Abstract
BACKGROUND AND PURPOSE: Neuroimaging techniques such as diffusion- and perfusion-weighted MR imaging have been proposed as tools for advanced diagnosis in hyperacute ischemic stroke. There is, however, substantial doubt regarding the feasibility and practicality of applying MR imaging for the diagnosis of stroke on a routine basis, especially with respect to possible delay for specific treatment such as thrombolysis. In this study, we tested whether MR imaging of stroke is safe, fast, and accurate, and whether the gain in additional information can be used in the daily routine without a loss of time and a risk of suboptimal treatment for the patient with stroke.
METHODS: Between September 1997 and August 1999, 64 patients with acute ischemic stroke were recruited for MR imaging (ie, diffusion-weighted imaging, perfusion-weighted imaging, MR angiography, T2-weighted imaging) after a baseline CT was performed. We evaluated practicality and feasibility of MR imaging of stroke by analyzing the intervals between symptom onset, arrival, CT, and MR imaging.
RESULTS: Sixty-four patients (mean age, 60.9 years) underwent routine CT and MR imaging within 12 hours after stroke onset (n=25, ≤3 hr; n=26, 3–6 hr; n=13, 6–12 hr). Median times to arrival, start of CT, MR imaging, and between CT and MR imaging were 1.625 hours, 2 hours, 3.875 hours, and 1 hour, respectively. Intervals between symptom onset and MR imaging (P<.005), arrival and MR imaging (P<.002), and CT and MR imaging (P=.0007) differed significantly between the early phase of the study and after November 1998, whereas the intervals between symptom onset and arrival, symptom onset and CT, and arrival and CT did not. Hemorrhage could be excluded in all; a perfusion/diffusion match or mismatch could be shown in 63 of 64 patients.
CONCLUSION: Practice and experience with MR imaging in a stroke team significantly reduce the time and effort required to perform this technique and thus make 24-hour availability for MR imaging of stroke practical. Assessment of patients with hyperacute stroke is rapid and comprehensive. Image quality can be substantially improved by head immobilization and by mild sedation, if necessary.
The advent of new MR techniques such as perfusion- and diffusion-weighted imaging in the early 1990s has added another dimension to diagnostic imaging of stroke (1–6). During the last few years, a growing body of evidence has accumulated, documenting the usefulness of this methodology in the clinical setting of acute ischemic stroke. Several investigators found a significant correlation of diffusion and perfusion changes with follow-up T2 changes, as well as with neurologic outcome (7–12). It is presumed that the difference (mismatch) between abnormal areas on diffusion-weighted and perfusion-weighted images (with perfusion-weighted images > diffusion-weighted images) shows the ischemic tissue at risk, which is potentially salvageable (7, 11, 12). Some authors have concluded that different infarct patterns can be identified by means of diffusion-weighted and perfusion-weighted imaging of hyperacute stroke, which may allow a more rational selection of therapeutic strategies based on the presence or absence of a tissue at risk. Also the sensitivity (up to 94%) and specifity (up to 100%) of diffusion and perfusion imaging is reported to be excellent within the first hours of stroke (13, 14). A recently published study showed that a subgroup of patients with stroke, defined by MR imaging, benefited significantly from early recanalization (15), whereas other authors found by using diffusion and perfusion imaging that reperfusion was more frequent with intravenous rt-PA therapy (16). Furthermore, MR imaging of stroke does show intracerebral hemorrhage, which must be excluded before any form of recanalization therapy using thrombolytic agents can be performed (17–19). Nevertheless, substantial doubts remain regarding the feasibility and practicality of MR imaging of stroke in the clinical setting (20). We aimed to show that MR imaging of stroke is feasible and practical in the setting of hyperacute stroke. We also sought to address the logistical problems that may arise, including clinical pathways, when this novel methodology is implemented routinely. Finally, we sought to demonstrate that, after successful implementation of these new techniques, the time needed to perform the studies in a routine setting can be shortened significantly.
Methods
Patients
Patient recruitment in this open nonrandomized study started in September 1997, and is still continuing. The last patient included was recruited in August 1999. The basic protocol was designed to identify patients who are suitable for specific forms of therapy within the first hours of stroke. We included patients who had had an ischemic stroke within the 12 hours of symptom onset and a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 3, but preferred a window of 6 hours after symptom onset. With approximately 1200 stroke patients/year seen at the neurology emergency room, at least 50% arrive outside of a window of 12 hours. Fifteen percent of the patients have intracerebral hemorrhages, and in another 20% of patients, stroke severity is not adequate for aggressive forms of therapy, or there is a marked improvement of more than 4 points on the NIHSS. The NIHSS is a clinical test battery, which consists of 10 items: consciousness, gaze palsy, facial palsy, hemianopia, arm and leg weakness, sensory deficits, dysarthria, ataxia, aphasia, and hemineglect. The minimum score is 0 points (no symptoms), the maximum score is 42: death is not coded. Finally, there are 10% of patients with good demarcation of the acute infarct on CT scans or signs of infarction exceeding one third of the middle cerebral artery (MCA) territory. All these patients were excluded from this study. Of the remaining 100 patients suitable for MR imaging, MR imaging of stroke was not performed in roughly one third of the patients because of nonavailability of the MR stroke team, because the stroke MR imaging team was not informed by the neurologist or neuroradiologist on call, or, in scarce cases, because of technical problems with the MR scanner. Stroke onset was defined as the last time the patient was known to be without neurologic deficit. Exclusion criteria were an age of less than 18 years or more than 85 years, a significant preexisting neurologic deficit (modified Rankin Scale > 1), a history or CT findings of severe cerebral microangiopathy or multiinfarct dementia, unstable vital signs, or general MR contraindications. All patients had a CT scan before they were enrolled into the MR study. We performed thrombolysis with 0.9mg/kg BW rt-PA according to the ECASS II study protocol (17) in eligible patients within a 6-hour window (n=24). Because MR imaging of stroke was the technique under investigation, we chose not to delay therapy in patients selected for thrombolysis. Therefore, the indication for thrombolysis was based exclusively on clinical status and CT findings (ECASS criteria), not on MR imaging of stroke imaging results. These inclusion criteria for thrombolysis are moderate-to-severe hemispheric ischemic stroke (NIHSS 7–15[-20]), which can be treated within 6 hours after symptom onset, and no or minor signs of infarction (≤ 1/3 MCA territory) on the initial CT scan (17). In addition to the general contraindications such as pregnancy, coagulation disorders, trauma, recent surgery, and recent gastrointestinal hemorrhage blood pressure exceeding 200/110 mm Hg, exclusion criteria were intracranial hemorrhage, including subarachnoid hemorrhage, coma or stupor, hemiplegia plus fixed head and eye deviation, rapid improvement of stroke symptoms (≥ 4 points NIHSS), or only minor stroke symptoms (17). CT was followed by MR imaging, after which the patient was admitted to the neurocritical care unit, or to the stroke unit for further monitoring and therapy. For patient safety, a neurologic stroke fellow experienced in neurologic critical care was present throughout the MR examination. The patients' oxygen saturation and electrocardiogram were monitored continuously, whereas blood pressure was measured intermittently. We obtained informed consent from all patients or their next of kin. The study protocol was approved by the local ethics committee. We applied ear plugs prior to imaging to reduce patient irritation from noise, especially with the echo-planar sequences (diffusion-weighted, perfusion-weighted, echo-planar fluid-attenuated inversion recovery [FLAIR]). Further, the patient's head was immobilized with pieces of foam rubber (5 × 2 × 8 in), which were stuffed between the coil and the head on both sides. When necessary, mild sedation was given with either continuous intravenous propofole 20–60 mg/hr or midazolam 5–10 mg as an intravenous bolus, repeatedly if necessary.
Imaging and Clinical Assessment
All patients, except one, with hyperacute-stage CT studies already done at the referring hospital were examined with a state-of-the-art CT scanner and immediately thereafter with a 1.5-T whole-body MR imager equipped with enhanced gradient hardware for echo-planar imaging. For the MR examination, we used a circular polarized head coil. The MR imaging protocol included an axial T2-weighted fast spin-echo sequence, an axial FLAIR echo-planar sequence, an isotropic axial diffusion-weighted spin-echo echo-planar sequence (b=0, 333, 666, 1000 s/mm2), time-of-flight MR angiography (MRA), and perfusion-weighted imaging with an axial T2*-weighted gradient-echo echo-planar sequence (40 data sets during and after injection of 25 mL Gd-DTPA with a power injector [5mL/s]). The complete MR imaging protocol required 15 to 20 minutes to perform, and an additional 10 minutes were necessary for patient positioning and transfer, as well as calculation of the apparent diffusion coefficient and mean transit time maps. All neuroimaging studies were crossread by staff neuroradiologists. Image quality was categorized as good quality/readable or low quality/not performed and thus unreadable.
Statistical Analysis
For statistical analysis, we used a standard software package. Demographic data, intervals between examinations, and other descriptive statistics are given as mean or median values and range. For the calculation of significance of reduction in the interval between examinations before and after November 1998, we used a chi-square test, or a Mann-Whitney U-test, as appropriate.
Results
Patients and Image Quality
We examined 64 patients (36 men, 28 women) with MR imaging. The mean age was 60.9 years (range, 29–83 years), with women being slightly older (mean, 63.5 years; range, 29–83 years) than men (mean 58.9 years; range, 34–78 years). There was an even distribution of left (n=31) and right (n=33) hemispheric infarctions; 28 of the 31 patients with left hemispheric stroke had a moderate or severe aphasia. The median NIHSS at baseline was 12 (range 3 to 25), with only 1 patient with a NIHSS score of less than 5. With the exception of one patient, the quality of all MR images obtained was satisfactory for interpretation and without disturbing motion artifacts. One patient did not tolerate the examination despite mild sedation so that only the diffusion-weighted sequences were interpretable (feasibility = 98.4%). Fixation of the patients' heads with foam rubber resulted in a marked noise reduction as well as an excellent reduction of motion artifacts. In some patients with hyperkinesia, the nonparetic arm had to be held by the physician inside the MR imaging room; leg motion did not influence image quality as long as the head was satisfactorily immobilized.
Logistics
At our institution, the routine call schedule requires the presence of at least three neurology fellows or residents (one neurointensivist, one emergency room neurologist, one stroke unit neurologist), one neuroradiologist, and one MR technologist at all times. The MR technologists are familiar with the procedure of MR imaging of stroke, because diffusion-weighted and perfusion-weighted imaging are also used in other areas of neuroimaging, such as multiple sclerosis and brain tumors. During this study, two additional stroke MR imaging physicians (one neurologist [P.D.S.] and one neuroradiologist [J.B.F.]) assisted by two doctoral students (O.P., H.R.) took weekly turns for stroke MR imaging call. When eligible stroke patients were referred to the neurology emergency room, the stroke MR imaging physician on call was paged, and the technologist on call prepared the MR scanner and the infusion pump. Ideally, during the day, the patient was directly moved from the CT to the MR suite, which are next to each other. An MR examination underway would not be interrupted, but the next time slot was claimed for the emergency stroke MR imaging, so that waiting times never exceeded 30 minutes except for logistical reasons other than MR capacity. At night, the interval between CT and MR imaging was slightly longer, except when the stroke MR imaging physician on call was available on site. This period, however, was not very extended, because the stroke MR imaging physician on call was paged from the emergency room before CT was performed, and both stroke MR imaging physicians and doctoral students live only within 10 minutes' distance to the hospital. All further therapy, such as thrombolysis, or randomization to phase III treatment trials, was initiated at the MR site after knowledge of the CT results, and started during MR imaging, without unacceptable loss of time. Thrombolytic therapy was based on CT results in order to avoid a bias in favor of an imaging method that has not been fully evaluated. Postprocessing of MR images, such as 3D reconstruction of MR angiography and calculation of apparent diffusion coefficient and perfusion maps was done online and parallel to the patient's transport from the MR suite to his or her final destination at the neurologic critical care or stroke unit. Secondary therapeutic intervention or escalating forms of therapy such as hypothermia or decompressive surgery were initiated depending on MR imaging results. Since November 1998, all neuroradiologists participating in the on-call schedule have been trained in MR imaging of stroke. Subsequently, MR imaging of stroke has become part of the clinical routine and thus available 24 hours a day, 7 days a week, without additional costs for a technologist or physician on call.
Time Windows
Of the 64 patients included in our study, 25 underwent MR imaging of stroke within 3 hours, 26 within the 3- to 6-hour window, and another 13 within the 6- to 12-hour window of symptom onset. The average time of symptom onset to arrival at the neurologic emergency room was 2.23 ± 1.64 hours (median, 1.625 hr; range, 0 hr to 8 hr). The mean time from symptom onset to CT was 2.744 ± 1.834 hours (median, 2.0 hr; range, 0.25 hr to 10 hr), the mean interval from arrival to CT was 0.603 ± 0.592 hours (median, 0.5 hr; range, 0.25 hr to 4.25 hr). One patient had a CT scan done already at an outside hospital. The mean time from symptom onset to MR imaging of stroke was 4.48 ± 2.662 hours (median, 3.875 hr; range, 1.25 hr to 11.5 hr), from arrival to MR imaging of stroke 2.262 ± 1.949 hours (median, 1.5 hr; range, 0.25 hr to 7.5 hr). The mean interval between CT and MR imaging of stroke was 1.734 ± 1.89 hours (median, 1 hr; range, 0.25 hr to 6.75 hr). The window between MR imaging of stroke and CT was significantly reduced with training and growing experience of the involved technical and medical personnel. Using the period before November 1998 as an arbitrarily chosen point of time, only 10 of 31 patients underwent MR imaging within 1 hour after CT, whereas from November 1998 on, 27 of 33 patients underwent MR imaging within 1 hour after baseline CT (P=.0001; chi-square test). Comparing the intervals before and since November 1998 with the Mann-Whitney U-test, we found a statistically signficant difference between the intervals from symptom onset to MR imaging (P=.0047), arrival to MR imaging (P=.0015), and between CT and MR imaging (P=.0007). The interval between symptom onset and arrival, as well as that between symptom onset and CT, did not differ at all (P=.48 and P=.4785) before and after November 1, 1998. There was a trend but no statistically significant difference in the interval between arrival and CT (P=.1119) before and after November 1, 1998. These observations (Fig 1) match the investigators' impression that the overall acceptance of the feasibility and practicality of MR imaging of stroke among colleagues of the departments of neurology and neuroradiology, who are part of the on-call schedule, has increased decidedly during the study. Subsequently, MR imaging was successfully implemented as a standard stroke emergency procedure in the clinical routine at all times.
fig 1.
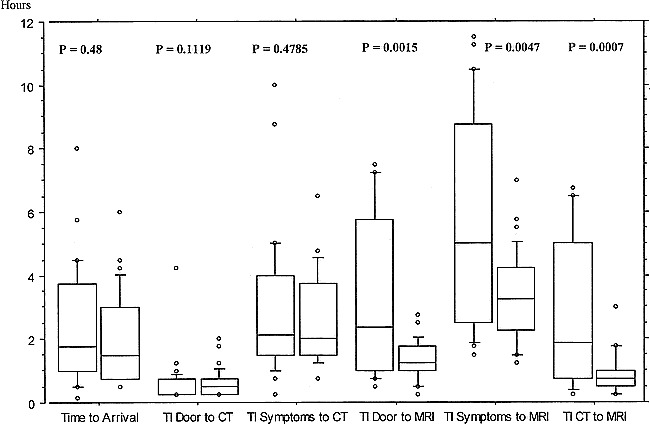
From left to right, box pairs delineate the time intervals (TI) between symptom onset and arrival, symptom onset and CT, arrival (door) to CT, symptom onset to MR, arrival (door) to MR, and in between CT and MR. The left box of each pair denotes the patients included before November 1998 and the right box those from November 1998 to date. Each box shows the 25th to 75th percentile, whiskers show the 10th to 90th percentile, dots denote all observations above 90th and below 10th percentile. The horizontal line represents median values. P values (significance of difference between TIs before and after November 1) are given for each subset, and were calculated with the Mann-Whitney U-test
Discussion
The need for a comprehensive diagnostic tool that allows one to look at all important pathophysiologic aspects of hyperacute stroke is evident. Such a method must be rapid, show actual irreversible ischemic brain damage as well as its age, show any tissue at risk and, if such tissue is present, its size, show any vessel occlusion and its location, and rule out intracerebral hemorrhage. During the last few years, there has been accumulating evidence of the superiority of novel MR sequences in not only the diagnosis of hyperacute ischemic but also hemorrhagic stroke (8, 11, 12, 15, 19, 21–26). MR imaging of stroke, the combination of diffusion-weighted and perfusion-weighted imaging with a fast T2-weighted and an MR angiography sequence, enables the stroke physician to investigate all important pathophysiologic aspects of hyperacute stroke. In spite of remaining doubts that MR imaging is useful to guide therapy for acute stroke (20), there is now sufficient evidence that diffusion-weighted imaging, perfusion-weighted imaging, and MR angiography do show irreversible ischemic infarction, tissue at risk, and presence as well as location of a vessel occlusion (11, 23, 27–29). In patients with transient ischemic attacks, reversibility of lesions on diffusion-weighted images has been described (30), although reversibility is not present in patients with completed stroke or in those with a grade of severity that calls for thrombolytic therapy. Earlier studies revealed a good correlation between baseline diffusion imaging findings and initial and outcome NIHSS (11, 12). The question, however, is whether a diagnostic approach with MR imaging is feasible and practical in a clinical setting, where the time span to a specific form of therapy such as thrombolysis is crucial (17).
It has been claimed that a method for the selection of patients that benefit most from effective recanalization therapy would be an improvement (31). Because of the narrow window for treatment, at present only scarce data exist about MR imaging findings in patients undergoing thrombolysis, especially with imaging performed before onset of therapy (15, 16). Our study shows that the performance of MR imaging before initiation of a specific therapy does not lead to an unacceptable loss of time to therapy, with a median window between CT and MR imaging of 1 hour. Approximately one third of the eligible patients during the investigation period was not included in the study mostly owing to nonavailability of the MR imaging team or because the MR imaging team was not informed by the neurologist or neuroradiologist on call. Although unlikely, this may have introduced a bias in favor of shorter intervals between CT and MR imaging. With the implementation of MR imaging of stroke into our routinely available diagnostic procedures, follow-up studies on the efficacy of MR imaging of stroke will not be subjected to such a bias. Also, 51 patients were examined within 6 hours of stroke onset, with the examination lasting 20 to 30 minutes. Although CT in hyperacute stroke is the standard of care, an accurate detection of infarct location and a rough estimation of infarct size (more or less than one third of the MCA territory) is achieved in only 50% to 67% of patients in an early time window (17, 32). Therefore, we believe that CT does not meet the present requirements of diagnostic sensitivity in hyperacute stroke patients to allow optimal patient selection for thrombolytic therapy or more invasive means to achieve recanalization. Detection of vessel occlusion with Doppler sonography is time consuming, highly dependent on examiner experience, and may technically not be possible, whereas a short fast MR angiography sequence offers this information within 3 minutes (15). Further, perfusion-weighted imaging has to meet the critique that it renders only a qualitative index of tissue perfusion, whereas other methods such as single-photon emission CT (SPECT) and positron emission tomography (PET) may offer accurate quantitative regional CBF measurements (20). However, neither PET nor SPECT have become a standard for patient management, as such quantification is not easy and most institutions rely on qualitative or semiquantitative SPECT and PET studies. We concur that the evaluation of perfusion status and identification of tissue at risk by xenon CT, SPECT, or PET is not practical for routine use. Furthermore, they do not deliver any visualization of vessel status or normal brain anatomy as opposed to MR imaging (29). A direct comparison of these different techniques, however, has yet to come. In addition to the high sensitivity and specifity of diffusion-weighted imaging findings, it has been shown that the difference in size of signal abnormality between diffusion and perfusion in hyperacute ischemic stroke reliably identifies that area of brain tissue at risk of irreversible infarction (15, 16). Salvage of the tissue at risk (for instance by recanalizaton) results in smaller infarcts and a better neurologic outcome as opposed to loss of the tissue at risk with subsequently large infarctions accompanied by clinical deterioration (15, 33).
A new diagnostic test must of course show that it can replace a more invasive or more expensive test and that the additional gain in information leads to an improvement of patient management and prognosis (20). Cost effectiveness of MR imaging of stroke is likely if one considers that multiple examinations such as CT, CT angiography, and Doppler sonography may be substituted by an at least equally accurate imaging technique that is also capable of excluding intracerebral hemorrhage (18, 19). It is known that optimal stroke care necessitates a lot of manpower (34, 35). Therefore, in a stroke center, there usually are two or more neurologists and an additional neuroradiologist plus a technologist present at all times, allowing for the performance of MR imaging of stroke protocols without the need for extra personnel. Furthermore, a gain in information is reached by the substantially higher sensitivity of diffusion-weighted imaging for stroke location and size (10). The particular advantage of MR imaging of stroke, however, besides that of rapid demonstration of infarct size and vessel occlusion as the rationale for thrombolytic therapy, is delineation of potential tissue at risk, which represents the aim for most therapeutic efforts (15, 36, 37). Not only can those patients be identified that profit most from successful recanalization therapy, but also those that probably will not, but have an excess risk of complications associated with thrombolysis (38). Our data show, however, that the interval to baseline diagnostic imaging (ie, CT) could not be shortened at all. Besides establishing MR imaging of stroke as an initial imaging technique, the interval from symptom onset, presentation at the emergency room, to initial diagnostic imaging must be further reduced. Several multicenter trials attempting to establish the efficacy and cost-benefit ratio of MR imaging of stroke further are underway.
Conclusion
In conclusion, MR imaging is a procedure that rapidly allows a comprehensive diagnostic evaluation of patients with acute stroke and provides all necessary and relevant information in the setting of hyperacute stroke. Image quality can be optimized by head immobilization and by mild sedation, if necessary. Practice and experience with MR imaging in a stroke team significantly reduces the time effort for this methodology and facilitates a 24-hour availability of MR imaging of stroke. Therefore, we postulate, in contrast to other investigators, that MR imaging of stroke is “ready for prime time” (20).
Acknowledgments
We want to express our gratitude to the medical and nursing staff of the neurocritical care unit, the stroke unit, and the intermediate-care unit, the Department of Neurology, as well as to all Department of Neuroradiology medical and technical staff. Without their help, this study would not have been accomplished.
Footnotes
Address reprint requests to Peter D. Schellinger, MD, Department of Neurology, University of Heidelberg, Medical School, Im Neuenheimer Feld 400, D-69120 Heidelberg, Germany.
References
- 1.Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401-407 [DOI] [PubMed] [Google Scholar]
- 2.Moseley ME, Kucharczyk J, Asgari HS, Norman D. Anisotropy in diffusion-weighted MRI. Magn Reson Med 1991;19:321-326 [DOI] [PubMed] [Google Scholar]
- 3.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423-429 [PMC free article] [PubMed] [Google Scholar]
- 4.Mintorovitch J, Moseley ME, Chileuitt L, et al. Comparison of diffusion- and T2-weighted MRI for the early detection of cerebral ischemia and reperfusion in rats. Magn Reson Med 1991;18:39-50 [DOI] [PubMed] [Google Scholar]
- 5.Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology 1992;42:1717-1723 [DOI] [PubMed] [Google Scholar]
- 6.Fisher M, Albers GW. Applications of diffusion-perfusion magnetic resonance imaging in acute ischemic stroke. Neurology 1999;52:1750-1756 [DOI] [PubMed] [Google Scholar]
- 7.Warach S, Boska M, Welch KM. Pitfalls and potential of clinical diffusion-weighted MR imaging in acute stroke. Stroke 1997;28:481-482 [PubMed] [Google Scholar]
- 8.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab 1996;16:53-59 [DOI] [PubMed] [Google Scholar]
- 9.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996;199:391-401 [DOI] [PubMed] [Google Scholar]
- 10.Lovblad KO, Baird AE, Schlaug G, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol 1997;42:164-170 [DOI] [PubMed] [Google Scholar]
- 11.Barber PA, Darby DG, Desmond PM, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 1998;51:418-426 [DOI] [PubMed] [Google Scholar]
- 12.Tong DC, Yenari MA, Albers GW, et al. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6.5 hour) ischemic stroke. Neurology 1998;50:864-870 [DOI] [PubMed] [Google Scholar]
- 13.Lovblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 1998;19:1061-1066 [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 1999;210:155-162 [DOI] [PubMed] [Google Scholar]
- 15.Jansen O, Schellinger PD, Fiebach JB, Hacke W, Sartor K. Early recanalization in acute ischemic stroke saves tissue at risk defined by MRI. Lancet 1999;353:2036-2037 [DOI] [PubMed] [Google Scholar]
- 16.Marks MP, Tong D, Beaulieu C, et al. Evaluation of early reperfusion and IV rt-PA therapy using diffusion- and perfusion-weighted MRI. Neurology 1999;52:1792-1798 [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998;352:1245-1251 [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Edelman RR, Warach S. Detection of hyperacute primary intraparenchymal hemorrhage by magnetic resonance imaging. Stroke 1996;27:2321-2324 [DOI] [PubMed] [Google Scholar]
- 19.Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke 1999;30:765-768 [DOI] [PubMed] [Google Scholar]
- 20.Powers WJ, Zivin J. Magnetic resonance imaging in acute stroke: not ready for prime time. Neurology 1998;50:842-843 [DOI] [PubMed] [Google Scholar]
- 21.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol 1997;41:581-589 [DOI] [PubMed] [Google Scholar]
- 22.Baron JC, von Kummer R, del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. Stroke 1995;26:2219-2221 [DOI] [PubMed] [Google Scholar]
- 23.Beaulieu C, de Crespigny A, Tong D, et al. Longitudinal MRI study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol 1999;46:568-578 [DOI] [PubMed] [Google Scholar]
- 24.Koroshetz WJ, Gonzalez G. Diffusion-weighted magnetic resonance imaging: an ECG for “brain attack”? Ann Neurol 1997;41:565-566 [DOI] [PubMed] [Google Scholar]
- 25.Minematsu K, Hasegawa Y, Yamaguchi T. Diffusion MRI for evaluating cerebrovascular disease. Rinsho Shinkeigaku 1995;35:1575-1577 [PubMed] [Google Scholar]
- 26.Mohr JP, Biller J, Hilal SK, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke 1995;26:807-812 [DOI] [PubMed] [Google Scholar]
- 27.Prichard JW, Grossman RI. New reasons for early use of MRI in stroke. Neurology 1999;52:1733-1736 [DOI] [PubMed] [Google Scholar]
- 28.Jansen O, Knauth M, Sartor K. Advances in clinical neuroradiology. Akt Neurologie 1999;26:1-7 [Google Scholar]
- 29.Jansen O, Heiland S, Schellinger P. Neuroradiological diagnosis in acute ischemic stroke. Value of modern techniques. Nervenarzt 1998;69:465-471 [DOI] [PubMed] [Google Scholar]
- 30.Kidwell CS, Alger JR, F Di, Salle et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999;30:1174-1180 [DOI] [PubMed] [Google Scholar]
- 31.Grotta J. Should thrombolytic therapy be the first-line treatment for acute ischemic stroke? N Engl J Med 1997;337:1309-1310 [PubMed] [Google Scholar]
- 32.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. JAMA 1999;282:2019-2026 [DOI] [PubMed] [Google Scholar]
- 33.Schellinger PD, Jansen O, Fiebach JB, Sartor K, Hacke W. Multimodal MRI in hyperacute ischemic stroke: associations of tissue at risk, vessel occlusion, recanalization, morphological and functional outcome. Neurology 1999;52:A87-A88 [Google Scholar]
- 34. The European Ad Hoc Consensus Group. Optimizing intensive care in stroke: a European perspective—a report of an ad hoc consensus group meeting. Cerebrovasc Dis 1997;7:113-128 [Google Scholar]
- 35. The European Ad Hoc Consensus Group. European strategies for early intervention in stroke. A report of an ad hoc consensus group meeting. Cerebrovasc Dis 1996;6:315-324 [Google Scholar]
- 36.Albers GW. Expanding the window for thrombolytic therapy in acute stroke—the potential role of acute MRI for patient selection. Stroke 1999;30:2230-2237 [DOI] [PubMed] [Google Scholar]
- 37.Barber PA, Davis SM, Darby DG, et al. Absent middle cerebral artery flow predicts the presence and evolution of the ischemic penumbra. Neurology 1999;52:1125-1132 [DOI] [PubMed] [Google Scholar]
- 38.Sunshine JL, Tarr RW, Lanzieri CF, et al. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology 1999;212:325-332 [DOI] [PubMed] [Google Scholar]


