Abstract
BACKGROUND AND PURPOSE: The developing fetal skull base has previously been studied via dissection and low-resolution CT. Most of the central skull base develops from endochondral ossification through an intermediary chondrocranium. We traced the development of the normal fetal skull base by using plain radiography, MR imaging, and CT.
METHODS: Twenty-nine formalin-fixed fetal specimens ranging from 9 to 24 weeks' gestational age were examined with mammographic plain radiography, CT, and MR imaging. Skull base development and ossification were assessed.
RESULTS: The postsphenoid cartilages enclose the pituitary and fuse to form the basisphenoid, from which the sella turcica and the posterior body of the sphenoid bone originate. The presphenoid cartilages will form the anterior body of the sphenoid bone. Portions of the presphenoid cartilage give rise to the mesethmoid cartilage, which forms the central portion of the anterior skull base. Ossification begins in the occipital bone (12 weeks) and progresses anteriorly. The postsphenoid (14 weeks) and then the presphenoid portion (17 weeks) of the sphenoid bone ossify. Ossification is seen laterally (16 weeks) in the orbitosphenoid, which contributes to the lesser wing of the sphenoid, and the alisphenoid (15 weeks), which forms the greater wing.
CONCLUSION: MR imaging can show early progressive ossification of the cartilaginous skull base and its relation to intracranial structures. The study of fetal developmental anatomy may lead to a better understanding of abnormalities of the skull base.
The skull base is a platform at the bottom of the cranium that cradles the brain, provides support and protection, and serves as the entrance and exit for major vascular and neural structures (1–4). Phylogenetically, the cranial base is one of the oldest skeletal components. The human skull base shares similarities with those of our primitive progenitors with smaller, simpler heads. A thorough understanding of fetal growth and development is the key to understanding both the completed normal anatomic product and the abnormal variations (3, 5).
The skull base separates the neurocranium (calvaria) from the facial viscerocranium (jaw and muscles of mastication). The skull base is called the chondrocranium, because most of these bones are preformed in cartilage and ossify by the process of endochondral ossification. Most of the facial bones and calvaria are of membranous origin (6). The calvaria expand rapidly owing to growth of the brain. The skull base, however, is like the keel of a ship, with a central, less-yielding foundation (7). The early-established connection of the cranial nerves, blood vessels, and spinal cord from their origins to their destinations is maintained by the relative stability of the skull base (3). The purpose of this study was to trace the development of the central fetal skull base nondestructively by using imaging.
Methods
Twenty-nine formalin-fixed fetal specimens from the Carnegie Collection of normal fetuses (Carnegie Laboratories of Embryology, University of California, Davis) were examined with mammographic plain radiography, CT, and MR imaging (eight fetuses were examined with plain radiography, 16 with CT, and 27 with MR imaging). Franklin P. Mall established the Carnegie Embryological Collection in 1914. Dr. Mall's successor, George L. Streeter, classified human embryos into stages. The Carnegie Collection forms the basis for the study and understanding of early human development, and has been called the Bureau of Standards for primate embryology (8). We determined gestational age on the basis of an average of the biparietal diameter, crown-rump length, and femoral length. The 29 fetuses ranged in age from 9 to 24.4 gestational weeks. One fetal specimen was dissected to compare anatomic relationships. Radiographs were made using a GE mammographic unit with magnification film screen technique. A GE 9800 CT unit was used to scan the specimens with 1-mm-thick contiguous slices. A Signa 1.5-T clinical unit was used for the MR imaging. Three-dimensional spoiled gradient-echo sequences were obtained with parameters of 40/8/4 (TR/TE/excitations) a 45° flip angle, a 512 × 256 matrix, and a contiguous section thickness of 0.7 mm. Spin-echo T1-weighted sequences had the following parameters: 500/90/3, 512 × 384 matrix, and 3.0-mm-thick sections with a 0.5-mm section gap. Fast spin-echo T2-weighted sequences had imaging parameters of 4000–5000/90–96/3–4, 512 × 384 matrix, 3.0-mm-thick sections with a 0.5-mm section gap, and an echo train length of 12. Skull base development and ossification were assessed.
Results
The T2-weighted fast spin-echo sequences produced the best-quality images, differentiating the intermediate signal intensity of unossified cartilage from the low-signal intensity of bone. At 11 weeks 5 days (Fig 1), the entire skull base is preformed in cartilage before it undergoes ossification. The pituitary is contained in the cartilaginous sella turcica, and the cerebral hemispheres are small and have not expanded to cover the midbrain and hindbrain. At 12 weeks 4 days, the first ossification was noted on T2-weighted MR images as a signal void in the supraoccipital and basioccipital portions of the occipital bone. The occipital bone is formed from the union of four primary cartilaginous centers that encircle the foramen magnum. In our study, the initial appearance of ossification in the lateral exoccipital centers was at 15 weeks 1 day. The pituitary is contained in the cartilaginous sella of the basisphenoid. The optic nerve is superior to the chiasmatic sulcus of the presphenoid, and the chiasmatic sulcus is posterior to the planum, defined posteriorly by the tuberculum sellae and anteriorly by the limbus (Figs 2 and 3).
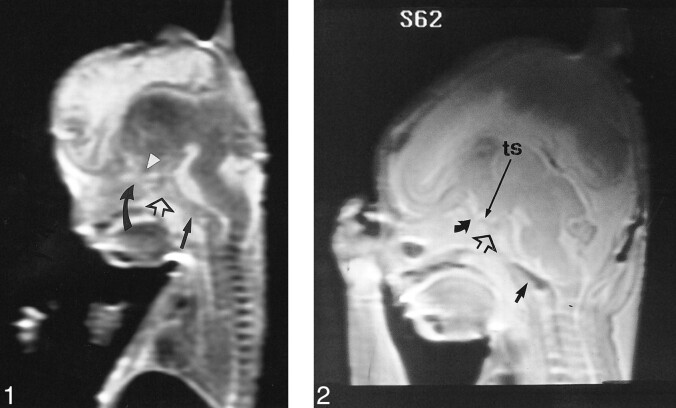
fig 1. T2-weighted fast spin-echo MR image (4000/90/4) of fetal specimen with a gestational age of 11 weeks 5 days shows that the skull base is formed in cartilage before ossification. The basiocciput (straight solid arrow), the postsphenoid (open arrow), containing the pituitary in the cartilaginous sella turcica, and the presphenoid (curved arrow) are seen. Note the optic chiasm (arrowhead). The cerebral hemispheres and anterior third ventricle are derived from the telencephalon. The cerebral hemispheres consist of lateral diverticula that have not yet expanded to cover the brain stem. Note ossification of palate and mandible. fig 2. T2-weighted fast spin-echo MR image (500/90/3) of a fetal specimen with a gestational age of 14 weeks 4 days shows ossification as low signal in the supraoccipital and basioccipital (short straight arrow) centers of the occipital bone. The cartilaginous sella turcica contains the pituitary (open arrow). Note the optic chiasm superior to the chiasmatic sulcus (curved arrow) of the presphenoid. The chiasmatic sulcus is delineated anteriorly by the limbus and posteriorly by the tuberculum sellae (ts)
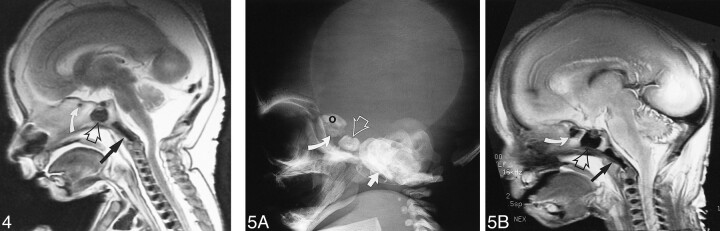
Ossification progresses from posterior to anterior, and the earliest postsphenoid ossification appears around the sella turcica at 13 weeks 5 days. The cerebral hemispheres have greatly enlarged, and the expanding brain is the stimulus for growth of the calvaria (Fig 3). The earliest ossification in the presphenoid is found in the region of the chiasmatic sulcus (Fig 4). Presphenoid ossification was first observed in our study at gestational age 17 weeks 4 days. At 24 weeks 4 days (Fig 5), progressive ossification of the basiocciput, basisphenoid, and presphenoid has occurred. The development of the cerebral hemispheres dominates the morphologic appearance of the head.
fig 3.
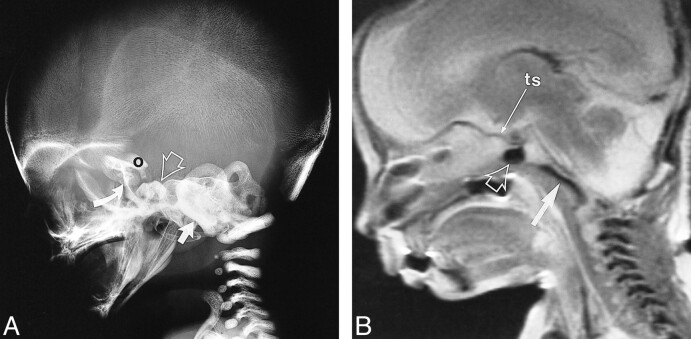
A and B, Lateral skull radiograph of a fetal specimen with a gestational age of 19 weeks 5 days (A) and T2-weighted fast spin-echo MR image (4500/90/4) of a fetal specimen with a gestational age of 19 weeks 3 days (B) show progressive ossification of the skull base from posterior to anterior. The ossification has extended to the postsphenoid (open arrow), which encloses the pituitary to form the sella turcica. The cerebral hemispheres have greatly expanded. Note ossification of presphenoid (curved arrow) on lateral radiograph (arrow, basiocciput; O, orbitosphenoid; ts, tuberculum sellae)
fig 3.
fig 4. T2-weighted fast spin-echo MR image (4500/87/4) of a fetal specimen with a gestational age of 20 weeks 5 days shows progressive skull base ossification that has extended from the basiocciput (straight arrow) to the postsphenoid (open arrow) to the presphenoid (curved arrow) in the area of the chiasmatic sulcus. fig 5. A and B, Lateral skull radiograph of a fetal specimen with a gestational age of 22 weeks 3 days (A) and T2-weighted fast spin-echo MR image (4000/90/3) of a fetal specimen with a gestational age of 24 weeks 4 days (B) show advancing ossification of the basiocciput (arrow), basisphenoid (postsphenoid, open arrow), and presphenoid (curved arrow). Most of the growth of the head is due to the dominant development of the cerebral hemispheres (O, orbitosphenoid)
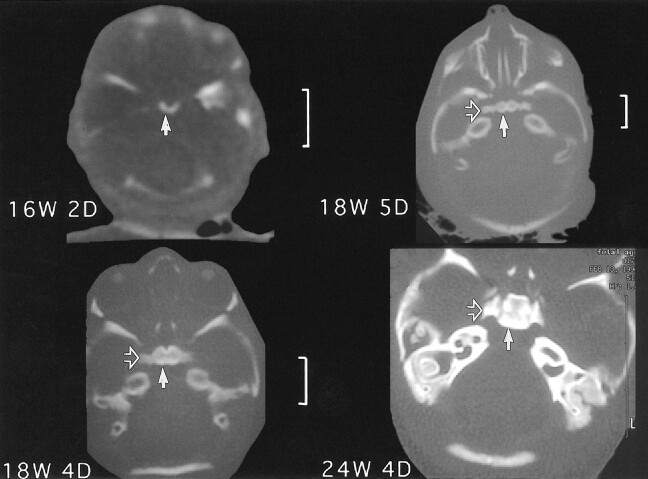
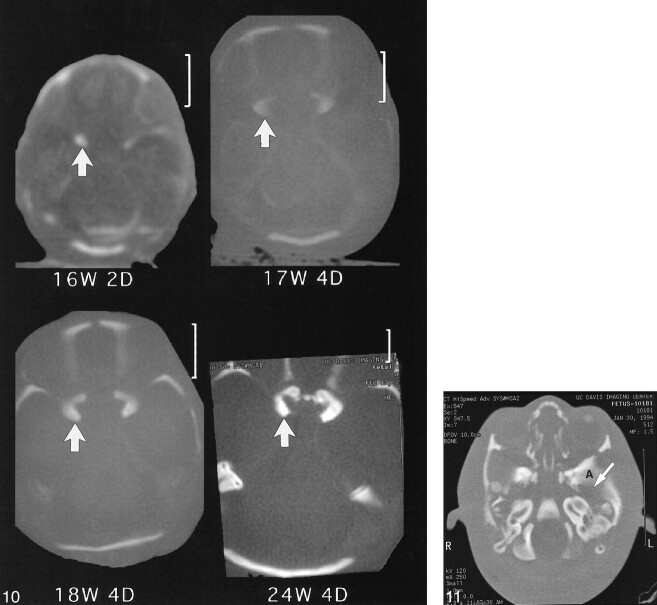
The MR images also showed that the anterior portion of the basioccipital cartilage ossifies earlier than the posterior part (Figs 3–5). Figure 6 shows progressive ossification of the postsphenoid in fetuses from 16 through 24 weeks. The two medial centers ossify first, followed by the lateral centers. The medial centers surround the pituitary to form the sella turcica. The medial centers usually fuse early in fetal life or may persist as two ossification centers (Fig 7). In Figure 6, there is greater ossification in the medial postsphenoid centers in the fetal specimen of 18 weeks 4 days than in the older specimen of 18 weeks 5 days. Ossification always progresses in an orderly pattern, but not strictly according to gestational age.
fig 6.
CT scans at 16 weeks 2 days, 18 weeks 4 days, 18 weeks 5 days, and 24 weeks 4 days show progressive ossification of the postsphenoid. Note that the medial ossification centers (solid arrows) appear first followed by ossification of the lateral centers (open arrows)
fig 6.
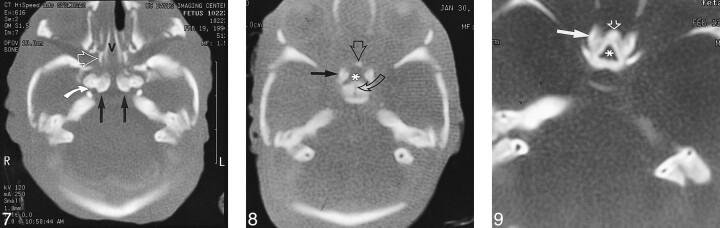
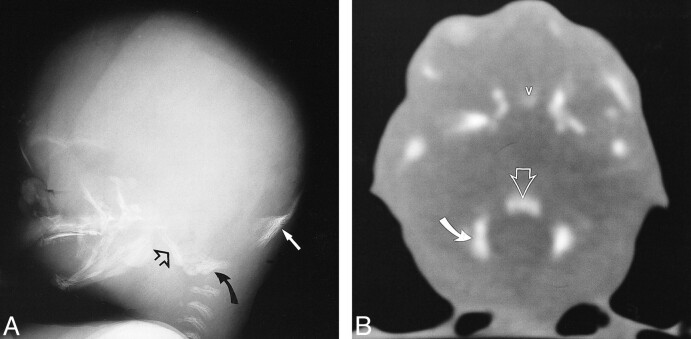
fig 7. CT scan of fetal specimen with a gestational age of 23 weeks 3 days shows persistence of the medial ossification centers of the postsphenoid as separate structures (straight solid arrows). Note the bones of Bertin (open arrow) adjacent to the vomer (v) and rostrum of the basisphenoid. This is the site of sphenoid pneumatization. Note also carotid groove in sphenoid (curved arrow). fig 8. CT scan of fetal specimen with a gestational age of 21 weeks 4 days shows early ossification of the main centers (solid arrow) and corporal middle centers (open straight arrow) of the presphenoid. Note small defect that represents the remnant of the obliterated craniopharyngeal canal (Rathke's pouch) in the postsphenoid (curved arrow). Asterisk indicates olivary eminence. fig 9. CT scan of fetal specimen with a gestational age of 24 weeks 4 days shows progressive ossification and fusion of the main (solid arrow) and corporal middle (open arrow) portions of the presphenoid. Note the olivary eminence (asterisk), which is a triangular area filled with unossified cartilage in the presphenoid
The presphenoid, which forms the body of the sphenoid anterior to the tuberculum sellae, shows progressive ossification forming at 21 weeks 4 days (Fig 8) and at 24 weeks 4 days (Fig 9). Figure 10 shows increasing ossification of the orbitosphenoid, which contributes to the formation of the anterior clinoids and lesser wing of the sphenoid in fetal specimens from the initial appearance at 16 weeks 2 days to 24 weeks 4 days. The alisphenoid will form the greater wings of the sphenoid bone, and ossification appeared at 15 weeks in our study. Figure 11 shows the greater wing of the sphenoid at 22 weeks 3 days. The foramen ovale and spinosum are not yet seen as discrete openings but are contained in an area of unossified cartilage.
fig 6.
fig 10. CT scans show progressive ossification of the orbitosphenoid (arrow) at 16 weeks 2 days, 17 weeks 4 days, 18 weeks 4 days, and 24 weeks 4 days. fig 11. CT scan of fetal specimen with a gestational age of 22 weeks 3 days shows ossification of alisphenoid (A), which forms the greater wing of the sphenoid. Foramen ovale and spinosum are seen as a large defect in the greater wing of the sphenoid (arrow)
Discussion
The skull base originates predominately from cartilaginous precursors with a small contribution from membranous bone (9). The components of the skull base are derived from neural crest cells and mesoderm during the fourth week of fetal life to form the cartilaginous and bony components of the cranial base (5). Mesoderm surrounding the notochord (paraxial mesoderm) plus neural crest cells located between the cranial part of the neural tube and the foregut consolidate to form a basal condensation called the desmocranium at 4 weeks' gestational age (5, 10). By 8 weeks' gestational age, the mesenchyme of the desmocranium has been almost totally replaced by chondrocranium (3, 10).
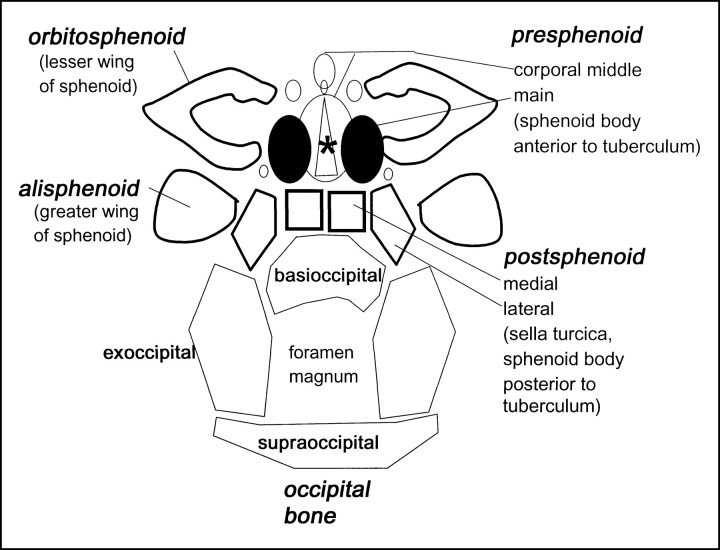
The chondrocranium forms from three pairs of central cartilaginous precursors, with contribution from lateral centers. The parachordal and prechordal formations are defined by their relationship with the notochord, which terminates posterior to the hypophysis. The parachordal formation develops on each side of the notochord. The prechordal formation is anterior to the notochord. The hypophyseal (postsphenoid) centers surround the pituitary (1, 3, 10). The lateral centers include the orbitosphenoid, which surrounds the optic nerve, and the alisphenoid, which encompasses the maxillary and mandibular divisions of the trigeminal nerve (Fig 12). The skull base is formed from the basal part of the occipital and sphenoid bones, the ethmoid bone, and the petrous part of the temporal bone (11). Our study was limited to the occipital and sphenoid bones.
fig 12.
Diagram of the skull base ossification centers. Asterisk indicates olivary eminence (anterior foramen)
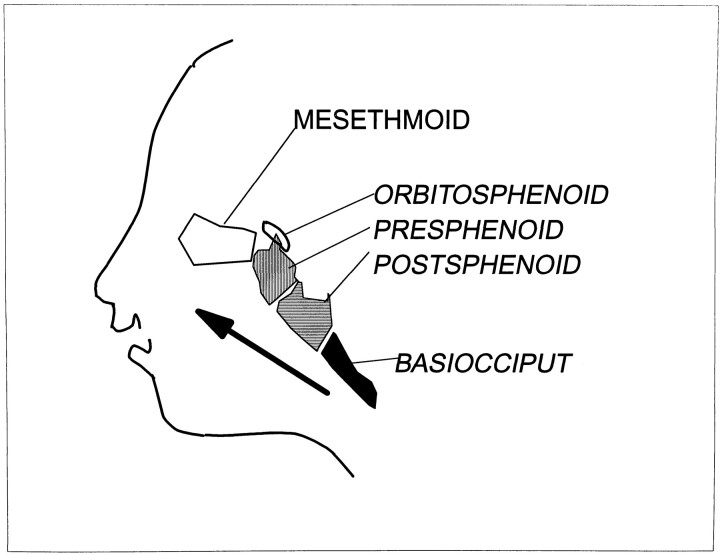
One hundred ten ossification centers have been identified in the fetal skull (5). The numerous centers of ossification and the multiplicity of terms describing them lead to confusion. Table 1 lists the major centers, their synonyms, and derivatives. The earliest ossification has been reported at 8 weeks in the frontal, parietal, occipital, squamous, temporal, zygomatic, mandible, maxilla, vomer, and palatine bones. These bones are derived from membrane (5, 7). Ossification of the skull base progresses in an orderly pattern from posterior to anterior (Fig 13 and Table 2). Ossification begins in the occipital bone (12 weeks 4 days) then proceeds to the postsphenoid (13 weeks 5 days) and presphenoid (17 weeks 4 days) portions of the sphenoid bone, and finally to the ethmoid bone (5, 12). The appearance and extent of ossification do not always correspond exactly with gestational age. Ossification may appear earlier and progress more rapidly in some fetuses (Fig 6), but the pattern always follows a well-defined sequence (13).
TABLE 1:
Skull base ossification
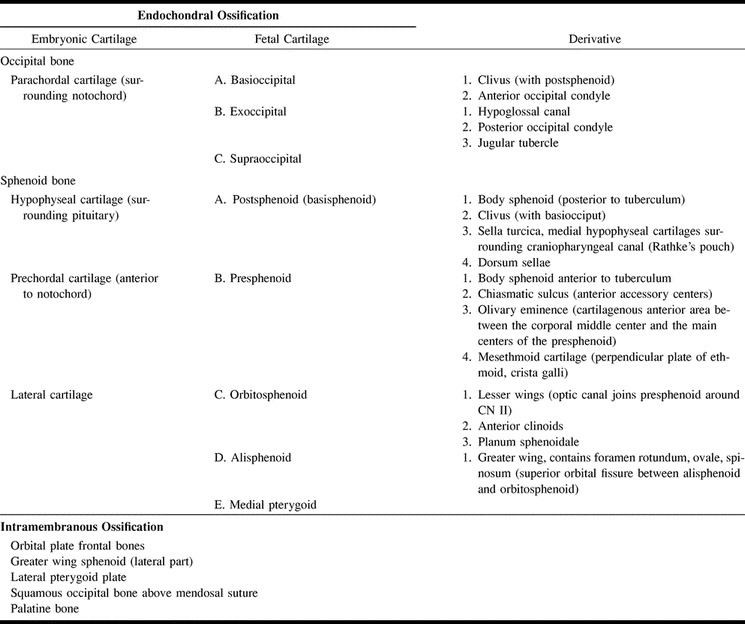
fig 13.
Diagram of progression of skull base ossification. Arrow indicates normal sequence of ossification of the skull base from posterior to anterior
TABLE 2:
Milestones in skull base development and corresponding imaging findings
The embryonic chondrification centers forming around the cranial end of the notochord are called parachordal (basioccipital) cartilages (3). Incorporation of these, plus the four fused occipital sclerotomes, are the forerunners of the basiocciput and the occipital condyles (3). The occipital bone is formed from the union of four primary cartilaginous centers that encircle the spinal cord to form the foramen magnum (Figs 12 and 14). These centers are the paired lateral exoccipital segments: the posterior squamous and the anterior basilar portion of the occipital bone, or basiocciput (14).
The sphenoid bone consists of the body (formed by the presphenoid and postsphenoid centers, with a contribution from the medial crus of the orbitosphenoid), the paired lesser wings (orbitosphenoids), and the greater wings (alisphenoids) (14–16). The hypophyseal (postsphenoid, basisphenoid) cartilages develop around the pituitary. The postsphenoid consists of two medial and two lateral ossification centers. The medial centers ossify first (Fig 6) and enclose the pituitary to create the sella turcica. The medial centers usually fuse to form a single ossification center (Fig 6). The two medial centers may not immediately coalesce (Fig 7), which may be associated with variable timing in the disappearance of the craniopharyngeal canal (17, 18). The postsphenoid becomes the dorsum sellae, and forms the body of the sphenoid posterior to the tuberculum. The bones of Bertin (Fig 7) are two paired ossification centers adjacent to the vomer, which enclose the unossified rostrum of the basisphenoid. This is the first site of sphenoid pneumatization (9, 19). The basisphenoid unites with the basiocciput at the sphenooccipital synchondrosis to form the clivus (14). The sphenooccipital synchondrosis is primarily responsible for postnatal growth of the skull base (15). The sphenooccipital synchondrosis fuses at 12 to 14 years in girls and at 13.5 to 15 years in boys (5, 14). A shallow groove is noted on the anterior aspect of the fused medial postsphenoid centers, which represents the obliterated craniopharyngeal canal (Fig 8). This canal marks the intersphenoid synchondrosis at the border of the tuberculum sellae.
The prechordal (presphenoid) cartilages are found just anterior to the notochord and are the precursor to the anterior body of the sphenoid bone (3). The presphenoid forms the sphenoid body anterior to the tuberculum sellae and the chiasmatic sulcus. Up to 19 separate ossification centers have been described (15) but only four were identified in the fetal specimens in this study. The lateral main centers ossify before the corporal middle centers (7, 20). Progressive fusion of the presphenoid centers creates a triangular area called the olivary eminence, or the anterior foramen, which is a triangular area filled with unossified cartilage (21) (Figs 8, 9, and 12). Since neither nerves nor vessels pass though this structure, the designation foramen is a misnomer. The olivary eminence is usually obliterated around the time of birth, and a small residual foramen in the newborn should not be mistaken for a sinus tract or encephalocele (22). The tuberculum sellae delineates the junction between the pre- and postsphenoid bones (7). Portions of the presphenoid cartilage give rise to the mesethmoid cartilage, which forms the central portion of the anterior skull base, including the perpendicular plate of the ethmoid bone and the crista galli (3, 23, 24). There was no ethmoid ossification in our specimens. The anterior skull base remains largely cartilaginous at birth (23). Like the otic capsule, the skull base is formed in cartilage before ossification begins (12). Unlike the otic capsule, however, ossification begins before the structure has attained adult size (25). In this study, MR imaging had the advantage of revealing not only the onset and progression of ossification but also the relationship between the skull base and adjacent soft-tissue structures.
At 3 weeks, the development of the pituitary begins. The anterior lobe of the pituitary (adenohypophysis) originates as a diverticulum from surface endoderm of the pharynx immediately anterior to the bucopharyngeal membrane. This endodermally lined invagination from the roof of the stomodeum is called Rathke's pouch (1, 7, 12). The posterior pituitary (neurohypophysis) is a downward extension of neuroectoderm derived from the infundibular process of the diencephalon. By 5 weeks, the invaginated endodermally derived adenohypophysis has lost its connection with the pharynx through Rathke's pouch (12). The MR image at 11 weeks 5 days (Fig 1) shows pituitary gland contained in the cartilaginous sella turcica. The attachment with Rathke's pouch and the fetal oral cavity have long been lost. The MR images also show that the anterior part of the basioccipital cartilage ossifies earlier than the posterior portion (Figs 3–5). The various holes and foramina of the skull base are created around preexisting structures. Therefore, the surrounding mesenchyme is induced to form cartilage around the structures it finds, namely, the spinal cord, vessels, and cranial nerves that have already formed.
Laterally, the orbitosphenoid forms the anterior clinoid and the lesser wing of the sphenoid (Figs 10 and 12). This center encloses the optic nerve, forming the optic canal (14). The alisphenoid contributes to the greater wing of the sphenoid. The foramen ovale and spinosum had not yet formed in our specimens and are seen as a dehiscence in the greater wing of the sphenoid (Fig 11). The space between the orbitosphenoid and alisphenoid creates the superior orbital fissure for the passage of cranial nerves III (oculomotor), IV (trochlear), VI (abducens), and V1 (ophthalmic division of the trigeminal nerve). The alisphenoid contains the foramen rotundum, ovale, and spinosum.
Conclusion
This study traces the development of the skull base using high-resolution CT, MR imaging, and plain radiography. The central skull base is preformed in cartilage and ossifies from posterior to anterior. Development of the skull base begins only after the spinal cord, cranial nerves, and blood vessels have formed. The cranial base is relatively stable during development as compared with the rapid growth and expansion of the calvaria. Study of fetal developmental anatomy may lead to a better understanding of congenital skull base disorders.
fig 14.
A and B, Lateral radiograph (A) and CT scan (B) of fetal specimen with a gestational age of 16 weeks 2 days show early ossification of the four primary centers of the occipital bone that surrounds the foramen magnum: supraoccipital (solid arrow), basioccipital (open arrows), and exoccipital (curved arrows). Note ossification of the frontal bone, vomer (v), pterygoid plates, zygoma, mandible, and maxilla
Acknowledgments
We thank Professor Ronan O'Rahilly and the Carnegie Laboratories of Embryology for supplying the normal fetal specimens and Kathy Sommers for invaluable help in preparing this manuscript.
Footnotes
Address reprint requests to William R. Nemzek, MD, Department of Radiology, University of California, Davis Medical Center, 4860 Y St, Suite 3100, Sacramento, CA 95817.
References
- 1.Larsen WJ. Human Embryology.. New York: Churchill Livingstone 1993;
- 2.Janecka IP. Introduction. In: Janecka IP, ed. Tiedemann, K. Philadelphia: Lippincott-Raven 1997;3-15
- 3.Sperber GH. Dental Handbooks, No 15: Craniofacial Embryology.. Boston: Wright 1989;
- 4.Curtin HD, Chavali R. Imaging of the skull base. Radiol Clin North Am 1998;36:801-817 [DOI] [PubMed] [Google Scholar]
- 5.Ricciardelli EJ. Embryology and anatomy of the cranial base. Clin Plast Surg 1995;22:361-372 [PubMed] [Google Scholar]
- 6.Stool S, Post J. Cranial growth, development and malformations: phylogenetic aspects and embryology. In: Bluestone C, Stool S, Kenna M, eds. Pediatric Otolaryngology. 3rd ed. Philadelphia: Saunders 1996;1:1-18 [Google Scholar]
- 7.Virapongse C, Sarwar M. Development of the skull. In: Newton TH, Hasso AN, Dillon WP, eds. Modern Neuroradiology, III: Computed Tomography of the Head and Neck. New York: Raven 1988;113-121
- 8.O'Rahilly R. Early human development and the chief sources of information on staged human embryos. Eur J Obstet Gynecol Reprod Biol 1979;9:273-280 [DOI] [PubMed] [Google Scholar]
- 9.Curtin HD, Som PM, Braun IF, Nadel L. Skull Base. In: Som PM, Curtin HD, eds. Head and Neck Imaging. St Louis: Mosby-Year Book 1996;2:1233-1299 [Google Scholar]
- 10.Gasser RF. Early formation of the basicranium in man. In: Bosma J, ed. Symposium on the Development of the Basicranium. Publication no. (NIH) 76–989. Bethesda, MD: U.S. Department of Health, Education, and Welfare 1976:29-43 [Google Scholar]
- 11.Stool S, Vig K, Petrone J. Postnatal craniofacial growth and development. In: Bluestone C, Stool S, Kenna M, eds. Pediatric Otolaryngology. 3rd ed. Philadelphia: Saunders 1996;1:19-32 [Google Scholar]
- 12.Kjaer I, Fischer-Hansen B. The adenohypophysis and the cranial base in early human development. J Craniofac Genet Dev Biol 1995;15:157-161 [PubMed] [Google Scholar]
- 13.Bach-Peterson S, Kjaer I. Ossification of lateral components in the prenatal cranial base. J Craniofac Genet Dev Biol 1993;13:76-82 [PubMed] [Google Scholar]
- 14.Madeline LA, Elster AD. Suture closure in the human chondrocranium: CT assessment. Radiology 1995;196:747-756 [DOI] [PubMed] [Google Scholar]
- 15.Laine FJ, Nadel L, Braun IF. CT and MR of the central skull base, 1: techniques, embryologic development, and anatomy. Radiographics 1990;10:591-602 [DOI] [PubMed] [Google Scholar]
- 16.Kodama G. Developmental studies on the orbitosphenoid of the human sphenoid bone. In: Bosma J, ed. Symposium on the Development of the Basicranium. Publication no. (NIH) 76–989. Bethesda, MD: U.S. Department of Health, Education, and Welfare 1976:166-176 [Google Scholar]
- 17.Kjaer I. Radiographic determination of prenatal basicranial ossification. J Craniofac Genet Dev Biol 1990;10:113-123 [PubMed] [Google Scholar]
- 18.Sasaki H, Kodama G. Developmental studies on the postsphenoid of the human sphenoid bone. In: Bosma J, ed. Symposium on the Development of the Basicranium. Publication no. (NIH) 76–989. Bethesda, MD: U.S. Department of Health, Education, and Welfare 1976:177-191 [Google Scholar]
- 19.Bosma J. Introduction to the symposium on development of the basicranium. In: Symposium on the Development of the Basicranium. Publication no. (NIH) 76–989. Bethesda, MD: U.S. Department of Health, Education, and Welfare 1976:3-43 [Google Scholar]
- 20.Kodama G. Developmental studies on the presphenoid of the human sphenoid bone. In: Bosma J, ed. Symposium on the Development of the Basicranium. Publication no. (NIH) 76–989. Bethesda, MD: U.S. Department of Health, Education, and Welfare 1976:141-155 [Google Scholar]
- 21.Kier E, Rothman SLG. Radiologically significant anatomic variations of the developing sphenoid in humans. In: Bosma J, ed. Symposium on the Development of the Basicranium. Publication no. (NIH) 76–989. Bethesda, MD: U.S. Department of Health, Education, and Welfare 1976:107-113 [Google Scholar]
- 22.Madeline L, Elster AD. Postnatal development of the central skull base: normal variants. Radiology 1995;196:757-763 [DOI] [PubMed] [Google Scholar]
- 23.Belden C, Mancuso A, Kotzur I. The developing anterior skull base: CT appearance from birth to 2 years of age. AJNR Am J Neuroradiol 1997;18:811-818 [PMC free article] [PubMed] [Google Scholar]
- 24.Scott J. The cartilage of the nasal septum (a contribution to the study of facial growth). Br Dent J 1953;95:37-43 [Google Scholar]
- 25.Nemzek WR, Brodie HA, Chong BW, et al. Imaging findings of the developing temporal bone in fetal specimens. AJNR Am J Neuroradiol 1996;17:1467-1477 [PMC free article] [PubMed] [Google Scholar]