Abstract
BACKGROUND AND PURPOSE: Surgical repair of spinal metastases from renal origin is often complicated by excessive bleeding. The purpose of this study was to assess the effect of preoperative particulate embolization on intraoperative blood loss.
METHODS: Twenty spinal metastases from renal origin (17 patients) treated by preoperative embolization with polyvinyl alcohol particles were analyzed retrospectively. Surgical decompression was performed within 2 days after embolization. A control group of 10 patients with 11 spinal metastases of renal origin underwent surgery without embolization. The effect of preoperative embolization, of completeness of embolization, and of particle size on the estimated intraoperative blood loss was analyzed using nonparametric statistical tests.
RESULTS: Complete embolization was achieved in 10 cases and partial embolization in the other 10. The estimated blood loss of 19 embolized and 11 control cases was available from the surgical report. Median intraoperative blood loss in 19 embolized lesions was 1500 mL (range, 300–8000 mL), compared with 5000 mL (range, 1440–15000 mL) in the control group. Even after partial embolization, blood loss (median, 2000 mL) was significantly lower than in the control group. No significant differences in estimated blood loss were noted between the use of particles smaller than 250 μm and those larger than 250 μm. No embolization-related permanent neurologic deficit or skin or muscle necrosis occurred.
CONCLUSION: Preoperative embolization of spinal metastases of renal origin with polyvinyl alcohol particles is safe and might reduce intraoperative blood loss significantly. Even partial embolization seems to be effective.
About 40% of patients with renal cell carcinoma develop bone metastases. Renal cell carcinoma is the fourth most common metastatic tumor of the spine and the most common cancer to present as a neurologic deficit secondary to an undetected primary malignancy (1). Chemotherapy and hormone therapy are ineffective, making radiation and surgery the mainstays of treatment. Failure to respond to or relapse after radiotherapy is common (2, 3). Osseous destruction is often associated with mechanical instability, intractable pain, radiculopathy, and symptoms of cord compression. Anterior surgical decompression and stabilization are the treatments of choice for radiation-resistant metastatic spinal tumors, resulting in improved neurologic function and pain reduction in more than 80% of patients (4, 5). Vertebral metastases of renal origin are highly vascular and often cause life-threatening intraoperative bleeding (6, 7). Embolization has been used as a presurgical maneuver in the treatment of a variety of primary and secondary spinal tumors (6–22). There is, however, limited data about the effect of preoperative embolization on intraoperative blood loss in spinal metastases of renal origin (6, 7, 15).
The purpose of this retrospective study was to evaluate the effects of state-of-the-art selective embolization with polyvinyl alcohol (PVA) particles on intraoperative blood loss in vertebral metastases of renal cell carcinoma. The effects of the completeness of embolization and the size of the embolization particles were evaluated.
Methods
Patients
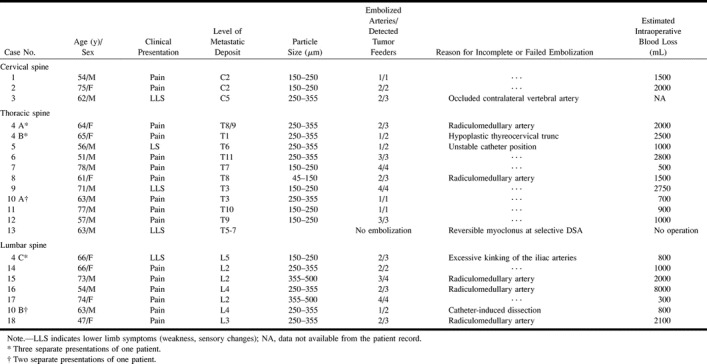
This retrospective study included 18 consecutive patients with 21 symptomatic spinal metastases who underwent preoperative spinal angiography and embolization at our institution between October 1995 and August 1999. All patients had histologically proved metastases of renal carcinoma origin. Patient data are further specified in Table 1. The 12 men and six women were between 47 and 78 years of age (mean age, 64 years) at their first presentation. The metastatic lesions were located in the cervical spine (n = 3, 14.3%), the thoracic spine (n = 11; 52.4%), and the lumbar spine (n = 7; 33.3%). In two (9.5%) of the 21 cases, the tumor extended to two adjacent levels. Primary symptoms were intractable back pain and/or radicular pain in 16 cases and lower limb weakness, spasticity, or sensory changes due to cord or nerve root compression in five cases (20.8%).
TABLE 1:
Surgical decompression of spinal metastases with preoperative embolization
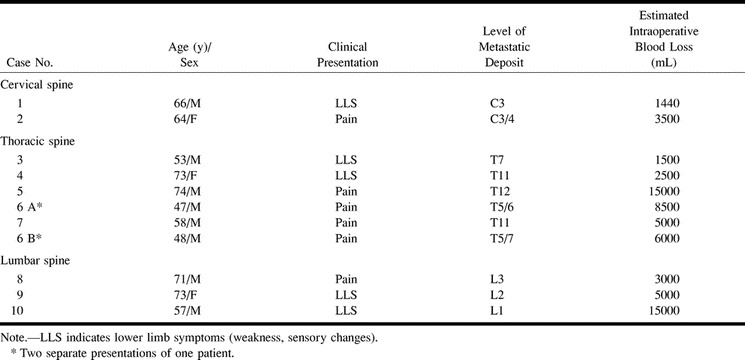
The control group consisted of 10 consecutive patients with 11 spinal metastases of renal carcinoma, which were treated surgically without preoperative embolization between April 1994 and April 1997. Control group data are further specified in Table 2. These patients underwent operation without preoperative embolization in seven instances because the method was not available at our institution before October 1995 and in four instances for reasons that defied explanation retrospectively. These seven men and three women were between 47 and 74 years of age (mean age, 64 years) at their first presentation. The metastatic lesions were located in the cervical spine (n = 2; 18.2%), the thoracic spine (n = 6; 54.5%), and the lumbar spine (n = 3; 27.3%). In three (27.3%) of 11 cases, the tumor extended to two adjacent levels. Primary symptoms were intractable pain in six cases (54.5%) and indications of cord or cauda compression in five cases (45.5%).
TABLE 2:
Surgical decompression of spinal metastases without preoperative embolization (control group)
Spinal Angiography and Embolization
All embolization procedures were performed under local anesthesia via a femoral sheath to facilitate catheter exchange. When necessary, midazolam hydrochloride, piritramide hydrogentartrat, or pethidine hydrochloride were administered intravenously for sedation and analgesia. Digital subtraction angiograms were carefully evaluated for a spinal cord supply and for tumor feeding pedicles using nonionic contrast material.
The angiographic technique has been described previously (23–25). After global aortography, selective bilateral catheterization of the corresponding segmental arteries (including one level above and below) was performed. For cervical and upper thoracic tumors, the vertebral artery and the thyrocervical and costocervical trunk were selectively catheterized. For lesions of the lower lumbar spine, both internal iliac arteries were catheterized additionally. Various preshaped 5F catheters were used (C2, JB1, RIM, or SIM1; Cook, Inc, Bloomington, IN), depending on the origin of the feeding arteries.
Feeding arteries were superselectively catheterized using a coaxial catheter system (Tracker 18, Target Therapeutics, Fremont, CA, or Rapid Transit, Cordis, Miami Lakes, FL). For embolization, PVA particles of 45- to 150-μm diameter were used in one case, 150- to 250-μm diameter in seven cases, 250- to 355-μm diameter in 10 cases, and 355- to 500-μm diameter in two cases (Contour, Target Therapeutics). One vial of particles was suspended in 50 mL normal strength nonionic contrast medium. Pedicles with a spinal cord vascular supply were not embolized. In one patient, embolization could not be performed owing to reversible neurologic symptoms during selective angiography. Of the remaining 20 lesions, 10 were embolized completely and 10 partially. Incomplete embolization was due to a radiculomedullary artery originating from the same pedicle as the tumor in five cases. In four cases, segmental arteries were not embolized because of catheter-induced dissection of the feeder, hypoplastic tumor feeders, unstable catheter position, or excessive kinking of the iliac arteries (Table 1). In the remaining case, tumor-supplying branches of a vertebral artery were not embolized after balloon occlusion of the contralateral vertebral artery.
To avoid downstream embolization of lumbar and intercostal arteries (17), the artery distal to the origins of tumor feeders was occluded using platinum (Target Therapeutics) or tungsten (Balt, Montmorency, France) coils in 11 cases (Fig 1). In three cases, cervical metastases were supplied by small branches of the vertebral artery. The vertebral artery was occluded temporarily with a latex balloon occlusion catheter (Meditech, Boston Scientific, Watertown, MA). When the patient remained neurologically asymptomatic for 20 minutes, the vertebral artery was occluded distal to the origins of the tumor feeders with a detachable latex balloon (GVB, Nycomed-Ingenor, Paris, France) and the tumor vessels were obliterated with PVA particles (Fig 2).
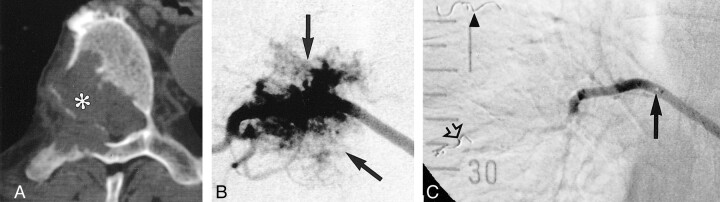
fig 1.
A, CT scan in a patient with metastasis involving the T7 vertebral body (asterisk).
B, Selective angiogram of the right 7th intercostal artery with a 5F Cobra-shaped catheter shows a hypervascular mass (arrows) and no arterial supply to the spinal cord.
C, Injection after further selective catheterization with a 3F Tracker catheter (solid arrow indicates catheter tip), protective embolization of intercostal artery distal to the tumor feeders with a platinum coil (open arrow), and embolization with PVA particles. No tumor blush is identified. A coil (arrowhead) is seen in the 6th right intercostal artery after previous embolization
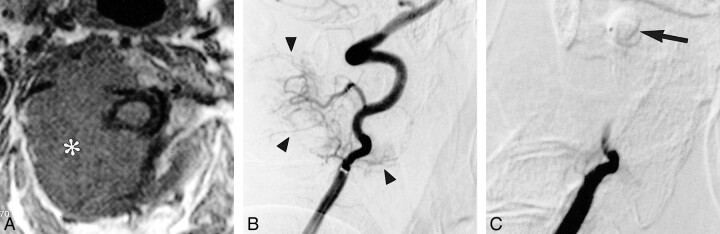
fig 2.
A, T1-weighted axial MR image shows a large metastasis (asterisk) involving the C2 vertebral body and extending into the surrounding soft tissues.
B, Early arterial phase of a preembolization right vertebral angiogram shows a hypervascular mass (arrowheads) supplied by small cervical branches of the vertebral artery.
C, Right vertebral angiogram obtained after balloon occlusion of the vertebral artery at the level of C1 (arrow) and embolization with PVA particles. No residual tumor stain is visible
Angiograms and angiographic reports were analyzed retrospectively for completeness of embolization. Embolization was judged to be complete if 75% of the tumor stain and all angiographically detectable feeding pedicles were obliterated. Embolization was judged to be partial if there was less than 75% obliteration of the tumor vascularity or at least one major feeding artery not embolized. Clinical success was defined as less than 3000 mL estimated blood loss (EBL) at surgery (13).
Surgical Resection
All patients were operated on by the same team of four experienced orthopedic surgeons using standardized techniques. Surgery was scheduled by the orthopedic surgeon within 2 days after embolization. Surgery was performed on the same day as embolization in five cases, on the following day in 14 cases, and on day 2 in one case. The surgical approach was dependent on the anatomic site of the osseous destruction and the soft tissue tumor. Usually, the lesions were decompressed anteriorly by vertebrectomy and stabilized by metal-reinforced polymethylmethacrylate and internal fixation (6). Laminectomy and posterolateral decompression were performed when the tumor had destroyed not only anterior but also lateral or posterior elements (26). Intraoperative blood loss was estimated by the anesthesiologist and noted in the surgical report.
Statistical Method
Results with P < .05 were considered significant. The occurrence rate of severe hemorrhage (≥3000 mL) among embolized lesions was compared with that in the control group using the Fisher exact test.
The EBL for control and embolized lesions was compared using the nonparametric Mann-Whitney rank sum test. Subgroups were compared by means of the same test: the intraoperative EBL of the control lesions was compared with the EBL of the partially embolized lesions. Intraoperative EBL of partially and completely embolized lesions was compared. The intraoperative EBL of lesions embolized with small and medium-size (45–250 μm) particles was compared with the EBL of lesions that were embolized with larger (250–500 μm) PVA particles.
Results
All patients who underwent spinal angiography had hypervascular metastases. One patient with an incomplete paraparesis and metastasis of the fifth to seventh thoracic vertebra experienced persisting myoclonus of the right leg after contrast injection into the fifth left intercostal artery, although no spinal-supplying artery was identified on the angiograms (case 13, Table 1). Neurologic symptoms subsided after selective intraarterial injection of 10 mg diazepam, and no further attempt to embolize the lesion was undertaken. The lesion was considered to be inoperable by the orthopedic surgeon and the patient was treated conservatively. The remaining 20 lesions were embolized completely in 10 cases and partially in 10 cases for reasons noted above. The median number of embolized arteries was two (range, one to four). No embolization-related permanent neurologic deficit nor skin or muscle necrosis was noted.
Data for completeness of embolization, size of particles used, and EBL are given in Tables 1 and 2. Data for intraoperative EBL were available for 19 of 20 embolized lesions and for all 11 nonembolized lesions. No intraoperative EBL was noted in the surgical report for one partially embolized cervical lesion. The rate of severe intraoperative hemorrhage (≥3000 mL) was significantly higher in the control group than in the embolized group (eight of 11 vs one of 19 cases, P < .001). Intraoperative EBL was significantly less among embolized lesions than among nonembolized lesions (median, 1500 mL vs 5000 mL; P < .001). Intraoperative EBL of partially embolized lesions (n = 9) was also significantly lower than among nonembolized lesions (median, 2000 mL vs 5000 mL; P = .02). Although no significant difference in EBL between complete (n = 10) and partial (n = 9) embolization was observed, there was a trend toward higher blood loss if lesions were embolized incompletely (median, 1000 mL vs 2000 mL; P = .287). The only severe intraoperative hemorrhage after embolization (EBL, 8000 mL) occurred after an L4 metastasis was embolized incompletely (two of three supplying lumbar arteries). One lumbar artery gave rise to a radiculomedullary artery and hence was not embolized. No significant differences in EBL between the use of PVA particles smaller than 250 μm (n = 8) and larger than 250 μm (n = 11) were noted (median, 1250 mL vs 2000 mL; P = .507).
In two of the 10 patients who underwent surgery without preoperative embolization, the operation had to be terminated prematurely due to a life-threatening intraoperative hemorrhage.
Discussion
Although surgical management of symptomatic spinal metastases is strictly palliative, it improves quality of life. Neurologic deficit improves after surgery in 58% to 80% of patients (4, 26). Vertebrectomy in spinal metastases requires intralesional curettage of the tumor, whereas peripheral lesions (eg, femoral neck metastases) are usually resected radically without opening the tumor. Stabilization of spinal metastases of renal origin is occasionally complicated by catastrophic intraoperative hemorrhage of more than 10 L (6, 7, 15). Although early studies failed to show significant effects (6, 16), preoperative embolization appeared to be an effective method to decrease intraoperative blood loss. The limitations of most studies on preoperative spinal embolization include inhomogeneous spinal diseases, the use of a variety of embolization agents, and the lack of a control group. Several retrospective studies have reported a mean or median intraoperative blood loss between 1540 and 4300 mL after embolization of spinal tumors of various origin (7, 9–11, 13, 15, 19, 22). In our homogeneous collective, the mean intraoperative blood loss of 1797 mL compares well with these published figures. In our patient group, intraoperative hemorrhage was significantly lower than in the control group, even with incomplete embolization of the lesion. However, there was a trend toward higher blood loss as compared with the completely embolized lesions, and the only severe hemorrhage (8000 mL) during operation occurred in a partially embolized lesion. Surgeons have to be aware of this risk, although partial embolization seems to reduce blood loss effectively in most patients. Several studies have described excessive intraoperative bleeding after incomplete preoperative embolization of spinal metastases when embolization of tumor feeders was impossible (16).
Retrospective studies are of limited value in determining the exact effect of a certain form of treatment. Blood loss depends on the individual skills of the surgeon and is difficult to estimate. In our study, intraoperative blood loss has been quantified by the anesthesiologist by means of cell savers, reinfusion of irradiated red blood cells (27), and estimation of the blood remaining on swabs and sheets. Our patients were operated on by the same team of four orthopedic surgeons using standardized techniques. Since all surgeons had had several years of experience with the repair of spinal lesions before the beginning of this study, the observed differences are unlikely to be only the result of increasing surgical experience. Although our two study groups were comparable in age and tumor location, the lower rate of lower limb symptoms and extension of tumor to more than one level in the embolized group suggests bias in patient selection: patients with pain and less extensive tumor masses, who would otherwise be treated conservatively, probably underwent surgery after being treated by preoperative embolization. Therefore, a prospective, randomized study would be desirable for an exact evaluation of the method. However, a randomized study comparing intraoperative hemorrhage with and without embolization would be impossible to perform from an ethical point of view. Embolization with particles is a safe and minimally invasive procedure in experienced hands. Most orthopedic surgeons agree that preoperative embolization is a prerequisite for vertebrectomy procedures in hypervascular and, especially, renal metastatic disease of the spine (6, 7, 15).
Different embolization agents have been used for preoperative embolization of spinal metastases, including alcohol, tissue adhesive, coils, gelatin sponges, and PVA particles. The use of liquid embolization agents (eg, ethanol, tissue adhesive) for spinal metastases is associated with a high rate of neurologic complications, even in experienced hands (17, 24). Berkefeld et al (9) found no significant effect of preoperative coil embolization on intraoperative blood loss in hypervascular spinal metastases as compared with a control group. The use of coils seems to be ineffective, since metastases from renal cell carcinoma show an angiomatous vascularization that may open collateral channels within hours. The same study suggested that the additional use of coils after PVA particle embolization provides no further benefit. Gelfoam particles have been used in early studies for preoperative embolization of spinal metastases. However, this gelatin sponge has no defined size, is degraded by enzymes, and results only in temporary occlusion. In one study, rapid revascularization of the tumor was observed within 3 days from adjacent collaterals, resulting in considerable intraoperative bleeding (13). Two other reports also suggested a less reliable control of intraoperative bleeding as compared with nonresorbable PVA particles of defined size (15, 19). Our findings suggest that the use of larger particles (250–355 μm or 355–500 μm) has no significant impact on intraoperative blood loss as compared with medium or small particles (<250 μm). We generally prefer the use of larger particles for this indication, since smaller particles may compromise arterial cord supply below the level of collaterals. Nonetheless, proper angiographic technique with frequent angiograms obtained during embolization is necessary to avoid inadvertent embolization of cord-supplying arteries via intersegmental anastomoses. These anastomoses usually open at the end of particle embolization (24).
Protective coil or balloon embolization of arteries distal to the origin of tumor feeders was applied to avoid inadvertent downstream embolization. This procedure is absolutely necessary for tumor feeding branches of the vertebral artery. Although the risk of skin or muscle necrosis is minimal with the use of PVA particles in thoracolumbar lesions, protective coil embolization might shorten the embolization procedure: in our experience, embolization of the tumor vessels was completed faster when there was a distal occlusion of the tumor supplying the segmental artery, and there was no need for superselective catheterization of tumor feeders.
Complete embolization could be achieved in only 50% of our cases, as compared with 75% to 86% in comparable studies (7, 9, 14, 16). These differences may be explained by our rigid criteria of complete embolization and cautious embolization technique: embolization was aborted when there was doubt concerning potential spinal feeders. This strategy is supported by our result that even partial preoperative embolization seemed to be effective. Aggressive embolization with the risk of paraplegia is not justified in these patients, since survival is not affected by surgery for metastases (6). No irreversible neurologic complication was noted in our study. A postembolization syndrome (fever, pain) as described by other authors (8, 20, 28) was difficult to separate retrospectively from the pain associated with the metastasis and the surgical procedure in our patients.
Conclusion
The preoperative embolization of spinal metastases of renal cell carcinoma with PVA particles is a safe and effective tool to reduce intraoperative blood loss. Even partial embolization seems to reduce intraoperative blood loss in most patients.
Footnotes
Address reprint requests to Christoph Manke MD, Department of Diagnostic Radiology, Klinikum der Universität, Franz-Josef-Strauss-Allee 11, D-93042 Regensburg, Germany.
References
- 1.Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine 1985;10:19-20 [DOI] [PubMed] [Google Scholar]
- 2.Lockich JJ, Hartwell Harrison J. Renal cell carcinoma: natural history and chemotherapeutic experience. J Urol 1975;114:371-374 [DOI] [PubMed] [Google Scholar]
- 3.Halperin EC, Harisiadis L. The role of radiation therapy in the management of metastatic renal cell carcinoma. Cancer 1983;51:614-617 [DOI] [PubMed] [Google Scholar]
- 4.Sundarasen N, Scher H, DiGiacinto GV, Yagoda A, Whitmore W, Choi IS. Surgical treatment of spinal cord compression in kidney cancer. J Clin Oncol 1986;4:1851-1856 [DOI] [PubMed] [Google Scholar]
- 5.Sundarasen N, Gailicich JH, Lane JM, Bains MS, McCormack P. Treatment of epidural cord compression by vertebral body resection and stabilization. J Neurosurg 1985;63:676-684 [DOI] [PubMed] [Google Scholar]
- 6.King GJ, Kostuik JP, McBroom RJ, Richardson W. Surgical management of metastatic renal carcinoma of the spine. Spine 1991;16:265-271 [DOI] [PubMed] [Google Scholar]
- 7.Sundarsan N, Choi IS, Hughes JEO, Sachdev VP, Berenstein A. Treatment of spinal metastases from kidney cancer by presurgical embolization and resection. J Neurosurg 1990;73:548-554 [DOI] [PubMed] [Google Scholar]
- 8.Barton PP, Waneck RE, Karnel FJ, Ritschl P, Kramer J, Lechner GL. Embolization of bone metastases. J Vasc Interv Radiol 1996;7:81-88 [DOI] [PubMed] [Google Scholar]
- 9.Berkefeld J, Scale D, Kirchner J, Heinrich T, Kollath J. Hypervascular spinal tumors: influence of the embolization technique on perioperative hemorrhage. AJNR Am J Neuroradiol 1999;20:757-763 [PMC free article] [PubMed] [Google Scholar]
- 10.Boudghene F, Zyllberstein C, Breittmayer F, LeBreton C, Bigot JM. Preoperative embolization of vertebral tumors: assessment in a retrospective study of 20 cases. Cardiovasc Intervent Radiol 1994;17: (Suppl 2) 79 [Google Scholar]
- 11.Breslau J, Eskridge JM. Preoperative embolization of spinal tumors. J Vasc Interv Radiol 1995;6:871-875 [DOI] [PubMed] [Google Scholar]
- 12.Broaddus WC, Grady MS, Delashaw JB, Ferguson RD, Jane JA. Preoperative superselective arteriolar embolization: a new approach to enhance resectability of spinal tumors. Neurosurgery 1990;27:755-759 [PubMed] [Google Scholar]
- 13.Gellad FE, Sadato N, Numaguchi Y, Levine AM. Vascular metastatic lesions of the spine: preoperative embolization. Radiology 1990;176:683-686 [DOI] [PubMed] [Google Scholar]
- 14.Lasty JLL, Missenard G, Raynaud A, et al. Preoperative embolization of vascular metastatic lesions of the spine. Cardiovasc Intervent Radiol 1997;20: (Suppl 1) S105 [Google Scholar]
- 15.Olerud C, Jonson H Jr, Lofberg AM, Lorelius LE, Sjostrom L. Embolization of spinal metastases reduces perioperative blood loss: 21 patients operated on for renal cell carcinoma. Acta Orthop Scand 1993;64:9-12 [DOI] [PubMed] [Google Scholar]
- 16.Roscoe MW, McBroom RJ, Louis ES, Grossman H, Perrin R. Preoperative embolization in the treatment of osseous metastases from renal cell carcinoma. Clin Orthop 1989;238:302-307 [PubMed] [Google Scholar]
- 17.Rossi C, Ricci S, Boriani S, et al. Percutaneous transcatheter arterial embolization of bone and soft tissue tumors. Skeletal Radiol 1990;19:555-560 [DOI] [PubMed] [Google Scholar]
- 18.Rowe DM, Becker GJ, Rabe FE, et al. Osseous metastases from renal cell carcinoma: embolization and surgery for restoration of function. Radiology 1984;150:673-676 [DOI] [PubMed] [Google Scholar]
- 19.Smith TP, Gray L, Weinstein JN, Richardson WJ, Payne CS. Preoperative transarterial embolization of spinal column neoplasms. J Vasc Interv Radiol 1995;6:863-869 [DOI] [PubMed] [Google Scholar]
- 20.Sun S, Lang EV. Bone metastases from renal cell carcinoma: preoperative embolization. J Vasc Interv Radiol 1998;9:263-269 [DOI] [PubMed] [Google Scholar]
- 21.Vetter SC, Strecker EP, Ackermann LW, et al. Preoperative embolization of cervical spine tumors. Cardiovasc Intervent Radiol 1997;20:343-347 [DOI] [PubMed] [Google Scholar]
- 22.Shi HB, Shu DC, Lee HK, et al. Preoperative transarterial embolization of spinal tumor: embolization techniques and results. AJNR Am J Neuroradiol 1999;20:2009-2015 [PMC free article] [PubMed] [Google Scholar]
- 23.Berenstein A, Lasjaunias P. Surgical Neuroangiography 3: Functional Vascular Anatomy of Brain, Spinal Cord and Spine.. Berlin: Springer 1992;15-83
- 24.Berenstein A, Lasjaunias P. Surgical Neuroangiography 5: Endovascular Treatment of Spine and Spinal Cord Lesions.. Berlin: Springer 1992;111-132
- 25.Choi IS, Berenstein A. Surgical neuroangiography of the spine and the spinal cord. Radiol Clin North Am 1998;26:1131-1141 [PubMed] [Google Scholar]
- 26.Weigl B, Maghsudi M, Neumann C, Kretschmer R, Müller FL, Nehrlich M. Surgical management of symptomatic spinal metastases. Spine 1999;24:2240-2246 [DOI] [PubMed] [Google Scholar]
- 27.Hansen E, Altmeppen J, Täger K. Practicability and safety of intra-operative autotransfusion with irradiated blood. Anaesthesia 1998;53: (Suppl 2) 42-43 [DOI] [PubMed] [Google Scholar]
- 28.Hemingway AP, Allison DJ. Complications of embolization: analysis of 410 procedures. Radiology 1988;166:669-672 [DOI] [PubMed] [Google Scholar]