Abstract
BACKGROUND AND PURPOSE: Tumor volume and cartilage invasion have been suggested as prognostic factors of glottic carcinomas following definitive radiation therapy. Radiologic examinations provide additional information regarding the deep extension of tumor. We determined whether dynamic helical CT can predict local control of early (T1 and T2 stage) glottic carcinomas treated with definitive radiation therapy.
METHODS: Sixty-eight patients with early glottic carcinoma evaluated on pretreatment dynamic helical CT were treated with definitive radiation therapy. Tumor detectability, maximum dimension, tumor volume, and involvement of anatomic subsites (anterior commissure, ventricle, subglottic region, and thyroid and arytenoid cartilages) were determined by consensus by three radiologists without previous knowledge of the clinical information. The CT findings were correlated with local control.
RESULTS: The two-year local control rate was 76%; 91% for T1 and 60% for T2 lesions. Univariate analysis revealed clinical T stage, tumor detectability, maximum dimension, tumor volume, anterior commissure involvement, ventricle involvement, and thyroid cartilage involvement as significant prognostic factors. Thyroid cartilage involvement was an independent predictor by multivariate analysis. The lesions separate from the thyroid cartilage had a 95% probability of local control, whereas the lesions adjacent to the cartilage had only a 42% control rate.
CONCLUSION: Dynamic helical CT provides prognostic information for the results of definitive radiation therapy. Patients with a tumor adjacent to the thyroid cartilage had an increased risk of local failure.
Squamous cell carcinoma of the glottis is the most common laryngeal cancer, and is often discovered at the early stage when the patient presents with hoarseness. Radiation therapy is preferred for early (T1- and T2-stage) glottic carcinomas in most institutions, because it has a high cure rate and the voice is retained following therapy (1–3). Many authors have published analyses evaluating a variety of prognostic factors in glottic carcinomas treated with radiation therapy (3–11). Tumor characteristics and treatment schedules may influence local control. It has been suggested that tumor volume is a significant factor in determining the outcome following definitive radiation therapy (8–11). Furthermore, the presence of cartilage invasion reduces the likelihood of local control and may preclude treatment with radiation therapy (1,10–13).
Although mucosal spread of glottic carcinomas is better detected on laryngoscopy, the radiologic findings may provide additional information regarding the deep extension of tumor (1, 14). Pretreatment CT has been shown to be an effective predictor of local control in laryngeal tumors treated with definitive radiation therapy (10, 15). Pameijer et al (10) demonstrated significant differences in local control rates for T3 glottic tumors based on the tumor volume and the lesion spread patterns. Glottic tumors with volumes of 3.5 cm3 or less had an 85% probability of local control, whereas those with volumes greater than 3.5 cm3 had only a 25% control rate. In a similar study of T2 glottic carcinomas, Mukherji et al (16) reported that tumor volume and lesion spread patterns determined by CT did not classify patients into groups more and less likely to be locally controlled with definitive radiation therapy. These results of the previous studies were based on conventional CT that considerably differed from examinations achieved currently with helical CT scanners and automated bolus injections.
Contrast-enhanced dynamic CT has been used for the evaluation of head and neck tumors and lymph nodes (17–19). Helical CT of the larynx is a new technique that permits rapid scanning of large volumes with decreased motion artifacts attributable to respiratory and swallowing movement (19–23). In this study, we evaluated contrast-enhanced dynamic study with helical CT (dynamic helical CT) of early glottic carcinomas in terms of its predictive value for local control with definitive radiation therapy.
Methods
We performed dynamic helical CT to evaluate the feasibility of laryngeal preservation therapies of glottic carcinomas based on the discussion of otolaryngologists, radiation oncologists, and radiologists. Informed consent was obtained from each patient. From 1995 through 1997, 81 patients with early (T1- and T2-stage) glottic squamous cell carcinomas were treated with definitive radiation therapy at our institution. Sixty-eight of these patients were evaluated with dynamic helical CT.
In this study, there were 68 men aged 43 to 93 years (mean, 68 years). Clinical T stage was determined by discussion among otolaryngologists, radiation oncologists, and radiologists. Using the criteria of the Union Internationale Contre le Cancer (24), 21 patients were diagnosed with T1a, 16 patients T1b, and 31 patients T2 tumors, respectively. Definitive radiation therapy with daily fractions ranging from 1.8 to 2.2 Gy delivered by 3 MV X-ray was started within 1 week following radiologic examination. Total radiation doses were determined by both the clinical T stage and the tumor regression during radiation therapy, and included 60–66 Gy for T1a lesions and 64–70 Gy for T1b and T2 lesions. Lower doses were used for tumors evaluated by otolaryngologists as complete remission at 40 Gy irradiation.
All CT scans were obtained with a helical CT scanner (Hispeed Advantage; General Electric Medical Systems, Milwaukee, WI). Images were oriented parallel to the plane of the true vocal cords. Using a power injector (Autoenhance; Nemoto Kyorindo, Tokyo, Japan), 2 mL iopamidol (iopamiron 300; Nihon Schering, Osaka, Japan)/kg body weight was infused at a rate of 2 mL/s. Forty-five seconds following the start of the infusion, a helical scan was initiated to evaluate the primary lesion from the false vocal cords to the base of the cricoid cartilage. Data were acquired using a collimation of 1 mm and a table speed of 1 mm/s (pitch, 1/s). The voltage was 120 kV, the dose was 220 mA, and the field of view was 15 cm. Each study required approximately 30 seconds. Subsequently, a delayed helical scan was performed to evaluate the neck lymph nodes from C1 through the thoracic inlet with contiguous 5-mm-thick sections. The patients were instructed to breathe quietly and to refrain from coughing or swallowing.
Tumor detectability, maximum dimension, tumor volume, and involvement of anatomic subsites (anterior commissure, ventricle, subglottic region, and thyroid and arytenoid cartilages) were determined by consensus of three radiologists (M.F., Y.B., R.N.) without previous knowledge of the clinical information. CT data sets were transmitted to a 3D workstation (Advantage Windows; General Electric Medical Systems) for the volumetric analysis. The lesion was visually outlined on each image in which a mass was present. Then, the tumor volume was calculated by the area on each image and the slice thickness of 1 mm. Patients with a mass on both the inner and outer aspects of the cartilage were considered to have T4 lesions with cartilage invasion and were not included in this study (25, 26). Tumors adjacent to the cartilage were classified as “adjacent,” and tumors separate from the cartilage were “intact.” Presence or absence of irregularity of the cartilage cortex was also recorded. For lesions undetected on dynamic helical CT, maximum dimension, tumor volume, and involvement of anatomic subsites were considered to be “≤10 mm,” “≤1000 mm3,” and “intact,” respectively.
To evaluate the efficacy of radiation therapy on local control, residual tumors following full-dose irradiation and local recurrences during the follow-up period were considered local failures. Patients received regular surveillance in the outpatient clinic every 2 weeks in the first year after finishing radiation therapy and then every 1 to 3 months thereafter. The mean follow-up time was 27 months (range, 12–45 months). When a suspicious lesion was found, biopsy was performed to confirm local failure. Local control was defined as no evidence of disease.
The clinical T stage and CT findings (tumor detectability, maximum dimension, tumor volume, and involvement of anatomic subsites) were correlated with local control. Kaplan-Meier methods were used to estimate the time to local recurrence distribution (27). Outcomes were measured from the first date of radiation therapy to the date of failure or the last date of the follow-up. Log-rank tests were used to determine which covariates were univariately predictive of time to local recurrence. Cox's step-by-step proportional hazards regression model was used for multivariate analysis (28). A value of P < .05 was considered as significant difference.
Results
Forty-three (63%) of 68 lesions, 16 (43%) of 37 T1 lesions, and 27 (87%) of 31 T2 lesions were detected as enhanced masses on dynamic helical CT (Fig 1–4). Sixteen lesions were determined to be local failures; three residual tumors after full-dose irradiation and thirteen local recurrences during the follow-up period. Laryngeal preservation was achieved after surgical salvage in five of 16 patients. Two patients died of the disease. Two-year local control rate with radiation therapy alone was 76%; 91% for T1 and 60% for T2 lesions. Two-year laryngeal preservation and disease-free survival rates after surgical salvage were 83% and 95%, respectively. No evidence of severe radiation-induced complications was seen.
fig 1.
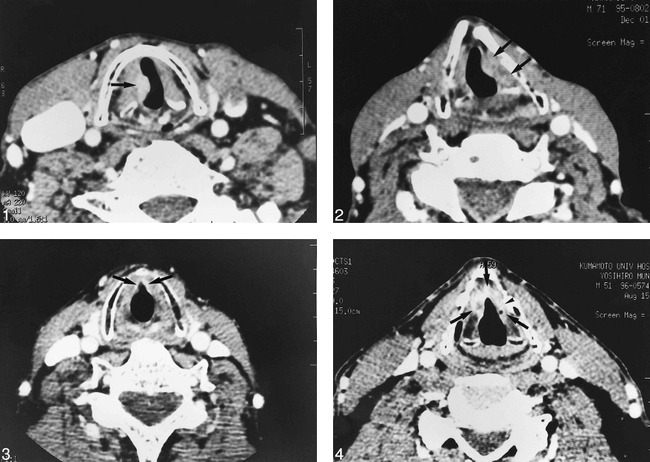
A 68-year-old man with glottic carcinoma of the right true cord (T2 stage). This patient was curatively treated with radiation therapy. Dynamic helical CT shows an enhanced mass in the right true cord with a volume of 545 mm3 (arrow). The thyroid cartilage is intact.fig 2. A 71-year-old man with glottic carcinoma of the left true cord (T2 stage). This patient had a local recurrence after radiation therapy. Dynamic helical CT shows an enhanced mass in the left true cord with a volume of 871 mm3 (arrows). The tumor is adjacent to the left thyroid cartilage with irregular cortex.fig 3. A 68-year-old man with an anterior commissure tumor (T2 stage). This patient was curatively treated with radiation therapy. Dynamic helical CT shows an enhanced area in the anterior third of the bilateral true cords with a volume of 152 mm3 (arrows). The lesion involves the anterior commissure, but the thyroid cartilage is intact.fig 4. A 51-year-old man with an anterior commissure tumor (T2 stage). This patient had a local recurrence after radiation therapy. Dynamic helical CT shows an enhanced mass of the bilateral true cords with a volume of 429 mm3 (arrows). The tumor is adjacent to the anterior half of the left thyroid cartilage with irregular cortex (arrowhead)
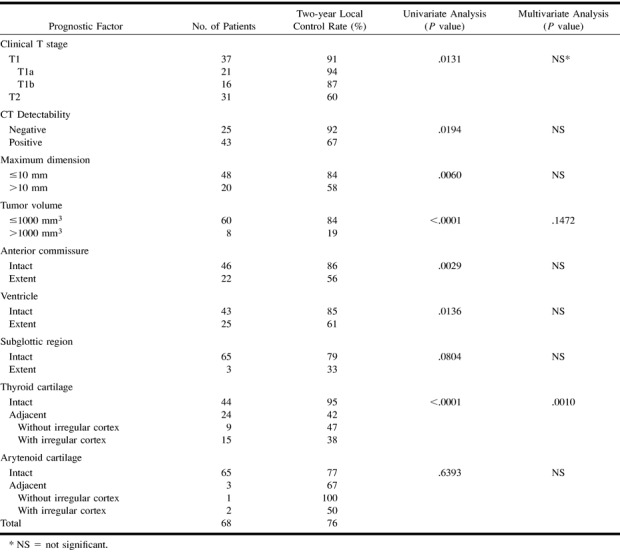
Local control rates with respect to the variables are given in Table 1. Univariate analysis revealed clinical T stage (P=.0131), tumor detectability (P=.0194), maximum dimension (P=.0060), tumor volume (P<.0001), anterior commissure involvement (P=.0029), ventricle involvement (P=.0136), and thyroid cartilage involvement (P<.0001) as significant prognostic factors. Thyroid cartilage involvement was an independent predictor by multivariate analysis. The lesions separate from the thyroid cartilage had a 95% probability of local control, whereas the lesions adjacent to the cartilage had only a 42% control rate. In 15 of 24 adjacent lesions, there was irregularity of the cartilage cortex and tendency of poor prognosis. Local control rates of adjacent lesions with and without irregular cortex were 38% and 47%, respectively (Fig 2 and 4).
TABLE 1:
Prognostic factors for local control of all lesions
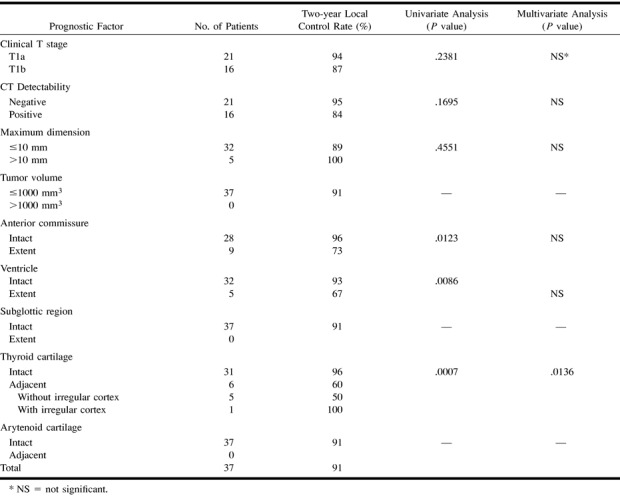
Prognostic factors for local control of each clinical T stage are given in Tables 2 and 3. For T1 lesions, factors found to have a significant impact were anterior commissure involvement (P=.0123), ventricle involvement (P=.0086), and thyroid cartilage involvement (P=.0007), and multivariate analysis revealed thyroid cartilage involvement as an independent predictor. In T2 lesions, significant prognostic factors were tumor volume (P=.0082) and thyroid cartilage involvement (P=.0045), and multivariate analysis revealed thyroid cartilage involvement as an independent predictor of local failure. T2 lesions with volumes of 1000 mm3 or less had a 73% probability of local control, whereas those with volumes greater than 1000 cm3 had only a 19% control rate. T2 lesions with intact thyroid cartilage had a 92% probability of local control, whereas those adjacent to the thyroid cartilage had only a 36% control rate.
TABLE 2:
Prognostic factors for local control of T1 lesions
Discussion
Dynamic CT has been used for the evaluation of head and neck tumors and lymph nodes (17–19). The method of contrast enhancement provides information about the boundaries of masses, their vascularity, and their relationship to surrounding structures (18). Helical CT is a new technique that permits rapid scanning of large volumes with minimal motion artifacts or respiratory misregistration (20–22). Furthermore, multiplanar and three-dimensional reconstructed images can easily demonstrate the anatomic structures (22, 23). We evaluated the early glottic carcinomas with dynamic helical CT to assess its predictive value for local control with radiation therapy.
Radiation therapy for early glottic carcinomas has been established with local control rates of 80% to 95% for T1 and 65% to 85% for T2 lesions (3–9). In our study population (Table 1), local control rates of 91% for the T1 lesions was compatible with previous reports of definitive radiation therapy. However, 60% for the T2 lesions was lower than that of other results. The T stage of the TNM system for glottic carcinomas is based on anatomic features by physical and laryngoscopic examination (24). Although T stage predicts the rate of local control with definitive radiation therapy, radiologic findings provide additional information (10–13). Our T2 lesions probably included more tumors with adverse factors such as thyroid cartilage involvement and large volume. In the radiologic evaluation, we must consider not only the tumor characteristics but also the patient outcome (29).
Early invasion of cartilage may be difficult to diagnose by CT because of the variability in ossification of the cartilage (25, 30). Becker et al (26) suggested that detection of neoplastic cartilage invasion with CT was greatly dependent on the appropriate use of individual and combined CT criteria. In this study, CT findings were correlated with local control after definitive radiation therapy and not with microscopic findings. The relationship between the tumor and cartilage was easily classified into “intact” and “adjacent” (Fig 1–4). This classification of thyroid cartilage involvement showed an independent predictor for local control with definitive radiation therapy. Furthermore, there was the tendency of poor prognosis in adjacent lesions with irregularity of the cartilage cortex (Table 1). There was a possibility of increased risk of local failure attributable to cartilage invasion in adjacent lesions. Further examinations were necessary to evaluate histopathologic correlation with dynamic helical CT findings because there was no histopathologic evidence in this study.
Some authors have demonstrated that risk factors of local failure appear to be associated with impaired mobility of the vocal cord, indicating tumor growth in the transverse direction (5–9). However, there is a lack of objective criteria for quantifying the cord mobility (7). Tumor adjacent to the thyroid cartilage may indicate not only possibility of cartilage invasion but also possibility of impaired cord mobility.
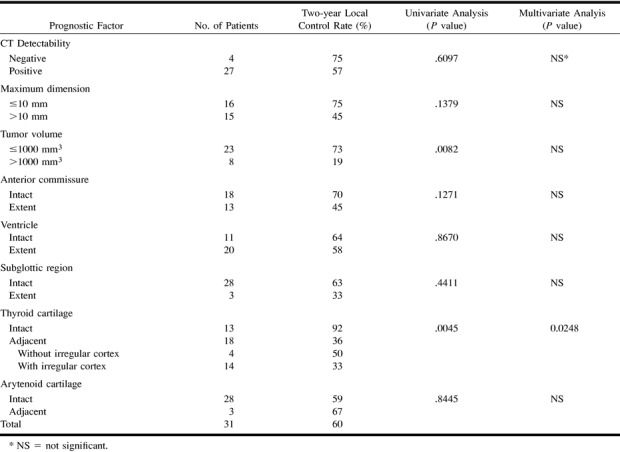
Tumor volume has been considered as an important prognostic factor for local control in glottic carcinomas treated with definitive radiation therapy (8–11). Pameijer et al (10) demonstrated that low-volume T3 glottic carcinomas revealed by CT, with the value of 3.5 cm3 for a volume cutoff, were significantly controlled with definitive radiation therapy. We classified early glottic carcinomas with the cutoff volume of 1.0 cm3. Although multivariate analysis in this study did not show the predictive value of tumor volume, univariate analysis revealed that tumor volume was a significant factor in outcome of T2 lesions (Table 3). T1 lesions limited to vocal cord are suggested to be small in volume, but T2 and T3 lesions can be either small or large in volume. Thyroid cartilage involvement is an important prognostic factor in T1 lesions that are small in volume (Table 2). T3 lesions with vocal cord fixation are suggested to be adjacent to the thyroid cartilage, but T1 and T2 lesions can be either separate from or adjacent to the cartilage. Tumor volume is an important prognostic factor in T3 lesions that are adjacent to the thyroid cartilage. In T2 lesions, both tumor volume and thyroid cartilage involvement can be considered to be significant prognostic factors (Table 3).
TABLE 3:
Prognostic factors for local control of T2 lesions
Involvement of the anterior commissure has been implicated as a poor prognostic factor in some reports (6, 9). Burke et al (6), however, suggested that anterior commissure involvement was no longer a significant factor after adjustment for cord mobility, fraction size, and field size. Mukherji et al (16) demonstrated that anterior commissure tumors without evidence of cartilage invasion or bulky mass were curatively treated with definitive radiation therapy. In this study, univariate analysis revealed that anterior commissure involvement was a significant factor, but multivariate analysis did not show the predictive value (Tables 1 and 2). Anterior commissure tumors with intact thyroid cartilage revealed by dynamic helical CT may be controlled with definitive radiation therapy (Fig 3).
Although clinical T stage was determined by discussion among otolaryngologists, radiation oncologists, and radiologists, our T1 lesions included five lesions with ventricle involvement on dynamic helical CT. Univariate analysis of T1 lesions revealed ventricle involvement as a significant factor (P=.0086) (Table 2). The lesions with a 67% control rate might have been considered as T2 stage.
In 25 (37%) of 68 our patients, tumors were not detected using dynamic helical CT. The tumors were probably superficial mucosal lesions without deep extension, and had a 92% chance of the local control with radiation therapy. There may be other prognostic factors that cannot be explained on the basis of CT findings. This is likely because of the biological characteristics and tumor-host interactions, which are still not sufficiently understood (16, 31).
Conclusion
Dynamic helical CT provides prognostic information for the patients with early (T1- and T2-stage) glottic carcinomas following definitive radiation therapy. Univariate analysis revealed tumor detectability, maximum dimension, tumor volume, anterior commissure involvement, ventricle involvement, and thyroid cartilage involvement as significant prognostic factors. Thyroid cartilage involvement was an independent predictor by multivariate analysis. The lesions separate from the thyroid cartilage had a 95% probability of local control, whereas the lesions adjacent to the cartilage had only a 42% control rate. Patients with a tumor adjacent to the thyroid cartilage had an increased risk of local failure following radiation therapy.
Footnotes
Address reprint requests to Ryuji Murakami, MD, Department of Radiology, Kumamoto University School of Medicine, 1–1–1 Honjo, Kumamoto 860, Japan.
References
- 1.Mukherji SK, Pillsbury HR, Castillo M. Imaging squamous cell carcinoma of the upper aerodigestive tract: what clinicians need to know. Radiology 1997;205:629-646 [DOI] [PubMed] [Google Scholar]
- 2.Million RR, Cassisi NJ, Mancuso AA. Larynx. In: Million RR, Cassisi NJ, eds. Management of Head and Neck Cancer: A Multidisciplinary Approach. 2nd ed. Philadelphia: Lippincott 1994 431-497
- 3.Million RR. The larynx, so to speak: everything I wanted to know about laryngeal cancer I learned in the last 32 years. Int J Radiat Oncol Biol Phys 1992;23:691-704 [DOI] [PubMed] [Google Scholar]
- 4.Le QT, Fu KK, Kroll S, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiation Oncol Biol Phys 1997;39:115-126 [DOI] [PubMed] [Google Scholar]
- 5.Fein DA, Mendenhall WM, Parsons JT, Million RR. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiotherapy: a multivariate analysis of variables potentially influencing local control. Int J Radiat Oncol Biol Phys 1993;25:605-611 [DOI] [PubMed] [Google Scholar]
- 6.Burke LS, Greven KM, McGuirt WT, Case D, Hoen HM, Raben M. Definitive radiotherapy for early glottic carcinoma: prognostic factors and implication for treatment. Int J Radiat Oncol Biol Phys 1997;38:1001-1006 [DOI] [PubMed] [Google Scholar]
- 7.Ang KK, Peters L. Vocal cord cancer: 2b worse than not 2b? Radiother Oncol 1990;8:365-366 [DOI] [PubMed] [Google Scholar]
- 8.Karim ABMF, Kralendonk JH, Yap LY, et al. Heterogeneity of stage II glottic carcinoma and its therapeutic implications. Int J Radiat Oncol Biol Phys 1987;13:313-317 [DOI] [PubMed] [Google Scholar]
- 9.Mantravadi RVP, Liebner EJ, Haas RE, Skolnik EM, Applebaum EL. Cancer of the glottis: prognostic factors in radiation therapy. Radiology 1983;149:311-314 [DOI] [PubMed] [Google Scholar]
- 10.Pameijer FA, Mancuso AA, Mendenhall WM, Parsons JT, Kubilis PS. Can pretreatment computed tomography predict local control in T3 squamous cell carcinoma of the glottic larynx treated wtih definitive radiotherapy alone? Int J Radiat Oncol Biol Phys 1997;37:1011-1021 [DOI] [PubMed] [Google Scholar]
- 11.Castelijns JA, van den Brekel MWM, Smit EMT, et al. Predictive value of MR imaging-dependent and non-MR imaging-dependent parameters for recurrence of laryngeal cancer after radiation therapy. Radiology 1995;196:735-739 [DOI] [PubMed] [Google Scholar]
- 12.Castelijns JA, van den Brekel MWM, Tobi H, Smit EMT, Golding RP, van Schaik C, Snow GB. Laryngeal carcinoma after radiation therapy: correlation of abnormal MR imaging signal patterns in laryngeal cartilage with the risk of recurrence. Radiology 1996;198:151-155 [DOI] [PubMed] [Google Scholar]
- 13.Isaacs JH, Mancuso AA, Mendenhall WM, Parsons JT. Deep spread patterns in CT staging of T2–4 squamous cell laryngeal carcinoma. Otolaryngol Head Neck Surg 1988;99:455-464 [DOI] [PubMed] [Google Scholar]
- 14.Curtin HD. Imaging of the larynx: current concepts. Radiology 1989;173:1-11 [DOI] [PubMed] [Google Scholar]
- 15.Freeman DE, Mancuso AA, Parsons JT, et al. Irradiation alone for supraglottic carcinoma: can CT findings predict treatment results? Int J Radiat Oncol Biol Phys 1990;19:485-490 [DOI] [PubMed] [Google Scholar]
- 16.Mukherji SK, Mancuso AA, Mendenhall W, Kotzur IM, Kubilis P. Can pretreatment CT predict local control of T2 glottic carcinomas treated wih radiation therapy alone? AJNR Am J Neuroradiol 1995;16:655-662 [PMC free article] [PubMed] [Google Scholar]
- 17.Michael AS, Mafee MF, Valvassori GE, Tan WS. Dynamic computed tomography of the head and neck: differential diagnostic value. Radiology 1985;154:413-419 [DOI] [PubMed] [Google Scholar]
- 18.Gay SB, Pevarski DR, Phillips CD, Levine PA. Dynamic CT of the neck. Radiology 1991;180:284-285 [DOI] [PubMed] [Google Scholar]
- 19.Mukherji SK, Castillo M, Huda W, et al. Comparison of dynamic and spiral CT for imaging the glottic larynx. J Comput Assist Tomogr 1995;19:899-904 [DOI] [PubMed] [Google Scholar]
- 20.Suojanen JN, Mukherji SK, Wippold FJ. Spiral CT of the larynx. AJNR Am J Neuroradiol 1994;15:1579-1582 [PMC free article] [PubMed] [Google Scholar]
- 21.Robert Y, Rocourt N, Chevalier D, Duhamel A, Carcasset S, Lemaitre L. Helical CT of the larynx: a comparative study with conventional CT scan. Clinical Radiol 1996;51:882-885 [DOI] [PubMed] [Google Scholar]
- 22.Silvrman PM, Zeiberg AS, Sessions RB, Troost TR, Davros WJ, Zeman RK. Helical CT of the upper airway: normal and abnormal findings on three-dimensional reconstructed images. AJR Am J Radiol 1995;165:541-546 [DOI] [PubMed] [Google Scholar]
- 23.Steinkamp HJ, Hosten N, Richter C, Schedel H, Felix R. Enlarged cervical lymph nodes at helical CT. Radiology 1994;191:795-798 [DOI] [PubMed] [Google Scholar]
- 24. International Union against Cancer (UICC). TNM Classification of Malignant Tumour.. 4th ed, 2nd revision. Heidelberg, Germany: Springer;1992
- 25.Becker M, Zbaren P, Laeng H, Stoupis C, Porcellini B, Vock P. Neoplastic invasion of the laryngeal cartilage: comparison of MR imaging and CT with histopathologic correlation. Radiology 1995;194:661-669 [DOI] [PubMed] [Google Scholar]
- 26.Becker M, Zbaren P, Delavelle J, et al. Neoplastic invasion of the laryngeal cartilage: reassessment of criteria for diagnosis at CT. Radiology 1995;194:661-669 [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc 1958;53:457-481 [Google Scholar]
- 28.Cox DR. Regression models and life table. J R Stat Soc 1972;34:187-220 [Google Scholar]
- 29.Curtin HD. Importance of imaging demonstration of neoplastic invasion of laryngeal cartilage. Radiology 1995;194:643-644 [DOI] [PubMed] [Google Scholar]
- 30.Mafee MF, Schild JA, Valvassori GE, Capek V. Computed tomography of the larynx: correlation with anatomic and pathologic studies in cases of laryngeal carcinoma. Radiology 1983;147:123-128 [DOI] [PubMed] [Google Scholar]
- 31.Walter MA, Peters GE, Peiper SC. Predicting radioresistance in early glottic squamous cell carcinoma by DNA content. Ann Otol Rhinol Laryngol 1991;100:523-526 [DOI] [PubMed] [Google Scholar]


