Abstract
BACKGROUND AND PURPOSE: Recent studies have shown the beneficial effect of highly active antiretroviral therapy (HAART) in AIDS-related progressive multifocal leukoencephalopathy (PML). The purpose of our study was to evaluate the initial and follow-up imaging findings and survival in patients with PML who were treated with HAART.
METHODS: The clinical course and MR imaging findings on initial and follow-up MR studies in four consecutive AIDS patients with PML who were treated with HAART are described.
RESULTS: Two patients were short-term survivors and died after 3 months. Two patients are still alive, with a survival time of 22 and 43 months, respectively. On initial MR studies, more extensive white matter changes were seen in the short-term survivors. Development of a mass effect and temporary enhancement (in one patient) was observed in two HAART responders on follow-up MR studies. Increased hypointensity on T1-weighted images with concomitant low signal on fluid-attenuated inversion-recovery fast spin-echo (FLAIR-FSE) images was seen in two responders, representing leukomalacia. Atrophic changes of the involved areas of the brain, consistent with burnt out PML lesions, were seen in two long-term survivors. In the short-term survivors, increased hypointensity was present on T1-weighted images with increased high signal on FLAIR-FSE images, representing progressive destructive disease.
CONCLUSION: Our results suggest that a clinical and radiologic response can be seen in some patients with AIDS-associated PML on HAART while in others there may be no beneficial response. Development of a mass effect and temporary enhancement on MR images in the early phase of treatment might represent positive predictive factors for prolonged survival.
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the CNS caused by JC Polyomavirus, and usually occurs in immunocompromised patients (1). At present, there is no efficacious therapy for PML, although regression of the disease with or without various drugs has been described in several reports (2–5). Recent studies have shown that potent highly active antiretroviral therapy (HAART) may improve survival, clinical status, and radiologic findings in AIDS patients with PML (6–10). The purpose of our study was to characterize the changes in MR imaging findings in four patients with AIDS-related PML participating in a HAART regimen.
Methods
The study group consisted of four consecutive AIDS patients in whom PML was diagnosed at our institution between January 1997 and April 2000. A HAART regimen, using different antiretroviral drugs, was initiated in all patients. The subjects included two women and two men, with a mean age of 32 years. Two patients died after a survival time of less than 4 months. Two patients are still alive, with survival exceeding 22 and 43 months, respectively.
The diagnosis of PML was established by clinical, laboratory (polymerase chain reaction [PCR] for JC virus [JCV]), and neuroimaging studies in two patients, by brain biopsy in one patient, and at autopsy in one patient.
MR imaging was performed initially and during the HAART regimen. We used a 1.0T or a 1.5T superconducting system, and the images were acquired with a slice thickness of 4 to 6 mm with an interslice gap of 1 mm, a field of view of 230 mm with a 70% rectangular field of view, and an image matrix of 192 × 256 pixels.
Axial T1-weighted spin-echo (SE), T2-weighted fast SE (FSE), and fluid-attenuated inversion-recovery (FLAIR) FSE sequences were performed in all patients. Magnetization transfer images were not obtained, nor were magnetization transfer ratios calculated. Contrast material was administered intravenously at the recommended doses. Lesion number, size, and location; MR signal intensity; and the presence of mass effect and contrast enhancement were determined on an initial MR study and compared with the findings on subsequent MR studies. All MR images were reviewed by two neuroradiologists.
The drug regimens consisted of the following antiretroviral drugs: 1) nucleoside reverse-transcriptase inhibitors (NRTIs), including zidovudine, stavudine, and lamivudine; 2) nonnucleoside reverse-transcriptase inhibitors (NNRTIs), including nevirapine and efavirenz; and 3) protease inhibitors, including indinavir, saquinavir, and nelfinavir. The drugs were given at the currently recommended doses.
Medical records were reviewed for neurologic signs and symptoms, results of CSF analysis, CD4+ cell count (cells/mm3), and HIV-1 viral load (copies/mL) at baseline and during antiretroviral treatment. JCV was detected by PCR in peripheral blood lymphocytes and CSF.
Results
Clinical information is summarized in Table 1. Two patients had tested positive for HIV for 14 years, and two were known to have been HIV-positive for 9 years. Both patients who are long-term responders were medication-naive as was one short-term survivor. One patient (case 4) had failed to respond on several NRTI and NRTI+NNRTI regimens. CSF examination was performed in all patients, and analyses were negative for virus and other opportunistic agents. PCR was positive for JCV in the two long-term survivors and in one of the short-term survivors. In one short-term survivor (case 3), PCR was negative for JCV. Stereotactic brain biopsy was performed in one patient (case 4), and specimens showed demyelinated areas with swollen oligodendrocytes, diagnostic for PML. Neuropathologic examination confirmed PML in the involved brain areas, with a large amount of JCV antigens in one patient (case 3). No unusual findings were observed at histopathologic examination.
TABLE 1:
Immunologic and virologic values in four patients with AIDS-associated PML receiving HAART
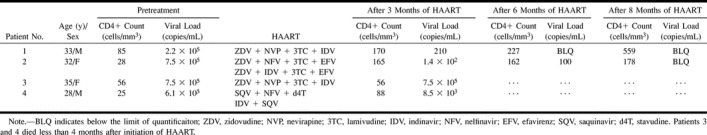
In all patients, the CD4+ count was below 100 cells/mm3 (mean, 48.5 cells/mm3) at the time of PML diagnosis. After 3 months of HAART, an increase above 150 cells/mm3 was observed in the long-term survivors. In the short-term survivors, the CD4+ count never exceeded 100 cells/mm3 (Table 1).
The mean HIV viral load level was 5.2 × 105 copies/mL. In the two long-term survivors, the viral load level decreased below the limit of quantification after 8 months of HAART, and was still below the limit of quantification at this writing. In the two short-term survivors, the viral load did not significantly decrease after 3 months of therapy (Table 1).
The findings on MR images on initial and sequential (posttreatment) studies are listed in Table 2. Both short-term survivors had more extensive white matter changes on initial (pretreatment) MR studies as compared with the long-term survivors. No differences in signal intensity were observed among any of the lesions, nor did we find enhancement or mass effect in either group (Table 2).
TABLE 2:
Initial and follow-up MR findings in four patients with AIDS-associated PML receiving HAART
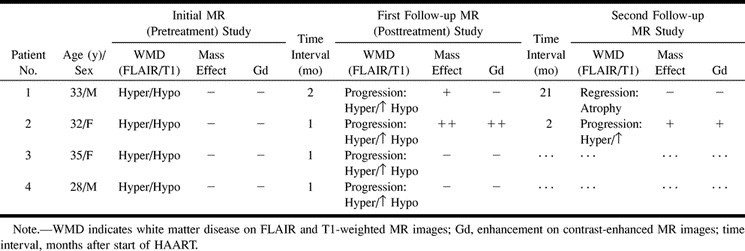
On follow-up MR studies during the HAART regimen, development of mass effect was seen only in the two long-term survivors (cases 1 and 2) and only on their posttreatment studies. Enhancement was observed in one of the responders after 4 weeks of HAART, and then subsequently resolved (case 2, Fig 1). In both long-term survivors, increased hypointensity was observed on T1-weighted images, with concomitant low signal on fast-FLAIR images, representing leukomalacia. In contrast, in the short-term survivors (cases 3 and 4), increased hypointensity was observed on T1-weighted images in combination with increased high signal on fast-FLAIR images, representing progressive destruction (Fig 2).
fig 1.
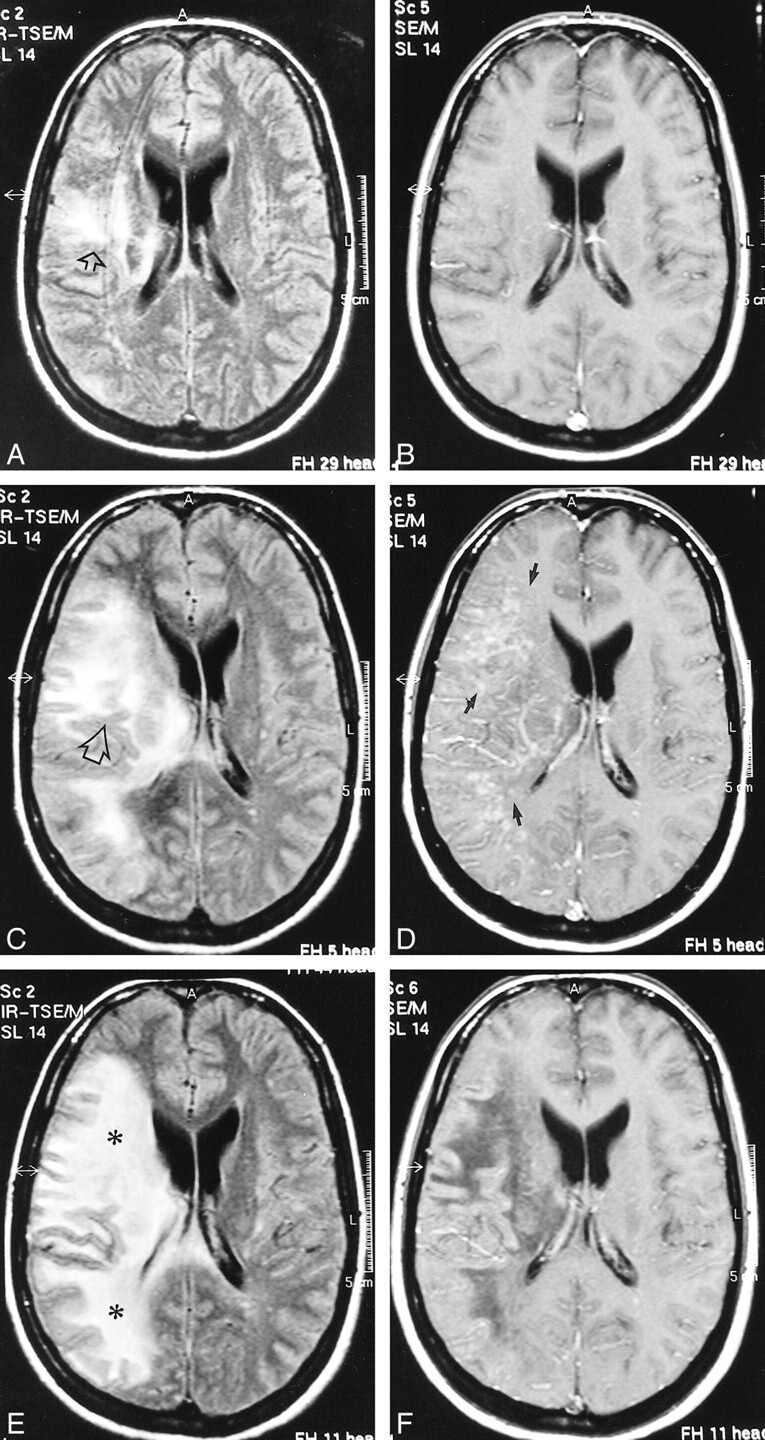
Case 2: 32-year-old HIV-positive woman with PML and survival time exceeding 22 months.
A and B, Initial MR study, December 1998. Axial FLAIR-FSE (TR/TE = 10000/150, TI = 2600) image (A) shows high-signal-intensity lesion in the white matter of the right centrum semiovale (arrow). There is no mass effect. No enhancement was present on contrast-enhanced T1-weighted (550/20) image (B).
C and D, Follow-up MR study, January 1999 (1 month after initiation of HAART). FLAIR-FSE (10000/150, TI = 2600) image (C) shows progression of white matter abnormalities (arrow) with mass effect and compression of right ventricle. Increased hypoattenuation of the lesion was evident on T1-weighted (550/20) images (not shown). On contrast-enhanced T1-weighted (550/20) image (D), diffuse enhancement of white matter abnormalities is present (arrows).
E and F, Subsequent MR study, February 1999 (2 months after initiation of HAART). FLAIR-FSE (10000/150, TI = 2600) image (E) shows further progression of the high-signal-intensity lesions of white matter disease (asterisks). Slight enhancement is still present on contrast-enhanced T1-weighted (550/20) image (F). Note increased hypoattenuation of the lesions and regression of the mass effect. Continued on page 982
fig 2.
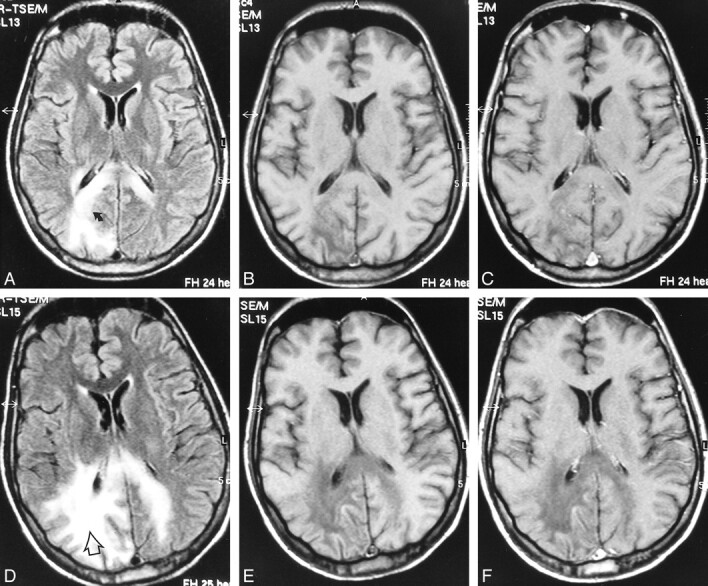
Case 4: 28-year-old man with AIDS who presented with psychomotor slowing and hemianopsia. Stereotactic biopsy revealed PML.
A–C, Initial MR study, March 1998. Axial FLAIR-FSE (10000/150, TI = 2600) image (A) shows scalloped, high-signal-intensity lesion in the parietooccipital white matter with extension to dorsal part of the corpus callosum on both sides (arrow). There is no mass effect. The lesion is hypointense on noncontrast T1-weighted SE (550/20) image (B). No enhancement is observed on contrast-enhanced T1-weighted SE (550/20) image (C).
D–F, Follow-up MR study, May 1998 (4 weeks after the start of HAART). FLAIR-FSE (10000/150, TI = 2600) image (D) shows progression of the white matter lesion (arrow) with involvement of the right parietal white matter and contralateral occipital white matter. Increased hypointensity is evident on noncontrast T1-weighted (550/20) image (E). Contrast-enhanced T1-weighted (550/20) image shows no enhancement (F)
Discussion
The prevalence of PML has increased greatly over the last 15 years, concomitantly with the rise of AIDS (1). Since the introduction of HAART, reports have indicated that AIDS-associated PML may show clinical and neuroradiologic improvement with longer survival (2–10). However, in our series of four consecutive AIDS patients with PML treated with HAART, two were short-term survivors and two were long-term survivors.
On MR images, PML typically appears as multifocal, scalloping lesions located in the white matter. The lesions are hypointense on T1-weighted images and show high signal intensity on T2-weighted images (11). Increased hypointensity on T1-weighted images has been observed on follow-up MR studies, and has been suggested to be indicative of an aggressive form of the disease (12, 13). This feature has also been described in pathologic series (14). It was suggested recently that such imaging findings as increased atrophy, confluence of lesions, and increased hypointensity on follow-up T1-weighted MR studies may be regarded as indicators of a poor prognosis in untreated AIDS patients with PML (12). In our HAART-treated patients, increased hypointensity on T1-weighted images was observed in both therapy responders and nonresponders. In the two long-term survivors, increased hypointensity on T1-weighted images and subsequent atrophic changes probably represented necrotic areas, which were a result of the demyelination before the process was halted by HAART. In these two patients, increases in low signal and atrophy were not associated with a concomitant increase in high signal on fast-FLAIR images. If we postulate that the initial progressive disease was halted under the HAART regimen at some point, atrophic changes and leukomalacia (increasing low signal on T1-weighted images and corresponding low signal on FLAIR images) may represent a burnt out process. In contrast to this evolution of MR findings in therapy responders, increased low signal on T1-weighted images with corresponding increases in high signal on FLAIR images would represent a progressive destructive process, which is a poor prognostic sign. This was suggested in a recently published study (15), and was also true for our two nonresponders. Thus, the evolution of imaging findings in AIDS patients with PML must be interpreted differently for those who are undergoing HAART.
A mild degree of mass effect associated with PML has been described infrequently in the published literature. In one study (13), marked mass effect was observed in a patient coinfected with HIV-1. Furthermore, another study found a correlation between the presence of a mass effect and shorter survival time (12). In our series, in the two long-term survivors on a HAART regimen, a mass effect was noted on follow-up MR studies that was not present on initial examinations. In the short-term survivors, a mass effect did not materialize. The development of mass effect in the immediate phase after institution of therapy, which was not evident on pretreatment images, may have resulted from transient edema rather than from progression of the PML, thus explaining the long-term survival in our patients.
Typical PML lesions do not usually enhance; however, faint, peripheral enhancement has been described (11, 16, 17). Recently, contrast enhancement has been thought to be the result of an intense inflammatory reaction and one of the predictive factors for prolonged survival (18, 19). In one study, enhancement was present in only 8.9% of the short-term survivors, in contrast to 50% in the long-term survivors (18). The enhancement was described as faint and peripheral in these cases. Of three patients with enhancement, two were receiving zidovudine and one was receiving zidovudine, indinavir, and lamivudine; however, no information was given concerning the presence of enhancement on follow-up neuroimaging studies. In our patients, the initial MR study showed no contrast enhancement, while marked enhancement was observed on a follow-up examination 4 weeks after the start of HAART in one long-term survivor. In contrast to the faint enhancement reported in other studies, our patient had intense, diffuse enhancement distributed in the involved white matter. The other long-term survivor as well as the short-term survivors on HAART did not show enhancement on sequential studies. The fact that contrast enhancement developed only after therapy and in the early phase in our patient may be explained as an effect of the therapy on the blood-brain barrier, which, with breakdown, causes edema, mass effect, and enhancement. The breakdown of the blood-brain barrier could be a result of improved immunocompetence and the ability to produce perivascular inflammatory reaction with inflammatory cells and mediator release and finally dysfunction of capillary integrity. Prominent perivascular mononuclear inflammation has been reported to be greatest in the contrast-enhancing region of the PML lesion (19, 20). After improvement, enhancement was no longer seen. In one study, development of contrast enhancement on MR studies was seen in patients with PML being treated with HAART only when their CD4+ count had increased (21). Interestingly, in these patients, the PML lesions turned from enhancing to nonenhancing on follow-up MR studies after 3 to 6 months of therapy. This was also true for one of our patients in whom temporary enhancement was noted. Larger series are needed to attempt to explain the relationship between the development of edema and contrast enhancement in PML lesions under HAART. In contrast to untreated PML cases, in the early phase of treatment the presence of mass effect and enhancement might represent a positive predictive factor for prolonged survival. The observations from our study as well as from others suggest that enhancing PML is characteristic of patients who experienced immunological reconstitution during the early phase of therapy.
It has been suggested recently that such imaging features as increasing atrophy, confluence of lesions, and increasing hypointensity on T1-weighted images and increasing high signal intensity on subsequent T1-weighted and fast-FLAIR images could be used as indicators of a poor prognosis in AIDS patients with PML (12). Our study's results indicate that differentiating between responders and nonresponders has to be based on findings on fast-FLAIR images. Findings of decreasing high signal on follow-up fast-FLAIR images, concomitant with increasing atrophy and increasing hypointensity on T1-weighted images, are signs compatible with longer survival. The low signal areas on T1-weighted and fast-FLAIR images would then represent the burnt out parts of the PML lesion, a leukomalacic area with secondary atrophic changes. On the other hand, follow-up findings of increasing hyperintensity on fast-FLAIR images as well as increasing atrophy and increasing hypointensity, are poor prognostic signs.
Clinically, PML is characterized by progressive neurologic deficits, leading to death (without therapy) in approximately 4 months. Improved patient survival has been reported with the use of a variety of drugs in a limited number of studies, mostly involving small patient cohorts (2–5). Recent studies have reported improvement in patients with AIDS-associated PML treated with a combination of antiretroviral regimens. In a study by Giudici et al (8), all patients with PML who received HAART were negative for JCV for a period of 4 or more months after therapy. In that study, MR images revealed a worsening of white matter lesions in five of seven patients 3 months after initiation of therapy, followed by stabilization or improvement after 12 months. This was also true for the long-term survivors in our study. Similarly, the beneficial effects of HAART were noticed after an initial deterioration in another study (6), in which 10 of 11 patients treated with HAART showed improvement or stabilization of PML lesions after 4 weeks of therapy. In another study that evaluated the efficacy of cytarabine, a temporary worsening of MR abnormalities was present simultaneously with clinical improvement, followed later by an improvement in MR imaging features (3, 4).
The drop in HIV viral load (below the limit of quantification) and increase in CD4+ count in our long-term survivors undergoing HAART supports the theory of an immune reconstitutive effect. The results of published reports and observations of ongoing studies suggest that a relatively preserved immune status and immunologic reconstitution can interrupt the JCV lytic cycle in oligodendrocytes and slow further demyelination, one of the important factors for prolonged survival (18). Furthermore, reduced replication of HIV in the brain and a reduced amount of HIV-encoded transactivator of transcription protein leads to reduced JCV gene expression (22). Thus, it would appear that therapeutic strategies should be directed against JCV as well as at the underlying immune function.
Present recommendations about the treatment of neurologic dysfunction in AIDS patients include the use of protease inhibitors as part of a combined therapy. Although the data concerning the ability of protease inhibitors to cross the blood-brain barrier are conflicting, HAART, consisting of NRTIs and protease inhibitors, causes sustained suppression of the viral load (22, 23). Indinavir has been shown to achieve favorable CSF concentrations (23). The results of a recent study in patients with PML showed that survival improved substantially among patients who received combination therapy, including NRTIs and protease inhibitors (7).
Only a few studies have reported a failure to observe benefit from HAART in AIDS patients with PML (25). In contrast to the majority of published articles, which state that HAART is effective in treating PML in AIDS patients, our experience indicates that there may be two (or more) sets of patients: those who respond to HAART and those who clearly do not. A comparison of the two groups of patients in our series showed some interesting differences. The initial degree of immunosuppression did not differ between the two groups. On the pretreatment images, the only difference between them was the extent of the white matter lesions, which were more extensive in the nonresponders. After 3 months of therapy, responders showed a better immunologic and virologic response. Follow-up MR studies showed development of a mass effect in both long-term survivors, one of whom also showed temporary enhancement. Among the nonresponders, no mass effect and no enhancement could be observed.
In one study, PML developed in three patients while receiving HAART, and, despite the virologic response, the PML did not improve (25). In another study, two patients showed symptoms of PML during the first weeks of HAART, with a strong CD4+ cell-driven inflammatory response to a subclinical CNS infection of JCV (26). Follow-up MR studies in these patients revealed a clear improvement. These observations suggest that HAART may not be effective in all patients with PML and may not prevent PML in patients undergoing the HAART regimen. Previously established JCV infection of the brain that antedates treatment with HAART is one possible explanation. On the other hand, PML appearing after the institution of HAART should be added to the differential diagnosis. In the future, we may see PML developing in AIDS patients who are undergoing HAART, making the interpretation of imaging findings even more challenging.
Conclusion
Our results suggest that a clinical and radiologic response to HAART may be seen in some patients with PML while in others there may be no beneficial response. Initial worsening of the MR imaging findings with development of temporary contrast enhancement, mass effect, and edema in our long-term survivors was probably due to the effect of a posttreatment inflammatory reaction. Atrophic changes and increased hypointensity on follow-up T1-weighted images with concomitant low signal on FLAIR images in those patients may represent leukomalacia and burnt out PML lesions. The persistent neurologic deficits in patients with PML who are responding to the potent antiretroviral therapy are due to the sequelae rather than to unremitting disease.
TABLE 2:
Continued
fig 1.
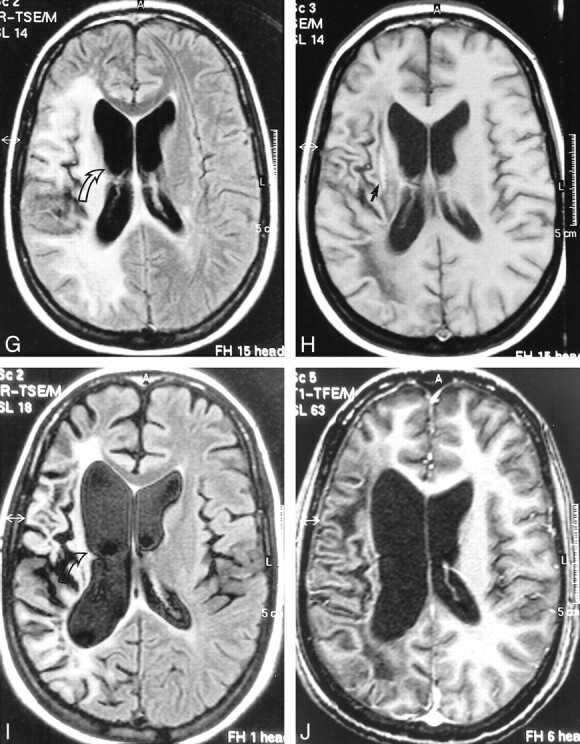
Continued.
G and H, Further follow-up MR study, April 1999 (4 months after initiation of HAART). FLAIR-FSE (10000/150, TI = 2600) image (G) reveals regression of the white matter changes with partially present low signal (corresponding to areas of increased hypoattenuation on T1-weighted images). Note atrophic changes of the right hemisphere with widening of the ventricle (arrow). Further increase in hypointensity is evident on T1-weighted (550/20) image (H). Elongated hyperintense area is seen medial to the sylvian fissure, representing subacute hemorrhage (arrow). Enhancement was not present on contrast-enhanced T1-weighted image (not shown).
I and J, Follow-up MR examination, September 2000 (21 months after initiation of HAART). Axial FLAIR-FSE (7384/130, TI = 2100) image (I) and contrast-enhanced T1-weighted (8/2.7, flip angle = 10°) image (J) show atrophic changes in the right hemisphere with widening of the sulci and further ex-vacuo widening of the right ventricle (arrow). Note low signal on the FLAIR-FSE image, representing leukomalacia
Footnotes
Address reprint requests to M. M. Thurnher, MD, Department of Radiology, University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria.
References
- 1.Berger JR, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. Neurovirol 1995;1:5-18 [DOI] [PubMed] [Google Scholar]
- 2.Happe S, Besselmann M, Matheja P, et al. Therapy of progressive multifocal leukoencephalopathy (PML) in AIDS with cidofovir (Vistide): review of the literature and two case reports. Nervenarzt 1999;70:935-943 [DOI] [PubMed] [Google Scholar]
- 3.Portegies P, Algra PR, Hollak CEM, et al. Response to cytarabine in progressive multifocal leukoencephalopathy in AIDS. Lancet 1991;337:680-681 [DOI] [PubMed] [Google Scholar]
- 4.Nicoli F, Chave B, Peragut JC, Gastaut JL. Efficacy of cytarabine in progressive multifocal leukoencephalopathy in AIDS. Lancet 1991;339:306. [DOI] [PubMed] [Google Scholar]
- 5.De Luca A, Giancola ML, Cingolani A, et al. Clinical and virological monitoring during treatment with intrathecal cytarabine in patients with AIDS-associated progressive multifocal leukoencephalopathy. Clin Infect Dis 1999;28:624-628 [DOI] [PubMed] [Google Scholar]
- 6.Miralles P, Berenguer J, Garcia de Viedma D, et al. Treatment of AIDS-associated progressive multifocal leukoencephalopathy with highly active antiretroviral therapy. AIDS 1998;12:2467-2472 [DOI] [PubMed] [Google Scholar]
- 7.Tassie JM, Gasnault J, Bentata M, et al. Survival improvement of AIDS-related progressive multifocal leukoencephalopathy in the era of protease inhibitors. AIDS 1999;13:1881-1887 [DOI] [PubMed] [Google Scholar]
- 8.Giudici B, Vaz B, Bossolasco S, et al. Highly active antiretroviral therapy and progressive multifocal leukoencephalopathy: effects on cerebrospinal fluid markers of JC virus replication and immune response. Clin Infect Dis 2000;30:95-99 [DOI] [PubMed] [Google Scholar]
- 9.Baqi M, Kucharczyk W, Walmsley SL. Regression of progressive multifocal leukoencephalopathy with highly active antiretroviral therapy. AIDS 1997;11:1526-1527 [PubMed] [Google Scholar]
- 10.Teofilo E, Gouveia J, Brotas V, da Costa P. Progressive multifocal leukoencephalopathy regression with highly active antiretroviral therapy. AIDS 1998;12:449. [PubMed] [Google Scholar]
- 11.Whiteman MLH, Post MJD, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology 1993;187:233-240 [DOI] [PubMed] [Google Scholar]
- 12.Post MJD, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AJNR Am J Neuroradiol 1999;20:1896-1906 [PMC free article] [PubMed] [Google Scholar]
- 13.Thurnher MM, Thurnher SA, Mühlbauer B, et al. Progressive multifocal leukoencephalopathy in AIDS: initial and follow-up CT and MRI. Neuroradiology 1997;39:611-618 [DOI] [PubMed] [Google Scholar]
- 14.Kuchelmeister K, Gullotta F, Bergmann M, Angeli G, Masini T. Progressive multifocal leukoencephalopathy (PML) in the acquired immunodeficiency syndrome (AIDS): a neuropathological autopsy study of 21 cases. Pathol Res Pract 1993;189:163-173 [DOI] [PubMed] [Google Scholar]
- 15.Dörries K. Virus-host interactions and diagnosis of human Polyomavirus-associated disease. Intervirology 1996;39:165-175 [DOI] [PubMed] [Google Scholar]
- 16.Wheeler AL, Truwit CL, Kleinschmidt-DeMasters BK, Byrne WR. Progressive multifocal leukoencephalopathy: contrast enhancement on CT scans and MR imaging. AJR Am J Roentgenol 1993;161:1049-1051 [DOI] [PubMed] [Google Scholar]
- 17.Port JD, Miseljic S, Lee RR, et al. Progressive multifocal leukoencephalopathy demonstrating contrast enhancement on MRI and uptake of thallium-201: a case report. Neuroradiology 1999;41:895-898 [DOI] [PubMed] [Google Scholar]
- 18.Berger JR, Levy RM, Flomenhoft D, Dobbs M. Predictive factors for prolonged survival in acquired immunodeficiency syndrome–associated progressive multifocal leukoencephalopathy. Ann Neurol 1998;44:341-349 [DOI] [PubMed] [Google Scholar]
- 19.Kotecha N, George MJ, Smith TW, Corvi F, Litofsky NS. Enhancing progressive multifocal leukoencephalopathy: an indicator of improved immune status. Am J Med 1998;105:541-543 [DOI] [PubMed] [Google Scholar]
- 20.Woo HH, Rezai AR, Knopp EA, et al. Contrast-enhancing progressive multifocal leukoencephalopathy: radiological and pathological correlations: case report. Neurosurgery 1996;39:1031-1035 [DOI] [PubMed] [Google Scholar]
- 21.Collazos J, Mayo J, Martinez E, Blanco MS. Contrast-enhancing progressive multifocal leukoencephalopathy as an immune reconstitution event in AIDS patients. AIDS 1999;13:1426-1428 [DOI] [PubMed] [Google Scholar]
- 22.Tada H, Rappaport J, Lashgari M, Amini S, Wong-Staal F, Khalili K. Transactivation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc Natl Acad Sci U S A 1990;87:3479-3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Entning RH, Hoetelmans MW, Lange JMA, Burger DM, Beijnen JH, Portehies P. Antiretroviral drugs and the central nervous system. AIDS 1998;12:1941-1955 [DOI] [PubMed] [Google Scholar]
- 24.Flexner C. HIV-protease inhibitors. N Engl J Med 1998;338:1281-1292 [DOI] [PubMed] [Google Scholar]
- 25.Tantisiriwat W, Tebas P, Clifford DB, Powderly WG, Fichtenbaum CJ. Progressive multifocal leukoencephalopathy in AIDS receiving highly active antiretroviral therapy. Clin Infect Dis 1999;28:1152-1154 [DOI] [PubMed] [Google Scholar]
- 26.Mayo J, Collazos J, Martinez E. Progressive multifocal leukoencephalopathy following initiation of highly active antiretroviral therapy. AIDS 1998;12:1720-1722 [PubMed] [Google Scholar]


