. A 6F guide catheter (Brite Tip, Cordis, Miami Lakes, FL) was placed into a vertebral artery (right and left vertebral arteries for patients 1 and 2, respectively). A 2.3F microcatheter (Rapid Transit Cordis, Miami Lakes, FL) primed with a 0.014-inch guidewire (Transend, Scimed, Maple Grove, MN) was navigated across the basilar artery stenosis under digital roadmap guidance. The tip of the microcatheter was positioned within the P1 segment of the left posterior cerebral artery, and the guidewire was exchanged for a 0.014-inch, 300-cm exchange-length guidewire (Balance, ACS, Redwood City, CA) and the microcatheter removed. The tip of the exchange guidewire was positioned beyond the P2 segment of the left posterior cerebral artery.
An AVE GFX-2 stent was then passed over the guidewire and positioned across the basilar stenosis. An angiographic run was performed to confirm optimum stent positioning. The stent was then deployed by a slow balloon inflation (30 seconds) to 8 atm (nominal diameter). In case 2, a second balloon inflation to 10 atm was performed. An immediate postdeployment angiographic run showed no evidence of residual stenosis, with all previously visible vertebrobasilar branches opacifying normally.
A careful neurologic examination, performed after the patients were awakened from anesthesia, revealed no evidence of deficit. Heparinization was continued overnight with a target activated partial clotting time of 60 to 80 seconds. The groin sheaths were removed immediately after the procedure with the patients fully heparinized using a percutaneous suture delivery device (Perclose, Redwood City, CA). Both patients were monitored for a period of 24 hours in the intensive care unit. Postprocedural recovery was uneventful in both cases, and the patients were discharged home on the third postoperative day on oral aspirin, 325 mg indefinitely, and oral clopidogrel, 75 mg for a period of 6 weeks. Both patients were neurologically stable with no further symptoms of vertebrobasilar ischemia at a mean clinical follow-up period of 6.5 months. Follow-up angiography at 12 months was planned.
Discussion
Patients with stenosis of the basilar artery have a significant risk of disabling stroke. Moufarrij et al (3) reported 44 patients with at least 50% stenosis of the intracranial vertebral (36%) or basilar (64%) artery who were followed up for an average of 6 years, 73% of whom had experienced a previous vertebrobasilar territory stroke or definite vertebrobasilar ischemic symptoms. During follow-up, 18% of patients sustained a stroke (37% of which were fatal), which was 17 times the expected stroke rate for a matched population.
The WASID study represents the most comprehensive investigation to date examining the therapeutic effect of antithrombotic agents for intracranial arterial stenosis. The authors reported on 68 patients with at least 50% stenosis of an intracranial vertebral (n = 31), basilar (n = 28), posterior cerebral (n = 6), or posterior inferior cerebellar (n = 3) artery who were followed up for a median period of 14 months. All patients had experienced a previous transient ischemic attack or stroke in the territory of the stenotic artery and were treated with warfarin (n = 42) or aspirin (n = 12). The stroke rate was highest in patients with basilar artery stenosis, reported as 15.0 and 10.7 per 100 patient-years of follow-up for strokes in any territory or in the corresponding vascular territory, respectively. The authors concluded that “the high rate of stroke in the territory of a severely stenotic vertebral or basilar artery with either antithrombotic agent suggests that adjunctive stroke-preventive therapies (eg, intracranial angioplasty) may be needed for patients with symptomatic high-grade vertebral or basilar artery stenosis” (1).
The prognosis for patients with basilar artery atherothrombotic occlusion is even less favorable, with a greater than 70% mortality rate if untreated (4–6). Even with modern local intraarterial fibrinolytic treatment, death is still a probable outcome for these patients (7–9).
Despite being first reported in 1980 (2), simple balloon angioplasty of the basilar artery has not gained widespread use owing to its significant limitations. The insubstantial muscularis and adventitial layers possessed by the basilar artery result in an elevated risk of vessel perforation during balloon angioplasty that is invariably fatal. Acute basilar occlusion due to elastic recoil, vasospasm, thrombosis, or intimal dissection may occur. Terada et al (10) performed balloon angioplasty in 12 patients with severe basilar (n = 5) or distal vertebral artery (n = 7) stenosis. The degree of intraluminal stenosis decreased from a mean of 84% to a mean of 44% after angioplasty. Four complications (33%) occurred: two intimal dissections resulting in abrupt vessel occlusion and two thromboembolic complications. The periprocedural rate of major stroke or death was 17%. Because of the risk of vessel perforation, particularly with eccentric plaque, some authors have advocated underdilating basilar artery stenotic lesions to no more than one half the normal midbasilar diameter (11, 12). Hence, balloon angioplasty rarely results in normalization of the vessel diameter.
The use of stents for the treatment of intracranial disease has previously been limited by rigid stent and stent delivery catheter designs that are unable to atraumatically maneuver acute vascular bends. The advent of flexible coronary stents has allowed safe and relatively reliable endovascular access of the intracranial arteries by using small-diameter delivery systems. In 1997, Higashida et al (13) reported the first clinical use of a stent (Palmaz-Schatz, Johnson & Johnson Interventional Systems, Warren, NJ) in the basilar artery; however, this was for treatment of a ruptured fusiform aneurysm, with the stent acting as a scaffold to support electrolytically detachable coils. In 1999, Phatouros et al (14) reported the first use of a percutaneous stent (Gianturcot-Roubin II, Cook Cardiology, Bloomington, NJ) to treat a basilar artery atherosclerotic stenosis after failed balloon angioplasty in a patient with acute basilar artery thrombosis. Although the patient eventually died of cardiogenic shock and sepsis, the stent was successful at restoring normal basilar artery flow. Lanzino et al (15) reported the successful use of a coronary stent (AVE GFX) for the treatment of a symptomatic lower basilar artery stenosis after attempted balloon angioplasty was unsuccessful in relieving the stenosis and furthermore resulted in an intraplaque dissection. Similarly, Joseph et al (16) used a stent to successfully treat a symptomatic basilar artery stenosis recalcitrant to balloon angioplasty. Horowitz et al (17) described three patients with symptomatic basilar artery stenosis (n = 2) or occlusion (n = 1) in whom stenting without preceding balloon angioplasty was successfully performed. However, one patient incurred a postprocedural hemiparesis subsequent to a pontine infarct, which resolved over 4 weeks.
In the largest series to date, Gomez et al (18) reported on the use of stents to electively treat 12 patients with symptomatic basilar artery stenosis. All patients either had undergone unsuccessful medical therapy or had contraindications to long-term anticoagulation. Predilatation with balloon angioplasty was used before stent deployment, and technical success was achieved in all cases. One patient incurred postprocedural sixth and seventh nerve pareses, which resolved within 8 weeks. During a mean clinical follow-up period of 5.9 months, one patient experienced a transient ischemic attack prompting cerebral angiography, which revealed a basilar artery occlusion proximal to the stent. This was successfully treated with balloon angioplasty.
Primary stenting is usually defined as the use of a stent intentionally to treat a vascular stenosis rather than as a bail-out measure in the case of failed balloon angioplasty (secondary stenting). However, primary stenting may require predilatation of the lesion with simple balloon angioplasty to allow safe and atraumatic crossing of the lesion by the stent delivery catheter. The AVE GFX-2 is a premounted, balloon-expandable stent requiring the minimal use of a 6F guide catheter. We aimed to closely match the stent diameter to the native basilar artery diameter. Oversizing of the balloon-mounted GFX stent may lead to an increased risk of intimal dissection at the stent margins owing to the phenomenon of “dumb-belling” or “dog-boning” of the balloon, which refers to the propensity for initial enlargement of the uncovered proximal and distal ends of the balloon before expansion of the stent, per se, occurs (19). Since in our cases, the minimum residual lumen measured approximately 1 mm in both patients, we chose to primarily cross the lesion with the stent delivery catheter rather than predilate with balloon angioplasty. There was no difficulty in navigating the stent to and/or across the lesion. Primary stenting has a lower risk of flow-limiting intimal dissection and distal thromboembolism than does simple balloon angioplasty. The risk of vessel perforation may also be reduced by the use of a stent, since the stent may provide additional wall support.
We prefer to initially traverse the lesion with a microcatheter primed with a soft-tip 0.14-inch guidewire (Transend Platinum Tip, Scimed, Maple Grove, MN) rather than an exchange-length 0.14-inch guidewire (Balance, ACS, Redwood City, CA) because of the reduced likelihood of plaque trauma and dislodgment. The stent in case 1 was placed above the level of the AICAs and hence a theoretical risk of covering the ostia of small perforating vessels of the brain stem was present. The magnitude of this risk and of the risk of an associated brain stem stroke is unknown. Experimental evidence in dogs suggests that small, lateral, carotid branches, which approximate intracranial perforating vessels relative to their diameter and angle of origin, remain patent if less than 50% of the ostial diameter is covered by the stent struts (15). In the aforementioned six reports of basilar artery stent placement (13–18), in which 19 patients were treated, one proved case of brain stem infarction possibly related to perforator occlusion by the stent metalwork was encountered. Indeed, the lower margin of the stent in our case 1 at least partially covered the origin of the right AICA, and in case 2, the superior margin of the stent covered the origins of both AICAs. However, all these vessels continued to opacify normally.
The use of adjunctive antithrombotic agents is critical in preventing acute stent thrombosis and maintaining patency, particularly at stent diameters of 4 mm or less. The concurrent use of aspirin and clopidogrel has been shown experimentally to have a synergistic effect on platelet antiaggregation (20, 21). We chose to use intravenous heparin intraprocedurally; however, the intraprocedural administration of platelet glycoprotein IIb/IIIa inhibitors, such as abciximab or eptifibatide, has been shown to decrease mortality and morbidity in coronary stent studies (22–24). Although the prophylactic or bail-out role of such platelet glycoprotein IIb/IIIa inhibitors remains undefined in intracranial stenting procedures, they may be used for the treatment of acute intraprocedural stent thrombosis, since platelet aggregation (white thrombus) represents the primary mechanism (25).
Conclusion
In two patients, primary stenting of high-grade symptomatic basilar artery stenosis refractory to medical therapy was performed without preliminary balloon angioplasty. Both procedures resulted in complete (100%) normalization of the basilar artery lumen without any intra- or periprocedural complications. Although the number of reported cases of stenting for basilar artery stenosis remains small, the advent of flexible stents that require small-diameter delivery systems may result in this being a potential therapeutic alternative to balloon angioplasty in patients with symptoms refractory to medical therapy or in whom anticoagulation is contraindicated. Long-term follow-up assessing the patency rates of these small-diameter intracranial stents is required.
Acknowledgments
We acknowledge the neurovascular neurologists D. Gress, W. S. Smith, and C. Johnstone for their invaluable assistance in the preprocedural clinical evaluation and postprocedural management of these patients.
Footnotes
Address reprint requests to Constantine C. Phatouros, MBBS, FRANZCR, Interventional Neuroradiology Unit, Royal Perth Hospital, GPO Box X2213, Perth, WA, 6001, Australia.
References
- 1. WASID Investigators. Prognosis of patients with symptomatic vertebral or basilar artery stenosis: the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study group. Stroke 1998;29:1389-1392 [DOI] [PubMed] [Google Scholar]
- 2.Sundt TM Jr, Smith HC, Campbell JK, Vlietstra RE, Cucchiara RF, Stanson AW. Transluminal angioplasty for basilar artery stenosis. Mayo Clin Proc 1980;55:673-680 [PubMed] [Google Scholar]
- 3.Moufarrij NA, Little JR, Furlan AJ, Leatherman JR, Williams GW. Basilar and distal vertebral artery stenosis: long-term follow-up. Stroke 1986;17:938-942 [DOI] [PubMed] [Google Scholar]
- 4.Fields WS, Ratinov G, Weibel J, Campos RJ. Survival following basilar artery occlusion. Arch Neurol 1966;15:463-471 [DOI] [PubMed] [Google Scholar]
- 5.Castaigne P, Lhermitte F, Gautier JC, et al. Arterial occlusions in the vertebro-basilar system: a study of 44 patients with post-mortem data. Brain 1973;96:133-154 [DOI] [PubMed] [Google Scholar]
- 6.Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke 1977;8:383-390 [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo GJ. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke 1988;19:1216-1222 [DOI] [PubMed] [Google Scholar]
- 8.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion: variables affecting recanalization and outcome. Stroke 1996;27:875-881 [DOI] [PubMed] [Google Scholar]
- 9.Cross DT III, Moran CJ, Akins PT, Angtuaco EE, Diringer MN. Relationship between clot location and outcome after basilar artery thrombolysis. AJNR Am J Neuroradiol 1997;18:1221-1228 [PMC free article] [PubMed] [Google Scholar]
- 10.Terada T, Higashida RT, Halbach VV, et al. Transluminal angioplasty for arteriosclerotic disease of the distal vertebral and basilar arteries. J Neurol Neurosurg Psychiatry 1996;60:377-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahuja A, Guterman LR, Hopkins LN. Angioplasty for basilar artery atherosclerosis: case report. J Neurosurg 1992;77:941-944 [DOI] [PubMed] [Google Scholar]
- 12.Nakatsuka H, Ueda T, Ohta S, Sakaki S. Successful percutaneous transluminal angioplasty for basilar artery stenosis: technical case report. Neurosurgery 1996;39:161-164 [DOI] [PubMed] [Google Scholar]
- 13.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery: case report and review of the literature. J Neurosurg 1997;87:944-949 [DOI] [PubMed] [Google Scholar]
- 14.Phatouros CC, Higashida RT, Malek AM, et al. Endovascular stenting of an acutely thrombosed basilar artery: technical case report and review of the literature. Neurosurgery 1999;44:667-673 [DOI] [PubMed] [Google Scholar]
- 15.Lanzino G, Fessler RD, Miletich RS, Guterman LR, Hopkins LN. Angioplasty and stenting of basilar artery stenosis: technical case report. Neurosurgery 1999;45:404-407 [DOI] [PubMed] [Google Scholar]
- 16.Joseph GJ, Goldstein J, Cloft H, Tong F, Dion J. Endovascular stenting of atherosclerotic stenosis in a basilar artery after unsuccessful angioplasty. AJR Am J Roentgenol 2000;174:383-385 [DOI] [PubMed] [Google Scholar]
- 17.Horowitz MB, Pride GL, Graybeal DF, Purdy PD. Percutaneous transluminal angioplasty and stenting of midbasilar stenoses: three technical case reports and literature review. Neurosurgery 1999;45:925-931 [DOI] [PubMed] [Google Scholar]
- 18.Gomez CR, Misra VK, Liu MW, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke 2000;31:95-99 [DOI] [PubMed] [Google Scholar]
- 19.Malek AM, Higashida RT, Halbach VV, Phatouros CC, Meyers PM, Dowd CF. Tandem intracranial stent deployment for treatment of an iatrogenic, flow-limiting, basilar artery dissection: technical case report. Neurosurgery 1999;45:919-924 [DOI] [PubMed] [Google Scholar]
- 20.Makkar RR, Eigler NL, Kaul S, et al. Effects of clopidogrel, aspirin and combined therapy in a porcine ex vivo model of high-shear induced stent thrombosis. Eur Heart J 1998;19:1538-1546 [DOI] [PubMed] [Google Scholar]
- 21.Herbert JM, Dol F, Bernat A, Falotico R, Lale A, Savi P. The antiaggregating and antithrombotic activity of clopidogrel is potentiated by aspirin in several experimental models in the rabbit. Thromb Haemost 1998;80:512-518 [PubMed] [Google Scholar]
- 22.Chronos N, Vahanian A, Betriu A, et al. Use of abciximab in interventional cardiology. Eur Heart J 1998;19::D31-D39 [PubMed] [Google Scholar]
- 23. The CAPTURE Investigators. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE study (see comments) [published erratum appears in Lancet 1997;6;350:744]. Lancet 1997;349:1429-1435 [PubMed] [Google Scholar]
- 24. The EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization: the EPILOG investigators (see comments). N Engl J Med 1997;336:1689-1696 [DOI] [PubMed] [Google Scholar]
- 25.Jeong MH, Owen WG, Staab ME, et al. Porcine model of stent thrombosis: platelets are the primary component of acute stent closure. Cathet Cardiovasc Diagn 1996;38:38-43 [DOI] [PubMed] [Google Scholar]