Abstract
BACKGROUND AND PURPOSE: Our purpose was to show changes in the diameter of the M1 segment of the middle cerebral artery (MCA) by using high-resolution MR imaging in patients with chronic internal carotid artery occlusion after IV administered acetazolamide challenge. Changes in blood flow velocity of the basal cerebral arteries are thought to correlate with changes of cerebral blood flow. Changes in the diameter of the basal cerebral arteries, however, might influence the validity of transcranial Doppler measurements.
METHODS: Eight patients with internal carotid artery occlusion who were undergoing acetazolamide testing for assessment of cerebrovascular vasomotor reactivity were included in the study. Blood flow velocities of both MCAs were measured with transcranial Doppler sonography before and 25 minutes after the administration of acetazolamide. Before and 15 minutes after the administration of medication, MR imaging was performed contralateral to the occlusion side. A T2-weighted turbo-gradient spin-echo sequence was chosen to show a cross section of the M1 segment in high resolution (pixels, 0.27 × 0.29 mm). Based on interpolated data, the smallest and greatest MCA diameters were determined.
RESULTS: We did not find changes in the diameter of the MCA after acetazolamide provocation testing with high-resolution MR imaging in patients with occlusive extracranial carotid artery disease.
CONCLUSION: The results of our study support the hypothesis that changes in MCA flow velocity measured by transcranial Doppler sonography reflect relative changes in cerebral blood flow after acetazolamide provocation testing.
The acetazolamide provocation test is an established diagnostic tool with which to assess the cerebral hemodynamic situation by determining the vasomotor reactivity (VMR) of cerebral blood vessels (1–4). It may provide valuable information of compromised cerebral perfusion and might contribute to the decision of performing an extracranial-intracranial bypass operation (5). Compromised VMR in patients with extracranial occlusive carotid artery disease has also been shown to correlate with the risk of developing hemodynamic ischemic stroke (6–9).
The IV administration of acetazolamide inhibits blood carboanhydrase activity, increasing blood and extracellular CO2 concentration. CO2 dilates cerebral arterioles and therefore increases cerebral blood flow, which can be measured as a rise in blood flow velocity in the basal cerebral arteries by use of transcranial Doppler sonography (1, 10).
The measurement, however, is correct only if the diameter of the insonated vessel remains unchanged. A diameter increase or decrease of the insonated basal cerebral arteries during acetazolamide provocation testing would alter the measured blood flow velocity according to the Hagen-Poiseuille law and could lead to clinical relevant under- or overestimation of the VMR.
In the present study, we measured the diameter of the M1 segment of the middle cerebral artery (MCA) by use of high-resolution MR imaging in patients with chronic internal carotid artery occlusion before and after the IV administered acetazolamide provocation test.
Methods
Eight patients (six male and two female patients; age range, 45–85 years; mean age, 62 years) with internal carotid artery occlusion (five left-sided and three right-sided occlusions) who were undergoing acetazolamide provocation testing for evaluation of cerebral VMR were assessed with high-resolution MR imaging for changes in the diameter of the M1 segment of the MCA, contralateral to the occluded side. In none of the patients was a stenosis greater than 40% shown on the side contralateral to the occlusion. All patients provided informed consent to the study.
Acetazolamide provocation testing was conducted by slow IV injection by use of our standard dose of 1000 mg of Diamox diluted in 10 mL of NaCl. Transcranial Doppler sonography was performed with the patient in the supine position. Blood flow velocities of both MCAs were measured simultaneously using a range-gated 2-MHz transducer (Multidop-X4; DWL, Sipplingen, Germany) fixed by a holder that was provided by the manufacturer. The proximal M1 segment of the MCA was located via transtemporal approach at a depth of approximately 50 mm. Pulse and blood pressure as well as mean flow velocities were recorded before and 25 minutes after the IV administration of the medication. In five of eight patients, additional recordings of respiratory frequency and end-tidal CO2 partial pressure were obtained during the transcranial Doppler sonography data acquisition by using an infrared analyzer (Datex SC-103; Hoyer, Bremen, Germany). Data were stored on a computer and were analyzed offline.
MR images were obtained using a 1.5-T unit (Siemens, Erlangen, Germany) with a circular polarized head coil. The standard protocol for all patients included T1-weighted spin-echo and T2-weighted turbo-gradient spin-echo imaging to evaluate brain parenchyma. MR arterial time-of-flight angiograms (Flash 2D; 39/6.5 [TR/TE]; degrees of freedom, 20°) were obtained in both the axial and coronal planes to localize the M1 segment (Figs 1 and 2). The representation in two planes allowed the exact perpendicular placement of a single sagittal section to measure the MCA diameter before and 15 minutes after the administration of Diamox. A T2-weigthed turbo-gradient spin-echo sequence (115/1500; number of acquisitions, six; no presaturation; field of view, 140 mm; matrix, 483 × 512; section thickness, 3 mm; pixel size, 0.27 × 0.29 mm) was chosen to show a cross section of the MCA in high resolution. Because we found an ideal circular shape in only two of our eight patients, the smallest and greatest MCA diameters were determined for each vessel. Data analysis was conducted on a video screen by use of an evaluation program that interpolates the dataset and allows the measurement of distances down to 0.1 mm (Figs 3 and 4).
fig 1.
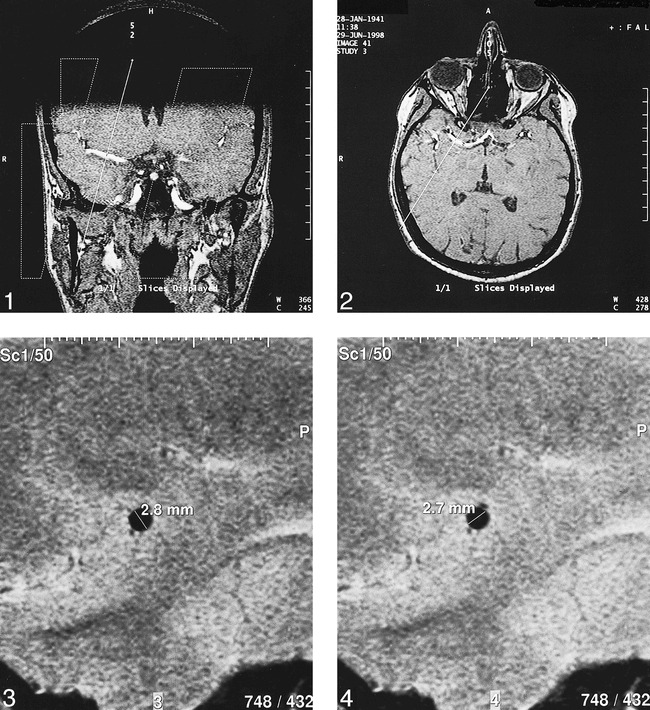
Axial-plane MR image (patient 5), chosen to localize the M1 segment and the optimal MCA cross-section plane.fig 2. Coronal-plane MR image (patient 5), chosen to localize the M1 segment and the optimal MCA cross-section plane.fig 3. Cross section of the MCA in high resolution and measurement of greatest diameter in patient 5.fig 4. Measurement of greatest and smallest diameters in patient 5
Results
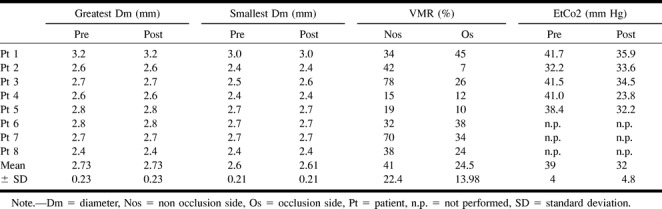
Complete visualization of the M1 segment and measurements of the MCA cross section on the side contralateral to the occlusion were achieved in all patients. The mean maximal diameter of the M1 segment of the MCA before acetazolamide testing was within the expected range, as previously published (11). The mean maximal diameter was 2.73 mm ± 0.23 (mean ± SD), and the mean minimal diameter was 2.6 mm ± 0.21. On the unoccluded side, all patients showed normal VMR after undergoing acetazolamide testing: 41% ± 22.4 (cut off = 10%). At the occlusion side, the VMR was significantly lower: 24.5 % ± 13.98 (one-tailed P value = .049, unpaired t test). Two patients showed reduced VMR at the occlusion side. Changes in the end-tidal CO2 partial pressure among the five available measurements varied between a maximum increase of 17.2 mm Hg and a minimum increase of 1.4 mm Hg.
Only one patient showed a nonsignificant change of 0.1 mm in the smallest diameter (+4%). All other measurements showed no changes in the diameter of the MCA after acetazolamide testing. All obtained results are presented in the Table.
Discussion
The assessment of cerebral VMR is a widely used test for evaluation of the cerebral hemodynamic situation in patients with extracranial occlusive carotid artery disease. Two methods, the CO2 challenge and the acetazolamide provocation test, are most commonly accepted. The postulated mechanism of action is, in both cases, the increase of blood and extracellular CO2 concentration, dilating cerebral arterioles and therefore leading to an increase of blood flow velocities in the main brain supplying vessels. The changes in blood flow velocities, which can easily be measured by using noninvasive transcranial Doppler sonography, are thought to correlate directly with cerebral blood flow.
Correct measurements, however, can be obtained only if the insonated vessels do not change their diameters during the testing period. This issue has been very controversially discussed in the past. As early as 1967, Huber and Handa (12) showed, in an angiographic study, that cerebral arteries smaller than 2.5 mm had diameter changes of up to 22.5% after induction of hypercapnic conditions, whereas arteries greater than 2.5 mm had no significant caliber changes. This hypothesis was supported by the more indirect approaches of Poulin and Robbins (13), who used the comparison of the measured Doppler power during hypercapnia and hypoxia. Kleiser et al (14) and others (15, 16) compared velocity changes of intra- and extracranial arteries before and after vasoactive stimulation.
More recently, however, an increasing number of publications have indirectly indicated a diameter variability of the MCA. Most of them draw their conclusions comparing changes in cerebral perfusion (as measured by single-photon emission CT) with cerebral blood flow velocity changes (as measured by transcranial Doppler sonography) after different vasoactive stimuli. Valdueza et al (17) calculated an MCA dilation in normal individuals ranging from –0.9% to 22% after CO2 inhalation stimulation by comparing the reaction pattern of MCA and the sphenoparietal sinus. Overall reported results vary between 4% and 10% average diameter change, depending on the method and test conditions (18–21).
Regarding the reaction of the MCA after acetazolamide provocation, results are not only controversial but contradictory. Some authors suspect a diameter increase of the MCA, and others assume a diameter decrease. Sorteberg et al (10) postulated a direct and site-specific narrowing effect of acetazolamide on the proximal MCA as well as on the anterior cerebral artery and posterior cerebral artery based on a comparison of flow velocitiy increases (mean increase, 36% to 42%) with changes in the regional cerebral blood flow as measured with xenon-133 inhalation and single-photon emission CT (mean increase, only 24% to 26%). In contrast, Eicke et al (22) showed an intracranial flow velocity increase of 39% and a simultaneous rise in common carotid artery flow of 50%, suggesting a diameter increase of the MCA of approximate-ly 4%.
The approach of our study was to visualize the vessel directly. High-resolution MR imaging cross-section studies allow the direct assessment of cerebral vessel diameter. Kane et al (23) were able to show that this method can deliver reliable results if the region of interest sections are well chosen (23). Niehaus et al (24) showed that changes in the diameter of the MCA after the administration of the strong vasodilator nitroglycerin are detectable in healthy volunteers by high-resolution MR imaging. No changes of the MCA were found in an MR study conducted by Valdueza et al (25) after inducing hyperventilation in healthy volunteers (21). The study, however, was limited by a low spatial MR resolution, which would have revealed only changes in diameter of more than 18%.
Despite a much higher resolution in our study, allowing the differentiation of changes of at least 10%, we were not able to show diameter differences in the M1 segment of the MCA after acetazolamide provocation. A 10% increase or decrease of the vessel diameter, however, could, according to the law of Hagen-Poiseuille, lead to a clinically relevant error between –17% and +23% in the estimation of cerebral blood flow. Based on the recent literature and assuming that the diameters of basal cerebral arteries can change under certain defined conditions, the following problems must be addressed:
If the diameter of the M1 segment of the MCA reacts to Diamox stimulation, the effect could have been counteracted by hyperventilation. In our study, only one patient showed a marked decrease of end-tidal CO2 partial pressure during the test, indicating that hypocapnia may not influence large vessel calibers as reported previously (25).
In two patients (patients 4 and 5), we found VMR bilaterally within the lower normal range of 10% to 19%. Because the cut-off value in healthy volunteers, however, can be assumed to be approximately 10% (mean –2 SD; published range between 3% and 18%) (10, 26), we do not expect an already existing maximal vasodilation at the time of acetazolamide challenge.
Considering the currently available spatial resolution, we were not able to detect changes smaller than 10% safely. Nonetheless, as some authors suggest, the diameter differences might vary approximately 4% to 10%, on average. Therefore, future investigations with new MR imagers using higher resolutions will be needed to help overcome this technical problem.
Most of the changes to date have been shown in young healthy volunteers. They usually do not suffer from atherosclerosis, which might lead to different reaction patterns of the vessel system toward vasoactive stimuli. In our study, we investigated only patients with ongoing major occlusive extracranial carotid artery disease undergoing acetazolamide testing to evaluate their VMR. Our patients might have had a more rigid vessel system, leading to constant MCA diameters.
In conclusion, we did not find changes in the diameter of the MCA after acetazolamide provocation testing with high-resolution MR imaging in patients with occlusive extracranial carotid artery disease. These findings suggest that the blood flow velocity measurement of the MCA after acetazolamide provocation testing is a reliable method with which to assess blood flow changes and to test the VMR in this population. Diameter measurements of the large basal cerebral arteries with high-resolution MR imaging may be an interesting tool with which to study physiological and pharmacologic reactions of the cerebral circulation.
Results of MR measurements, vasomotor reactivity, and EtCO2
Footnotes
Address reprint requests to Stephan J. Schreiber, MD, Department of Neurology, University Hospital Charité, Schumannstr. 20/21, 10098 Berlin, Germany.
References
- 1.Piepgras A, Schmiedek P, Leinsinger G, Haberl RL, Kirsch CM, Einhäupl KM. A simple test to assess cerebrovascular reserve capacity using transcranial Doppler sonography and acetazolamide. Stroke 1990;21:1306-1311 [DOI] [PubMed] [Google Scholar]
- 2.Dahl A, Lindegaard KF, Russell D, et al. A comparison of transcranial Doppler and cerebral blood flow studies to assess cerebral vasoreactivity. Stroke 1992;23:15-19 [DOI] [PubMed] [Google Scholar]
- 3.Okudaira Y, Bandoh K, Arai H, Sato K. Evaluation of the acetazolamide test: vasoreactivity and cerebral blood volume. Stroke 1995;26:1234-1239 [DOI] [PubMed] [Google Scholar]
- 4.Dahl A, Russell D, Rootwelt K, Nyberg-Hansen R, Kerty E. Cerebral vasoreactivity assessed with transcranial Doppler and regional cerebral blood flow measurements: dose, serum concentration, and time course of the response to acetazolamide. Stroke 1995;26:2302-2306 [DOI] [PubMed] [Google Scholar]
- 5.Schmiedek P, Piepgras A, Leinsinger G, Kirsch CM, Einhäupl K. Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg 1994;81:236-244 [DOI] [PubMed] [Google Scholar]
- 6.Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke 1992;23:171-174 [DOI] [PubMed] [Google Scholar]
- 7.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993;79:483-489 [DOI] [PubMed] [Google Scholar]
- 8.Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 1993;32:912-918 [DOI] [PubMed] [Google Scholar]
- 9.Chimowitz MI, Furlan AJ, Jones SC, et al. Transcranial Doppler assessment of cerebral perfusion reserve in patients with carotid occlusive disease and no evidence of cerebral infarction. Neurology 1993;43:353-357 [DOI] [PubMed] [Google Scholar]
- 10.Sorteberg W, Lindegaard KF, Rootwelt K, et al. Effect of acetazolamide on cerebral artery blood velocity and regional cerebral blood flow in normal subjects. Acta Neurochir (Wien) 1989;97:139-145 [DOI] [PubMed] [Google Scholar]
- 11.Lang J. Skull Base and Related Structures: Atlas of Clinical Anatomy. Stuttgart: Schattauer;1995
- 12.Huber P, Handa J. Effect of contrast material, hypercapnia, hyperventilation, hypertonic glucose and papaverine on the diameter of the cerebral arteries: angiographic determination in man. Invest Radiol 1967;2:17-32 [DOI] [PubMed] [Google Scholar]
- 13.Poulin MJ, Robbins PA. Indexes of flow and cross-sectional area of the middle cerebral artery using doppler ultrasound during hypoxia and hypercapnia in humans. Stroke 1996;27:2244-2250 [DOI] [PubMed] [Google Scholar]
- 14.Kleiser B, Scholl D, Widder B. Doppler CO2 and Diamox test: decreased reliability by changes of the vessel diameter? Cerebrovasc Dis 1995;5:397-402 [Google Scholar]
- 15.Lindegaard KF, Lundar T, Wiberg J, Sjoberg D, Aaslid R, Nornes H. Variations in middle cerebral artery blood flow investigated with noninvasive transcranial blood velocity measurements. Stroke 1987;18:1025-1030 [DOI] [PubMed] [Google Scholar]
- 16.Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke 1994;25:793-797 [DOI] [PubMed] [Google Scholar]
- 17.Valdueza JM, Draganski B, Hoffmann O, Dirnagl U, Einhäupl KM. Analysis of CO2 vasomotor reactivity and vessel diameter changes by simultaneous venous and arterial Doppler recordings. Stroke 1999;30:81-86 [DOI] [PubMed] [Google Scholar]
- 18.Kontos HA. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke 1989;20:1-3 [DOI] [PubMed] [Google Scholar]
- 19.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 1993;32:737-741 [PubMed] [Google Scholar]
- 20.Brooks DJ, Redmond S, Mathias CJ, Bannister R, Symon L. The effect of orthostatic hypotension on cerebral blood flow and middle cerebral artery velocity in autonomic failure, with observations on the action of ephedrine. J Neurol Neurosurg Psychiatry 1989;52:962-966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caekebeke JF, Ferrari MD, Zwetsloot CP, Jansen J, Saxena PR. Antimigraine drug sumatriptan increases blood flow velocity in large cerebral arteries during migraine attacks. Neurology 1992;42:1522-1526 [DOI] [PubMed] [Google Scholar]
- 22.Eicke BM, Buss E, Bahr RR, Hajak G, Paulus W. Influence of acetazolamide and CO2 on extracranial flow volume and intracranial blood flow velocity. Stroke 1999;30:76-80 [DOI] [PubMed] [Google Scholar]
- 23.Kane AG, Dillon WP, Barkovich AJ, Norman D, Dowd CF, Kane TT. Reduced caliber of the internal carotid artery: a normal finding with ipsilateral absence or hypoplasia of the A1 segment [comment]. AJNR Am J Neuroradiol 1996;17:1295-1301 [PMC free article] [PubMed] [Google Scholar]
- 24.Niehaus L, Gottschalk S, Weber U. Effect of drug-induced vasodilatation of basal brain arteries with nitroglycerin on blood flow velocity and volume flow in the middle cerebral artery. Ultraschall Med 1998;19:225-229 [DOI] [PubMed] [Google Scholar]
- 25.Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhäupl KM. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. AJNR Am J Neuroradiol 1997;18:1929-1934 [PMC free article] [PubMed] [Google Scholar]
- 26.Kleiser B, Scholl D, Widder B. Assessment of cerebrovascular reactivity by Doppler CO2 and Diamox testing: which is the apropriate method? Cerebrovasc Dis 1994;4:134-138 [Google Scholar]


