Abstract
Summary: A 73-year-old man was admitted with invasive aspergillus of the sphenoid sinus. Endoscopic debridement of the sphenoid sinus was complicated by rupture of a mycotic cavernous carotid artery aneurysm with severe epistaxis. The aneurysm was closed emergently by endovascular coil placement. Subsequently, the mycotic aneurysm extended intradurally and caused fatal subarachnoid hemorrhage.
The radiologic-pathologic data illustrate the mechanism of fungal mycotic aneurysm formation and growth. This case emphasizes the need for rapid diagnosis of potential fungal involvement of the central nervous system and suggests the necessity for aggressive treatment once fungal cerebrovascular involvement is identified.
Infectious aneurysms involving the cerebral vasculature are uncommon lesions, believed to represent only 2% to 5% of all intracranial aneurysms (1). Although the causative agent is usually bacterial, intracranial aneurysms may also result from fungal infection, causing true “mycotic”, or fungal, aneurysms.
Intracranial fungal aneurysms are extremely rare, reported less than 15 times since their first description in 1968 (1, 2). However, increasing numbers and prolonged survival of patients with immunosupression from pathologic or iatrogenic causes means that the incidence of CNS fungal infections is increasing, making recognition of this devastating cerebrovascular lesion of increasing importance (3).
We report the case of an internal carotid artery aneurysm resulting from aspergillus sinusitis that caused epistaxis, repeated subarachnoid hemorrhage, and cerebral infarction. This case illustrates the imaging-pathologic correlation of the diagnosis and progression of fungal aneurysms. Understanding the pathophysiology of aspergillus aneurysm development highlights the combination of hemorrhagic and ischemic cerebrovascular complications that may result from angioinvasive fungal infections.
Case Report
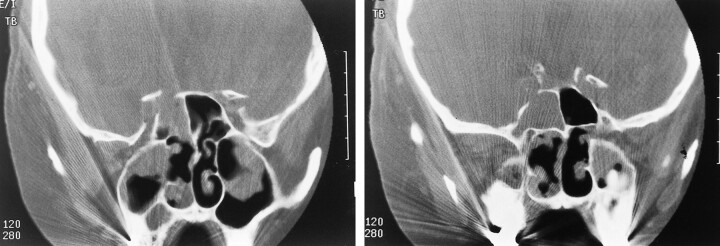
A 73-year-old man with a history of increasing headaches over 1 month, decreased vision in the right eye, and epistaxis was admitted to an outlying community hospital. The epistaxis was controlled with anterior and posterior packing of the right nostril. CT showed right sphenoid sinus opacification with erosion of the lateral wall and extension of soft tissue into the right cavernous sinus and orbital apex (Fig 1). The patient was transferred to our institution for further evaluation. The differential diagnoses included tumor, bacterial sinusitis, and invasive fungal sinusitis.
fig 1.
A and B, Coronal CT scan shows soft tissue density within the sphenoid sinus, with bony destruction in the region of the cavernous portion of the right internal carotid artery
The patient's past medical history was relevant for idiopathic thrombocytopenic purpura, treated with splenectomy and steroids. He also had a history of coronary artery disease, myocardial infarction, and hyperthyroidism.
Neurologic examination was remarkable for decreased extraocular motion of the right eye referable to cranial nerves III, IV, and VI, as well as decreasd visual acuity on the right to light perception only. A decreased direct and consensual pupillary response to light was present on the right. Laboratory evaluation showed a white blood cell count of 28,000, hemoglobin 9 g/dL, and a platelet count of 78,000.
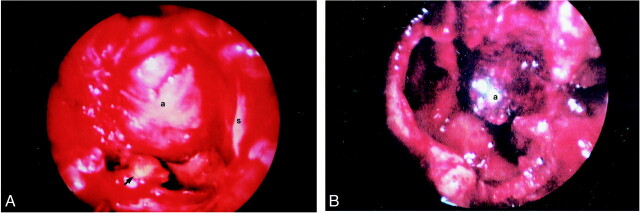
Endoscopic approach to the sphenoid sinus revealed inspissated secretions and debris, which were sent for pathologic examination and culture. A superiorly based, slightly pulsatile mass was also identified within the sphenoid cavity (Fig 2). After debridement, the sphenoid sinus and sinonasal cavity were lightly packed anterior to the mass and surgery was terminated. After extubation and transport to the recovery room, the patient was noted to have right-sided epistaxis following an episode of coughing.
fig 2.
A, Endoscopic view of the sphenoid sinus following removal of the inspissated mucus and fungal debris. The most lateral and inferior portions of the sinus are evident (arrowhead). The aneurysm (a) projects from the superior aspect of the sphenoid, to nearly fill the sinus (s = midline nasal septum).
B, Endoscopic view of the sphenoid sinus approximately 5 days after embolization. There is no evidence of recurrent fungal debris and the embolized aneurysm is much smaller
The patient was returned to the operating room, where endoscopic examination revealed that the bleeding orignated from a pinpoint area on the anterior surface of the previously identified mass. Suction electrocautery was briefly tried, but was ineffective in stopping the hemorrhage. The bleeding site and entire sinonasal cavity were tightly packed and the bleeding abated. The patient had become hypotensive with a systolic blood pressure of 70–80 mm Hg.
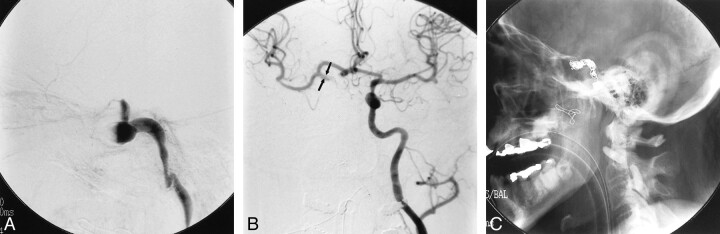
The patient was taken from the operating room to the interventional neuroradiology suite where angiography revealed a fusiform aneurysm involving the entire cavernous segment of the right internal carotid artery (Fig 3). The surgical packing of the sphenoid sinus had occluded the vessel, preventing distal filling of the carotid artery, and the supraclinoid portion was not visualized on the right internal carotid injection. The aneurysmally dilated cavernous carotid was packed with detachable coils and the cervical internal carotid artery was occluded using detachable balloons.
fig 3.
A, Lateral view, angiogram of the right internal carotid artery following initial debridement and packing of the sphenoid sinus shows fusiform dilation of the cavernous portion of the vessel. Occlusion of the carotid artery resulted from packing of the sphenoid sinus at the time of surgery.
B, Injection of the left internal carotid artery shows good filling across the anterior communicating artery. The visualized portions of the supraclinoid right internal carotid artery (arrows) and the right middle cerebral artery are of normal caliber.
C, Postembolization plain film shows microcoils within the lumen of the aneurysm and proximal detachable balloons occluding the right internal carotid artery
Postembolization injection of the left internal carotid artery showed good cross filling with reflux into the supraclinoid internal carotid artery on the right. The visualized portion of the right supraclinoid internal carotid artery and the right middle cerebral artery were of normal caliber. The patient awoke with no recurrence of hemorrhage and no new neurologic deficits.
Cultures of the sphenoid sinus revealed aspergillus and the patient was treated with intravenous amphotericin B.
Follow-up endoscopic examination of the sphenoid sinus 5 days following carotid occlusion demonstrated considerable shrinkage of the aneurysm. There was no recurrence of fungal debris and no bleeding from the aneurysm.
Approximately 10 days later, the patient complained of a severe headache. CT scanning revealed subarachnoid hemorrhage.
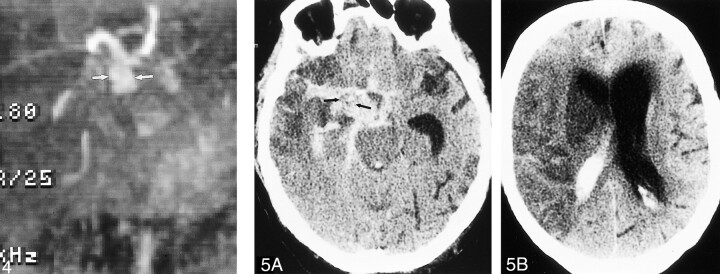
MR and MR angiography were performed to evaluate the etiology of the hemorrhage. Although signal loss from the intralumenal coils obscured the right cavernous carotid, fusiform enlargement of the supraclinoid right internal carotid artery was present (Fig 4). The findings represented a change from the configuration of the vessel seen earlier on the angiogram and indicated extension of the aneurysm distally to involve the supraclinoid internal carotid artery.
fig 4.
MR angiogram (time of flight, 48/5.9 [TR/TE]) shows dilated supraclinoid right internal carotid artery (arrows) representing aneurysmal progression from angiogram performed at the time of embolization.
fig 5. A, Unenhanced CT scan shows extensive subarachnoid hemorrhage outlining soft tissue mass (arrows) in the right basilar cistern, which represents the supraclinoid portion of the aneurysm.
B, Hypodense cerebral infarct with mass effect is present throughout the right middle cerebral artery distribution
Antifungal therapy was continued. No surgical therapy was considered possible and the patient developed left hemiparesis asociated with loss of consciousness 2 days later. CT showed a second episode of subarachnoid hemorrhage outlining the fusiform enlargement of the supraclinoid right internal carotid artery (Fig 5). An acute right middle cerebral artery infarct was present. Life support was withdrawn and the patient died 2 days later.
Postmortem Examination
Postmortem examination of the right sphenoid sinus showed a collection of pus that extended through the lateral sinus wall to involve the right internal carotid artery. The cavernous carotid artery was markedly dilated and covered with pus. Embolization coils were present within this segment of the vessel (Fig 6).
fig 6.
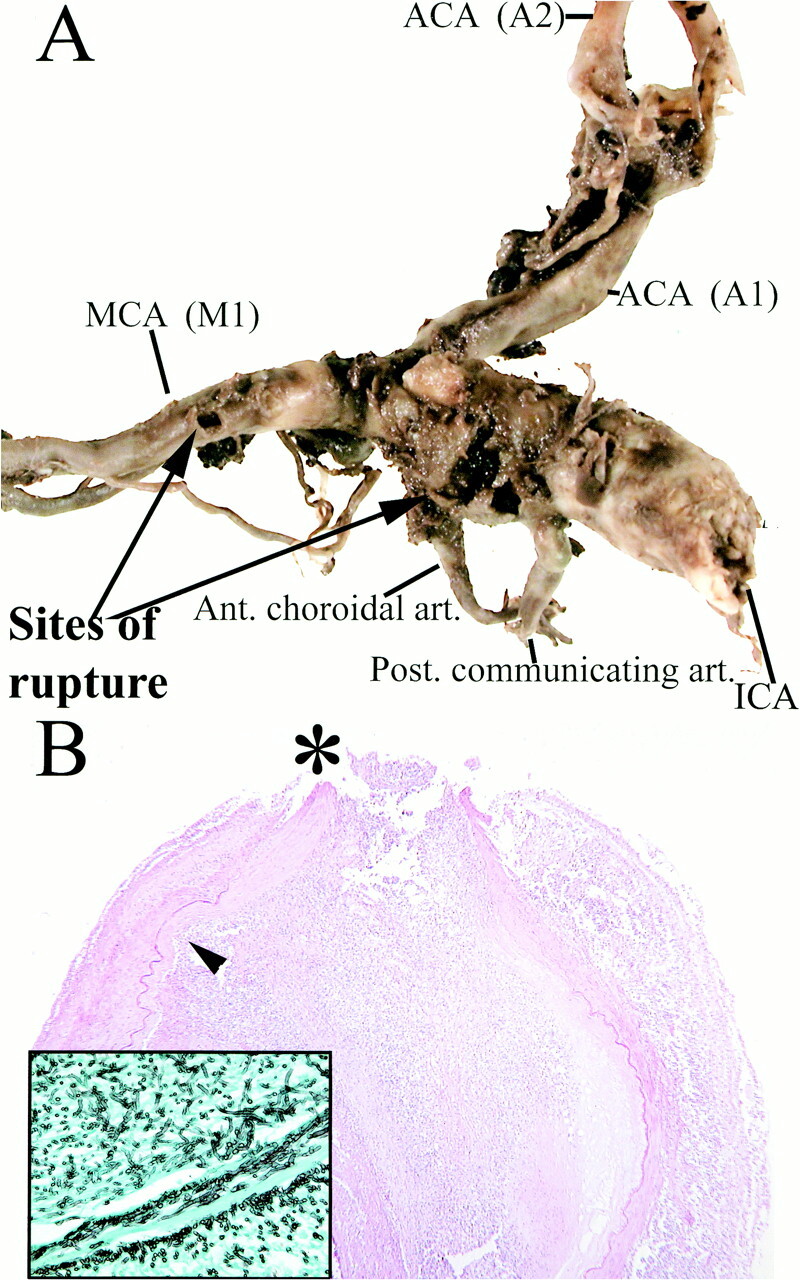
A, Aneurysmal dilation of the supraclinoid segment of the right internal carotid artery. Rupture sites (arrows) are present between the posterior communicating and anterior choroidal arteries, as well as along the M1 segment of the right middle cerebral artery.
B, Histopathologic examination shows severe inflammatory change, necrosis of the arterial wall and elastica, intralumenal thrombosis, and focal rupture site (asterisk). Inset: (Grocott stain) Septated hyphae branching at acute angles, morphologically consistent with aspergillus species, located predominantely in the vessel wall and adventitia are seen
On microscopic examination, aneurysmal ruptures were identified in both the intracavernous and supraclinoid segments of the right internal carotid artery. There was extensive basal subarachnoid hemorrhage. Fusiform dilation of the supraclinoid right internal carotid artery was present, tapering toward the M1 segment of the middle cerebral artery. Ruptures were grossly identified between the posterior communicating and anterior choroidal arteries, as well as along the M1 segment of the right middle cerebral artery.
Histopathologic examination of these arterial segments showed severe acute and chronic inflammation, necrosis of the arterial wall, and elastica, thrombosis, and focal rupture. Grocott stains identified abundant septated fungal hyphae branching at acute angles, morphologically consistent with aspergillus species. These were located predominantly in the vessel wall and adventitia. Acute infarction was present in representative sections from the right middle cerebral artery distribution.
Discussion
We report the case of an immunocompromised patient with invasive aspergillus sinusitis who developed a mycotic aneurysm of the cavernous carotid artery. Intrasphenoid hemorrhage following surgical debridement of the sinusitis was initially controlled by endovascular closure of the aneurysm and internal carotid artery. Despite intravenous treatment with amphotericin B, progressive distal involvement of the vessel wall caused intradural extension of the aneurysm, with subsequent fatal subarachnoid hemorrhage and cerebral infarction.
Autopsy identified the mechanism of subarachnoid hemorrhage as carotid artery invasion and aneurysm formation by aspergillus. The ischemic stroke resulted from intralumenal extension of fungal hyphae, causing occlusion of the internal carotid artery bifurcation and middle cerebral artery.
This case provides pathologic-imaging correlation of one of the most devastating types of fungal involvement affecting the CNS—invasive aspergillosis with fungal aneurysm.
Aspergillus is a ubiquitous fungus that commonly causes disease in debilitated or immunocompromised patients. Inhalation of airborne spores is the usual mechanism of infection and gives the organism entry to the bronchopulmonary system or paranasal sinuses. Any of three manifestations of aspergillosis may develop: allergic aspergillosis, colonization or aspergilloma, and invasive aspergillosis.
Immune responses to aspergillus antigens give rise to allergic aspergillosis, a condition of inflammation and mucosal hypertrophy, most often without mucosal invasion. Surgical debridement and postoperative corticosteriod treatment is recommended. Antifungal therapy is usually not necessary.
Aspergillomas are localized masses of aspergillus, necrotic material, and granulation tissue, including multinucleated Langhans cells. Aspergillomas usually arise in preexisting cavitary lung lesions or, less often, in the paranasal sinuses. They may exist for long periods of time without invasion or evidence of systemic infection.
Invasive aspergillosis is the most common form of the disease to affect the immunocompromised host. The lung is the most frequent organ of primary involvement in a sequence of events characterized by endobronchial invasion, ulceration, and parenchymal lung infection.
Involvement of the CNS by aspergillus, first reported in 1943, occurs secondarily, usually as a result of hematogenous spread from a pulmonary focus. The CNS may also be affected by direct extension from skull base structures including the ear, orbit, or as seen in this patient, the paranasal sinuses. Other less frequently reported mechanisms of invasion include introduction of the organism during surgery or via blood transfusion (4, 5).
Primary invasive aspergillus sinusitis is characterized by extension of hyphae through the sinus mucosa and walls, followed by invasive spread into contiguous structures. Sphenoid sinusitis, as seen in this case, provides particular opportunities for a broad range of vascular, skull base, and CNS effects. The central location of the sphenoid sinus in the skull base provides multiple pathways for intracranial spread to critical regions of the CNS and cerebral vasculature. Direct bony involvement of the skull base with osteomyelitis, cranial nerve extension via the skull base foramina, invasion of the sella, extension into the cavernous sinus, and, as exemplified in this case, invasion of the carotid arteries, have all been reported. Intracranial complications resulting from invasive aspergillus sinusitis, therefore, may include skull base osteomyelitis, meningitis, meningoencephalitis, brain abscess, cavernous sinus thrombosis, infarcts, and rarely, fungal aneurysms (6–8).
Fewer than 15 reports of this rare type of aneurysm have been published, although there is evidence that fungal infection of the CNS, including vascular involvement, is increasing in frequency (9). Fungal aneurysms involving the intracranial vessels have been described to result from either hematogenous seeding or direct spread of infection. The rarity of fungal aneurysms, their tendency to occur in the immunocompromised patient in a broad range of clinical settings, their nonspecific clinical picture, and their usually rapid progression to a fatal outcome provide considerable challenges for both diagnosis and management.
In our patient, fungal infection of the sphenoid sinus placed the organism into close proximity to the cavernous carotid artery, where bony destruction provided an opportunity for invasive arteritis. In contrast to most bacterial infections, from which arterial walls provide a relatively protective barrier, hyphal fungal agents, including both aspergillus and the phycomycetes (eg, mucormycosis), have a tendency to directly invade the walls of blood vessels (10). The vascular invasiveness of aspergillus is frequently seen in the lungs, where pulmonary vascular invasion has been well documented.
The angioinvasive nature of aspergillus is directly related to its ability to digest elastic tissue, a characteristic mediated by production of the enzyme elastase. This enzyme facilitates destruction of potential barriers to fungal infection. Many of the most effective barriers, including arterial walls, contain significant elastin components. Strains of aspergillus that produce elastase exhibit much higher mortality rates in animal infection studies than elastase negative strains (11). Human studies have also found the aggressiveness of clinical infection to be related to elastase production by the responsible strain of fungus (12). The increased aggressiveness and mortality associated with elastase-producing aspergillus suggests that the ability to cross elastic barriers contributes greatly to the organism's virulence, including its ability to invade and infect arterial walls.
Hyphal growth extends through the vessel wall, compromising the structural integrity of relatively long segments of the vessel. As was seen in this patient, involvement of long segments of the vascular wall predisposes to extension of the aneurysmally dilated portion of the vessel, as well as to repeated episodes of vascular rupture and hemorrhage (13).
The tendency of aspergillus hyphae to intramural growth results in a configuration and location of fungal aneurysms that differs from the more common infectious bacterial aneurysms. Fungal aneurysms are characteristically fusiform in shape and tend to involve longer, more proximal segments of the intracranial vessels. The intradural portion of the internal carotid artery, from which our patient's fatal hemorrhage arose, is the most commonly reported area of fungal aneurysm formation.
These morphologic features of fungal aneurysms contrast with those of bacterial aneurysms and can aid in differential diagnosis. Bacterial aneurysms are usually spherical in shape, have relatively small diameters (ranging from 2–5 mm), arise distally in the cerebral vasculature, and are frequently multiple (14).
Cerebral infarction also represents a frequent and characteristic complication of CNS vascular involvement by aspergillosis. Vessel invasion and extension of hyphae into the lumen may cause in situ thrombosis or embolization of hyphal masses. The tendency to invade proximal vessels means that the resulting infarcts frequently affect large vascular distributions.
The close proximity of vital structures of the skull base and aggressive progression of disease that characterizes invasive aspergillosis means that life-threatening complications may develop hours to days after presentation. This relatively rapid course was exemplified by our patient, whose cavernous fungal aneurysm was identified less than a week after the initial diagnosis of sinusitis. The potential for rapid progression to a poor outcome requires a high index of suspicion and prompt consideration of fungal sinusitis in immunocompromised patients.
Imaging studies in sinusitis may be relatively nonspecific with respect to the causative agent in the early stages of infection. Early findings include mucosal thickening involving the nasal cavity, as well as the maxillary or ethmoid sinuses. The sphenoid sinus is less frequently affected.
As infection progresses, imaging often reveals findings more specific for a fungal etiology. CT may demonstrate bony changes including sclerotic thickening, erosion, or remodeling, which are rare in acute bacterial infections. A central area of high density within the sinus cavity on CT may also suggest fungal disease, although chronic inspissated secretions and hemorrhage may give a similar appearance. The presence of paramagnetic substances within fungal mycetomas may give rise to very low signal intensity on all MR imaging sequences. Either imaging modality may demonstrate the aggressive extension of fungal disease from the sinuses into the orbit, facial tissues, or intracranial cavity. As demonstrated in this patient, any evidence for vascular invasion provides strong evidence for a fungal etiology.
In the absence of specific imaging findings, diagnosis of aspergillus sinusitis is often difficult, since blood and cerebrospinal fluid (CSF) cultures are frequently negative. Suspicion of the diagnosis is often confirmed at the time of surgical debridement of the involved sinuses, the initial management step.
Intravenous amphotericin following debridement is generally considered the treatment of choice. In our patient, extension of fungal invasion from the cavernous carotid artery into the supraclinoid portion of the vessel and subsequent rupture occurred despite debridement and intravenous antifungal therapy. Similar progression and extension of intramural vascular involvement has also been noted in pulmonary aspergillosis. In the pulmonary circulation, resection of involved vascular structures has been accomplished for curative treatment, a procedure obviously not feasible in the intracranial circulation (15).
Nevertheless, the 85%–100% mortality of patients with CNS aspergillosis suggests that more effective treatment algorithms need to be developed. Fatal outcomes are the uniform result when invasive aspergillus aneurysms develop, reflecting an even greater need for early diagnosis and more effective treatment (16).
Management of CNS fungal infections is often a complex problem in neurologic therapeutics. Intravenous amphotericin treatment following debridement is usually considered appropriate therapy in invasive sinusitis. Inadequate CNS penetration of amphotericin usually mandates additional intrathecal therapy when fungal meningitis is identified. Recent experience indicates that direct intracavitary instillation of amphotericin in CNS fungal abscesses can also result in improved survival (17). The use of hyperbaric oxygen therapy has also been reported to significantly decrease mortality in cases of direct extension from sinus infections caused by mucormycosis and suggests a possible role for this modality in aspergillus aneurysms (18, 19).
The uniformly poor outcomes reported following cerebrovascular invasion by fungus might indicate that the development of a fungal aneurysm is a clinical marker requiring more aggressive therapy than simple intravenous treatment, perhaps including intrathecal medication or hyperbaric therapy.
Conclusion
Mycotic aneurysm as a result of direct extension of sinusitis to the intracranial circulation is a rare but devastating complication of invasive aspergillosis. The clinical data and radiologic-pathologic correlation in this case illustrate the mechanism, rapid progression, and devastating effects of fungal cerebrovascular aneurysms. The difficulty in preventing a fatal outcome of CNS fungal infections once vascular involvement has occurred places increased emphasis on suspicion of the diagnosis to initiate aggressive therapy as early as possible. This entity should be considered early in the management of sinusitis in immunocompromised patients.
Footnotes
Address reprint requests to Robert W. Hurst, MD, Associate Professor of Radiology, Neurosurgery, and Neurology, Department of Radiology-Neuroradiology, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 19104.
References
- 1.Frazee J. Inflammatory aneurysms. In: Wilkins R, Rengachary S, eds. Neurosurgery. New York: McGraw Hill; 1996:2378–2382
- 2.Mahaley M, Spock A. An ususual case of intracranial aneurysm. In: Smith J, ed. Neuro-Ophthalmology. St. Louis: Mosby; 1968:158–166
- 3.Beal M, O'Carroll C, Kleinman G, Grossman R. Aspergillosis of the nervous system. Neurology 1982;32:473-479 [DOI] [PubMed] [Google Scholar]
- 4.Sekhar L, Dujovny M, Rao G. Carotid-cavernous sinus thrombosis caused by aspergillus fumigatus. Case report. J Neurosurg 1980;52:120-125 [DOI] [PubMed] [Google Scholar]
- 5.Takeshita M, Izawa M, Kubo O, et al. Aspergillotic aneurysm formation of cerebral artery following neurosurgical operation. Surg Neurol 1992;38:146-151 [DOI] [PubMed] [Google Scholar]
- 6.Bazan C, Rinaldi M, Rauch R, Jinkins J. Fungal infections of the brain. Neuroradiol Clin N Am 1991;1:57-88 [Google Scholar]
- 7.Horten B, Abbott G, Porro R. Fungal aneurysms of intracranial vessels. Arch Neurol 1976;33:577-579 [DOI] [PubMed] [Google Scholar]
- 8.Kountakis S, Kemper J, Chang C, DiMaio D, Stiernberg C. Osteomyelitis of the base of skull secondary to Aspergillus. Am J Otolaryngol 1997;18:19-22 [DOI] [PubMed] [Google Scholar]
- 9.Fraser D, Ward J, Ajello L, Plikaytis B. Aspergillosis and other systemic mycoses. The growing problem. JAMA 1979;242:1631-1635 [PubMed] [Google Scholar]
- 10.Scaravilli F. Parasitic and fungal infections of the nervous system. In: Adams J, Corsellis J, Duchen L, eds. Greenfieid's Neuropathology. New York: Wiley;1984:
- 11.Kothary M, Chase T, MacMillan J. Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect Immun 1984;43:320-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes J, Bode R, McCuan-Kirsch C. Elastase production in clinical isolates of Aspergillus. Diagn Microbiol Infect Dis 1988;10:165-170 [DOI] [PubMed] [Google Scholar]
- 13.Ahuja G, Jani N, Vijayaraghavan M, Roy S. Cerebral mycotic aneurysm of fungal origin. Case report. J Neurosurg 1978;49:107-110 [DOI] [PubMed] [Google Scholar]
- 14.Lau A, Takeshita M, Ishii N. Mycotic (aspergillus) arteritis resulting in fatal subarachnoid hemorrhage: a case report. Angiology 1991;42:251-255 [DOI] [PubMed] [Google Scholar]
- 15.Buescher T, Moritz D, Killyon G. Resection of chest wall and central veins for invasive cutaneous aspergillus infection in an immunocompromised patient. Chest 1994;105:1283-1285 [DOI] [PubMed] [Google Scholar]
- 16.Scully R. Case records of the Massachusetts General Hospital. N Engl J Med 1988;317:427-440 [Google Scholar]
- 17.Elgamal E, Murshid W. Intracavitary administration of amphotericin B in the treatment of cerebral aspergillosis in a non Immune-compromised patient: case report and review of the literature. Br J Neurosurg 2000;14:137-141 [DOI] [PubMed] [Google Scholar]
- 18.Couch L, Theilen F, Mader JT. Rhinocerebral mucormycosis with cerebral extension successfully treated with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol Head Neck Surg 1988;114:791-794 [DOI] [PubMed] [Google Scholar]
- 19.Yohai R, Bullock J, Aziz A, Markert RJ. Survival factors in rhino-orbital-cerebral mucormycosis. Surv of Ophthalmol 1994;39:3-22 [DOI] [PubMed] [Google Scholar]