Abstract
BACKGROUND AND PURPOSE: The ability to identify patients at increased risk for stroke from cerebral hemodynamic ischemia may help guide treatment planning. We tested the correlation between regional cerebrovascular reserve (rCVR) on acetazolamide-challenged single-photon emission CT (SPECT) brain scans and intracranial collateral pathways as well as extra- or intracranial (EC-IC) arterial stenosis on cerebral angiography.
METHODS: A retrospective analysis of 27 patients who underwent cerebral angiography and acetazolamide-challenged SPECT brain imaging was performed. With cerebral angiography, the anterior, middle, and posterior cerebral artery (ACA, MCA, PCA) territories were evaluated for patterns of flow, including the ipsilateral carotid or basilar arteries, the circle of Willis collaterals, the EC-IC collaterals, and the leptomeningeal collaterals. With acetazolamide-challenged SPECT, the ACA, MCA, and PCA territories were classified as either showing or not showing evidence of decreased rCVR. Statistical significance was determined by the χ2 test.
RESULTS: Patients with decreased rCVR had significantly greater dependence on either the EC-IC or leptomeningeal collaterals (42%) than did patients without decreased rCVR (7%). Similarly, the cerebral hemispheres with decreased rCVR showed a higher prevalence of 70% or greater stenosis or occlusion of the ipsilateral EC-IC arteries in the anterior circulation (74%) than did hemispheres with no evidence of decreased rCVR (16%), and this difference was also statistically significant.
CONCLUSION: Acetazolamide-challenged SPECT brain scanning provides additional information regarding rCVR that is not reliably provided by cerebral angiography.
Although the number one cause of stroke in symptomatic patients with high-grade carotid artery stenosis is thromboembolism, cerebral hemodynamic insufficiency also plays an important role (1–4). Evaluation of the hemodynamic status of the brain may identify an increased risk of stroke in patients with occlusion or high-grade stenosis of the intra- or extracranial (EC-IC) arteries (5, 6). Such stenoses or occlusions, as well as those of alternative collateral pathways, can be readily detected with cerebral angiography (7). However, perfusion of the brain cannot be adequately evaluated with angiography.
Perfusion refers to the flow through the capillary network, which delivers oxygen and nutrients to the cerebral tissue (8). Cerebral blood flow (CBF), which reflects the perfusion of the brain, is the volume of blood flow through the vascular network in milliliters per second per 100 g of brain tissue. Cerebral blood volume (CBV) refers to the volume of blood in 100 g of brain tissue (9). Regional CBF (rCBF) has a high correlation with regional cerebral metabolism. Metabolically active areas in the brain have higher rCBF. Cerebral perfusion pressure (CPP) is the difference between the arterial and the venous pressures. When CPP falls, CBV increases by means of arteriolar vasodilatation in order to keep the CBF constant. When vasodilatation reaches its maximum, CBF begins to drop. The oxygen extraction fraction (OEF) increases at this point to maintain the metabolic needs of the cerebral tissues. If CPP keeps falling, cerebral metabolism becomes impaired, resulting in either reversible or irreversible deficits (10). Regional cerebrovascular reserve (rCVR) measures the vasodilatory capacity of the cerebral arterioles. Acetazolamide or CO2 is commonly used as a vasodilatory stimulus. rCVR is defined as [(rCBF after vasodilatory stimulus − rCBF resting) / rCBF resting] × 100 (11).
Different imaging techniques currently used to measure perfusion include positron emission tomography (PET) (12), dynamic first-pass perfusion CT (13), xenon-enhanced CT (14), perfusion-weighted MR imaging (15), and single-photon emission CT (SPECT) (16, 17). Recently, acetazolamide has been used with different imaging techniques to enhance the detection of decreased rCVR (7, 11, 18, 19).
Early diagnosis of cerebral hemodynamic ischemia could guide treatment decisions regarding medical therapy, angioplasty and stenting, carotid endarterectomy, or EC-IC bypass surgery and thus potentially improve prognosis. Smith et al (20) reported good correlation between rCVR measured with acetazolamide-challenged xenon-enhanced CT and leptomeningeal collateralization at cerebral angiography. They concluded that xenon-enhanced CT combined the anatomic information with pathophysiological states, and thus was helpful in identifying patients who were at risk for hemodynamic stroke. Another study with PET found that ophthalmic and leptomeningeal collateralization at cerebral angiography correlated closely with reduced CPP and reduced CBF, respectively (3). We therefore conducted a retrospective study to test the hypothesis that rCVR measured with technetium-99m ethylene cysteine dimer (99mTc-ECD) SPECT, a less expensive and more available imaging method than PET, might correlate with intracranial collateralization patterns as shown on cerebral angiography. We also tested the correlation between rCVR and the degree of stenosis of the carotid artery, anterior cerebral artery (ACA), and middle cerebral artery (MCA).
Methods
Subjects
A retrospective review of 27 patients who underwent both acetazolamide-challenged SPECT of the brain and cerebral angiography between December 1996 and July 1999 was performed (Table 1). The study group included 26 men and one woman (reflecting the Veterans Affairs population) with ages ranging from 51 to 83 years (mean age, 66 years). In eight patients, the studies were done within 1 week of each other; in 10 patients, within 3 months; and in nine patients, within 5 months. None of the patients experienced new neurologic deficits between studies.
TABLE 1:
Cerebral vascular reserve and angiographic findings in 27 patients

Nineteen patients (70%) presented with neurologic symptoms. Eight of them (30%) had had a transient ischemic attack, five (19%) had suffered stroke, and six (22%) had experienced a presyncopal attack. The other eight patients (30%) were either clinically asymptomatic or had symptoms that were inconclusive.
Echocardiography was obtained in 16 patients (59%), and objective cardiac ejection fractions were reported in 14 of them. Six of those had ejection fractions above 50%, five had ejection fractions between 35% and 49%, and three had ejection fractions between 30% and 34%. No patient had an ejection fraction below 30%.
SPECT Imaging
SPECT imaging was performed with a commercially available triple-head camera (Trionix, Triad, Twinsburg, OH). The spatial resolution was 3.3 mm full width at half-maximum intensity in the center of the field of view. Approximately 20 mCi 99mTc-ECD (Neurolite, Dupont, Billerica, MA) was injected intravenously as the perfusion radiotracer after the patient had been in a quiet, dimly lit room for 15 minutes. The initial baseline (no acetazolamide) imaging was performed 60 to 120 minutes after injection of the radiotracer. One to five days later, the patient was placed in the same environment as for the baseline study for 15 minutes. Then 1 g of acetazolamide (Diamox, Lederle Lab Division, Pearl River, NY) was injected intravenously over 2 minutes. After an additional 15 minutes, the radiotracer was injected and images were obtained 60 to 120 minutes later. The patients had their eyes open at the time of injection for both the baseline and acetazolamide studies.
Image data were acquired on the triple-detector gamma camera using high-resolution fan beam collimators with an acquisition matrix of 128 by 128. The acquisition was 30 stops at 30 seconds per stop, and the orbit was circular. Postacquisition computer processing was done using a Hamming filter with a 0.9 high frequency cut-off and a Ramp filter with a high frequency cut-off of 1.4.
Axial, coronal, and sagittal images were reconstructed and the axial images were printed with the baseline study in the upper row and anatomically matched to the corresponding acetazolamide study in the lower row. Each study was scaled independently using the cerebellum as the reference of 100% maximal counts or perfusion. For the interpretations, all SPECT brain scans were displayed as 16 × 20-cm color prints. The prints were reviewed by two experienced nuclear medicine physicians, who were blinded to the patient identification as well as to clinical status and cerebral angiography results. The two physicians decided by consensus whether a specific vascular territory showed normal rCVR or decreased rCVR.The images were scored for relative perfusion abnormalities using the 10-level color scale on the baseline studies and then the acetazolamide studies. This was recorded on a form dividing the brain into ACA, MCA, and posterior cerebral artery (PCA) vascular territories. A comparison was then made on the relative perfusion changes between the baseline and acetazolamide studies. An abnormal rCVR was defined as a 10% (one color change) or more decrease in perfusion on the acetazolamide study as compared with the baseline study. Abnormalities that did not show a decrease in perfusion by 10% or that showed an increase in perfusion on the acetazolamide study were scored as showing no evidence of abnormal rCVR.
In 23 patients, SPECT brain scans were correlated with a recent CT or MR study of the brain by a neuroradiologist. If there was an infarct on the CT or MR study corresponding to the perfusion defect on the SPECT brain scan, then the size of the defect was compared. If the size of the perfusion defect on the SPECT brain scan was similar to the size of the infarct on the CT or MR study, and the perfusion defect did not change with acetazolamide challenge, then this lesion was not considered as decreased rCVR. Likewise, if the perfusion defect on the SPECT brain scan was larger than the infarct seen on the CT or MR study, or if the perfusion defect became larger with acetazolamide challenge, then this lesion was considered positive for decreased rCVR.
Cerebral Angiography
All 27 patients had bilateral carotid angiography. Seventeen patients (63%) also had arch aortography, and 12 (44%) had either unilateral or bilateral vertebral angiography. The visualized arteries were evaluated as being normal, less than 70% stenotic, or showing 70% or greater stenosis. Flow patterns in the ACA, MCA, and, where applicable, the PCA were classified as type 1, normal (ipsilateral carotid or basilar); type 2, circle of Willis collaterals; type 3, EC-IC collaterals; or type 4, leptomeningeal collaterals. If a vascular territory had more than one pattern of flow, the most compromised pattern was counted, with type 1 being the least compromised and type 4 the most. Normal anatomic variations of the circle of Willis were counted as type 1 flow pattern. All angiograms were reviewed by two experienced neuroradiologists to reach a consensus.
Data Analysis
Angiographic patterns of intracranial collateral flow were compared with the results of acetazolamide-challenged SPECT brain scans, and the statistical significance of any differences was determined by χ2 test. Stenoses of the common and internal carotid artery (CCA, ICA) and the ACA and MCA were similarly compared.
Results
Cerebral Angiography
Seventeen patients (63%) had unilateral occlusion of the ICA, 10 on the right and seven on the left. Of these 17 patients, 16 had the occlusion at the origin or cervical portion of the ICA and one had an occlusion of the CCA origin with no reconstitution of the ICA. Three patients (11%) had severe (70% or greater) unilateral ICA stenosis, all on the right side. One patient had severe stenosis of the basilar artery, as well as severe stenosis of the right MCA and occlusion of both ACAs and of the right PCA. One patient had occlusion of the right ACA and MCA in addition to complete occlusion of the ipsilateral ICA (Table 1).
CVR and Flow Patterns to the ACA, MCA, and PCA
Of the 132 major vascular territories (ACA, MCA, and PCA) examined by both SPECT brain imaging and angiography, 106 (80%) showed no evidence of decreased rCVR and 26 (20%) showed decreased rCVR. In the 26 vascular territories with decreased rCVR, 8 (31%) had a type 1 flow pattern at cerebral angiography, seven (27%) had a type 2 (circle of Willis) pattern, and 11 (42%) had either a type 3 (EC-IC) or a type 4 (leptomeningeal) pattern. Of those with no evidence of decreased CVR, 82 (77%) had type 1, 17 (16%) had type 2, and only seven (7%) had either type 3 or type 4 flow patterns (Table 2). The vascular territories with decreased rCVR on SPECT brain scans had a significantly higher dependence on leptomeningeal or EC-IC collaterals on the cerebral angiograms (χ2 = 24.11, df = 3, P < .0001).
TABLE 2:
Comparison of angiographic cerebral flow patterns in the anterior, middle, and posterior cerebral artery territories with rCVR

CVR and Stenosis or Occlusion of the Carotid Artery, the ACA, and the MCA
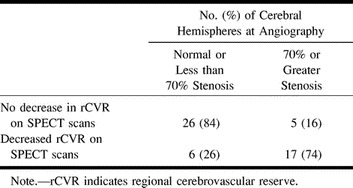
Fifty-four cerebral hemispheres were examined by both SPECT brain imaging and cerebral angiography. Twenty-three (43%) of the cerebral hemispheres showed decreased rCVR in either the ACA or MCA territory and 31 (57%) showed no evidence of decreased rCVR. Of the 23 cerebral hemispheres with decreased rCVR by SPECT brain scan, 17 (74%) had angiographic evidence of 70% or greater stenosis or occlusion of the ipsilateral CCA, the ICA, or either the ACA or MCA, whereas only six (26%) were normal or had less than 70% stenosis. In the remaining 31 cerebral hemispheres with no SPECT evidence of decreased rCVR, 26 (84%) had no evidence of significant arterial stenosis in the anterior circulation and only five (16%) had significant stenosis (Table 3). The difference was statistically significant (χ2 = 20.2, df = 1, P < .0001). Only one of the evaluated PCA territories showed evidence of decreased CVR to acetazolamide by SPECT. This patient had type 4 (leptomeningeal) flow to the ipsilateral PCA territory on angiography and had more than 70% stenosis of the basilar artery as well as occlusion of the ipsilateral PCA.
TABLE 3:
Comparison of angiographic anterior circulation arterial stenosis or occlusion with rCVR
Twenty-three patients had either a CT or MR study of the brain after the SPECT studies. Fifteen patients had a total of 22 infarcts. Ten of the infarcts (45%) were of a watershed type, eight (36%) were cortical, and four (18%) were lacunar.
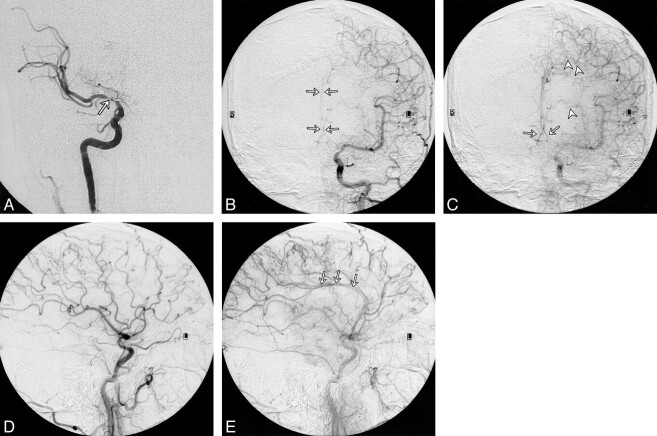
As an example, patient 11 in our study was admitted to the hospital with symptoms referable to both cerebral hemispheres. His SPECT brain scan showed large areas with abnormal rCVR response in the right ACA, MCA, and PCA territories (Fig 1). A diagnosis of neurosyphilis was established with CSF analysis. Cerebral angiography revealed a high-grade stenosis of the right MCA and occlusions of both ACAs and of the right PCA (Fig 2). The right MCA lesion was treated with percutaneous transluminal angioplasty. Postangioplasty angiography showed almost complete resolution of the MCA stenosis (Fig 2). A follow-up SPECT study showed significant improvement of rCVR in the right ACA and MCA territories, but continued decreased rCVR in the right PCA distribution (Fig 3). The patient's bilateral ACA and right PCA occlusions were clearly compromising the patient's rCVR. It is quite probable that the significant stenosis of the right MCA affected not only the right MCA territory but other vascular territories as well. This could explain the improvement of rCVR not only in the right MCA territory but also in the right ACA territory.
fig 1.
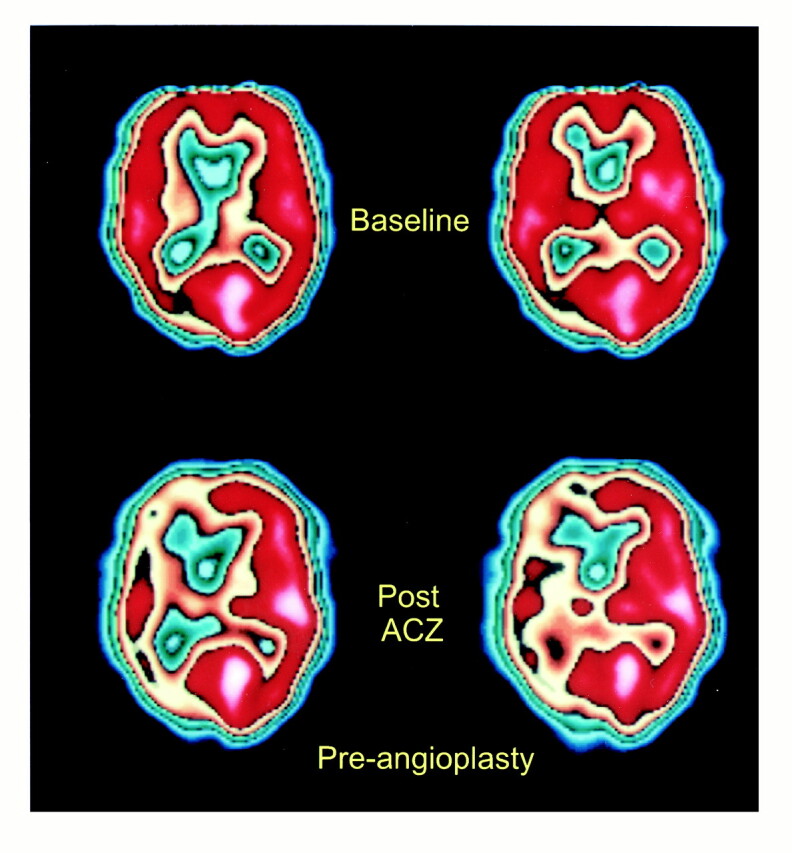
58-year-old man with neurosyphilis and progressive neurologic symptoms. SPECT scans before right MCA angioplasty show relatively normal baseline perfusion (top row) but significantly decreased perfusion in the right ACA, MCA, and PCA territories after acetazolamide administration (bottom row), consistent with decreased rCVR. (The printed color scale representing brain perfusion is different from the linear 10-level color scale used for interpreting nuclear medicine studies; however, it allows easier comparison with fig 3.)
fig 2.
A, Anteroposterior view of right ICA angiogram shows occlusion of the right ACA and high-grade stenosis of the right MCA (arrow).
B and C, Anteroposterior views of early (B) and late (C) arterial phases of left CCA angiogram show complete occlusion of the proximal left ACA and reconstitution of both pericallosal arteries (arrows) and the anterior communicating artery from leptomeningeal collaterals (arrowheads).
D and E, Lateral views of early (D) and late (E) arterial phases of left CCA angiogram show reconstitution of the pericallosal artery (arrows) in the late arterial phase by leptomeningeal collaterals
fig 3.
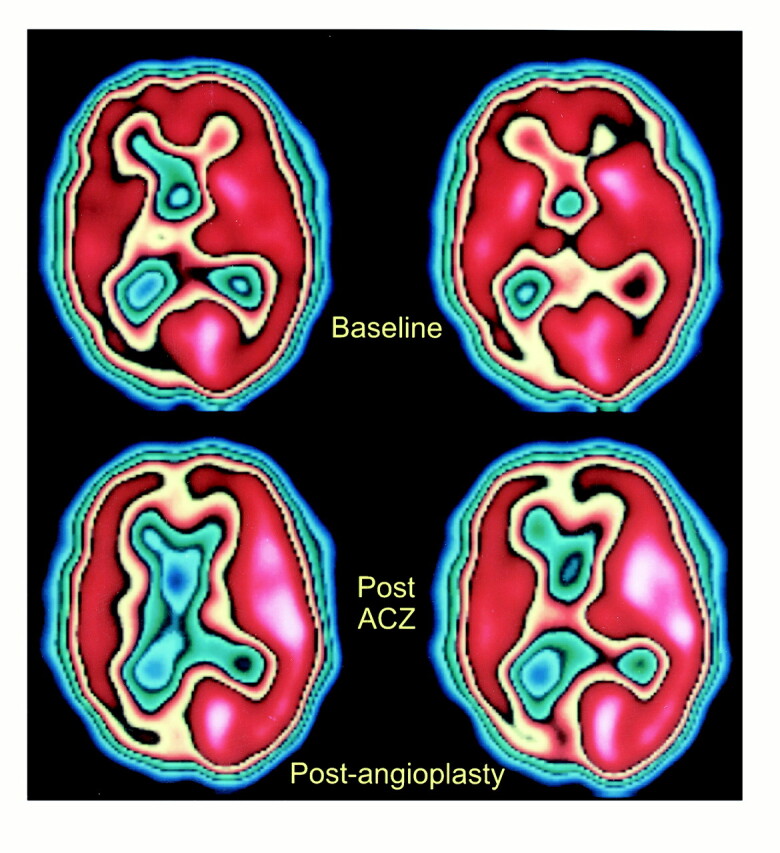
SPECT scans after right MCA angioplasty show improved CBF in right ACA and MCA territories on postacetazolamide study (bottom row), consistent with improved rCVR. The rCVR in the right PCA territory appears to be worse as compared with baseline study (top row)
Discussion
Identifying patients who are at increased risk for hemodynamic stroke is important, because they may benefit from flow augmentation procedures, such as carotid endarterectomy, EC-IC bypass (7, 11, 18, 19, 21, 22), or even angioplasty. However, evaluation of the hemodynamic status of the brain requires physiological imaging. In addition, inadequate perfusion of the brain may be caused by multiple pathophysiological mechanisms, including cardiac disease, resulting in decreased ejection fraction, atherosclerosis, or other vasculopathy involving the EC-IC vasculature and the status of collateral circulation in the brain.
The cerebral hemodynamic status can be determined by measuring CBF before and after vasodilatory challenge, which can be done with either hypercapnia or acetazolamide. Acetazolamide, a carbonic anhydrase inhibitor, has been shown to increase CBF in healthy subjects by vasodilatation (11, 23, 24). In the early stages of compromised cerebral perfusion, the baseline CBF is decreased, but it normalizes after vasodilatory challenge by recruitment of previously mentioned compensatory mechanisms. In this stage, the compromised flow is well compensated. When the cerebral hemodynamic status worsens, the decreased CBF either does not change by vasodilatory challenge or is further reduced relative to the area of normal vascularity. If there already is hypoperfusion with maximal compensatory vasodilatation, the hypoperfused area cannot further vasodilate to the acetazolamide challenge. Even in the presence of a normal baseline perfusion, imaging with acetazolamide may detect perfusion abnormalities that would otherwise be missed (21, 25). Conversely, an abnormal area in the baseline study may become normal after acetazolamide challenge if the CVR is preserved. Thus, the acetazolamide challenge may decrease both the rate of false-positive and false-negative results in CBF imaging.
In acute occlusions resulting in cerebral ischemia, areas of hypoperfusion can be detected within minutes on SPECT scans (26). In the subacute phase of cerebral infarction, hypoperfusion may continue, although luxury perfusion may result in hyperperfusion. Chronic infarction is seen as a hypoperfused area on SPECT brain scans. The role of acetazolamide SPECT brain imaging in the setting of acute stroke is limited, because the time it takes to complete the examination may delay the start of therapy. However, for patients who have had cerebrovascular accidents in the past and are at increased risk for stroke, acetazolamide-challenged SPECT brain imaging may provide valuable information to identify impaired CVR.
All the high-grade carotid stenoses or occlusions seen in our patients were chronic. Among the patients with decreased rCVR, 42% of the vascular territories showed dependence on either EC-IC or leptomeningeal collaterals, whereas only 7% of the vascular territories in the group with no evidence of decreased CVR showed EC-IC or leptomeningeal collaterals on angiography. Our results are comparable to those of Smith et al (20), who found an increased number of leptomeningeal collaterals in patients with a steal response to acetazolamide. We also found that more of the patients with decreased rCVR in the ipsilateral cerebral hemisphere had 70% or greater stenosis or occlusion in the anterior circulation (CCA, ICA, ACA, or MCA). These findings are compatible with those of Hosoda et al (11), who reported that the degree of ICA stenosis is a major determinant of CVR. There were more (45%) watershed type than cortical or lacunar type infarcts in our 23 patients who had a CT or MR study of the brain, which probably reflects the cerebral hemodynamic compromise in our patient population.
Current studies on the hemodynamics of the brain show mixed findings. Some reports describe good correlation of cerebral hemodynamic status with different types of intracranial collateral flow (3, 20) while others conclude there is no good correlation (11, 27). Similarly, some studies have found strong correlation between the degree of carotid artery stenosis and cerebral hemodynamic status (7, 11) while others have not (3). These differing results may reflect, at least in part, differences in techniques, imaging methods, study designs, and, possibly, presence or absence of physiological or pharmacological interventions, such as CO2 or acetazolamide challenge. For example, some studies only examined the ophthalmic artery collaterals but not other EC-IC collaterals, such as those between the middle meningeal or even the ascending pharyngeal artery and the ICA (3, 27). In some studies, leptomeningeal collaterals were not examined at all (27). Some investigators examined only the anterior and posterior communicating arteries as collaterals and did not address the EC-IC or leptomeningeal collaterals (11).
A recent study by Grubb et al (28) showed an increased risk of stroke in patients with carotid artery occlusion and increased OEF (stage 2 hemodynamic failure) as shown by PET. Eleven (28%) of 39 patients with increased OEF had a stroke during a mean follow-up period of 31 months. Similar results of increased stroke rates have been described in patients with abnormal rCVR detected by other imaging techniques, such as xenon-enhanced CT and transcranial Doppler sonography (29, 30). Yonas et al (29) followed 68 patients who had acetazolamide-challenged xenon-enhanced CT for a mean of 24 months and found that the group with decreased CVR had a stroke rate of 36%. Kleiser and Widder (30) reported 37 patients with abnormal vasodilatory response on transcranial Doppler sonography who showed a 22% stroke rate in a mean follow-up period of 38 months.
Treatment of symptomatic patients who have imaging evidence of decreased rCVR may include maintaining the blood pressure at normal or only slightly elevated levels or even revascularization surgery (20). Schmiedek et al (18) studied 28 patients who had clinical evidence of hemodynamic ischemia as well as impaired rCVR by SPECT brain scans. All patients underwent EC-IC bypass surgery and 23 patients had an uneventful postoperative course. During a mean follow-up of 3 years, none of these patients had a stroke and all of the abnormal SPECT scans with acetazolamide were either normalized or improved. Kuroda et al (19) reported nine patients with normal CBF and abnormal rCVR on acetazolamide-challenged SPECT studies, eight of whom underwent EC-IC bypass surgery. All remained symptom free after the surgery and their rCVRs normalized. Eleven other patients in that series had both decreased CBF and CVR, nine of whom underwent EC-IC bypass surgery. In this group, seven had a normal postoperative course and all seven showed normalization of rCVR with no cerebral ischemic attacks after surgery. However, there were no good control groups in any of the above-mentioned studies.
A potential limitation of our study is the semiquantitative method of using a linear 10-band scale to define abnormal rCVR on SPECT scans. Some of the studies in the literature measuring CBF used quantitative techniques that involved measuring multiple regions of the brain for relative radioactivity counts. On the other hand, our technique is easier to implement in daily work and does not require any significant data processing to calculate the arithmetic values. Another limitation of our study is that SPECT imaging can be used to evaluate only CBF and not OEF or glucose utilization. Our study was retrospective and did not evaluate costs or outcomes; our sole purpose was to correlate between rCVR determined by acetazolamide-challenged SPECT brain scanning and vascular flow patterns and arterial stenoses as determined by angiography.
Conclusion
Patients who had decreased rCVR on acetazolamide-challenged SPECT brain studies showed increased dependence on EC-IC and leptomeningeal collaterals. Also, patients who had 70% or greater stenosis or occlusion of the carotid artery, the ACA, or the MCA had a higher rate of decreased CVR of the ipsilateral cerebral hemisphere after acetazolamide-challenged SPECT. Acetazolamide-challenged SPECT brain scanning provides additional information regarding rCVR that is not reliably provided by cerebral angiography. Even though angiographic observations of collateral circulation and high-grade stenosis or occlusion are statistically significant in predicting decreased rCVR in this population of patients, individual variability necessitates an additional measure of rCVR. Prospective, well-controlled, longitudinal studies are necessary to further understand the natural history of patients who have different flow patterns at acetazolamide-challenged CBF imaging as well as to ascertain how different treatments may change the clinical course and imaging findings in patients with cerebral hemodynamic compromise.
fig 2.
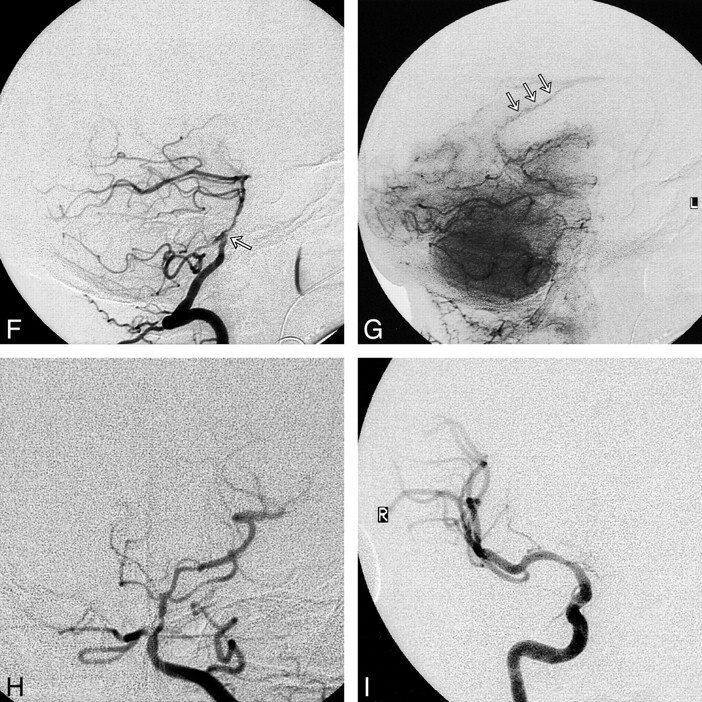
F and G, Lateral views of early arterial (F) and capillary (G) phases of left vertebral angiogram show high-grade stenosis of the mid-basilar artery (arrow, F) and retrograde filling of the pericallosal artery (arrows, G) via PCA collaterals.
H, Anteroposterior view of left vertebral angiogram shows an occluded right PCA.
I, Anteroposterior view of right ICA angiogram after right MCA angioplasty shows almost complete resolution of the right MCA stenosis
Acknowledgments
We thank Dennis Patton for his editorial comments and Denise Bruck for her assistance with chart review.
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, Atlanta, April 2000.
Address reprint requests to Hasan T. Ozgur, MD, Longview Radiologists, 1020 11th Ave, Longview, WA 98632.
References
- 1.Gibbs JM, Wise RJS, Leenders KL, Jones T. Evaluation of cerebral perfusion reserve in patients with carotid artery occlusion. Lancet 1984;1:310-314 [DOI] [PubMed] [Google Scholar]
- 2.Kistler JP, Ropper AH, Heros RC. Therapy of ischemic cerebral vascular disease due to atherothrombosis. N Engl J Med 1984;311:27-34 [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Press GA, Grubb RL, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med 1987;106:27-35 [DOI] [PubMed] [Google Scholar]
- 4.Schroeder T. Cerebrovascular reactivity to acetazolamide in carotid artery disease: enhancement of side-to-side CBF asymmetry indicates critically reduced perfusion pressure. Neurol Res 1986;8:231-236 [DOI] [PubMed] [Google Scholar]
- 5.Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996;27:2188-2190 [DOI] [PubMed] [Google Scholar]
- 6.Laloux P, Jamart J, Meurisse H, De Coster P, Laterre C. Persistent perfusion defect in transient ischemic attacks: a new clinically useful subgroup? Stroke 1996;27:425-430 [DOI] [PubMed] [Google Scholar]
- 7.Cikrit DF, Dalsing MC, Harting PS, et al. Cerebral vascular reactivity assessed with acetazolamide single photon emission computer tomography scans before and after carotid endarterectomy. Am J Surg 1997;174:193-197 [DOI] [PubMed] [Google Scholar]
- 8.Warach S, Chien D. Clinical perfusion and diffusion imaging. In: Anderson CM, Edelman RR, Turski PA, eds. Clinical Magnetic Resonance Angiography. New York: Raven 1993;143-159
- 9.Cenic A, Nabavi DG, Craen RA, Gelb AW, Lee TY. A CT method to measure hemodynamics in brain tumors: validation and application of cerebral blood flow maps. AJNR Am J Neuroradiol 2000;21:462-470 [PMC free article] [PubMed] [Google Scholar]
- 10.Powers WJ, Grubb RL. Hemodynamic and metabolic relationships in cerebral ischemia and subarachnoid hemorrhage. In: Wood JH, eds. Cerebral Blood Flow: Physiologic and Clinical Aspects. New York: McGraw-Hill 1987;387-401
- 11.Hosoda K, Fujita S, Kawaguchi T, Shose Y, Shibata Y, Tamaki N. Influence of degree of carotid artery stenosis and collateral pathways and effect of carotid endarterectomy on cerebral vasoreactivity. Neurosurgery 1998;42:988-995 [DOI] [PubMed] [Google Scholar]
- 12.Ter-Pogossian MM, Herscovitch P. Radioactive oxygen-15 in the study of cerebral blood flow, blood volume, and oxygen metabolism. Semin Nucl Med 1985;15:377-394 [DOI] [PubMed] [Google Scholar]
- 13.Reichenbach JR, Rother J, Jonetz-Mentzel L, et al. Acute stroke evaluated by time-to-peak mapping during initial and early follow-up perfusion CT studies. AJNR Am J Neuroradiol 1999;20:1842-1850 [PMC free article] [PubMed] [Google Scholar]
- 14.Gur D, Good WF, Wolfson SK, et al. In vivo mapping of local cerebral blood flow by xenon-enhanced computer tomography. Science 1982;215:1267-1268 [DOI] [PubMed] [Google Scholar]
- 15.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar imaging. Radiology 1996;199:391-401 [DOI] [PubMed] [Google Scholar]
- 16.Stokely EM, Sveinsdottir E, Lassen NA, et al. A single photon dynamic computer assisted tomograph (DCAT) for imaging brain function in multiple cross sections. J Comput Assist Tomogr 1980;4:230-240 [DOI] [PubMed] [Google Scholar]
- 17.Celsis P, Goldman T, Henriksen L, et al. A method of calculating regional cerebral blood flow from emission computed tomography of inert gas concentrations. J Comput Assist Tomogr 1981;5:641-645 [DOI] [PubMed] [Google Scholar]
- 18.Schmiedek P, Piepgras A, Leinsinger G, Kirsch CM, Einhaupl K. Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg 1994;81:236-244 [DOI] [PubMed] [Google Scholar]
- 19.Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery 1993;32:912-918 [DOI] [PubMed] [Google Scholar]
- 20.Smith HA, Thompson-Dobkin J, Yonas H, Flint E. Correlation of xenon-enhanced computed tomography-defined cerebral blood flow reactivity and collateral flow patterns. Stroke 1994;25:1784-1787 [DOI] [PubMed] [Google Scholar]
- 21.Russel D, Dybevold S, Kjartansson O, Nyberg-Hansen R, Rootwelt K, Wiberg J. Cerebral vasoreactivity and blood flow before and 3 months after carotid endarterectomy. Stroke 1990;21:1029-1032 [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T, Houkin K, Abe H, Isobe M, Kamiyama H. Cerebral haemodynamics and long term prognosis after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatry 1995;59:625-628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan HG, Allison JD, Kingsburry TB IV, et al. Analysis of inhalation rCBF data. Stroke 1987;18:495-502 [DOI] [PubMed] [Google Scholar]
- 24.Sullivan HG, Kingsburry TB IV, Morgan ME, et al. The rCBF response to Diamox in normal subjects and cerebrovascular disease patients. J Neurosurg 1987;67:525-534 [DOI] [PubMed] [Google Scholar]
- 25.Schroeder T, Sillesen H, Engell HC. Hemodynamic effect of carotid endarterectomy. Stroke 1987;18:204-209 [DOI] [PubMed] [Google Scholar]
- 26.Mountz JM, Deutsch G, Khan SH. Regional cerebral blood flow changes in stroke imaged by Tc-99m HMPAO SPECT with corresponding anatomic image comparison. Clin Nucl Med 1993;18:1067-1082 [DOI] [PubMed] [Google Scholar]
- 27.van Everdingen KJ, Visser GH, Klijn CJM, Kappelle LJ, van der Grond J. Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol 1998;44:167-176 [DOI] [PubMed] [Google Scholar]
- 28.Grubb RL, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055-1060 [DOI] [PubMed] [Google Scholar]
- 29.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993;79:483-489 [DOI] [PubMed] [Google Scholar]
- 30.Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke 1992;23:171-174 [DOI] [PubMed] [Google Scholar]