Abstract
Summary: We describe a 35-year-old man with a history of remote closed head injury who presented with new neurologic deficits. A noncontrast head CT scan showed hyperattenuation involving both temporal lobes, frontal lobes, basal meninges, and cerebellum. A subsequent contrast-enhanced MR examination of the brain showed enhancement in the basal cisterns extending into the upper cervical spine and throughout the spinal canal. Gross, histologic, and immunohistochemical analysis revealed meningeal melanocytoma.
Primary pigmented tumors of the leptomeninges are an uncommon and varied group of entities, and include pigmented meningioma, malignant melanoma, meningeal melanocytoma, melanotic schwannoma, and melanoblastosis. The differential diagnosis is often confusing owing to their similar appearance on CT and MR studies, thus necessitating additional diagnostic confirmation with electron microscopy and immunohistochemical analysis. Furthermore, since the biological behavior, treatment, and prognosis of these lesions is different, it is important to make the correct pathologic diagnosis. A review of the literature through 1998 revealed approximately 100 reported cases of meningeal melanocytoma (1–8). We present an additional and uncommon case of meningeal melanocytoma with concomitant involvement of the intracranial and intraspinal meninges.
Case Report
A 35-year-old man with a medical history significant for a severe closed head injury sustained approximately 11 years earlier presented with cortical blindness and ventriculomegaly requiring ventriculoperitoneal shunt placement with multiple revisions. The exact pathogenesis of the blindness and ventriculomegaly was not known, as the patient had been transferred to our hospital from an outside institution. However, his medical records indicated that he had continued to have persistent headaches and seizures pursuant to the earlier trauma, despite treatment, and that he had been admitted to the hospital on numerous occasions. Multiple CT scans of the head and all laboratory analyses were unremarkable. Multiple lumbar punctures revealed no abnormality except for mildly elevated opening CSF pressure, ranging from 30 to 40 cm of water.
Approximately 1 year later, the patient was readmitted to our hospital with another episode of recurrent headache and numerous neurologic changes, including subjective right-sided numbness, slight pronator drift of the right upper extremity, a pronounced expressive aphasia, and perseveration of both speech and movement. Physical examination was unremarkable except for the previously described expressive aphasia and cortical blindness. Specifically, examination of the skin revealed no large or irregular pigmented intradermal nevi. A noncontrast head CT scan showed areas of hyperattenuation, involving both temporal lobes, frontal lobes, basal meninges, and cerebellum (Fig 1A), which had not been present on a noncontrast head CT study obtained approximately 4 years earlier (Fig 1B). The exact pathogenesis of the previously described areas of hyperattenuation was uncertain at the time. A subsequent MR examination of the brain with and without contrast material showed enhancement in the basal cisterns with extension into the upper cervical spine, but no indication of hydrocephalus (Fig 1C and D). The intraparenchymal enhancing lesions were seen extending into both temporal lobes on T1-weighted contrast-enhanced MR images (Fig 1E). Corresponding axial fast spin-echo (FSE) T2-weighted images showed heterogeneous signal in the parasellar area with areas of decreased signal intensity associated with melanin deposition (Fig 1F). A contrast-enhanced MR study of the entire spine, performed to evaluate the extent of disease, showed extensive intradural enhancement throughout the spinal canal (Fig 1G–M). These findings were most prominent from C4 to T1, where an intraspinal mass was causing marked cord compression.
fig 1.
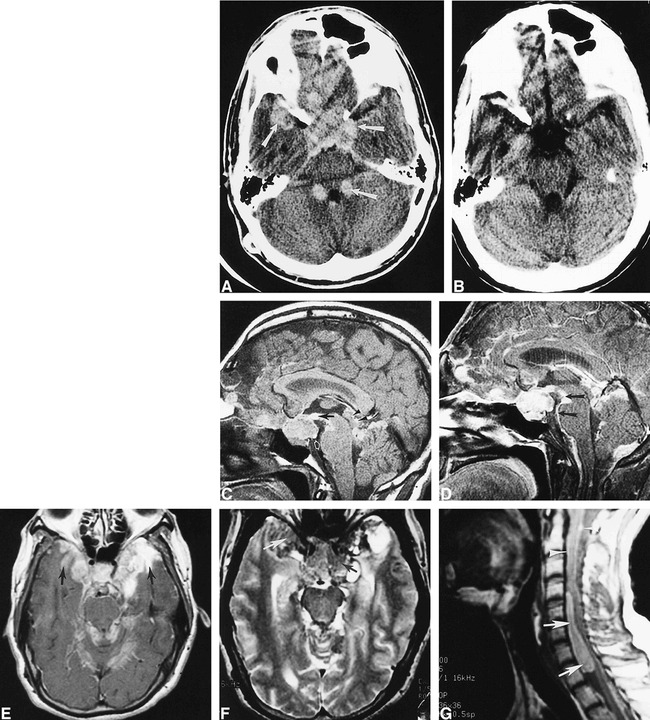
35-year-old man with a history of remote closed head injury who presented with recurrent headache, cortical blindness, and numerous new neurologic deficits.
A, Axial noncontrast CT scan shows increased attenuation of the right temporal lobe, frontal lobes, suprasellar cistern, cerebellum, basal cisterns, dentate nuclei, and an area adjacent to the fourth ventricle (arrows).
B, Axial noncontrast CT scan obtained 4 years earlier shows no areas of increased attenuation in the parasellar regions.
C, Sagittal noncontrast T1-weighted MR image depicts a suprasellar mass along with areas of increased signal in the tuber cinereum (black arrow) and tectal region (arrowheads), consistent with T1 shortening from melanin. Note the subtle area of increased signal in the pons (white arrow).
D, Contrast-enhanced MR image shows marked basal cistern involvement (arrows).
E, Axial contrast-enhanced T1-weighted MR image of the brain shows enhancement of the basal meninges and intraparenchymal lesions of both temporal lobes (arrows).
F, Corresponding axial FSE T2-weighted MR image shows heterogeneous signal intensity in the parasellar region with decreased signal intensity from areas of melanin (arrows).
G, Sagittal noncontrast T1-weighted MR image reveals a large, hyperintense, oblong, anterior intraspinal mass (arrows). Note subtle areas of increased signal intensity surrounding the adjacent spinal cord anteriorly and posteriorly (arrowheads).
The possibility was entertained that the right temporal lobe intraparenchymal lesion could have resulted from rupture of a right middle cerebral artery aneurysm. Two cerebral angiograms obtained over a 2-week period showed no evidence of an aneurysm or arteriovenous fistula. The possibility of meningeal carcinomatosis, meningioma, and chronic granulomatous disease were considered in the differential diagnosis. Leptomeningeal carcinomatosis was considered unlikely in the absence of newly identified or prior malignant lesions.
An elective right pterional craniotomy was performed, and included exploration, microdissection, biopsy, and partial tumor debulking. Surgical specimens consisting of several leptomeningeal-based fragments of soft brown tissue were submitted for frozen section. No characteristic features of any particular type of meningeal tumor were noted on examination of the gross specimen. Light microscopic examination showed the typical appearance of a pigmented lesion (Fig 1N). Immunohistochemical stains were positive for S-100 protein, HMB-45, and vimentin, suggesting a pigmented neoplasm of melanocytic origin (Fig 1O and P). The patient elected to seek a second opinion and possible treatment at an international cancer center and received no further treatment at our institution.
Discussion
The differential diagnosis of primary pigmented lesions of the leptomeninges includes pigmented meningioma, metastatic malignant melanoma, primary malignant melanoma, meningeal melanocytoma, melanotic schwannoma, and melanoblastosis (4, 6). Primary melanocytic neoplasms are rare lesions arising from normally occurring leptomeningeal melanocytes (9). Current embryologic evidence suggests a common origin of melanocytes originating from the neural crest elements normally found within the basal layer of the epidermis and the leptomeninges covering the base of the brain and the brain stem (8, 10, 11). Consequently, the areas most commonly involved are the pons, cerebellum, cerebral peduncles, medulla, interpeduncular fossa, and inferior surfaces of the frontal, temporal, and occipital lobes (10, 12). These neoplasms are generally divided into three main types, including diffuse melanosis, meningeal melanocytoma, and primary malignant melanoma.
Diffuse melanosis occurs more frequently in children and may be associated with congenital intradermal benign pigmented nevi in a rare nonfamilial disorder known as neurocutaneous melanosis (8, 11, 13). The diagnostic criteria described by Fox et al (14) require that the abnormal skin pigmentation consisting of giant or multiple pigmented nevi must occur in the absence of malignant change in the skin lesion and in the absence of malignant melanoma in all other organ systems except for the leptomeninges. However, Leaney et al (11) have stated that the frequency of malignant degeneration of skin nevi ranges from 2% to 13%. Additionally, a case of distant organ metastases from leptomeningeal melanoma has been reported by Kaplan et al (13). Current evidence indicates that neurocutaneous melanosis is actually a phakomatosis caused by congenital dysplasia of the neuroectodermal melanocyte precursors, resulting in excessive (focal or diffuse) proliferation of melanin-producing cells in the skin and leptomeninges.
This pattern of leptomeningeal involvement is unlike metastatic melanoma, which typically produces focal pigmented lesions of the leptomeninges. The dura mater is usually not involved, while involvement of the ventricular ependyma, choroid plexus, and cerebral parenchyma has been reported (13).
Meningeal melanocytoma and primary malignant melanoma of the leptomeninges are similar in their origin from leptomeningeal melanocytes, but actually represent both ends of the spectrum, ranging from a lesion that is benign in appearance and behavior to one that is malignant. However, neither of these entities is associated with pigmented lesions elsewhere, including benign congenital pigmented nevi or frank cutaneous malignant melanoma. Meningeal melanocytomas have a much better prognosis than their malignant counterparts. A variety of neurologic and clinical features may be seen with meningeal melanocytoma, including the frequent occurrence of hydrocephalus. Hydrocephalus is usually treated with placement of a ventriculoperitoneal shunt, but a filter must be added to the apparatus to prevent spread in the rare event of malignant transformation (10). Other clinical features include seizures, chronic basal meningitis, multiple cranial nerve palsies, chronic spinal arachnoiditis, psychiatric disturbances, stillbirth, intracranial hemorrhage of the meninges or subdural space, myelopathy, and radiculopathy (10, 13).
Considering the good postoperative survival rate of patients with meningeal melanocytoma, the surgeon should be advised of this possible diagnosis in suspected cases of meningioma, especially when it involves the posterior fossa or Meckel's cave (4). Accordingly, a maximal effort at tumor resection should be made, with expectations of a good postoperative outcome. However, preoperative diagnosis of meningeal melanocytoma is often difficult, since the gross pathologic, histologic, and radiologic features of the many varied meningeal lesions are not definitive. Fortunately, the MR imaging appearance may often suggest a diagnosis of a melanocytic leptomeningeal process by the characteristic shortening of T1 and T2 relaxation times. Additionally, the ultrastructural and immunohistochemical characteristics of these varied lesions are unique.
The gross appearance of meningeal melanocytoma as seen during surgery or at autopsy is that of a well-encapsulated, nodular, dark brown or black lesion that is firmly attached to the underlying leptomeninges (6). A meningioma may mimic this gross appearance if large amounts of hemosiderin are present within the lesion from previous episodes of hemorrhage (6).
Histologic examination of cells obtained from pigmented tumors is of limited value other than to demonstrate intracytoplasmic melanin. The tumor cells may be variously arranged and show differing degrees of melanization. The cells have a uniform cytologic appearance, without areas of anaplasia, necrosis, or significant mitotic activity. Ultrastructurally, meningeal melanocytomas contain a large number of melanosomes and premelanosomes at different stages of differentiation (2, 6). Additionally, meningeal melanocytoma cells lack desmosomes and interdigitating cytoplasmic processes characteristic of meningiomas (6).
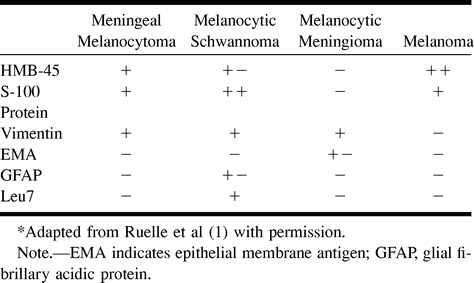
Immunohistochemical analysis is indispensable in differentiating meningeal melanocytoma from other similar pigmented lesions (1) (Table). Meningeal melanocytoma is characterized by a positive immunohistochemical reaction to antimelanoma antibody (HMB-45), S-100 protein, and vimentin antibodies, and by a negative reaction to epithelial membrane antigen (EMA) (1, 4, 6). Vimentin is the predominate intermediate filament of the cytoskeleton in cells of mesenchymal origin. It is important here because it usually appears in meningeal melanocytoma but is only rarely present in malignant melanoma. Melanocytic meningioma is characterized by a positive reaction to EMA and vimentin, and by a negative reaction to HMB-45 and S-100 protein. A positive reaction to HMB-45 and S-100 protein in the absence of a reaction to EMA provides a reliable diagnostic criterion for the diagnosis of a pigmented lesion, excluding meningioma (4). The histologic differentiation between malignant melanoma and melanocytoma can be even more difficult. The overall lack of mitotic activity, the lack of nuclear pleomorphism and hyperchromaticity, and the indolent growth of the mass spanning more than 4 years all point to melanocytoma versus melanoma. The presence of prominent nucleoli, as observed in our case, also is a regular feature of meningeal melanocytoma. The absence of reactivity to Leu7 excludes a diagnosis of melanocytic schwannoma (6).
Immunohistochemical features of pigmented tumors of the meninges*
The imaging appearance of meningeal melanocytoma is variable depending on the degree of melanization, and is thus of limited value in the differential diagnosis. The CT appearance is characterized by iso- to hyperattenuating lesions with variable contrast enhancement. The MR appearance is strongly influenced by the paramagnetic effects of melanin, which causes shortening of T1 and T2 relaxation times (13). Therefore, the MR appearance of these lesions is generally that of high signal intensity on T1-weighted images and diminished signal on T2-weighted images, with enhancement after contrast administration (14). Additionally, either imaging sequence may show complications, such as hydrocephalus, depending on the location and size of the lesion.
fig 1.
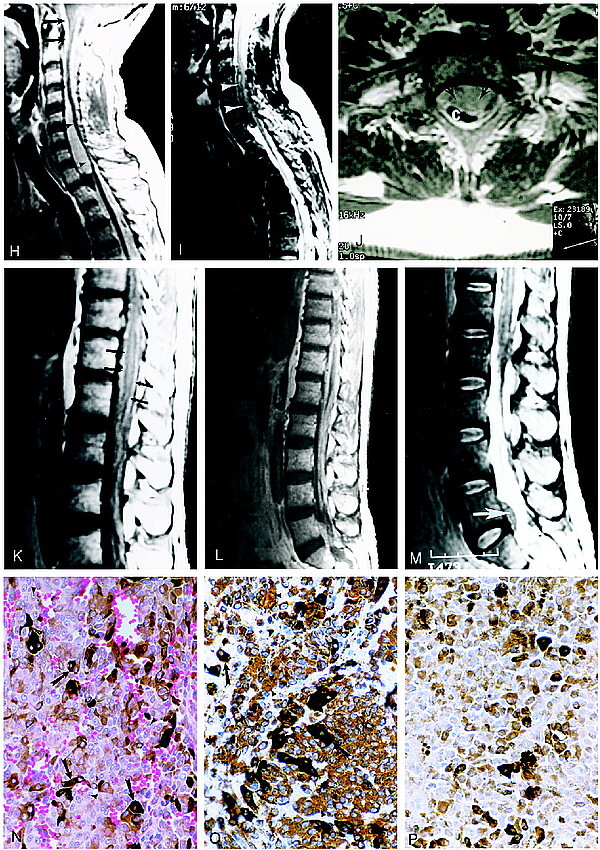
Continued. H, Sagittal contrast-enhanced T1-weighted MR image of the cervicothoracic spine shows extensive meningeal enhancement (arrows) with mass effect and spinal cord compression at the C4–T1 level (arrowheads).
I, Sagittal FSE T2-weighted MR image reveals decreased signal intensity within the intraspinal mass caused by melanin deposits (arrowheads).
J, Axial contrast-enhanced MR image shows marked compression of the cervical spinal cord by the mass (arrows). C indicates cervical spinal cord.
K, Sagittal noncontrast T1-weighted MR image of the thoracic and lumbar spine shows increased signal intensity surrounding the conus medullaris (arrows).
L, Sagittal contrast-enhanced T1-weighted MR image of the thoracolumbar spine shows extensive intradural enhancement and an anterior intraspinal mass at the L5–S1 level.
M, Sagittal FSE T2-weighted MR image of the thoracic and lumbar spine shows decreased signal intensity in the intraspinal mass at the L5–S1 level (arrow).
N, Photomicrograph of a mass in the left middle cranial fossa. The neoplasm contains sheets of cells, many of which contain dark brown melanin pigment (arrows). The nuclei are large and homogeneous with prominent nucleoli (arrowheads). Mitotic figures were rarely observed in the neoplasm (hematoxylin-eosin, original magnification ×560).
O, Photomicrograph shows immunohistochemistry for vimentin. The tumor cells contain abundant intracytoplasmic staining for this intermediate filament, which is characteristic of melanocytoma (arrowheads). Other cells are darkly pigmented owing to melanin deposits (arrows).
P, For comparison, this photomicrograph shows a control section containing only melanin deposits in which the primary antibody against vimentin was omitted. Immunohistochemical staining for HMB-45 and S-100 is not shown here (hematoxylin counterstain, original magnification ×560).
Footnotes
Address reprint requests to Gregory Chaljub, MD, Department of Radiology, The University of Texas Medical Branch, 301 University Blvd, Galveston, TX 77555.
References
- 1.Ruelle A, Tunesi G, Andrioli G. Spinal meningeal melanocytoma: case report and analysis of diagnostic criteria. Neurosurg Rev 1996;19:39-42 [DOI] [PubMed] [Google Scholar]
- 2.Jellinger K, Bock H, Brenner H. Meningeal melanocytoma: report of a case and review of the literature. Acta Neurochir 1988;94:78-87 [DOI] [PubMed] [Google Scholar]
- 3.Lach B, Russel N, Benoit B, Atack D. Cellular blue nevus (“melanocytoma”) of the spinal meninges: electron microscope and immunohistochemical features. Neurosurgery 1988;22:773-780 [DOI] [PubMed] [Google Scholar]
- 4.Litofsky NS, Zee CS, Breeze RE, Chandrasoma PT. Meningeal melanocytoma: diagnostic criteria for a rare lesion. Neurosurgery 1992;31:945-947 [DOI] [PubMed] [Google Scholar]
- 5.Naul LG, Hise JH, Bauserman SC, Todd FD. CT and MR of meningeal melanocytoma. AJNR Am J Neuroradiol 1991;12:315-316 [PMC free article] [PubMed] [Google Scholar]
- 6.Tatagiba M, Boker DK, Brandis A, Samii M, Ostertag H, Babu R. Meningeal melanocytoma of the C8 nerve root: case report. Neurosurgery 1992;31:958-961 [DOI] [PubMed] [Google Scholar]
- 7.Uematsu Y, Yukawa S, Yokote H, Itakura T, Hayashi N, Komai N. Meningeal melanocytoma: magnetic resonance imaging characteristics and pathological features. J Neurosurg 1992;76:705-709 [DOI] [PubMed] [Google Scholar]
- 8.Vanzieleghem BD, Lemmerling MM, Van Coster RN. Neurocutaneous melanosis presenting with intracranial amelanotic melanoma. AJNR Am J Neuroradiol 1999;20:457-460 [PMC free article] [PubMed] [Google Scholar]
- 9.Aichner F, Schuler G. Primary leptomeningeal melanoma: diagnosis by ultrastructural cytology or cerebrospinal fluid and cranial computed tomography. Cancer 1982;50:1751-1756 [DOI] [PubMed] [Google Scholar]
- 10.Faillace W, Okawara SH, McDonald JV. Neurocutaneous melanosis with extensive intracerebral and spinal cord involvement. J Neurosurg 1984;61:782-785 [DOI] [PubMed] [Google Scholar]
- 11.Leaney B, Rowe P, Klug G. Neurocutaneous melanosis with hydrocephalus and syringomyelia. J Neurosurg 1985;62:148-152 [DOI] [PubMed] [Google Scholar]
- 12.Alwatban J, Tampieri D, Salazar A, Melancon D, Duong H. MR of leptomeningeal melanocytosis in a patient with neurofibromatosis. J Comput Assist Tomogr 1997;21:38-40 [DOI] [PubMed] [Google Scholar]
- 13.Demirci A, Kawamura Y, Sze G, Duncan C. MR of parenchymal neurocutaneous melanosis. AJNR Am J Neuroradiol 1995;16:603-606 [PMC free article] [PubMed] [Google Scholar]
- 14.Fox H, Emery JL, Goodbody RA, Yates PO. Neurocutaneous melanosis. Arch Dis Child 1964;39:508-516 [DOI] [PMC free article] [PubMed] [Google Scholar]


