Abstract
Summary: Tumefactive fibroinflammatory lesions of the head and neck are rare. CNS involvement has not been reported. We present a histologically proven case of a tumefactive fibroinflammatory lesion that originated in the left side of the neck and progressed over 2 years to involve the meninges, the cavernous sinuses, the right temporal lobe, and the right orbit. The lesion caused destruction of the skull base and a subdural hematoma. The relationship of the present lesion to idiopathic hypertrophic pachymeningitis and Tolosa-Hunt syndrome is discussed.
Tumefactive fibroinflammatory lesions (TFILs) are histologically benign entities that present with local invasion but do not metastasize. Although common in other parts of the body, they rarely occur in the head and neck. As of this writing, only 24 such cases have been reported (1). These lesions can arise from virtually any extracranial head or neck location, most commonly from the sinonasal tract, parotid glands, and neck; and less commonly from the tongue, face, mandible, and cheek. One recent report detailed a TFIL that had originated in the infratemporal fossa (2). TFILs can invade vascular structures and muscle and cause extensive bony destruction (1, 2). To our knowledge, no report has previously documented extension into the middle cranial fossa or any direct form of brain involvement aside from cranial neuropathy.
Case Report
A 23-year-old man first presented 26 months before the current admission with a 2-year history of trismus and dysphagia. Related complaints included a left-sided headache and neck mass. His primary care provider attempted to treat him with antibiotics; however, the condition not only persisted but progressed. There was no direct evidence of neurologic involvement at this time.
Imaging examinations included contrast-enhanced CT of the neck, Gd-DTPA-enhanced (0.1 mmol/kg) MR imaging of the neck, MR angiography of the neck, and MR imaging of the temporomandibular joint. The imaging studies revealed a diffusely infiltrating mass arising from the left masticator space and extending from the cranial vault to the hypopharynx (Fig 1). The mass infiltrated the temporalis, the longus colli and medial and lateral pterygoid muscles, the anterior aspect of the sternocleidomastoid muscle and surrounding fascia, the parapharyngeal space, and the submandibular triangle. The lesion encased both the left carotid artery and the jugular vein in the neck without causing narrowing. The mass was hypointense relative to muscle on T2-weighted (4000/105/1 [TR/TE/excitations]) images, and showed a mild degree of enhancement as well as infiltration through the foramen ovale into the middle cranial fossa on the left side. Enlargement of the left cavernous sinus suggested invasion. Meningeal enhancement without thickening was noted in the anterior and posterior cranial fossae and along the tentorium cerebelli. No abnormalities were noted within the brain parenchyma or in the temporomandibular joints.
fig 1.
23-year-old man with 2-year history of trismus and dysphagia. A–C, Imaging studies at the time of the first admission. A, Axial contrast-enhanced CT scan through the neck at the level of the tongue and the submandibular triangle. The mildly enhancing lesion has infiltrated into the left carotid space (curved arrow) and the left submandibular triangle (straight arrows). B and C, Coronal contrast-enhanced T1-weighted (600/14/1) (B) and axial T2-weighted (4000/105/1) (C) MR images show infiltration by the lesion of the left nasopharynx (wide arrow). It invades into the left middle cranial fossa and the left cavernous sinus (double arrows) through the foramen ovale (single arrow). It is mildly enhancing on the T1-weighted image and is hypointense on the T2-weighted image. The lesion replaces adipose tissue in the left parapharyngeal space. fig 2. Contrast-enhanced CT study at the time of the second admission shows thickening and enhancement of the tentorium cerebelli, greater on the left (arrows). This finding was not seen previously
An extensive biopsy was performed, which included the neck mass itself, the sternocleidomastoid muscle, and the area of the jugular foramen. Tissue from all these areas was histologically uniform and revealed a dense sclerosing, fibrous proliferative process, accompanied by a lymphoplasmacytic inflammatory infiltrate. Immunohistochemical staining revealed these cells to be polyclonal, composed of both B- and T-lymphocytes and also histiocytes (KP-1+). During this admission, the patient was treated with a course of IV methylprednisolone sodium succinate (Solu-Medrol, Upjohn Co, Kalamazoo, MI). He continued to report problems with opening his mouth and chewing and of left-sided headache, but said that his swallowing had improved. However, he noted decreased hearing and tinnitus in the left ear.
The patient was readmitted 4 months later (22 months before the current admission), at which time a contrast-enhanced CT scan of the neck revealed a decrease in the size and degree of contrast enhancement of the left-sided neck mass. The left jugular vein was obliterated at the level of the piriform sinuses. It was unclear whether this actually represented encasement as opposed to thrombosis of the vein. A contrast-enhanced CT scan of the brain showed marked increase in the degree of thickening and enhancement of the tentorium cerebelli, especially on the left (Fig 2). He was treated with IV Solu-Medrol, as before, for 3 days and discharged on a course of oral steroids (prednisone 20-mg orally four times a day). Unfortunately, the patient was then lost to follow-up until the penultimate admission. He later revealed that he had stopped taking this medication after approximately 2 months owing to hearing problems and skin lesions.
Three months before the current admission, the patient sought medical attention for headaches, which had previously resolved after steroid treatment. More significantly, a right lateral rectus palsy had now developed, which manifest as horizontal diplopia on rightward gaze. The patient stated that these symptoms had been intermittently present for some 5 months and had recently worsened.
An MR study of the brain and orbits showed significant progression of the disease, especially on the right side (Fig 3). Axial Gd-DTPA-enhanced (0.1 mmol/kg), fat-suppressed T1-weighted (600/20/1) images showed marked thickening and enhancement of the right cavernous sinus. This led to significant narrowing (approximately 90%) of the cavernous portion of the carotid artery, which had not been seen earlier. The stenosis was confirmed by MR angiography.
There was also marked thickening and enhancement of the meninges of the right middle cranial fossa. This enhancement extended into and widened the sulci of the temporal lobe. Streaks of abnormal enhancement were also noted in the right temporal lobe parenchyma. T2-weighted (4000/102/1) images showed increased signal in the medial temporal lobe, which involved both the gray and white matter. A diffusion-weighted scan failed to show evidence of acute infarction in this area. The enhancing mass now infiltrated through the right foramen ovale and foramen spinosum. The pituitary stalk was thickened and also presumed to be involved. The mass extended along the planum sphenoidale with frank invasion into the sphenoid sinus. A CT scan showed extensive bony erosion of the planum sphenoidale, dorsum sellae, and tuberculum sellae. There was also thickening and enhancement of the right lateral rectus muscle, presumably a result of involvement with the TFIL. All these findings were new, and had been not observed on the prior studies.
Given the rarity of the diagnosis in this case, and what seemed potentially to be a treatment failure, another attempt was made to obtain confirmation and rule out other entities before attempting retreatment with steroids. A gallium scan was performed to look for signs of abnormal peripheral (ie, lymph node) uptake that might suggest sarcoidosis and offer a potential biopsy site. However, uptake was limited to the region of the right skull base, sphenoid wing, sphenoid sinus, and temporal lobe. CSF cultures and stains were negative, as were results of a specimen sent for tuberculosis polymerase chain reaction. The CSF glucose was normal (44 mg/dL), but the protein level (83 mg/dL) and the white cell count (42 cells/μL) were elevated, suggesting an inflammatory process. These cells were mostly mononuclear cells (4% polymorphonuclear cells, 94% lymphocytes, 2% monocytes). Finally, a CT-guided needle biopsy was performed with a tissue sample obtained from the left parapharyngeal space. This revealed only dense fibroconnective tissue, essentially identical to that found previously. The patient was treated with a 5-day course of Solu-Medrol and then discharged again on oral steroids.
The last admission was precipitated by the presence of a large subdural hematoma over the right convexity, which was seen on a routine follow-up MR scan (Fig 4). The patient stated that he had been having an unremitting headache, and that he had again stopped taking his steroids. He was again treated with high-dose steroids and released after 10 days when the subdural hematoma had decreased in size.
Discussion
This report describes a case of a TFIL of the neck, which spread to involve the meninges and the brain. The case is unique both in terms of the anatomic structures involved and the pattern of spread of the disease. To the best of our knowledge, there are no reports of a TFIL involving the brain or orbit or causing destruction of the base of the skull or a subdural hematoma. In terms of the pattern of spread, it appears from the first set of imaging studies that the center of the lesion was in the left masticator space. At that time, there was also involvement of other structures of the neck as far caudal as the submandibular triangle. The lesion also invaded cephalad through the left foramen ovale, which resulted in meningeal enhancement (Fig 1). Studies 4 months later showed increased involvement of the left tentorium cerebelli (Fig 2). Nineteen months later (after partial treatment) the lesion showed marked progression with extensive involvement of the contralateral (right) middle cranial fossa, meninges, and brain. The process then extended extracranially via the right foramen ovale and spinosum (Fig 3).
The involvement of the brain is of special interest. The cause of the enhancement and increased signal on the long-TR images of the right temporal lobe remains unclear. The most likely possibility is reaction of the brain to direct invasion by the inflammatory lesion. Another possibility is ischemic changes. Although diffusion scans were negative, this does not exclude reversible ischemic changes, such as venous congestion, which did not result in infarction. The venous drainage of the anterior temporal lobe is toward the cavernous sinus, which in this patient was obliterated by the inflammatory lesion. A possible contributing factor to the proposed ischemia may be the stenosis of the right internal carotid artery or possible involvement of the anterior temporal artery.
The cause of the subdural hematoma in this patient is unclear. TFILs have been known to invade vascular structures. A possible scenario is that the TFIL invaded a vein, causing extravasation, which led to the subdural hematoma.
It is interesting to speculate on the relationship of TFILs to other inflammatory disorders of the head and neck, such as Tolosa-Hunt syndrome and idiopathic hypertrophic pachymeningitis. These diseases are of unknown pathogenesis and essentially identical, histologically (2, 3). However, they have traditionally been classified as separate entities on the basis of their clinical presentation and anatomic distribution. Tolosa-Hunt syndrome (painful external ophthalmoplegia) is a regional variant of idiopathic orbital pseudotumor and is characterized by involvement of the cavernous sinus and the posterior portion of the orbital muscle cone with occlusion of the superior division of the ophthalmic vein and occasional narrowing of the cavernous carotid artery (4). Clinically, patients present with painful ophthalmoplegia with involvement of cranial nerves III, IV, VI, and V1. Our case exhibits some of the characteristics of Tolosa-Hunt syndrome not previously described in TFILs.
Idiopathic hypertrophic pachymeningitis presents with chronic, progressive thickening of the meninges, multiple cranial neuropathies, and headache (5, 6); carotid artery occlusion has also been reported (7). However, subdural hematomas and involvement of the brain, orbit, or neck have not been described. The processes that occurred in our patient originated in the neck and only later progressively spread into the cranial vault to involve the meninges.
Two recent articles reported idiopathic hypertrophic pachymeningitis or Tolosa-Hunt syndrome in the same patients and postulated a common pathogenesis (6, 8). In addition, a number of authors have noted that TFILs of the head and neck appear histologically essentially identical to sclerosing cholangitis, Riedel thyroiditis, retroperitoneal fibrosis, orbital pseudotumor, and other chronic inflammatory disorders (2, 3). Indeed, five of the reported cases of TFIL with head and neck involvement were found to harbor these other processes as well (2, 9–11).
Although the present case does not represent either idiopathic hypertrophic pachymeningitis or Tolosa-Hunt syndrome, our patient exhibited a number of findings not previously described in TFIL, which are characteristic of idiopathic hypertrophic pachymeningitis and Tolosa-Hunt. The clinical, histologic, and radiologic similarities of these disorders to the present case raise the possibility that these chronic inflammatory processes are spectra or variants of a single entity as opposed to discrete disorders.
fig 3.

A–E, Imaging studies at the time of the third admission. A and B, Coronal T1-weighted (600/23/1) (A) and coronal contrast-enhanced T1-weighted (500/14/1) (B) MR images show that the lesion has progressed to involve the contralateral cavernous sinus and the meninges of the right cranial fossa (double arrows, B). The lesion significantly narrows the right cavernous carotid artery and has invaded the right masticator space from the right middle cranial fossa through the foramen ovale (single arrow, B). The unenhanced image shows that the lesion has replaced the normally seen adipose tissue in the right infratemporal fossa. C–E, Coronal T2-weighted (4000/102/1) MR image (C) shows abnormal signal intensity in theright temporal lobe, which involves both the gray and white matter. Axial fat-suppressed, contrast-enhanced T1-weighted (600/20/1) MR images (D and E) show marked thickening and enhancement of the meninges of the right middle cranial fossa extending into the sulci as well as wisps of enhancement of the temporal lobe itself. There is marked narrowing of the cavernous carotid artery. The right lateral rectus muscle is enlarged and enhancing more than the other extraocular muscles, indicating probable involvement by the lesion
fig 4.
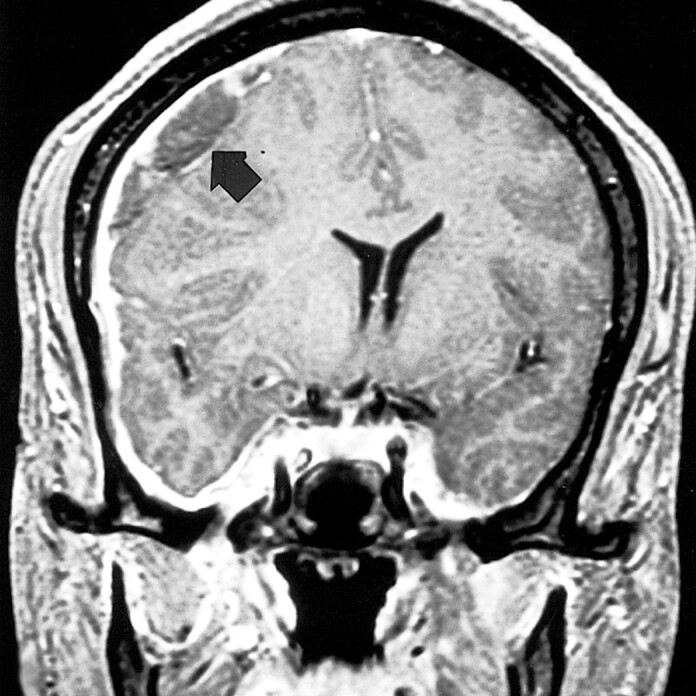
Coronal contrast-enhanced T1-weighted (500/5.7/1) MR image at the time of the fourth admission shows a large right convexity subdural hematoma (arrow), leading to a large midline shift
Footnotes
Address reprint requests to Andrei I. Holodny, MD, Department of Radiology C-320, University Hospital, 150 Bergen Street, Newark, NJ 07103.
References
- 1.Schulte DL, Wold LE, Kern EB, Olsen KD. Pathologic quiz case 1: tumefactive fibroinflammatory lesion of the nasal cavity. Arch Otolaryngol Head Neck Surg 1999;125:228-229 [DOI] [PubMed] [Google Scholar]
- 2.Patel PC, Pellitteri PK, Vrabec DP, Syzmanski M. Tumefactive fibroinflammatory lesion of the head and neck originating in the infratemporal fossa. Am J Otol 1998;19:216-219 [DOI] [PubMed] [Google Scholar]
- 3.Ritter JH, Humphrey PA, Wick MR. Malignant neoplasms capable of simulating inflammatory (myofibroblastic) pseudotumors and tumefactive fibroinflammatory lesions: pseudopseudotumors. Semin Diagn Pathol 1998;15:111-132 [PubMed] [Google Scholar]
- 4.Sondheimer FK, Knapp J. Angiographic findings in the Tolosa-Hunt syndrome. Radiology 1973;106:105-111 [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Malik A, Mishra NK, Gaikwad SB. Idiopathic hypertrophic pachymeningitis: spectrum of the disease. Neuroradiology 1997;39:619-623 [DOI] [PubMed] [Google Scholar]
- 6.Miwa H, Koshimura I, Mizuno Y. Recurrent cranial neuropathy as a clinical presentation of idiopathic inflammation of the dura mater: a possible relationship to Tolosa-Hunt syndrome and cranial pachymeningitis. J Neurol Sci 1998;154:101-105 [DOI] [PubMed] [Google Scholar]
- 7.Willing SJ, Broghamer W. Internal carotid artery occlusion due to idiopathic cranial pachymeningitis. AJNR Am J Neuroradiol 1992;13:1594-1596 [PMC free article] [PubMed] [Google Scholar]
- 8.Hatano N, Behari S, Nagatani T, et al. Idiopathic hypertrophic cranial pachymeningitis: clinicoradiological spectrum and therapeutic options. Neurosurgery 1999;45:1336-1342 [DOI] [PubMed] [Google Scholar]
- 9.Husband P, Knudsen A. Idiopathic cervical and retroperitoneal fibrosis: report of a case treated with steroids. Postgrad Med J 1976;52:788-793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen KD, DeSanto LW, Wold LE, et al. Tumefactive fibroinflammatory lesions of the head and neck. Laryngoscope 1986;96:940-944 [PubMed] [Google Scholar]
- 11.Pritchard AJN, Colloby P, Barton RPE, et al. Tumefactive fibroinflammatory lesions of the head and neck. J Laryngol Otol 1988;102:1064-1067 [DOI] [PubMed] [Google Scholar]



