Abstract
Summary: Diplopia, nystagmus, visual hallucinations, and internuclear ophthalmoplegia developed in a 30-year-old woman 84 days after she received a matched, unrelated bone marrow transplant for chronic myeloid leukemia. A regimen of tacrolimus had been administered since the transplantation was performed. MR imaging revealed bilaterally symmetric regions of signal abnormality with abnormal contrast enhancement in the brain stem. No supratentorial abnormality was present. Tacrolimus therapy was discontinued, and the symptoms resolved. MR imaging that was performed 10 days after tacrolimus was discontinued showed resolution of the abnormalities.
Tacrolimus (FK506; Fujisawa, Deerfield, IL) is an immunosuppressive agent used in bone marrow and solid organ transplantation. Tacrolimus exerts potent inhibitory effects on T-lymphocyte activation by inhibiting calcineurin phosphatase activity (1). Reversible neurotoxic effects have been reported in association with several drugs, including tacrolimus (2–6). This case is a noteworthy manifestation of tacrolimus-induced neurotoxicity for three reasons: 1) large regions of the brain stem were involved in a symmetric fashion, with no supratentorial involvement; 2) the involved portions of the brain stem showed abnormal contrast enhancement; and 3) there was complete resolution of all the imaging and clinical findings after withdrawal of tacrolimus.
Case Report
A 30-year-old woman with chronic myeloid leukemia was treated with 6/6 human lymphocyte antigens matched, unrelated bone marrow from a cytomegalovirus-positive donor. The patient was conditioned with total-body irradiation and cyclophosphamide. Tacrolimus therapy (0.02 mg/kg/day) was administered for graft-versus-host disease prophylaxis. Plasma levels of tacrolimus were maintained within the therapeutic range. Additional graft-versus-host disease prophylaxis was provided by methotrexate and Solu-Medrol. At the time of engraftment, ganciclovir and Bactrim were administered as prophylaxes against infection.
The patient developed a skin rash 39 days after undergoing bone marrow transplantation. The results of a skin biopsy were consistent with graft-versus-host disease. This was accompanied by bone marrow suppression, and the patient required support with blood products, cytokines, and high-dose steroids to sustain her blood counts.
The patient was readmitted to the hospital 80 days after receiving the transplant because of cough and dyspnea. CT of the chest showed diffuse interstitial infiltrates. A bronchoalveolar lavage revealed Aspergillus on special stains; cultures were negative. The lavage was positive for Rous sarcoma virus antigen, a few gram-positive cocci and gram-negative rods, and Candida. Trovafloxacin, single-strength Bactrim, and amphotericin B were administered as empiric antibiotics.
Four days later, the patient experienced an abrupt onset of diplopia and weakness. The results of a fundoscopic examination were normal. The pupils were unequal in size but equally reactive to light. A bilateral sixth cranial nerve palsy was noted. Horizontal nystagmus and a mild proximal weakness (4/5 muscle strength) were observed. The patient was areflexic. She exhibited mild bilateral dysmetria and a positive Babinski's sign on the right side. The results of a sensory examination were normal. There was no seizure activity, the patient was afebrile, and the blood pressure was 142/94 mm Hg.
Laboratory tests revealed thrombocytopenia (27 K/mm3), mild neutropenia (2.5 K/mm3), and mild anemia (hematocrit, 26.1%). There was mild elevation of serum creatinine (144.9 μmol/L), transaminases (aspartate aminotransferase, 21 U/L and alanine aminotransferase, 82 U/L), and prothrombin time (16.4 s). Albumin was low (25 g/L), and the serum sodium concentration was normal at 139 mmol/L.
Conventional MR imaging of the brain was performed using a 1.5-T imager (Magnatom Vision; Siemens Medical Systems, Islin, NJ) with 5-mm contiguous axial sections and the following sequences: spin-echo T1-weighted (700/20/1 [TR/TE/excitations]), fluid-attenuated inversion recovery (9000/110/1), fast spin-echo T2-weighted (4000/99/1), and T1-weighted (700/20/1) after the administration of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ). The images revealed patchy, bilaterally symmetric areas of abnormally increased signal on the T2-weighted and fluid-attenuated inversion recovery images within the midbrain, pons, and medulla oblongata (Fig 1A). These areas of signal abnormality showed abnormal contrast enhancement (Fig 1B). There was no abnormality within the cerebral hemispheres.
fig 1.
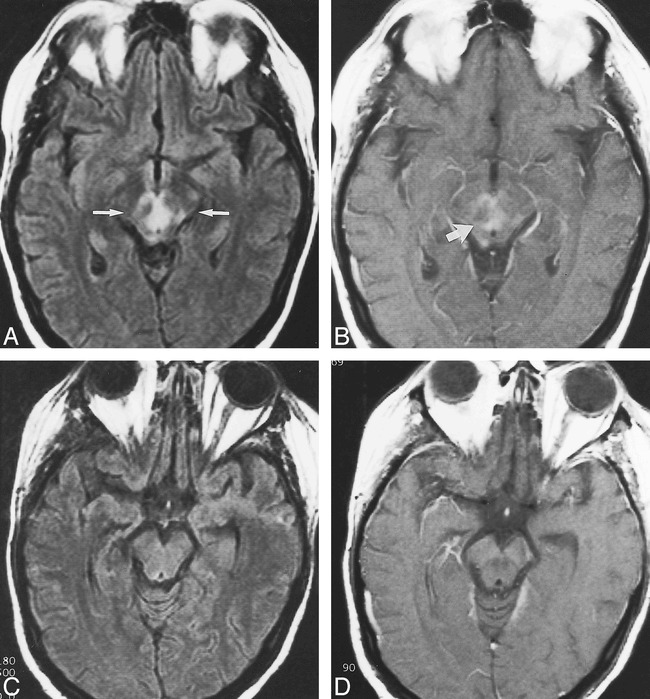
Images from the case of a 30-year-old woman who developed leukoencephalopathy in the brain stem while receiving tacrolimus therapy.
A, Fluid-attenuated inversion recovery (9000/110/1) image from the patient's initial study shows abnormally increased signal intensity bilaterally in the midbrain (between arrows), including the areas of the medial longitudinal fasciculus, trochlear nucleus, and the decussation of the superior cerebellar peduncle.
B, T1-weighted (700/20/1) contrast-enhanced image from the same study, obtained through the same level, shows diffuse abnormal enhancement (arrow) in the same regions.
C, Fluid-attenuated inversion recovery (9000/110/1) image from the follow-up study, after withdrawal of tacrolimus and resolution of symptoms, shows resolution of the previously noted abnormalities. The signal pattern has normalized.
D, T1-weighted (700/20/1) contrast-enhanced image from the follow-up study shows a normal enhancement pattern.
Two days after the MR images were obtained, the patient's condition worsened. She developed visual hallucinations, severe agitation, vertical nystagmus, and internuclear ophthalmoplegia. She experienced a self-limited episode (several seconds in duration) of a few clonic beats of her extremities. No additional seizure activity occurred. Blood pressure at this time was 112/79 mm Hg. Laboratory analysis conducted immediately afterward revealed a mild hyponatremia with a serum sodium concentration of 125 mmol/L. A lumbar puncture revealed acellular CSF with a mild elevation of protein (75.8 g/L) and a normal electrophoretic pattern. Cytomegalovirus proportionate mortality ratio findings were negative. Results of fungal, bacterial, and viral cultures were negative. Amphotericin B continued to be administered while the cultures were pending.
Although the plasma levels of tacrolimus were therapeutic, it was decided to discontinue this medication because a variant of tacrolimus neurotoxicity was suspected. Twenty-four hours after tacrolimus was discontinued, the patient's hallucinations, agitation, and internuclear ophthalmoplegia subsided. The vertical nystagmus resolved 48 hours after tacrolimus was discontinued. Four days after tacrolimus was discontinued, the patient no longer complained of diplopia, and her extraocular movements were intact. The nystagmus had resolved, and the proximal muscle weakness had improved. An MR image of the brain obtained 10 days after withdrawal of tacrolimus showed complete resolution of the previously noted abnormalities (Fig 1C and D).
Discussion
Tacrolimus is a potent immunosuppressive agent used to prevent graft-versus-host disease after bone marrow and other organ transplantation. It is a metabolite produced by the fungus Streptomyces tsukbaenis. Although structurally unrelated to cyclosporin, the cellular effects of these two agents are similar. Tacrolimus exerts potent inhibitory effects on T-lymphocyte activation by inhibiting calcineurin phosphatase activity. Signal transduction pathways in T cells are thus interrupted, reducing the production of interleukin-2, other cytokines, and regulatory proteins necessary for T-cell activation and proliferation (1, 2). The level of drug in the plasma is only an approximate indicator of the effect of the drug. Tacrolimus is erratically and incompletely absorbed. Further, the biological half-life can vary from 3.5 to 40.5 hours. Metabolic by-products of the drug retain activity. For these reasons, it is thought that there is a poor relationship between the emergence of neurologic side effects and plasma levels of the drug; rather, side effects of tacrolimus are thought to be related to the total amount of drug in the body (2, 3).
Both mild and major neurologic adverse effects have been reported in patients receiving tacrolimus. Mild neurologic symptoms, including headache, paresthesias, tremor, sleep disturbances, photophobia, and dysesthesias, have been reported in 40% to 60% of patients, and major neurologic complications, such as confusion, seizures, cortical blindness, encephalopathy, and coma, occur in 5% to 8% of patients receiving the drug (2).
Reversible posterior leukoencephalopathy syndrome is an appellation applied by some authors to the neurologic syndrome characterized by altered mental status, visual loss, stupor, and seizures that are manifested on imaging studies by regions of signal abnormality in the posterior aspects of the cerebral hemispheres (2–4). Dillon and Rowley (5) favor the term reversible posterior cerebral edema syndrome (RPCES) because it more accurately reflects the underlying pathologic abnormality. RPCES has been described as a sequela of generalized, severe seizures, hypertensive crisis, postpartum eclampsia, intermittent porphyria, and blood transfusion. (2–13). RPCES has also been associated with a toxic response to tacrolimus, cyclosporin, and cisplatin. Tacrolimus (FK506)-induced neurotoxicity has been reported in patients who have received several types of transplants (2, 8–10).
The lesions of RPCES, which are hyperintense on T2-weighted and fluid-attenuated inversion recovery images, are thought to be due to extracellular edema. There is a reported predilection for the lesions to occur in the subcortical arcuate fibers of the white matter in the occipital and parietal lobes. The enhancement pattern in RPCES is usually reported to be normal (2–5, 9–12). There is a single report of a patient on tacrolimus who developed a unilateral lesion in the pons (8). That report does not describe any abnormal contrast enhancement or MR imaging–documented resolution of the lesion. We found no previously reported cases of RPCES manifest by symmetric, abnormally enhancing brain stem involvement that showed complete resolution after withdrawal of tacrolimus.
There are several proposed explanations for the development of the lesions seen in RPCES. Hypertension is the most thoroughly studied cause of this syndrome. It is not known whether the primary cause of the edema is an ischemic process triggered by vasospasm and loss of autoregulation in response to increased blood pressure or fluid extravasation through arterioles and capillaries caused by a pressure-driven state (12). Hinchey et al (4) think that the hypertension associated with fluid overload in patients with altered blood-brain barriers explains the findings associated with RPCES (4). If untreated, the syndrome can progress to infarction or hemorrhage (12).
RPCES induced by tacrolimus and cyclosporin occurs at normal systemic blood pressures, and a loss of autoregulation does not seem to apply. The toxic effects of tacrolimus have not yet been defined, but a direct neurotoxicity may be the cause of the white matter edema in these patients. The drug itself may exert a direct neurotoxic effect, disrupt the blood-brain barrier, or interfere with cerebrovascular autoregulation (2, 3). This neurotoxicity may be secondary to local vasoconstriction mediated by endothelin, a neuropeptide (3, 6, 9).
The differential diagnosis of the findings in this case included extrapontine myelinolysis, a demyelinating process, and an inflammatory process. Depending on the pattern of signal abnormalities present, an opportunistic infection (eg, progressive multifocal leukoencephalopathy) or neoplasm (eg, lymphoma) might warrant consideration. In some cases, based on the imaging findings, ischemia/infarction would enter the differential diagnosis. Current data suggest that diffusion-weighted imaging and apparent diffusion coefficient maps can be useful in differentiating reversible posterior leukoencephalopathy syndrome from ischemic stroke because of their ability to separate extracellular edema from cytotoxic edema (12).
This case of acute reversible brain stem edema is of interest because a distribution of lesions in a bilaterally symmetric fashion throughout the brain stem, with sparing of the cerebral hemispheres, has not previously been reported as a manifestation of neurotoxicity secondary to tacrolimus. Also, the lesions in this case showed marked abnormal contrast enhancement, which is unusual in RPCES (2, 4, 5). The lesions in this case are thought to have been secondary to tacrolimus neurotoxicity because of the dramatic and rapid improvement that occurred once tacrolimus was discontinued. Further, there was no antecedent history of hypertensive crisis, fever, transfusion, or seizure activity.
Although the patient had a brief, mild seizure during her clinical course, this was not thought to be the cause of the neurologic deficits and the imaging findings. This single seizure occurred 2 days after initial MR imaging identified the lesions, and there was no seizure activity before the onset of symptoms or documentation of the imaging abnormalities. The cause of the seizure is uncertain but may have been neurotoxicity of tacrolimus or mild hyponatremia, which developed during the time after the initial MR images were obtained. The hyponatremia itself is unexplained and may have been related to the toxic effect of the tacrolimus on the brain stem. That there was rapid and complete resolution of the symptoms and MR signal abnormalities after tacrolimus was discontinued is compelling evidence that it was the cause of the lesions.
Perhaps as more clinical experience with tacrolimus is gained, lesions in other nontypical patterns will be encountered. It is therefore incumbent on those interpreting images to consider an acute “reversible edema syndrome” whenever unusual MR findings are encountered for patients who are receiving immunosuppressive medications or when other serious medical conditions are present.
Footnotes
Address reprint requests to Patrick J. Oliverio, MD, Assistant Professor of Radiology, Georgetown University School of Medicine, Department of Radiology, Neuroradiology Section, 3800 Reservoir Road, NW, Washington, DC 20007.
References
- 1.Spencer CM, Goa KL, Gillis JC. Tacrolimus: an update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs 1997;54:925-975 [DOI] [PubMed] [Google Scholar]
- 2.Appignani BA, Bhadelia RA, Blacklow SC, Wang AK, Roland SF, Freeman RB Jr. Neuroimaging findings in patients on immunosuppressive therapy: experience with tacrolimus toxicity. AJR Am J Roentgenol 1996;166:683-688 [DOI] [PubMed] [Google Scholar]
- 3.Small SL, Fukui MB, Bramblett GT, Eidelman BH. Immunosuppression-induced leukoencephalopathy from tacrolimus. Ann Neurol 1996;40:575-580 [DOI] [PubMed] [Google Scholar]
- 4.Hinchey J, Chaves C, Appignani B, et al. A reversible leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500 [DOI] [PubMed] [Google Scholar]
- 5.Dillon WP, Rowley H. The reversible posterior cerebral edema syndrome [comment]. AJNR Am J Neuroradiol 1998;19:591. [PMC free article] [PubMed] [Google Scholar]
- 6.Tomura N, Kurosawa R, Kato K, et al. Transient neurotoxicity associated with FK506: MR findings. J Comput Assist Tomogr 1998;22:505-507 [DOI] [PubMed] [Google Scholar]
- 7.Steg RE, Kessinger A, Wszolek ZK. Cortical blindness and seizures in a patient receiving FK506 after bone marrow transplantation. Bone Marrow Transplant 1999;23:959-962 [DOI] [PubMed] [Google Scholar]
- 8.Reyes J, Gayowski T, Fung J, Todo S, Alessiani M, Starzl TE. Expressive dysphasia possibly related to FK506 in two liver transplant recipients. Transplantation 1990;50:1043-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shutter LA, Green JP, Newman NJ, Hooks MA, Gordon RD. Cortical blindness and white matter lesions in a patient receiving FK506 after liver transplantation. Neurology 1993;43:2417-2418 [DOI] [PubMed] [Google Scholar]
- 10.Truwit CL, Denaro CP, Lake JR, DeMarco T. MR imaging of reversible cyclosporin A-induced neurotoxicity. AJNR Am J Neuroradiol 1991;12:651-659 [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Arahata Y, Goto Y, et al. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol 1998;19:415-417 [PMC free article] [PubMed] [Google Scholar]
- 12.Ay H, Buonanno FS, Schaefer PW, Le DA, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology 1998;15:1369-1376 [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Niwa H, Iida T, et al. Post-transfusion reversible posterior leukoencephalopathy syndrome with vasoconstriction. Neurology 1997;49:1174-1175 [DOI] [PubMed] [Google Scholar]


