Abstract
BACKGROUND AND PURPOSE: Sonographic criteria of the lymph node have been found to be good indicators for metastatic lymph nodes. We determined which sonographic features are most predictive of metastasis in cervical lymph nodes among patients with head and neck cancer.
METHODS: Gray-scale and power Doppler sonograms were retrospectively analyzed in 133 cervical lymph nodes (57 metastatic and 76 reactive nodes) from 52 patients with head and neck cancer. The gray-scale sonographic features of the presence or absence of hilar echoes, parenchymal echogenicity, and short and long axis lengths as well as the power Doppler features of normal hilar flow and abnormal parenchymal flow were evaluated. Univariate and multivariate logistic regression analyses were conducted to determine the relative value of each sonographic feature.
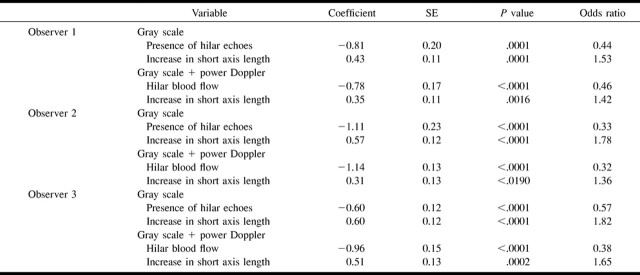
RESULTS: At univariate analysis, all sonographic features assessed were found to be important. Multivariate analysis, however, suggested that the presence or absence of hilar echoes, increases in short axis length, and the presence of normal hilar flow were the only sonographic features that were predictive of reactive (presence of hilar echoes and hilar flow) and metastatic (increases in short axis length) lymph nodes. Although multivariate analysis did not indicate any significant contribution of the color-flow criteria for predicting metastatic nodes, the color-flow criteria appeared to improve the overall diagnostic accuracy for the less experienced observer.
CONCLUSION: The sonographic criteria most predictive of metastatic cervical lymph nodes were absent hilar echoes and increases in short axis length, as assessed by logistic regression analysis. Compared with these gray-scale criteria, color-flow criteria had fewer predictive advantages.
Cervical lymph node status has been shown to be prognostically important in head and neck cancer outcome (1, 2). Several studies have attempted to establish diagnostic criteria for differentiation of metastatic from non-metastatic cervical lymph nodes in these patients (3, 4). Sonographic criteria based on gray-scale images of the lymph node have been found to be good indicators for metastatic lymph nodes (5). These included the absence of hilar echoes, increases in nodal size as assessed by short and long axis lengths, and abnormal echogenicity in the nodal parenchyma. More recently, Doppler sonographic evaluation of lymph nodes for patients with head and neck cancers showed that certain intranodal angioarchitectural changes were reliable and reproducible indicators of metastatic cervical lymph nodes (6, 7). These studies showed that the presence of parenchymal or peripheral blood flow signals in nodes were potentially suggestive of metastatic nodes. The relative contribution of each of the sonographic findings in diagnosing metastatic lymph nodes has, however, not been evaluated.
Previous studies have shown that multivariate logistic regression analysis is useful for indicating clinical data that can be efficiently and independently predictive of prognosis. Successful attempts using this method have been performed for patients with breast or prostate cancer (8–10). Therefore, we studied sonographic features, such as the presence or absence of hilar echoes, parenchymal echogenicity, increases in the short and long axis lengths of the node, and the presence or absence of hilar and parenchymal flows. Using multivariate feature analysis, we sought to determine which of the features is most predictive of metastasis in the cervical lymph nodes among patients with head and neck cancers.
Methods
Patients
The study was retrospectively conducted using sonographic images obtained from 52 patients (20 women and 32 men; average age, 63 years; age range, 44–85 years) with head and neck cancers. These malignant carcinomas had originated from the oral cavity (n = 31), hypopharynx (n = 8), larynx (n = 8), oropharynx (n = 3), nasopharynx (n = 1), and tonsil (n = 1). Primary sites in the oral cavity encompassed the tongue (n = 11), buccal mucosa (n = 7), oral floor (n = 4), lower gingiva (n = 5), and upper gingiva (n = 4). All patients had squamous cell carcinomas. These carcinomas were reported to be associated with rich vascular supplies (6, 7). A total of 133 lymph nodes were assessed by gray-scale and power Doppler sonography. Of these nodes, 57 were metastatic and 76 were reactive. All metastatic nodes were confirmed by histologic anlaysis after neck dissection (n = 52) or needle biopsy (n = 5). Of the reactive lymph nodes, 23 were histologically proved.
Histologic examinations of the excised nodes were first performed by bivalving the large nodes. The specimens were then fixed and embedded in paraffin. Six-micrometer sections were obtained at 20- to 30-μm intervals from either half of each of these nodes. The small nodes were fixed without bivalving and were embedded in paraffin, and sections were obtained at 20- to 30-μm intervals from each half. Although most of the histopathologic sections were re-evaluated by one of us, we could not rule out the possibility that there were micrometastases in the nodes that were diagnosed as having no metastasis. Thus, one should consider the possible potential flaw in these techniques. The non-malignant nature of the remaining nodes was confirmed by a follow-up study using sonography. During the follow-up periods, these nodes were periodically examined by sonography for at least 1 year to confirm that they did not increase in size. We had omitted all lymph nodes that were not histopathologically examined or that were obtained from the patients treated by radiation therapy or chemotherapy or both.
Correlation of Dissected Lymph Nodes to Sonograms
Using a previously described reporting system (6), topographical correlation between dissected nodes and sonograms was performed by node. The report includes data concerning the approximate location relative to the surrounding anatomic structures, such as to vessels and muscles, and the sizes of the enlarged nodes on sonograms. During surgery, the lymph nodes were excised en bloc along with the adjacent reference structures to ascertain more easily the spatial relationship between the excised nodes and surrounding structures such as muscles, salivary glands, and veins. The size of the node was also used for comparison with nodes studied sonographically. Surgeons and at least one of the radiologists who performed sonographic examination together compared the excised nodes with the nodes studied sonographically. Final decisions were reached by consensus. The excised nodes that matched those on sonograms were then examined histopathologically. These procedures enabled the surgeons to correlate the dissected nodes to the nodes evaluated by sonography. The average time course between sonographic examination and surgery was 37 days (range, 14–58 days).
Sonography
Gray-scale and power Doppler sonography were performed using a Logiq 700 unit (General Electric Yokogawa Medical Systems; Tokyo, Japan) equipped with a wide bandwidth (range, 6–13 MHz) transducer. All examinations were performed by a single examiner experienced in head and neck sonography and power Doppler sonography techniques. Gray-scale sonography was performed at 11 MHz. Power Doppler sonography was performed at 8 MHz, and the standard Doppler settings were chosen for optimal detection of the signals from the lymph node vessels, which had low-velocity flow. Common settings of pulse repetition frequency (500 MHz) and of wall filter (75 or 62 MHz) were used. Representative images by gray-scale and power Doppler sonography were obtained from each lymph node so that the maximal area of the lymph node appeared on the gray-scale sonographic images.
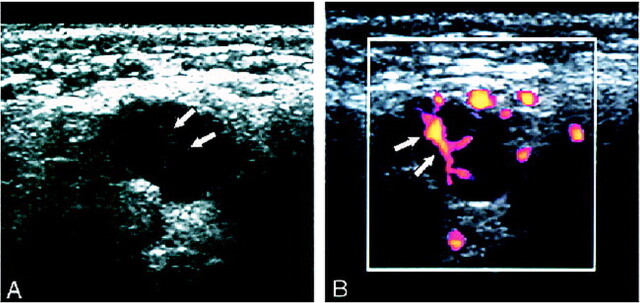
The sonographic criteria used in this study were: 1) the presence or absence (obliteration) of hilar echoes, 2) parenchymal echogenicity, 3) short axis length, 4) long axis length, 5) the presence or absence of normal hilar blood flow, and 6) the presence or absence of abnormal parenchymal blood flow. The normal parenchyma exhibits homogeneous and low echogenicity because of the predominance of a homogeneous cell population of lymphocytes without much tissue interface (6). On the other hand, the hilum is identified as a highly echogenic structure in the central part of the node (Fig 1). A preceding report showed that abnormal echogenic structures in the parenchyma could be caused by metastatic disease (11). Therefore, we also assessed the parenchymal heterogeneity. The parenchymal heterogeneity appeared as echogenic streaks (Fig 2) or concomitant presence of echogenic (eg, metastatic tumor) and hypoechoic (eg, necrosis) areas.
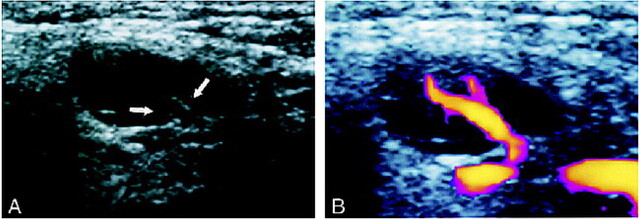
fig 1.
Case of a 61-year-old man with squamous cell carcinoma of the hard palate.
A, Gray-scale sonogram of a reactive cervical lymph node shows homogeneous and low echogenicity of the parenchyma and normal hilar echoes (arrows).
B, Power Doppler sonogram of the same node as that shown in panel A shows a normal reactive hilar blood flow.
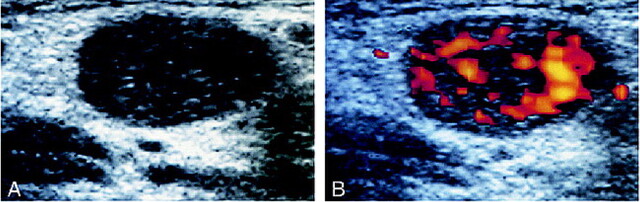
fig 2.
Case of a 53-year-old man with squamous cell carcinoma of the hypopharynx.
A, Gray-scale sonogram of a metastatic cervical lymph node shows abnormal, heterogeneous echogenicity of the parenchyma.
B, Power Doppler sonogram of the same node as that shown in panel A shows abnormal parenchymal blood flow, characteristic of metastasis.
Alterations of the intranodal angioarchitecture were postulated as potential pathognomic indicators of metastatic lymph nodes (6, 7). Therefore, power Doppler sonography was used to evaluate the maximal area of blood in the lymph node. Sonographic images were documented on color print papers by using a Mavigraph video printer (Sony Co.; Tokyo, Japan). The angioarchitecture of the lymph node was classified as hilar flow (Fig 1), parenchymal flow (including subcapsular flow [7]) (Fig 2), and no color flow (6). In non-metastatic nodes, the normal hilum exhibits hilar flow signal on Doppler sonogram, and normally, one sees the echoes (hilar echoes) on a gray-scale image.
The lymph node size was assessed by measuring with a calliper the smallest (short axis length) and greatest (long axis length) diameters on the same image, expressed as millimeters (5) (Fig 3). These length measurements were then entered directly into the logistic regression model, as described in the following section, to evaluate whether the increase in size contributed significantly to the determination of metastatic nodes.
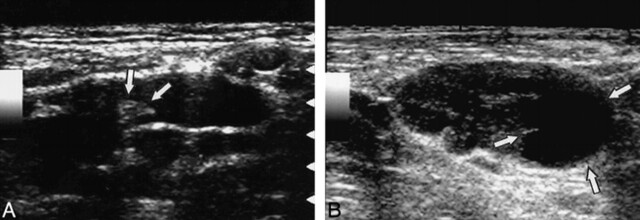
fig 3.
Sonograms of a reactive node from a 54-year-old man with squamous cell carcinoma of the larynx (A) and a metastatic node from 64-year-old man with squamous cell carcinoma of the hypopharynx (B) show a preferential increase in short axis diameter of the metastatic node.
A, Gray-scale sonogram shows reactive cervical lymph node with relatively flat shape. Arrows indicate hilum.
B, Gray-scale sonogram shows metastatic cervical lymph node. Note that the lymph nodes in panels A and B exhibit similar longitudinal axis lengths, but that the metastatic node in B has a greater short axis length. The metastatic node also shows a focal area of necrosis (arrows).
For sonographic criteria other than size measurements, the three observers were independently asked to assess the sonographic features and to decide whether a particular node was metastatic or reactive. For the length measurements, they were asked simply to measure the previously described short and long axis lengths for each node. The observers had varying degrees of clinical experience in head and neck sonography; they had 12 years (observer 1), 6 years (observer 2), and 2 years (observer 3) of experience with interpreting head and neck sonographic images. The observers had no knowledge of the clinical stage of disease in the neck, findings based on the other technique, or interpretations of the other observers. The observers were first asked to evaluate the lymph nodes only on gray-scale sonographic images. Then, at least 3 weeks after the first assessment, the observers were asked again to evaluate each lymph node on the gray-scale and power Doppler sonographic images.
The likelihood that each of the sonographic findings (the hilar echoes, parenchymal echogenicity, hilar flow, and parenchymal flow) was present was estimated by the observers with a 5-point rating scale (1 = definitely not present, 2 = probably not present, 3 = possibly present, 4 = probably present, 5 = definitely present) (12). Overall impressions by the observers were also estimated with a 5-point rating scale (1 = definitely not metastatic, 2 = probably not metastatic, 3 = questionable, 4 = probably metastatic, 5 = definitely metastatic). The 5-point rating system was not applied on the length measurements.
Multivariate Logistic Regression Analysis and Receiver Operating Characteristic Curve Analysis
Logistic analysis was conducted to identify sonographic imaging characteristics that could be used as predictive indicators for differentiating metastatic from non-metastatic cervical lymph nodes (12). Sonographic features on gray-scale and power Doppler images that were found to be important for univariate analysis were entered into multivariate models to determine their independent predictive value (13).
The results were also evaluated by calculating odds ratios per unit increase in the rating scale or per millimeter increase in length measurements. An odds ratio of 1.0 indicated no correlation between a particular sonographic imaging feature with the presence or absence of metastasis. A ratio greater than 1.0 indicated a positive predictive value for metastasis. A ratio smaller than 1.0 indicated a negative predictive value for metastasis.
Stepwise analysis was conducted as a forward stepping procedure based on a likelihood ratio test, with P < .05 for variable inclusion and P > .1 for exclusion from the model. Stepwise logistic regression analysis was conducted with the statistical software package SPSS for Macintosh, version 6.1 (SPSS; Chicago, IL).
Descriptive statistical data, including accuracy, sensitivity, and specificity, were determined for each sonographic finding by presuming that P > .5 was indicative of a metastatic lymph node. Areas (Az) under the receiver operating characteristic (ROC) curves for each observer were calculated to compare the discriminative ability of each observer based on a fitted probability calculated from data on the multivariate model. ROC curve analysis was also conducted of data obtained from observers' overall impressions. A univariate z-score test was performed to evaluate statistically significant differences between the areas under two ROC curves (area test). A true-positive fraction value at 0.1 of false-positive fraction was also calculated to compare different ROC curves (true positive fraction test). Interobserver agreement was assessed by quantifying kappa statistics; in general, a kappa value less than 0.40 indicates poor agreement and a kappa value of 0.40 to 0.75 indicates moderate agreement (12).
Results
Logistic Regression Analysis
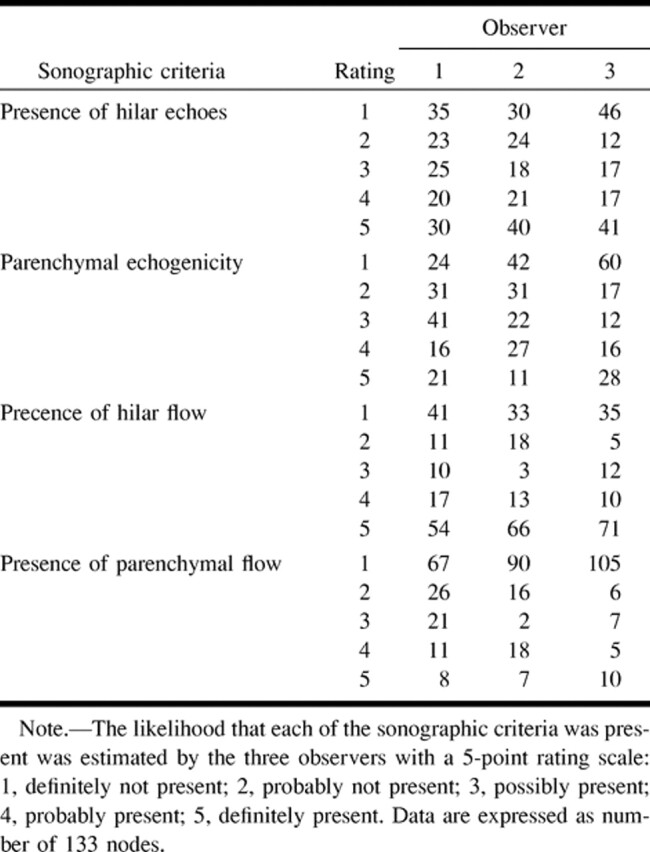
The incidences of lymph nodes assessed at a rating of 2 to 4 with a 5-point rating scale were lower than those of the nodes rated at 1 or 5 for the percentage of hilar and parenchymal blood flows (Table 1). In contrast, the incidence was approximately even among the ratings for the presence or absence of hilar echoes and parenchymal echogenicity. These findings may indicate that Doppler sonographic features are easy to interpret.
TABLE 1:
Estimation by three observers of the presence or absence of sonographic criteria in 133 nodes
Univariate logistic regression analysis showed that the six sonographic features were correlated with reactive or metastatic cervical lymph nodes (Table 2), indicating that these six sonographic features, if used alone, could be effectively predictive of the reactive or metastatic nodes. The presence of hilar echoes and hilar blood flow yielded odds ratios smaller than 1.0 among the three observers. Thus, these features were indicative of normal (reactive) nodes. On the other hand, the presence of parenchymal echogenicity, increases in short and long axis lengths of the lymph node, and the presence of parenchymal blood flow yielded odds ratios greater than 1.0 among the three observers, indicating features of metastatic nodes. When P > .5 was considered to indicate a metastatic node, descriptive statistical data showed that the presence of hilar blood flow on power Doppler sonographic images yielded the highest accuracy among the three observers (81.2%, 88.7%, and 84.2%). The other sonographic features also showed good descriptive statistical data, indicating consistent detection of the predictive sonographic features by univariate analysis.
TABLE 2:
Univariate logistic regression analysis of lymph node sonographic features
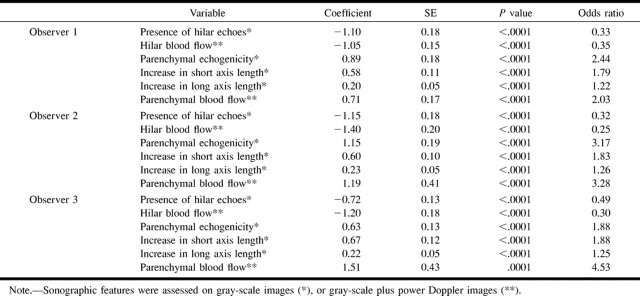
Multivariate analysis, however, indicated that only three of these sonographic features identified by the three observers (presence or absence of hilar echoes, increases in short axis length of the lymph node on gray-scale sonographic images, and hilar blood flow on gray-scale plus power Doppler sonographic images) showed statistically significant correlation with reactive (hilar echoes and hilar blood flow) and metastatic (increases in short axis length) lymph nodes (Table 3). These findings suggest that, if used in combination, the sonographic features such as the presence of abnormal parenchymal echogenicity, the presence of abnormal parenchymal flow, or increases in long axis length may not significantly contribute to the prediction of metastatic nodes. The odds ratio indicated that the presence of hilar echoes and hilar blood flow were indicative of reactive nodes (<1), and increases in short axis length were suggestive of metastatic nodes (>1).
TABLE 3:
Multivariate logistic regression analysis of sonographic features of the lymph nodes
Discriminative Power Analysis
Considering three gray-scale and power Doppler sonographic features that are independently and significantly predictive of metastatic lymph nodes, we next assessed whether the resultant bivariate models actually reflected the overall impressions of the observers. To this end, we determined the fitted probabilities from the bivariate models. ROC curves and Az values estimated from the bivariate models for each of the three observers closely approximated those of the overall impressions by the same observers, indicating that the bivariate models effectively simulated the discriminative ability of each of these observers. Close approximation between the Az values for ROC curves from the bivariate models and from the overall impressions by the observers was observed both in the results with gray-scale sonography and gray-scale plus power Doppler sonography. Az values for gray-scale sonography in the bivariate model and the overall impression, respectively, were 0.89 and 0.92 (observer 1), 0.94 and 0.90 (observer 2), and 0.92 and 0.89 (observer 3). Az values for the gray-scale plus power Doppler sonography in the bivariate model and the overall impression, respectively, were 0.90 and 0.92 (observer 1), 0.95 and 0.93 (observer 2), and 0.91 and 0.94 (observer 3). There was no significant difference in the Az values of each of the observers between gray-scale sonography and gray-scale plus power Doppler sonography, indicating that blood flow features depicted by power Doppler sonography did not provide additional predictive value over that of gray-scale sonography in the multivariate model. As an overall impression, however, observers 2 and 3, who showed relatively lower discriminative ability with gray-scale evaluation, showed a small but statistically significant improvement in discriminative ability when using gray-scale plus power Doppler sonographic evaluation, as evidenced by the true positive fraction test.
Interobserver Variability
In general, the interobserver agreement is low (kappa value is low) when the determinations by the more experienced observers are compared with those by the less experienced observers, and agreement is relatively high (kappa value is high) when compared among the equally experienced observers. The present study showed that the interobserver agreement was higher when gray-scale and power Doppler sonography were combined. Kappa values for gray-scale sonography alone were 0.60 (observer 1 versus observer 2), 0.57 (observer 1 versus observer 3), and 0.59 (observer 2 versus observer 3), and kappa values for gray-scale plus power Doppler sonography were 0.69 (observer 1 versus observer 2), 0.75 (observer 1 versus observer 3), and 0.75 (observer 2 versus observer 3).
Effects of Doppler Sonography on the Rate At Which the Presence of Hilar Echoes Is Observable
The rate at which the hilar echoes were interpreted to be present on the sonograms was different among the three observers. The rates for observers 1, 2, and 3, respectively, were 58%, 71%, and 64% for reactive nodes and 8%, 9%, and 12% for metastatic nodes. These rates were increased when the interpretation was performed on gray-scale plus Doppler images. The rates for observers 1, 2, and 3, respectively, were 83%, 92%, and 89% for reactive nodes and 14%, 16%, and 23% for metastatic nodes.
Discussion
To evaluate each of the sonographic features on gray-scale and power Doppler sonographic images for differentiating metastatic from reactive lymph nodes, we have conducted multivariate feature analysis to identify the sonographic features that are most predictive of metastasis to the cervical lymph nodes in patients with head and neck cancers. Univariate logistic regression analysis has shown that the six sonographic features assessed show significant correlations with the reactive or metastatic lymph nodes. By using multivariate logistic regression model, however, only three of these sonographic features (ie, the presence or absence of hilar echoes, increases in short axis length of the lymph node, and the presence or absence of hilar blood flow) were identified as predictive of reactive or metastatic lymph nodes. In addition, ROC curve analysis did not support the additional predictive value of power Doppler sonography.
The presence of the hilum, as depicted by sonography, has been reported to be highly indicative of reactive lymph nodes among patients with head and neck cancers (5). Nevertheless, the predictive value of the hilar blood flow remains controversial. For example, Ariji et al (6) reported that the hilar blood flow was shown only in reactive lymph nodes and never appeared in the metastatic nodes. In contrast, Tschammler et al (7) found that hilar blood flow appeared both in the reactive and metastatic lymph nodes at equivalent rates. The discrepancy between these two studies may be owing to the heterogeneity in their study populations. Tschammler et al (7) studied patients with several types of malignant tumors, including lymphomas, malignant melanomas, and squamous cell carcinomas. In the present study of squamous cell carcinomas, we confirmed that hilar blood flow was shown exclusively in reactive lymph nodes. The possibility raised by Tschammler et al (7) that parenchymal blood flow patterns (or subcapsular vessels) indicate metastatic lymph nodes is consistent with the findings presented by Ariji et al (6), who reported that when a metastasis was diagnosed on the basis of the presence of parenchymal blood flow, high sensitivity (83%) and specificity (98%) were obtained. Despite these previous findings, however, the present study showed that neither Doppler features could provide significant predictive value in differentiating metastatic from reactive nodes.
Considered together with the previous results, the present findings suggest that the presence of hilar echoes is a major predictive indicator of reactive nodes, whereas enlargement of the short axis diameter is predictive of metastatic cervical lymph nodes. Doppler flow patterns may not contribute significantly to differentiating metastatic from reactive lymph nodes. These findings may in part reflect that one sonographic feature might be dependent on another (5). In other words, Doppler flow patterns in the lymph node may be influenced by the structural changes in the node and must be interpreted with consideration of such changes.
The present findings are consistent with the notion that power Doppler sonographic features do not provide any significant contribution to differentiating metastatic from reactive lymph nodes, as evidenced by multivariate analysis. On the other hand, the ROC curve study on the observers' overall impression of sonographic features showed a small but significant increase (as evidenced by the true positive fraction test) in diagnostic performance of observers 2 and 3, who showed lower diagnostic performance among the three observers. No improvement, however, was noted for the remaining observer. Furthermore, interobserver agreement was higher when gray-scale and Doppler sonography were combined. These results may indicate that power Doppler sonographic features could assist observers who are less experienced in sonographic diagnosis of the lymph node by providing information in interpreting gray-scale sonographic findings that are critical for predicting metastatic or benign lymph nodes, such as the presence or absence of hilar echoes. In this regard, hilar blood flow signals on power Doppler images may facilitate detection of hilar echoes (Fig 4).
fig 4.
Case of a 66-year-old man with squamous cell carcinoma of the upper gingiva. This case shows the value of the presence of hilar echoes over that of increased short axis diameter. The presence of normal hilar flow as detected by Doppler sonography is helpful for confirming the presence of normal hilar echoes.
A, Gray-scale sonogram of a reactive cervical lymph. The short axis length is increased, suggestive of metastatic node; however, the normal hilar echo is present (arrows).
B, Power Doppler sonogram of the same reactive node as that shown in panel A shows a normal hilar flow (arrows).
The present results may be affected by the possible microscopic metastasis in non-enlarged nodes. Detailed studies may be required for the incidence of and prognosis with the microscopic metastasis in non-enlarged nodes of patients with head and neck cancer. In this regard, long-term follow-up studies should be conducted to examine whether possible microscopic metastasis may become detectable in enlarged nodes that were diagnosed as non-metastatic and were not excised.
Among the advantages of sonographic examination are its low cost and no radiation. When the enlargement of short axis diameter was used as a predictive factor for metastatic cervical nodes, we (unpublished data) and van den Brekel et al (14) found that sonographic evaluation yielded good sensitivity and specificity for predicting metastatic nodes in patients with head and neck cancer. In such conditions, sonography was predictive of metastatic cervical nodes with accuracy equivalent to that reported with CT and MR imaging (15). Furthermore, sonography may be useful for examination of nodes in the submental and submandibular regions, where artifacts from the mandible and metals may result in a poor study. Therefore, despite several limitations (6), sonography could be an adjunct, if not a main, tool to CT and MR imaging in predicting metastatic nodes among patients with head and neck cancer.
In conclusion, we have verified the gray-scale criteria for lymph node metastasis, partly diminishing the credibility of color flow Doppler analysis. The most predictive criteria of metastatic cervical lymph nodes were absence of the normal hilar echoes and increases in the short axis length (Table 4). Compared with these gray-scale criteria, color-flow criteria had less predictive values. In this regard, color-flow criteria may alter psychological confidence but may not alter the conclusion reached by gray-scale criteria.
TABLE 4:
A summary of the most important criteria for detecting cervical lymph node metastasis on sonography
Footnotes
Address reprint requests to Dr. Takashi Nakamura, Department of Radiology and Cancer Biology, Nagasaki University School of Dentistry, 1-7-1 Sakamoto, Nagasaki 852-8588, Japan.
References
- 1.Savoury LW, Gluckman JL. Cervical metastasis. In: Paparella MM, Shumrick DA, Gluckman JL, Myerhoff WL, eds. Otolaryngology. 3rd ed. Philadelphia: Saunders; 1991: 2565-2578
- 2.Farr HW, Goldfarb PM, Farr CM. Epidermoid carcinoma of the mouth and pharynx at Memorial Sloan-Kettering Cancer Center, 1965 to 1969. Am J Surg 1980;140:563-567 [DOI] [PubMed] [Google Scholar]
- 3.Van den Brekel MWM, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990;177:379-384 [DOI] [PubMed] [Google Scholar]
- 4.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol 1992;158:961-969 [DOI] [PubMed] [Google Scholar]
- 5.Vassallo PV, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology 1992;183:215-220 [DOI] [PubMed] [Google Scholar]
- 6.Ariji Y, Kimura Y, Hayashi N, et al. Power Doppler sonography of cervical lymph nodes in patients with head and neck cancer. AJNR Am J Neuroradiol 1998;19:303-307 [PMC free article] [PubMed] [Google Scholar]
- 7.Tschammler A, Ott G, Schang T, Seelback-Goebel B, Schwager K, Hahn D. Lymphadenopathy: differentiation of benign from malignant disease: color Doppler US assessment of intranodal angioarchitecture. Radiology 1998;208:117-123 [DOI] [PubMed] [Google Scholar]
- 8.Wolberg WH, Street MN, Mangasarian OL. Computer-derived nuclear features compared with axillary lymph node status for breast carcinoma prognosis. Cancer 1997;81:172-179 [DOI] [PubMed] [Google Scholar]
- 9.Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer 1995;75:1343-1353 [DOI] [PubMed] [Google Scholar]
- 10.Ravdin PM, De Laurentiis M, Vendely T, Clark GM. Prediction of axillary lymph node status in breast cancer patients by use of prognostic indicators. J Natl Cancer Inst 1994;86:1771-1775 [DOI] [PubMed] [Google Scholar]
- 11.Rubaltelli L, Proto E, Salmaso R, Bortoletto P, Candiani F, Cagol P. Sonography of abnormal lymph nodes in vitro: correlation of sonographic and histologic findings. AJR Am J Roentgenol 1990;155:1241-1244 [DOI] [PubMed] [Google Scholar]
- 12.Yu KK, Hricak H, Alagappan R, Chernoff DM, Bacchetti P, Zaloudek CJ. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology 1997;202:697-702 [DOI] [PubMed] [Google Scholar]
- 13.Mussurakis S, Buckley DL, Horsman A. Prediction of axillary lymph node status in invasive breast cancer with dynamic contrast-enhanced MR imaging. Radiology 1997;203:317-321 [DOI] [PubMed] [Google Scholar]
- 14.van den Brekel Castelijins JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: how reliable is it? AJNR Am J Neuroradiol 1998;19:695-700 [PMC free article] [PubMed] [Google Scholar]
- 15.Curtin HD, Ishwaran H, Mancuso AA, Dalley RW, Caudry DJ, McNeil BJ. Comparison of CT and MR imaging in staging neck metastasis. Radiology 1998;207:123-130 [DOI] [PubMed] [Google Scholar]