Abstract
BACKGROUND AND PURPOSE: Dissection of the carotid artery can, in certain cases, lead to significant stenosis, occlusion, or pseudoaneurysm formation, with subsequent hemodynamic and embolic infarcts, despite anticoagulant therapy. We sought to determine the therapeutic value of stent-supported angioplasty retrospectively in this subset of patients who are poor candidates for medical therapy.
METHODS: Five men and five women (age range, 37–83 years; mean age, 51.2 years) with dissection of the internal (n=9) and common (n=1) carotid artery were successfully treated with percutaneous endovascular balloon angioplasty and stent placement. The etiology was spontaneous in five, iatrogenic in three, and traumatic in two. Seven of the treated lesions were left-sided and three were right-sided.
RESULTS: The treatment significantly improved dissection-related stenosis from 74±5.5% to 5.5±2.8%. Two occlusive dissections were successfully recanalized using microcatheter techniques during the acute phase. Multiple overlapping stents were needed in four patients to eliminate the inflow zone and false lumen and establish an angiographically smooth outline within the true lumen. There was one case of retroperitoneal hemorrhage, but there were no procedural transient ischemic attacks (TIAs), minor or major strokes, or deaths (0%). Clinical outcome at latest follow-up (16.5±1.9 months) showed significant improvements compared with pretreatment modified Rankin score (0.7±0.3 vs 1.8±0.44) and Barthel index (99.5±0.5 vs 80.5±8.9). One delayed stroke occurred in a treated patient with contralateral carotid occlusion following a hypotensive uterine hemorrhage at 8 months; the remaining nine patients have remained free of TIA or stroke.
CONCLUSION: In select cases of carotid dissection associated with critical hemodynamic insufficiency or thromboembolic events that occur despite medical therapy, endovascular stent placement appears to be a safe and effective method of restoring vessel lumen integrity, with good clinical outcome.
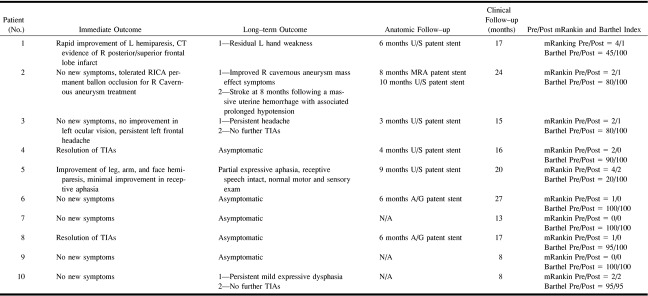
Dissection of the cervical carotid arteries occurs when the integrity of the intima and media layers of the vessel wall is compromised, resulting in hemorrhage in the vessel wall and consequent compromise of the native vessel lumen (1–4) (Fig 1). The formation of a false channel and associated anatomic disruption of the endothelial monolayer results in conditions favoring local thrombus formation, which may then embolize distally, and result in brain infarction (5). The accepted medical standard of therapy has consisted of systemic anticoagulation, and has been observed to lead to a significant rate of subsequent resolution and healing (6, 7). In a subset of patients, spontaneous healing, with restoration of native vessel anatomy, fails to occur (1, 8–10). Instead, the arterial dissection may result in total occlusion of the true vessel lumen by compression from the false lumen, created by entry of pressurized blood in the dissected medial defect (2, 9). In another subset of patients, the dissection site may develop into a focal or extensive stenosis, with formation of an associated pseudoaneurysm (1, 2, 8). In such cases, anticoagulation, although critical to prevent thromboembolic events in the initial stages of the disease, may be insufficient to overcome the flow-limiting nature of the dissecting lesion or may be contraindicated because of risk of pseudoaneurysm rupture (3). Endovascular stents have been shown to be of benefit for the treatment of coronary atherosclerosis (11), and have been considered for the treatment of carotid atherosclerosis in certain high-risk patient subgroups (12). Stents also have been used to treat intimal dissection resulting from balloon angioplasty and trauma (13–16). By virtue of their design, stents provide the necessary centrifugal force to permit apposition of the dissected segment to the vessel wall to obliterate the false lumen and resolve the stenosis (13, 17). Stents can also provide mechanical support to serve as a scaffold for coil embolization of dissection-related wide-necked pseudoaneurysms (18, 19) (Fig 1). In order to determine the clinical and angiographic outcome of this form of therapy, we present our endovascular management of cervical carotid dissection, achieved using percutaneous stent angioplasty in a retrospective series of 10 patients.
fig 1.
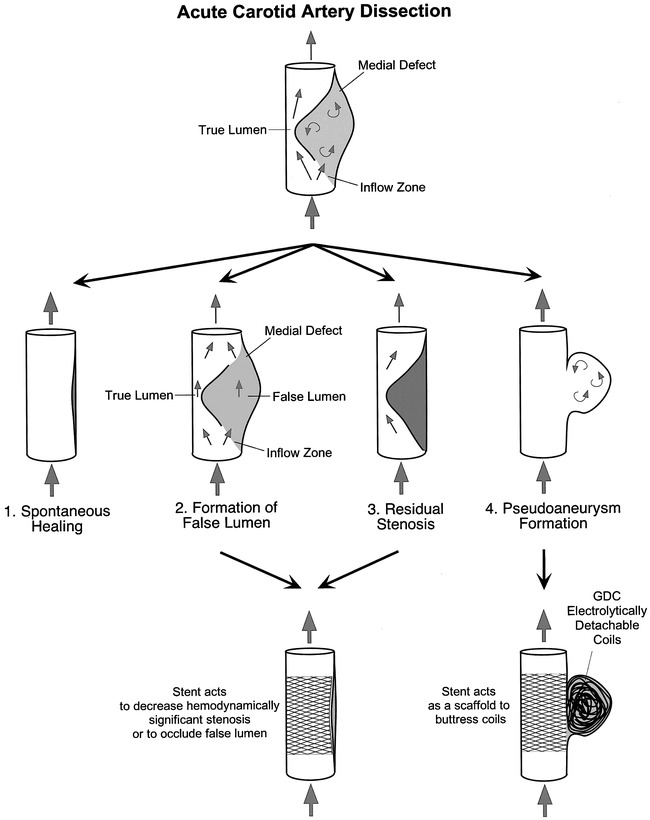
Simplified schematic illustration of the pathophysiologic process of carotid artery dissection proceeding from the acute stage to either spontaneous healing (1), formation of false lumen (2), residual stenosis of varying degree or complete occlusion (3), and formation of a pseudoaneurysm (4). A stent is used in cases that have not responded to medical therapy either to relieve a hemodynamically significant stenosis, to occlude a false lumen, or to serve as a scaffold to enable coil embolization of a wide-necked pseudoaneurysm
Methods
Patient Selection Criteria
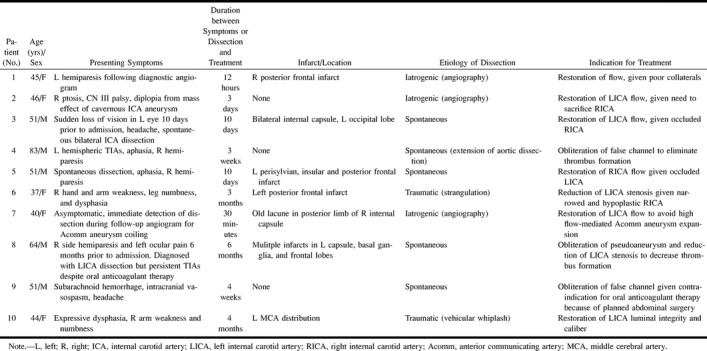
During the 18-month period between October 1997 and March 1999, 10 consecutive patients underwent endovascular treatment of carotid dissection performed using intraluminal stent placement. Informed consent was obtained from patients or medical guardians. Patients were considered eligible for stent therapy of carotid dissection only if they had either failed optimal medical therapy, were unable to undergo anticoagulation, or if they had no other therapeutic option of acceptably low risk. The specific indications (Table 1) included the presence of transient ischemic attacks (TIAs) despite anticoagulant or antiplatelet therapy (patients 4, 8, 10), contraindication to anticoagulation (patient 9), contralateral carotid occlusion or stenosis in a patient who was neurologically unstable or had clinical evidence of hemodynamic insufficiency (patients 3, 5, 5), documented poor collateral circulation (patient 1), need for elective occlusion of the contralateral internal carotid artery for aneurysm treatment (patient 2), and need to avoid flow increase through the anterior communicating artery because of the presence of an aneurysm (patient 7). Patients who underwent stent placement for angioplasty-induced intimal dissection during endovascular treatment of carotid atherosclerosis were excluded from the study.
TABLE 1:
Summary of the clinical presentation, coexisting medical problems, duration of symptoms until treatment, presence and location of any infarct, etiology of the dissection, and indication for endovascular treatment
Procedure
All patients underwent complete digital subtraction angiography of both anterior and posterior cerebral circulations, including the external carotid arteries. Patients were administered intravenous heparin to achieve an activated clotting time of greater than 250 seconds. A 7F or 9F guide catheter (Brite Tip; Cordis Endovascular, Miami Lakes, FL) was placed in the common carotid artery via a femoral vascular sheath (Avanti; Cordis). A 2.3F microcatheter (Rapid Transit; Cordis) was used coaxially over a 0.014- to 0.016-in microguidewire (Instinct 10; Cordis/Transend 14, Scimed, Maple Grove, MN) to enter the true arterial lumen under real-time high-resolution digital roadmap angiography (Toshiba, Tustin, CA). A 300-cm-long exchange microguidewire (Stabilizer; Cordis) was then passed through the microcatheter and used to exchange the latter for the stent delivery catheter. Three types of stents were employed in this study: 1) a self-expanding stainless steel stent (Wallstent; Schneider, Plymouth, MN); 2) a premounted balloon-expandable stainless steel stent (GFX; Arterial Vascular Engineering, Santa Rosa, CA); and 3) a Nitinol shape-memory alloy self-expanding stent (S.M.A.R.T.; Cordis). After stent deployment, the patients were administered ticlopidine (250 mg po bid) or clopidogrel (75 mg po qd) for 6 weeks and aspirin (325 mg po qd) indefinitely. The vascular access sheath was removed on the day after the procedure, with the aid of an external femoral compression device (FemoStop; Radi, Uppsala, Sweden).
Outcome Analysis
Follow-up was performed using both neurologic examination and telephone interview. The modified Rankin score and Barthel index were used, as previously described (20–22). Evaluation of stent patency was obtained by sonography or repeat digital subtraction angiography.
Statistics
Severity of stenosis was computed using the NASCET method, with dimensions obtained either by using the relative fluorographic size of a reference object or the angiographic digital computer system (Toshiba). Specifically, the diameter of maximal stenosis (DStenosis) was measured along with the most proximal diameter of the distal normal vessel (DNormal), and the degree of stenosis computed as Stenosis=[1−(DStenosis / DNormal)] ×100. The paired Student's t test and analysis of variance were used in the comparison of numerical variables. For categorical analysis, a Pearson's chi-square test was used. Statistical significance was assumed for P<.05.
Results
Patient Population and Lesion Characteristics
There were five male and five female patients, ranging in age from 37 to 83 years (mean age, 51.2±4.2 years) (Table 1). Seven (70%) of the 10 patients suffered from coexisting hypertension and seven patients (70%) had suffered a stroke prior to treatment as a result of the initial arterial dissection. The location of dissections treated was as follows: one in the left common carotid artery, six in the left internal carotid artery, and three in the right internal carotid artery. Two patients were treated within 12 hours of the initial angiographic or symptomatic dissection, three patients were treated between 3 and 10 days of symptomatic dissection, and the remaining five patients were treated for chronic dissection between 3 weeks and 10 years after initial detection of their dissection.
Therapeutic Interventions
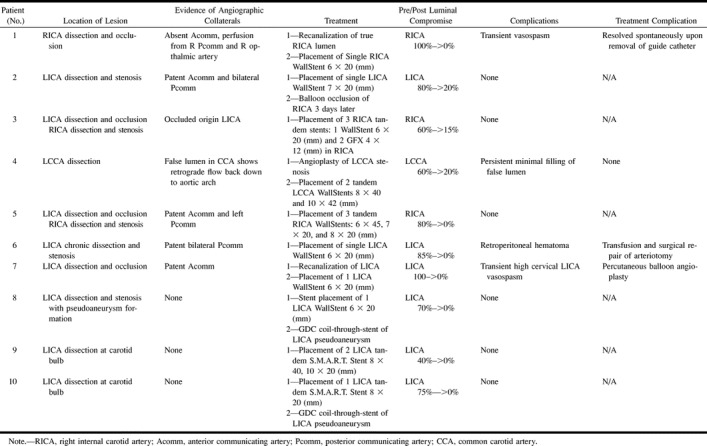
The etiology of the carotid dissections encountered in this study was spontaneous, iatrogenic, or traumatic. Five patients (50%) had spontaneous dissections, one of which was chronic (discovered 10 years prior to admission), and three had iatrogenic dissections (30%) resulting during diagnostic angiography. Of these three patients, two underwent recanalization and stent angioplasty of a completely occluded internal carotid artery, with complete restoration of flow (patients 1, 7). The third (patient 2) underwent stent angioplasty of a narrowed and dissected left internal carotid artery in order to enable treatment of a contralateral giant cavernous carotid artery aneurysm that required balloon occlusion and carotid sacrifice. Two patients (20%) presented with traumatic dissection, one as a result of manual strangulation by her spouse (patient 6), and the other after vehicular whiplash (patient 10) (21) (Table 2). The angioplasty and stent treatment improved dissection-induced stenosis significantly from 74±5.5% to 5.5±2.8% (P<.001).
TABLE 2:
Summary of the angiographic characteristics of the lesion, including location, presence of collaterals, method of treatment, stenosis, and complication related to treatment
Two patients (patients 3 and 5) had bilateral involvement of the internal carotid artery. These patients suffered spontaneous dissection and had undergone diagnostic angiography at an outside hospital 2 to 3 days prior to admission, which showed unilateral flow-limiting dissection on one side and slight irregularity in the contralateral carotid artery. After transfer, repeat angiography revealed progression of the disease to unilateral complete occlusion of the previously dissected internal carotid artery and a severe flow-limiting lesion on the contralateral side. Eight lesions were left-sided and two were right-sided.
In four patients, multiple stents were deployed in an overlapping fashion in order to enable the elimination of the false lumen and to treat the residual stenosis. Two of these patients, with a severe form of bilateral dissection leading to unilateral occlusion at the time of treatment, required three tandem stents to reconstitute an angiographically smooth luminal outline (patients 3 and 5). In the other two patients, two tandem stents were used in both cases to allow spanning the entire length of the dissection, which included the carotid bulb with its greater diameter compared with the proximal common carotid (patient 4) or the cervical internal carotid artery (patient 9). In two patients (patients 8 and 10), a stent was deployed to serve as a scaffold through which a wide-necked pseudoaneurysm was subsequently treated using Guglielmi electrolytically detachable coils, in a manner similar to that reported on in previous reports (18, 19). These aneurysmal dilatations were located in the cervicopetrous junction (patient 8) and in the petrocavernous segment (patient 10) of the internal carotid artery.
Complications
There were no instances of postprocedural transient ischemic attacks (TIAs), new neurologic deficits, and no new minor or major strokes (0%) prior to patient discharge (Table 3). Two patients had intraprocedural vasospasm of the high cervical carotid artery; this vasospasm was severe enough to necessitate angioplasty in one case (patient 7) and resolved spontaneously after removal of the microguidewire in the other (patient 1). One patient suffered a retroperitoneal hemorrhage after removal of the vascular sheath and placement of an external compression device (patient 6) on postoperative day 1; this required blood transfusion (2 U) and was surgically repaired without complication. A single, major, delayed complication occurred in patient 2, who was referred with a left internal carotid dissection that occurred during angiographic workup of a giant symptomatic cavernous aneurysm. The flow-limiting left internal carotid artery dissection was treated successfully using stent deployment. Three days later, permanent sacrifice of the right internal carotid artery was performed using detachable silicone balloons after the patient had tolerated successful balloon test occlusion (30 minutes with hypotensive challenge). The patient had an uncomplicated postprocedural course and was discharged home. Eight months later, the patient had a massive uterine hemorrhage with an associated prolonged hypotensive episode, which led to an ischemic stroke of the right hemisphere (contralateral to stent, ipsilateral to balloon occlusion), resulting in left-arm weakness. The deficit has mostly resolved at latest clinical follow-up (24 months).
TABLE 3:
Outcome of treated patients immediately after the procedure and at latest follow–up
Outcome
During the acute postprocedural period, no patients developed worsening of symptoms. Two patients treated in the acute phase of dissection showed significant and rapid improvement in symptoms (patients 1 and 5). Clinical follow-up was obtained from all patients at 16.5±1.9 months (range, 8–27 months) and angiographic or sonographic follow-up was available for seven patients at 6.3±0.9 months (range, 3–10 months). There was no evidence of stent occlusion or stenosis in any of the follow-up radiographic studies (0%). Clinical outcome was evaluated using the modified Rankin scale and was improved at latest follow-up (0.7±0.26) compared with pretreatment values (1.8±.0.44) (P<.008). Similarly, the Barthel index showed improvement at latest follow-up (99.5±0.5) compared with pretreatment values (80.5±8.6). Except for patient 2 presented above, no other patient in this high-risk subset has suffered any delayed TIA or stroke since treatment at latest follow-up.
Illustrative Cases
Acute Symptomatic Occlusive Dissection Treated with Recanalization and Stent Placement
The patient (patient 1) is a 45-year-old woman with a history of hypertension, lupus erythematosus, and migraine headaches, who underwent diagnostic angiography at an outside institution for evaluation of recurrent headaches with associated left-arm weakness and numbness. During the procedure, the operator noted that an acute dissection had been induced by contrast injection in a spastic vessel. The procedure was halted and intravenous heparin was immediately administered, but the patient developed hemiparesis 1 hour later. She was transferred to our hospital, where emergent angiography revealed that the previously dissected left internal carotid artery now showed tapering to a complete occlusion (Fig 2). Collateral evaluation revealed no anterior communicating flow across the midline from the left internal carotid artery injection, weak flow from the posterior circulation via the posterior communicating artery, and retrograde flow through the right ophthalmic artery from the right external carotid artery. A Rapid Transit microcatheter was used in combination with an Instinct-10 microguidewire to navigate through the true lumen carefully and to reestablish recanalization of the internal carotid artery, which was noted to harbor a tonsillar loop, a condition previously described to predispose to dissection (24). Evaluation of the major intracranial vessels by contrast injection through the microcatheter revealed no evidence of a large thrombus, and showed that most of the distal branches remained patent. A decision was thus made to treat the dissection flap by elimination of the inflow to the subintimal dissection. A single 8-mm × 2-cm Wallstent was deployed at the proximal inflow zone, after which control angiography revealed that blood flow had resumed to normal. The patient's left hemiparesis improved rapidly after the procedure, and she was discharged 3 days later with a mild left pronator drift. The patient has had no further TIAs or stroke, and follow-up sonography at 6 months revealed the stent to be patent, with no abnormal flow characteristics. At latest neurologic follow-up (17 months), the patient still complained of poor fine-finger movement, numbness of the left face, and subjective left-leg hypesthesia, but is otherwise intact.
fig 2.
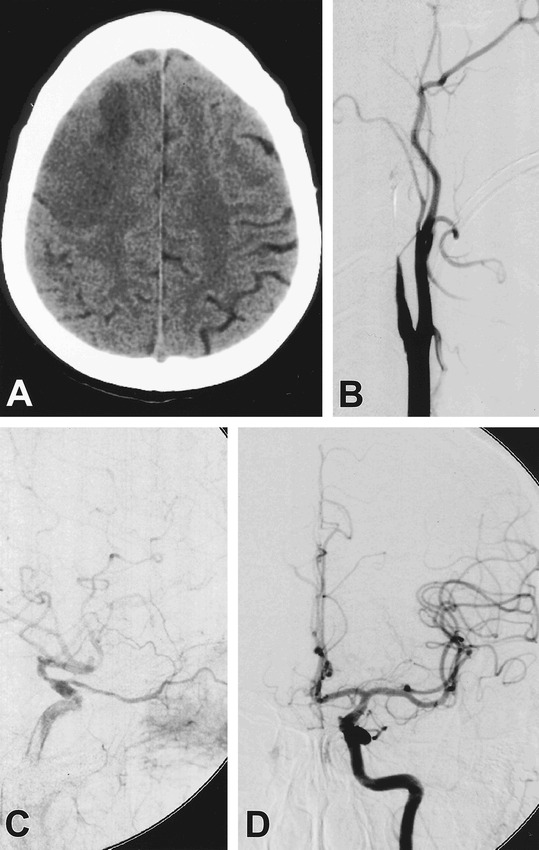
A 45-year-old woman (patient 1) noted to have developed left hemiparesis after diagnostic angiography at an outside hospital. Head CT scan shows evidence of a previous focal infarct as well as diffuse edema in the right posterior frontal lobe (A). Digital subtraction angiography of the right common carotid artery reveals tapering of the right internal carotid artery, to a complete occlusion, with appearance consistent of dissection (B). Injection of the right external carotid artery (C) shows retrograde collateral flow through the right ophthalmic artery, with filling of the cavernous segment of the right ICA. Injection of the left internal carotid artery shows no significant flow across the anterior communicating artery (D)
Bilateral, Subacute, Spontaneous Carotid Dissection Progressing to Unilateral Occlusion and Flow-limiting Contralateral Stenosis
This patient (patient 5) is a 51-year-old man, with no significant past medical history, who noted left arm and hand numbness while chopping wood. Four days later, he was found lying in bed, aphasic and disoriented, with right hemiparesis. He was admitted to an outside hospital, and imaging was performed. A head CT scan was unrevealing, but a brain MR scan showed a left posterior frontal infarct, and carotid sonography showed little to no flow in the left internal carotid artery (Fig 3). An angiogram obtained at an outside hospital showed a flow-limiting dissection of the left internal carotid artery and an irregular contour of a patent right internal carotid artery. Intravenous anticoagulation with heparin was begun, and the patient was transferred to our center. Examination disclosed that the patient was aphasic and had a right upper motor facial and right upper and lower extremity hemiparesis, with no response to visual threat from right-sided confrontation. A repeat diagnostic angiogram was obtained 2 days after the previous study. The new angiogram revealed progression of the previously noted left internal carotid artery dissection to complete occlusion. In addition, it showed the previous contour abnormality of the right internal carotid artery had progressed to a long-segment stenosis with an associated distal pseudoaneurysmal dilatation. Given the disease progression in both internal carotid arteries despite anticoagulation, a decision was made to proceed with securing a patent luminal conduit to prevent further progression of the right-sided dissection to complete occlusion. Recanalization of the left internal carotid artery was not attempted, given the duration and extent of the established left middle cerebral artery distribution infarct. Microcatheterization of the true lumen was performed with a Rapid Transit microcatheter over an Instinct-10 microguidewire. A 6-mm × 4.5-cm Wallstent was deployed over the Stabilizer exchange microguidewire at the proximal inflow zone to tack down the dissection flap, followed by balloon angioplasty of the long- segment stenosis. An additional two Wallstents (7 mm × 2 cm and 8 mm × 2 cm) were needed to reconstruct the vascular channel to the same size as the native vessel. After the procedure, the patient had progressive improvement of his right-sided face and arm weakness and of his receptive aphasia. Neurologic follow-up at 20 months revealed persistent, moderate, expressive aphasia with naming difficulty, normal comprehension, symmetric face, absent pronator drift, and intact sensation with normal gait. Sonography confirmed persistent patency of the right internal carotid artery and stents, with no abnormal flow characteristics within the deployed stents.
fig 3.
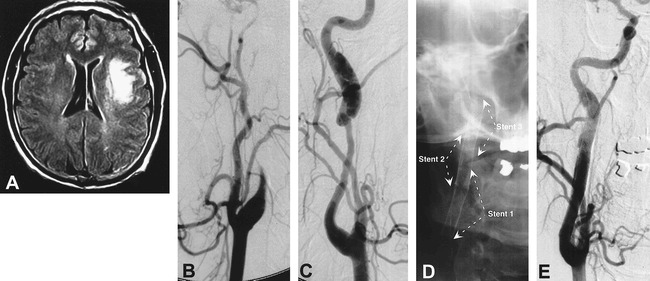
A 51-year-old man (patient 5) with a 10-day history of left-sided TIAs now presents with sudden onset of aphasia and right hemiparesis. Axial T1-weighted contrast-enhanced MR imaging shows a left frontal infarct (A). Digital subtraction angiography of the left common carotid artery showed progression of a left internal carotid dissection from a partial stenosis, 2 days prior, to a complete occlusion despite systemic anticoagulation (B). Digital subtraction angiography of the right common carotid artery revealed a dissection of the right internal carotid artery with an associated long-segment stenosis and an expansile pseudoaneurysm at the distal end (C). A Rapid Transit microcatheter was used to navigate the patent lumen of the right internal carotid artery and to deploy three Wallstents (6 mm × 45 mm, 7 mm × 20 mm, and 8 mm × 20 mm) in a tandem overlapping fashion with postdeployment balloon angioplasty (D). The procedure resulted in reconstitution of the normal lumen of the right internal carotid artery (E)
Treatment of a Symptomatic Carotid Dissection Related to an Aortic Dissection
The patient (patient 4) is an 83-year-old right-handed man with previous type A aortic dissection (involving both the ascending and descending aorta) 10 years prior to admission, which was surgically repaired using a Dacron graft. Angiographic study of the arch after an episode of transient aphasia revealed extension of the repaired aortic dissection into the left common carotid artery. The patient was begun on aspirin and Coumadin and had been neurologically without symptoms until 3 weeks prior to admission. The patient began to have left hemispheric TIAs, resulting in episodes of right-hand weakness, with poor fine-finger movement, despite therapeutic oral anticoagulant therapy. The patient was begun on intravenous anticoagulation. Angiography of the aortic arch revealed an extensive left common carotid artery dissection involving the origin of the common carotid artery, the cervical carotid bifurcation, and the proximal internal carotid artery to C3 (Fig 4). Flow was antegrade in the true lumen of the left common carotid artery and retrograde in the false lumen, which exited at a poorly visualized area in the aortic arch. The retrograde flow back to the arch was the result of a pressure gradient from a Venturi effect at the false lumen exit into the aorta. A 5F Simmons-1 catheter was used to catheterize the left common carotid artery selectively and was exchanged for a 9 F guide catheter. Two Wallstent devices were deployed in an overlapping fashion. The proximal stent measured 8 mm × 4 cm and was deployed in the common carotid artery across the inflow zone. The second stent measured 10 mm × 4.2 cm and was deployed distally in the carotid bulb and into the origin of the cervical internal carotid artery. Postdeployment angioplasty was performed for each stent by using an 8-mm then a 10-mm angioplasty balloon (Powerflex; Cordis) (Fig 4). Postprocedure angiography showed near-complete elimination of the false lumen and the retrograde flow component. The patient tolerated the procedure well, without further symptoms, and was maintained on Coumadin until 1.5 months after discharge, when he underwent open prostatectomy without complication. Carotid sonography performed at 6 months showed persistent patency of the stent, without evidence of retrograde flow in the previous false lumen. Neurologic follow-up at 16 months revealed no further TIAs.
fig 4.
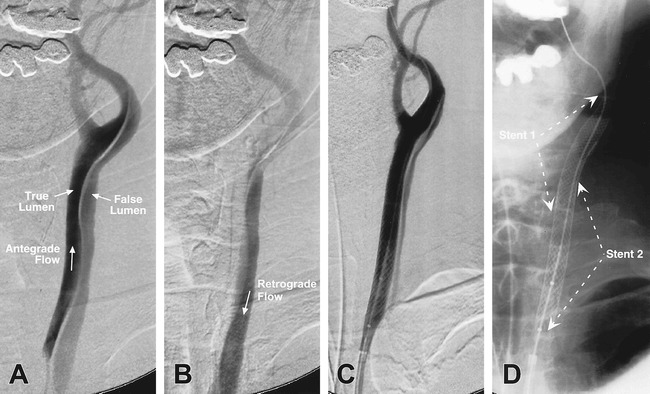
An 83-year-old man (patient 4) with a history of aortic dissection, which was surgically repaired 10 years previously, presents with left hemispheric TIAs and episodes of aphasia. Digital subtraction angiography of the left common carotid artery outlines a chronic extensive dissection, with antegrade flow through the true lumen and retrograde flow in the false lumen down to the aorta, by virtue of the Venturi effect at the aortic arch (A, early injection; B, late injection). Two stents were placed in tandem overlapping fashion, followed by postdeployment angioplasty, resulting in a near-complete elimination of the retrograde flow in the now-reduced false lumen (C, D)
Discussion
The annual incidence of spontaneous carotid dissection has been reported to be 2.6 per 100 000 (27), and has been estimated to account for up to 20% of strokes in the younger population (28). Most infarcts associated with dissection have been proposed to be embolic in nature, although a nonnegligible proportion consists of hemodynamic flow-related infarcts (5, 6). Despite anticoagulation, a number of patients progress to have hemodynamically significant residual stenosis or develop pseudoaneurysms (2), leading to a risk of hemodynamically significant stenosis or to a danger of delayed distal embolization (10). Although the rate of repeat embolization in unilateral spontaneous carotid dissection lesions is low (6, 29), and, as such, does not justify placement of an intravascular stent as primary treatment, the patients presented in this series underwent stent placement only after they had fulfilled stringent criteria such as failure of medical therapy and absence of low-risk surgical options. Although surgery has been performed for direct repair of carotid dissection, it is difficult to identify the inflow zone or repair the entire extent of the dissection (1). Consequently, surgery for carotid dissection is associated with a significantly greater risk than is carotid endarterectomy for atherosclerosis (1).
Surgical options for the treatment of hemodynamic insufficiency as a result of carotid dissection have included an extracranial-to-intracranial bypass procedure for patients with hemodynamic insufficiency who have not responded to anticoagulant therapy (29). The surgical treatment of dissection-induced cervical carotid aneurysm has included bypass grafting (30) and ligation of the internal carotid artery for pseudoaneurysm repair (29).
One of the drawbacks of conventional and stent-supported balloon angioplasty of the carotid artery for atherosclerosis has been the risk of distal embolization of atheromatous debris during the balloon dilatation phase of the procedure. We did not encounter in this series any cases of distal embolization, perioperative stroke, or new deficits attributable to stent deployment despite performing angioplasty of the deployed stent in the majority of cases. Unlike atherosclerotic stenoses, which require higher-pressure angioplasty and necessitate fracturing the plaque, the stent deployment for dissection is less traumatic and is performed at a lower pressure because the lesions are more compliant and are not usually atheromatous or calcified.
Additional advantages of endovascular therapy of carotid dissection are that it enables the identification of the true and false lumens by superselective catheterization and angiography, and further allows the recanalization of completely occluded vessels by use of microcatheter techniques, provided the thrombus burden is not prohibitive (Fig 2). The availability of flexible stents that conform to the contour of the artery enables the sequential reconstruction of an angiographically smooth luminal outline through the narrowed true lumen and obliteration of the false lumen (Fig 5). In addition, the endovascular approach circumvents the need for blood flow occlusion, which is required during direct surgical bypass procedures, a crucial factor that most patients in the current report would not have tolerated because of contralateral involvement or poor collateral flow. Finally, the endovascular approach enables the simultaneous treatment of any coexistent pseudoaneurysmal dilatations by embolization using Guglielmi electrolytically detachable coils, by the coil-through-stent technique, which was employed in two cases (patients 8 and 10) (Fig 1). Although the current series is the largest reported to date, the number of patients treated remains small. Nonetheless, the procedure has proved to be safe, because it was not associated with any periprocedural TIA or stroke. The success in reducing dissection-induced stenosis, the patency rate obtained at follow-up, and the lack of TIAs suggest that stent angioplasty offers a viable alternative to complex surgical bypass procedures. The long-term efficacy and durability of stent placement for carotid dissection remains to be determined. It is unknown whether intimal hyperplasia, which was not detected during the anatomic follow-up periods described herein, will become of concern in the distant future.
fig 5.
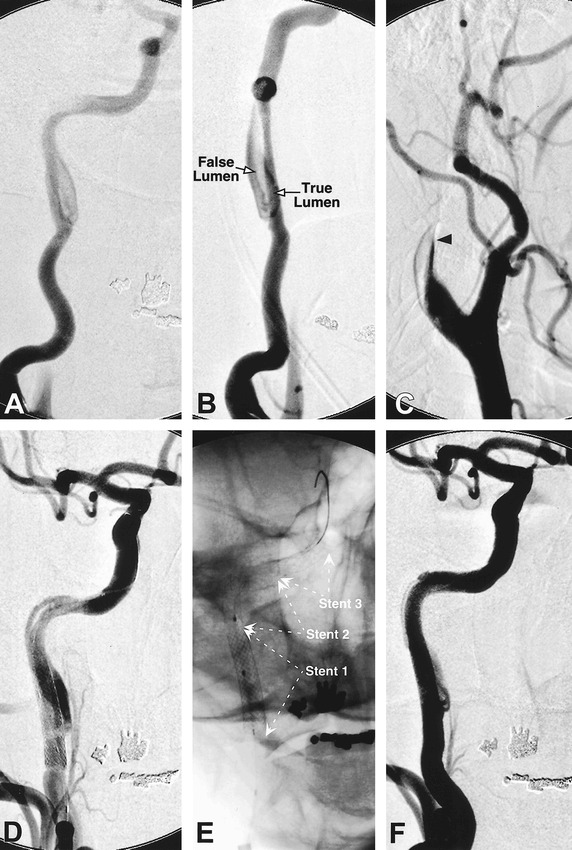
A 51-year-old man (patient 3) developed sudden loss of vision in the left eye, a left Horner's syndrome, and orthostatic lightheadedness. Digital subtraction angiography with injection of the right common carotid artery shows intimal dissection of the right internal carotid artery in the high cervical region, with delineation of the true and false lumen (A, B). Injection of the left common carotid artery, which had evidence of dissection but was shown to be patent on an angiogram obtained 4 days earlier, now reveals complete left internal carotid artery occlusion (arrowhead) despite systemic anticoagulation (C). A Rapid Transit microcatheter was used to catheterize the true lumen, followed by deployment of a Wallstent (6 mm × 20 mm) in the proximal portion of the dissected segment (D). Persistent filling of the false lumen, however, required the tandem placement of two additional GFX stents (4 mm × 12 mm) in the petrous segment of the right internal carotid artery (E), with reconstitution of the normal luminal diameter (F)
Conclusion
The results of the intermediate-term follow-up provided in this report suggest that treatment of carotid dissection is a useful technique for select high-risk cases, such as when the lesion is hemodynamically significant from severe stenosis or contralateral occlusion, or when anticoagulation has failed to prevent TIAs or is contraindicated. Additional, extended, long-term angiographic and clinical follow-up will help define the potential importance and relative value of this technique for the treatment of carotid dissection.
fig 2.
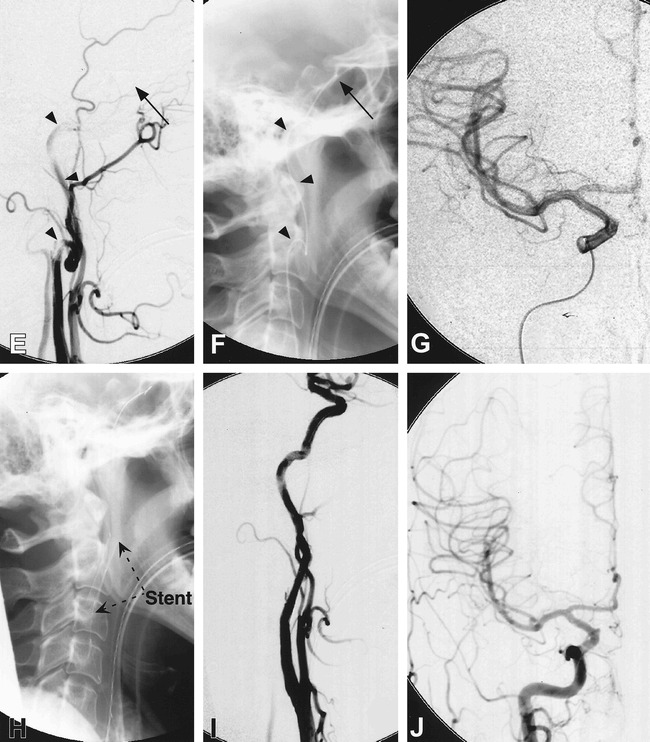
fig. 2 (continued). Treatment approach consisted of initial recanalization of the dissected right internal carotid artery achieved by entering the true lumen by using a Rapid Transit microcatheter and Instinct-10 microguidewire (arrowheads) (E), and advancing up to the cavernous portion of the right internal carotid artery (arrow) (F). Superselective injection shows a patent right middle and anterior cerebral artery (G). An 8-mm × 2-cm Wallstent was then deployed at the dissection site over a Stabilizer exchange microguidewire at the C2 level (H). The reconstitution of the lumen of the right internal carotid artery is shown by injection of the right common carotid artery (I), with resumption of intracranial perfusion (J)
Acknowledgments
We would like to thank the assistance of Drs. Vineeta Singh, Clay Johnston, and Daryl Gress.
Footnotes
A.M.M. was supported by a grant from the Boston Neurosurgical Foundation.
Address reprint requests to Adel M. Malek, M.D., Ph.D., Neurosurgery, Brigham and Women's and Children's Hospitals, 300 Longwood Avenue, Bader 3, Boston, MA 02115.
References
- 1.Ehrenfeld WK, Wylie EJ. Spontaneous dissection of the internal carotid artery. Arch Surg 1976;111:1294-1301 [DOI] [PubMed] [Google Scholar]
- 2.Mokri B, Sundt TM Jr, Houser OW, Piepgras DG. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol 1986;19:126-138 [DOI] [PubMed] [Google Scholar]
- 3.Anson J, Crowell RM. Cervicocranial arterial dissection. Neurosurgery 1991;29:89-96 [DOI] [PubMed] [Google Scholar]
- 4.Desfontaines P, Despland PA. Dissection of the internal carotid artery: aetiology, symptomatology, clinical and neurosonological follow-up and treatment in 60 consecutive cases. Acta Neurol Belg 1995;95:226-234 [PubMed] [Google Scholar]
- 5.Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke 1998;29:2646-2648 [DOI] [PubMed] [Google Scholar]
- 6.Sturzenegger M. Spontaneous internal carotid artery dissection: early diagnosis and management in 44 patients. J Neurol 1995;242:231-238 [DOI] [PubMed] [Google Scholar]
- 7.Rothrock JF, Lim V, Press G, Gosink B. Serial magnetic resonance and carotid duplex examinations in the management of carotid dissection. Neurology 1989;39:686-692 [DOI] [PubMed] [Google Scholar]
- 8.Ast G, Woimant F, Georges B, Laurian C, Haguenau M. Spontaneous dissection of the internal carotid artery in 68 patients. Eur J Med 1993;2:466-472 [PubMed] [Google Scholar]
- 9.Pozzati E, Giuliani G, Acciarri N, Nuzzo G. Long-term follow-up of occlusive cervical carotid dissection. Stroke 1990;21:528-531 [DOI] [PubMed] [Google Scholar]
- 10.Martin PJ, Humphrey PR. Disabling stroke arising five months after internal carotid artery dissection [letter]. J Neurol Neurosurg Psychiatry 1998;65:136-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macaya C, Serruys PW, Ruygrok P, et al. Continued benefit of coronary stenting versus balloon angioplasty: one- year clinical follow-up of Benestent trial. Benestent Study Group. J Am Coll Cardiol 1996;27:255-261 [DOI] [PubMed] [Google Scholar]
- 12.Yadav JS, Roubin GS, King P, Iyer S, Vitek J. Angioplasty and stenting for restenosis after carotid endarterectomy. Initial experience. Stroke 1996;27:2075-2079 [DOI] [PubMed] [Google Scholar]
- 13.Hong MK, Satler LF, Gallino R, Leon MB. Intravascular stenting as a definitive treatment of spontaneous carotid artery dissection. Am J Cardiol 1997;79:538. [DOI] [PubMed] [Google Scholar]
- 14.Dorros G, Cohn JM, Palmer LE. Stent deployment resolves a petrous carotid artery angioplasty dissection. AJNR Am J Neuroradiol 1998;19:392-394 [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuura JH, Rosenthal D, Jerius H, Clark MD, Owens DS. Traumatic carotid artery dissection and pseudoaneurysm treated with endovascular coils and stent. J Endovasc Surg 1997;4:339-343 [DOI] [PubMed] [Google Scholar]
- 16.Bejjani GK, Monsein LH, Laird JR, Satler LF, Starnes BW, Aulisi EF. Treatment of symptomatic cervical carotid dissections with endovascular stents. Neurosurgery 1999;44:755-761 [DOI] [PubMed] [Google Scholar]
- 17.DeOcampo J, Brillman J, Levy DI. Stenting: a new approach to carotid dissection. J Neuroimaging 1997;7:187-190 [DOI] [PubMed] [Google Scholar]
- 18.Mericle RA, Lanzino G, Wakhloo AK, Guterman LR, Hopkins LN. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery 1998;43:1229-1234 [DOI] [PubMed] [Google Scholar]
- 19.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. Case report and review of the literature. J Neurosurg 1997;87:944-949 [DOI] [PubMed] [Google Scholar]
- 20.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-607 [DOI] [PubMed] [Google Scholar]
- 21.Mahoney F, Barthel DW. Functional evaluation: the Barthel Index. Stroke Med J 1965;14:61-65 [PubMed] [Google Scholar]
- 22.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Study 1988;10:61-63 [DOI] [PubMed] [Google Scholar]
- 23. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade stenosis. N Engl J Med 1991;325:455-453 [Google Scholar]
- 24.Malek AM, Higashida RT, Phatouros CP, Halbach VV. A strangled wife. Lancet 1999;353:1324. [DOI] [PubMed] [Google Scholar]
- 25.Janjua KJ, Goswami V, Sagar G. Whiplash injury associated with acute bilateral internal carotid arterial dissection. J Trauma 1996;40:456-458 [DOI] [PubMed] [Google Scholar]
- 26.Ben Hamouda-M'Rad I, Biousse V, Bousser MG, et al. Internal carotid artery redundancy is significantly associated with dissection [letter]. Stroke 1995;26:1962. [PubMed] [Google Scholar]
- 27.Schievink WI, Mokri B, Whisnant JP. Internal carotid artery dissection in a community. Rochester, Minnesota 1987–1992. Stroke 1993;24:1678-1680 [DOI] [PubMed] [Google Scholar]
- 28.Bougousslavsky J, Regli F. Ischemic stroke in adults younger than 30 years of age. Cause and prognosis [published erratum appears in Arch Neurol 1987;44:817]. Arch Neurol 1987;44:479-482 [DOI] [PubMed] [Google Scholar]
- 29.Treiman GS, Treiman RL, Foran RF, et al. Spontaneous dissection of the internal carotid artery: a nineteen-year clinical experience. J Vasc Surg 1996;24:597-605 ; discussion 605–607 [DOI] [PubMed] [Google Scholar]
- 30.Coffin O, Maiza D, Galateau-Salle F, et al. Results of surgical management of internal carotid artery aneurysm by the cervical approach. Ann Vasc Surg 1997;11:482-490 [DOI] [PubMed] [Google Scholar]