Abstract
BACKGROUND AND PURPOSE: Iatrogenic dissections are an uncommon complication of cerebral angiography. We retrospectively reviewed 12 cases of arterial dissections complicating cerebral angiography and cerebrovascular interventions to evaluate the clinical course of these dissections.
METHODS: Cases from a large tertiary center performing a large number of neurovascular procedures were collected retrospectively. The patients' medical records and imaging studies were reviewed, with particular attention given to the cause of the dissection, the development of ischemic events resulting from the dissection, and the treatment used.
RESULTS: Each of nine dissections affected a vertebral artery, each of two affected an internal carotid artery, and one affected a common carotid artery. The prevalence of iatrogenic dissections was 0.4%. Seven of the dissections were noted at the time of contrast material injection for the filming of cerebral angiograms. The other five dissections occurred during catheter or wire manipulations for interventional neuroradiologic procedures. Five of the patients in our series were treated with IV administered heparin for 24 to 48 hours. The other seven patients had recently suffered acute intracranial hemorrhage or undergone neurosurgery and could not undergo anticoagulant therapy. None of the patients developed symptoms of ischemia, but one was later found to have an asymptomatic infarct in the territory supplied by the dissected artery.
CONCLUSION: Arterial dissections are an uncommon complication of cerebral angiography and cerebrovascular interventions and usually have a benign clinical course.
Because cerebral angiography involves the placement of catheters and guidewires into the carotid and vertebral arteries, there is an inherent risk of arterial dissection. The carotid and vertebral arteries provide blood to the brain, and injury to them poses a risk of ischemic injury to the brain. Arterial dissections can cause ischemic symptoms by limiting flow secondary to severe stenosis or occlusion or by acting as a source of thromboembolism. We report a retrospective review of patients suffering iatrogenic carotid and vertebral artery dissections during cerebral angiography and cerebrovascular interventions. We evaluated the specific circumstances surrounding the occurrence of each dissection and the subsequent clinical course in each case.
Methods
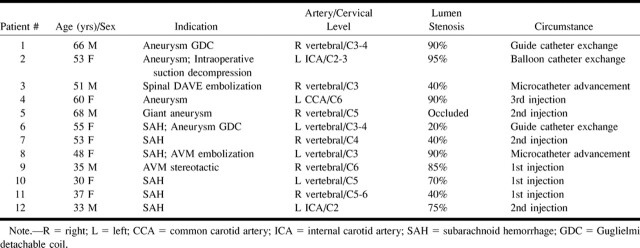
Cases were retrospectively collected from a tertiary care center for the period of June 1994 to July 1998. The cases were identified through a computerized review of the angiography records, using the search term “dissection,” and through a search of the quality assurance records, which record complications. Angiograms of the patients were reviewed, with particular attention given to the artery dissected, the length of the dissected segment, and the degree of stenosis (percent diameter) or occlusion caused by the dissection (Table 1).
TABLE 1:
Patients with arterial dissection complicating cerebral angiography
In all cases, the angiographic catheterizations were performed using a standard “over-the-wire” catheter technique (guidewire advanced into artery first, then into the catheter); contrast material was then test-injected. Records of injection rates and types of catheters and wires were generally not kept, so precise data regarding these factors are not available. A hand-injection technique using a 10-mL syringe was used in approximately half of the cases, and a power injector was used in the other half. The power injection rates that were typically used were 6 mL per second for the internal carotid artery, 8 mL per second for the common carotid artery, and 3 to 6 mL per second for the vertebral artery. The vast majority of diagnostic catheters used were braided, not hydrophilic, and curved. The guidewires that were used consisted mainly of the 0.035-inch Bentson (Cook; Bloomington, IN) and the 0.035 Glidewire (Meditech; Watertown, MA).
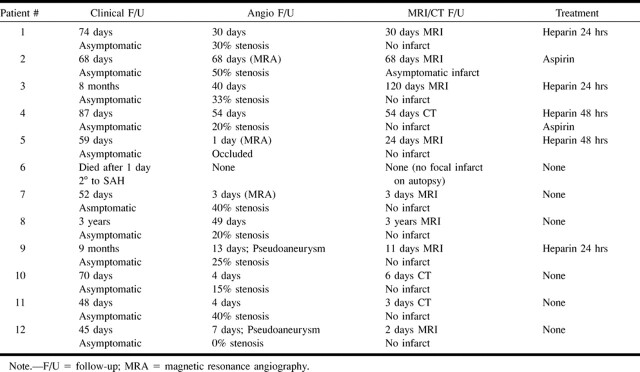
All patients had undergone clinical follow-up, angiographic follow-up, and cross-sectional imaging with CT or MR imaging of the brain (Table 2). Because this was a retrospective study, the clinical and imaging follow-up periods were variable. One patient died as a result of complications associated with subarachnoid hemorrhage the day after the dissection occurred. Excluding this patient who died, the clinical follow-up period ranged from 45 days to 3 years, the angiographic follow-up period ranged from 4 to 68 days, and the cross-sectional imaging follow-up period ranged from 2 days to 3 years.
TABLE 2:
Follow-up information for patients with arterial dissection complicating cerebral angiography
Results
The data regarding the occurrence and follow-up of the dissections are summarized in Tables 1 and 2. During the 4-year period, 2437 diagnostic cerebral angiograms were obtained and 675 arterial interventional neuroradiology procedures were performed. The total number of neuroradiologic procedures was 3112. Twelve cases of iatrogenic dissection were identified (seven female and five male patients). The prevalence of dissection was 0.4% for all neuroradiologic procedures. The prevalence of dissection was 0.3% for diagnostic cerebral angiograms. For interventional neuroradiology procedures, the prevalence was 0.7%. There was, however, no statistically significant difference between the prevalences of iatrogenic dissections for diagnostic and interventional neuroradiologic procedures (χ2 test; P > .10). The ages of the patients ranged from 30 to 68 years.
Each of nine dissections affected a vertebral artery, each of two affected an internal carotid artery, and one affected a common carotid artery. One patient (patient 4) had angiographic evidence of fibromuscular dysplasia. All of the dissections were focal, measuring less than 3 cm. No arterial tortuosity or other predisposing feature was identified. The arterial segments at which the dissections occurred were not more than gently curved.
In the seven diagnostic cerebral angiography cases, the dissections were not noted until after the injection of contrast material for filming. The five dissections that occurred during interventional neuroradiologic procedures were caused by catheter and wire manipulations.
Five patients were treated with IV administered heparin for 24 to 48 hours. One of these patients was treated with aspirin. The other seven patients were not treated with heparin because they had recently suffered intracranial hemorrhage or undergone neurosurgery. A patient who had undergone a craniotomy was treated with aspirin.
None of the patients developed transient or permanent ischemic symptoms referable to the territory of the dissected artery. Only one patient had an infarction in the territory of the dissection shown on follow-up cross-sectional images, and this small embolic infarction was asymptomatic (patient 2).
Initial lumen compromise ranged from 20% to complete occlusion. Nine patients had improvement in luminal compromise revealed by follow-up angiography, and the remaining three had conditions that remained unchanged. Two patients (patients 9 and 12) had a pseudoaneurysm. These patients with pseudoaneurysms remained asymptomatic during follow-up periods of 45 days and 9 months, respectively.
Discussion
Relatively little has been reported in the literature regarding dissections complicating transfemoral cerebral angiography. Olivecrona (1) reported three cases of intramural injection of contrast material into the wall of the carotid artery in a retrospective study of 3978 transfemoral cerebral angiograms (0.1%). Huckman et al (2) reported two cases of vascular injury during the injection of contrast material among 361 transfemoral cerebral angiograms (0.6%). Neither of these reports provided details regarding the outcome of these vascular injuries. Vitek (3) reported seven cases of injury to the internal carotid artery that were caused by subintimal injection of contrast material among 2000 angiograms (0.35%), from which no neurologic complication resulted. The prevalence of dissection in our series (0.4%) is similar to that reported in these other series (1–3).
Five modern studies have prospectively evaluated the risk of cerebral angiography, but none of these five reports recorded any iatrogenic dissections (4–8). More than 5000 patients were included in these five studies (approximately 1000 patients in each study). The lack of reported dissections in these studies may be because these studies were not specifically designed to record iatrogenic dissections. Also, some operators may consider small, asymptomatic dissections to be “technical events” rather than complications and therefore not report them. In these five studies, the risk of transient neurologic deficit was 0.55% to 2.2% and the risk of permanent neurologic deficit was 0.1% to 0.5%. These neurologic deficits complicating cerebral angiography seem to be due to thromboembolic events rather than dissection.
The great majority of dissections in our series involved the vertebral artery rather than the carotid artery. We do not have precise data available regarding the relative frequency of catheterization of the vertebral and carotid arteries in our practice. We do know, however, that in our practice, we selectively perform catheterization of the internal carotid artery more frequently than of the vertebral artery. This indicates that the vertebral artery is probably more prone to iatrogenic injury than the carotid artery. Factors that may make the vertebral artery more prone to injury are: 1) it is usually smaller than the internal carotid artery, such that the guidewire and catheter are more constrained and generate relatively more tension against the arterial wall; 2) it is constrained in a canal made up of the transverse foramina of the vertebral bodies, which limits its ability to conform to the shape of the catheter and the guidewire; and 3) it often has a tortuous origin, especially in elderly patients. This third factor apparently did not play a role in any of the dissections in our series, considering that all of the dissections occurred well above the proximal segment. Nevertheless, the distal location of all of the vertebral dissections suggests that it may be safest to place catheters low in the vertebral artery.
In the seven diagnostic cerebral angiography cases, the dissections were not noted until after injection of contrast material for filming and may have been caused by the jet of contrast material exiting the catheter during the injection. They might also have been initiated by an intimal injury during catheter and guidewire manipulation that was too small to be seen during a test injection, after which they were enlarged by the jet of contrast material exiting the catheter. They might also have occurred as a result of the catheter tip applying tension against the arterial wall and the catheter tip eventually eroding through the intima with the passage of time and the cardiovascular pulsatile motion. Tension on the catheter tip can be minimized by pulling back on the catheter after it is placed in the artery of interest to eliminate any forward tension built up on the catheter when it is initially advanced. Causation or enlargement of dissections by injection of contrast material can be minimized by careful attention to the vessel appearance and catheter position during the test injection.
Iatrogenic dissections occurred more frequently during interventional neuroradiologic procedures than during diagnostic cerebral angiography, although this was not statistically significant. There may, however, be a slightly elevated risk of iatrogenic dissection in interventional neuroradiologic procedures because of an increased number of vascular manipulations (eg, catheter exchanges, coaxial microcatheter placement) and the necessity for more distal placement of catheters compared with diagnostic angiography. The small increase in prevalence in interventional procedures may not have reached statistical significance because of the overall low prevalence of dissections for both diagnostic and interventional procedures.
The natural history of dissections complicating cerebral angiography was generally benign in our series. The natural history of spontaneous and traumatic dissections cannot be precisely known because many patients who do not have serious symptoms, such as stroke or transient ischemic attack (TIA), are probably never identified (9). There may be a large number of spontaneous or traumatic dissections that are never diagnosed because they remain asymptomatic or because they produce nonspecific symptoms, such as head or neck pain. In one study of 80 patients with carotid dissection, only 14 (18%) did not develop stroke or TIA either at presentation or within 1 month after presentation (10). An interesting group with which to compare our patients with iatrogenic dissection would be patients with proven dissection presenting with only local symptoms (ie, headache, neck pain, or Horner's syndrome, but not stroke or TIA). To our knowledge, however, no study of the occurrence of subsequent stroke or TIA in the population of patients presenting with only local symptoms has been conducted.
Dissections can have various angiographic appearances, depending on which layers of the artery are involved. Subintimal dissections tend to cause stenosis from blood located in the false lumen, whereas subadventitial dissections tend to produce saccular outpouchings of adventitia (11). Pseudoaneurysms in our series of dissections complicating angiography were unusual (two cases), whereas narrowing of the arterial true lumen angiographically was typical. This is likely because dissection complicating angiography usually causes only an intimal injury. The tendency for improvement of the stenosis on angiographic follow-up in our series is consistent with reports in the literature of angiographic follow-up of nonocclusive spontaneous dissections, the majority of which go on to improvement or resolution (9).
Many of the patients in our series underwent angiography for evaluation of a suspected cerebral aneurysm. This might tempt one to conclude that patients with aneurysms are more likely than other patients to suffer iatrogenic dissection during cerebral angiography. A higher prevalence of cerebral aneurysms has been reported among patients with spontaneous dissection (11). Nevertheless, there is selection bias in our series of patients that results in a higher prevalence of iatrogenic dissections in patients with aneurysms. The iatrogenic dissections generally occur in the vertebral and internal carotid arteries. Selective catheterization of the vertebral and internal carotid arteries is performed much more frequently in patients with aneurysms and arteriovenous malformations compared with patients who present with TIA or stroke, in whom only the common carotid arteries are usually selected. Therefore, we doubt that patients with aneurysms are significantly predisposed to iatrogenic dissection.
Angiographic findings of fibromuscular dysplasia have been found in 15% of patients with spontaneous craniocervical artery dissection (12), indicating that fibromuscular dysplasia is a risk factor for arterial dissection. Only one patient in our series (patient 4) had angiographic evidence of fibromuscular dysplasia.
No specific therapy for dissection has proved efficacy. The IV administration of heparin is the most common therapy for symptomatic spontaneous or traumatic dissections, and good outcomes have been reported (9, 10, 13–16). Many of the patients in our series were treated with a short course of IV administered heparin. Those patients who had recently suffered acute intracranial hemorrhage or had undergone neurosurgery and could consequently not undergo anticoagulant therapy did not fare significantly worse than did those patients who did undergo anticoagulant therapy. Those patients treated with heparin in our series were treated for only 24 to 48 hours, which is a shorter period of anticoagulation than is typically recommended for spontaneous and iatrogenic dissections. Stroke resulting from spontaneous and traumatic dissection usually occurs within the first week but can occur as late as 1 month after dissection, prompting a recommendation to administer anticoagulant therapy for at least 1 month (10). Aspirin has also been recommended for dissections, particularly in the absence of ischemic signs (12, 17). Surgery is rarely indicated and is reserved for patients who are refractory to medical therapy (3).
This study is somewhat limited in that it is a retrospective analysis. As is typical of retrospective studies, the patients were treated in a variety of ways and there was no systematic follow-up. The number of patients is small, so the incidence of ischemic infarction after iatrogenic dissection cannot be precisely determined. The data retrospectively derived from this series of patients, however, does indicate that iatrogenic dissections tend to have a benign course.
Footnotes
Address reprint requests to Harry J. Cloft, MD, PhD, Department of Radiology, Emory University Hospital, 1364 Clifton Road N.E., Atlanta, GA 30322.
References
- 1.Olivecrona H. Complications of cerebral angiography. Neuroradiology 1977;1977:175-181 [DOI] [PubMed] [Google Scholar]
- 2.Huckman MS, Shenk GI, Neems RL, Tinor T. Transfemoral cerebral angiography versus direct percutaneous carotid and brachial arteriography: a comparison of complication rates. Radiology 1979;132:93-97 [DOI] [PubMed] [Google Scholar]
- 3.Vitek JJ. Femoro-cerebral angiography: analysis of 2000 consecutive examinations, special emphasis on carotid artery catheterization in older patients. AJR Am J Roentgenol 1973;130:1097-1103 [DOI] [PubMed] [Google Scholar]
- 4.Ernest F, Forbes G, Sandok BA, et al. Complications of cerebral angiography: prospective assessment of risk. AJR Am J Roentgenol 1984;142:247-253 [DOI] [PubMed] [Google Scholar]
- 5.Dion JE, Gates PC, Fox AJ, Barnett HJM, Blorn RJ. Clinical events following neuroangiography: a prospective study. Stroke 1987;18:997-1004 [DOI] [PubMed] [Google Scholar]
- 6.Grzyska U, Freitag J, Zeumer H. Selective cerebral intraarterial DSA: complication rate and control of risk factors. Neuroradiology 1990;32:296-299 [DOI] [PubMed] [Google Scholar]
- 7.Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology 1992;182:243-246 [DOI] [PubMed] [Google Scholar]
- 8.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401-1407 [PMC free article] [PubMed] [Google Scholar]
- 9.Anson J, Crowell RM. Cervicocranial arterial dissection. Neurosurgery 1991;29:89-96 [DOI] [PubMed] [Google Scholar]
- 10.Biousse V, D'Angelejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. Stroke 1995;26:235-239 [DOI] [PubMed] [Google Scholar]
- 11.Schievink WI, Mokri B, Piepgras DG. Angiographic frequency of saccular intracranial aneurysms in patients with spontaneous cervical artery dissection. J Neurosurg 1992;76:62-66 [DOI] [PubMed] [Google Scholar]
- 12.Mokri B, Sundt TM, Houser OW, Piepgras DG. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol 1986;19:126-138 [DOI] [PubMed] [Google Scholar]
- 13.Hugenholtz H, Pokupa R, Montpetit VJA, Nelson R, Richard MT. Spontaneous dissecting aneurysm of the extracranial vertebral artery. Neurosurgery 1982;10:96-100 [PubMed] [Google Scholar]
- 14.Luken MG, Ascherl GF, Correll JW. Spontaneous dissecting aneurysms of the internal carotid arteries. Am J Surg 1971;122:549-5515098664 [Google Scholar]
- 15.Markwalder TM, Starrett RW, Mumemthaler M. Spontaneous bilateral recanalization in bilateral internal carotid artery occlusion. Stroke 1980;11:95-98 [DOI] [PubMed] [Google Scholar]
- 16.McNeill DH, Dreisbach J, Marsden RJ. Spontaneous dissecting aneurysm of the internal carotid artery, its conservative management with heparin sodium. Arch Neurol 1980;37:54-55 [DOI] [PubMed] [Google Scholar]
- 17.Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin 1983;1:155-182 [PubMed] [Google Scholar]