Abstract
Purpose of the review
Hypertension is the foremost risk factor for cardiovascular disease (CVD) and death. This review highlights recent findings that apply to the prevention, detection and management of high BP, in the context of the 2017 American College of Cardiology/American Heart Association blood pressure (BP) guideline.
Recent findings
Several new findings on the association of BP measurement with CVD outcomes are now available. (1) Beginning with a systolic BP (SBP) as low as 90 mm Hg, coronary artery calcium deposition and the risk of incident ASCVD increased in stepwise fashion with increasing SBP levels within the normal range in adults at low risk for ASCVD. (2) Isolated diastolic hypertension was not associated with atherosclerotic cardiovascular disease (ASCVD), heart failure or chronic kidney disease. (3) Nocturnal BP appeared to be better associated with CVD outcomes than office or daytime BP. (4) In a head-to-head comparison, home BP monitoring had higher reliability and predictive value than office or ambulatory BP to detect left ventricular hypertrophy, an intermediate form of hypertension-related target organ damage. In addition, new information indicates that autonomous aldosterone production is present in a substantially larger percentage of adults with hypertension than previously recognized. Finally, intensive BP lowering is associated with a significant reduction in the incidence of mild cognitive impairment, a precursor of dementia.
Summary
Ongoing research has made significant progress in the prevention, detection, and management of high blood pressure, clarifying, amplifying and/or supporting the 2017 ACC/AHA BP guideline recommendations.
Keywords: Hypertension, blood pressure, cardiovascular outcomes, primary aldosteronism, cognitive decline
Introduction
High blood pressure (BP) is the most important risk factor for cardiovascular disease (CVD), ranking first in global disability-adjusted life-years and associated with approximately 1,000 deaths per day [1]. This review highlights new findings that apply to the prevention, detection and management of hypertension in the context of the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) adult BP clinical practice guideline [2]. First, we highlight new reports on the association of BP with other CVD risk factors and atherosclerotic CVD (ASCVD). Second, we review new information on BP measurement, including the associations of out-of-office BP, isolated diastolic hypertension and the nocturnal hypertension phenotype with CVD. Next, we discuss secondary hypertension focusing on the unexpectedly high prevalence of primary aldosteronism and its ramifications for management of hypertension. Finally, we explore the results of randomized controlled trials demonstrating the effects of intensive BP lowering on cognitive function and implications for the therapeutic BP goal in hypertension.
Normal range systolic BP and ASCVD risk in “healthy’ adults
The risk of ASCVD in adults with an SBP at the lower end of the BP distribution is not well defined [2]. To examine this issue, a cohort of 1,457 adults (mean age 58 years) who were free of ASCVD and its traditional risk factors, and who had a baseline SBP that ranged from 90 to129 mm Hg was followed for 14.5 years (Figure 1) [3**]. Increasing levels of SBP were associated with prevalent coronary artery calcium (CAC) and with the incidence of traditional CVD risk factors as well as ASCVD events. For each 10 mm Hg increase in SBP, the adjusted HR (aHR) for ASCVD events was 1.53 (95% CI, 1.17–1.99). Compared with those who had a baseline SBP 90–99 mm Hg, the participants with an SBP 100–109, 110–119, and 120–129 mm Hg had an aHR of 3.00, 3.10, and 4.58, respectively. These results define the optimal SBP level in healthy adults for the first time and highlight the importance of primordial prevention to avert the development of atherosclerotic plaque and its consequences. The findings indicate that ASCVD begins early in life when BPs are much lower than is the case later in life and well below the ACC/AHA SBP definition for stage 1 hypertension in adults (130–139 mm Hg) [4]. The results emphatically suggest the need for a population-based strategy for primordial prevention by means of healthy lifestyle, especially a healthy dietary pattern, physical activity, and abstinence or moderation in alcohol consumption [5].
Figure 1:
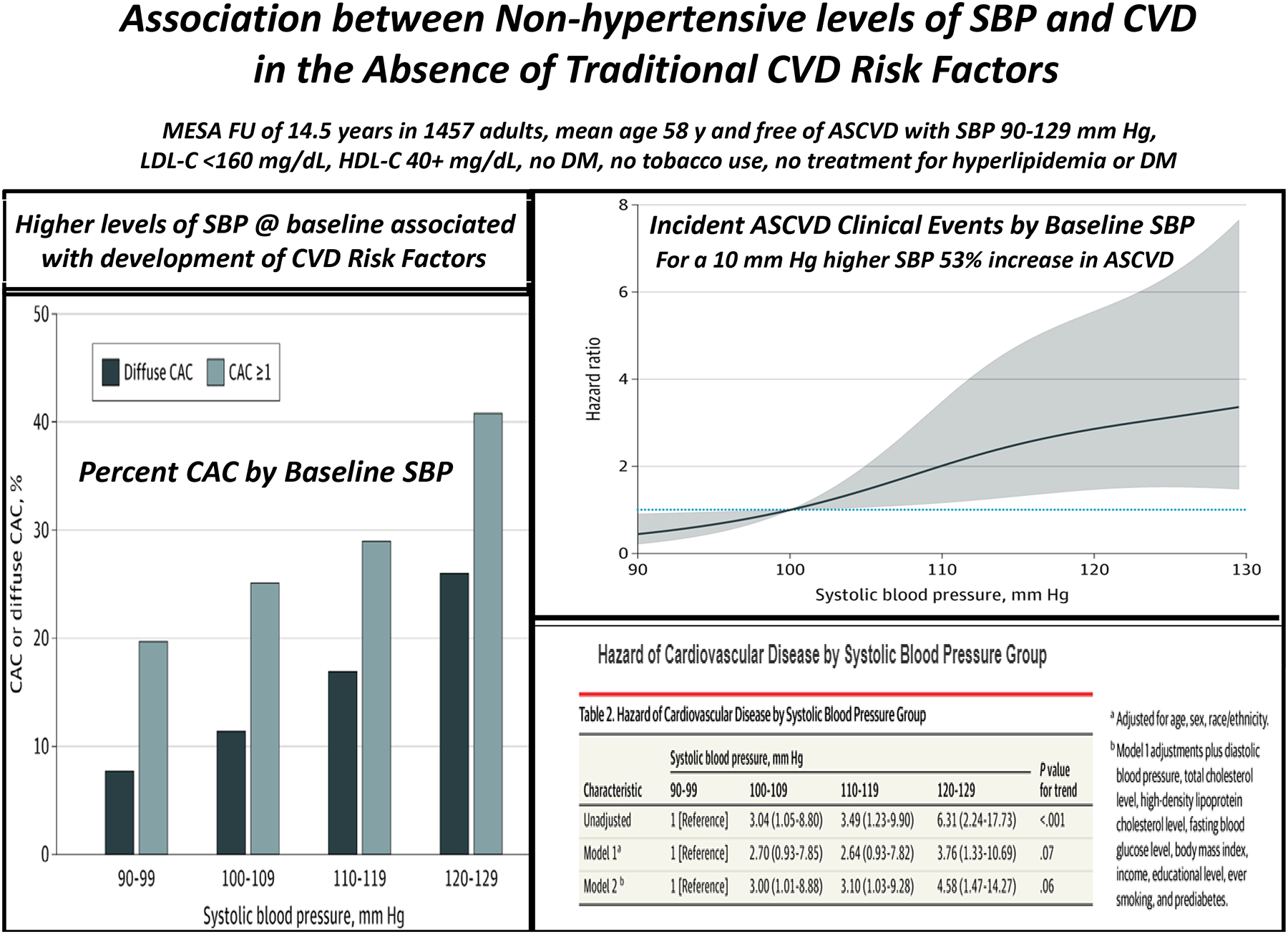
Summary of data from Reference 3**. Left: Proportion of participants with coronary artery calcium (CAC) and diffuse CAC by systolic blood pressure group. Top right: Adjusted cubic spline for the hazard of incident cardiovascular disease by systolic blood pressure. Bottom right: Hazard of cardiovascular disease by systolic blood pressure group. Adapted from Reference 3** with permission.
BP measurement
Substantial progress has been made during the past year in assessing the association of various BP measurement techniques and parameters with CVD outcomes. The 2017 ACC/AHA BP guideline [2] emphasizes the importance of accurate BP measurement using proper technique and use of out-of-office BP measurements in the diagnosis and management of high BP. Out-of-office measurement, either ambulatory BP monitoring (ABPM) or home BP monitoring (HBPM), is required to detect white coat and masked hypertension in patients not taking antihypertensive medication and the white coat effect and masked uncontrolled hypertension in patients taking antihypertensive drugs [2,6]. The distinction is important because masked and masked uncontrolled hypertension are associated with high CVD morbidity and mortality rates, similar to sustained hypertension, whereas white coat hypertension and white coat effect carry minimal excess CVD risk over normal BP and controlled hypertension, respectively [2,6]. Parenthetically, a 2018 analysis from the Spanish ABPM Registry reporting an unexpectedly high CVD risk in adults with white coat hypertension was withdrawn in 2020 due to discovery of inaccuracies (7,8). ABPM is a fully automated technique in which BP is monitored noninvasively for a prolonged period, usually 24 hours, and has the unique benefit of detecting dynamic daytime BP responses (eg. to exercise, meals, drugs) and BP patterns (eg. nocturnal hypertension, non-dipping or reverse-dipping, morning BP surge) [6]. In contrast, HBPM detects only resting daytime BP [6]. Both ABPM and HBPM have a stronger association with hypertension-related target-organ damage and clinical CVD outcomes than does office BP measurement, in part due to greater precision with multiple readings [6]. Due to the larger number of studies using ABPM than HBPM, ABPM is preferentially recommended over HBPM, unless ABPM is unavailable [2]. However, there is little evidence supporting the superiority of ABPM over HBPM in predicting future CVD events [9,10]. Continuing progress in improving the accuracy of BP measurements is being made by the AHA/AMA Target BP and other initiatives in the US and worldwide by complementary WHO Global Hearts, Resolve to Save Lives, World Hypertension League, and other initiatives [11].
A small (400 participants) but well-performed non-randomized prospective cohort study of the reliability (intraclass correlation coefficient) and predictive value of BP measured in the office or by ABPM or HBPM on left ventricular mass index (LVMI), an index of left ventricular hypertrophy, was reported by Schwartz et al. [12*]. The study population was relatively young (mean age 41.2 years) and excluded adults with severe hypertension (BP ≥ 160/105 mm Hg) and those taking antihypertensive or other pharmacologic agents which would predictably alter BP. Although the mean BP values were relatively low, 30–50% of the population would have been classified as having hypertension by 2017 ACC/AHA BP guideline criteria [2]. While all three BP measurement techniques were significantly related to left ventricular mass index (LVMI), HBPM unexpectedly demonstrated the best reliability and strongest association with LVMI [12*].
In the United States, ABPM is largely unavailable to primary care practitioners, who manage the majority of patients with hypertension. Thus, the demonstration that HBPM is reliable and associates closely with LVMI, if confirmed, would have the potential to change clinical practice. The results [12*] indicate that HBPM could be especially important for detecting elevated BP and hypertension in young adults at relatively low short-term but high lifetime risk. The findings of this study, if confirmed in older adults and those taking antihypertensive medication, encourage routine implementation of HBPM as the preferred method of out-of-office BP monitoring.
Hypertension can be diagnosed based on elevated systolic BP (SBP), elevated diastolic BP (DBP), or both [2]. The ACC/AHA BP guideline changed the definition of hypertension from a cutoff of greater than or equal to 140/90 mm Hg [as recommended in the 2003 7th Joint National Committee report (JNC-7) [13] ] to a lower threshold of greater than or equal to 130/80 mm Hg. The recommendation to lower the threshold has ramifications for an uncommon diagnostic category known as isolated diastolic hypertension (IDH), defined as a SBP <130 mm Hg with a BP ≥80 mm Hg by new criteria (2017 ACC/AHA BP guidelines), but an SBP <140 mm Hg with a DBP ≥90 mm Hg by the JNC-7 criteria. Previous studies suggested that IDH (based on JNC-7 diagnostic criteria) is more common in younger individuals and associated with future systolic hypertension but is generally not associated with ASCVD outcomes independently of baseline SBP [14–16]. In a large (9,590 participants), nationally representative US cross-sectional study [17**], the estimated prevalence of IDH based on the 2017 ACC/AHA guideline vs the JNC-7 guideline was 6.5% vs 1.3%, respectively. In a longitudinal analysis, there was no significant association between IDH as defined by the 2017 ACC/AHA guideline and incident ASCVD, heart failure, or chronic kidney disease. These results may change clinical practice in that, while patients with IDH should be monitored regularly for the development of systolic hypertension, they should not be treated with antihypertensive medication. This study does not, however, imply that elevated DBP is not associated with significant harm; the results only indicate that the IDH phenotype per se is not associated with clinical or subclinical disease.
ABPM and HBPM are better predictors of CVD events than is office BP measurement. In a population-based cohort study of 11,135 adults, a higher statistical significance was reported for the association between 24-h and nighttime ABBP readings and CVD events or death compared to office BP measurements [18*]. The JAMP (Japan Ambulatory Blood Pressure Monitoring Prospective) study [19*] studied the association between nocturnal hypertension and nighttime BP dipping patterns and the occurrence of CVD events, including heart failure (HF), in patients with hypertension. A total of 6,359 patients were included in the study analysis. During a follow up period of 4.5 ± 2.4 years, nighttime SBP was significantly associated with the risk of ASCVD and HF [hazard ratio (HR) adjusted for demographic and clinical risk factors per 20 mm Hg increase: 1.18 (95% CI, 1.02–1.37), P=0.029; and 1.25 [95% CI, 1.00–1.55], P=0.048, respectively]. Disrupted circadian BP rhythm (nocturnal riser pattern) was significantly associated with higher overall CVD risk [HR 1.48 (95% CI, 1.05–2.08); P=0.024], and especially HF [HR 2.45 (95% CI, 1.34–4.48); P=0.004)] compared with normal circadian BP rhythm. Thus, nighttime BP levels and a riser pattern were independently associated with total CVD event rate, especially for HF. These findings suggest the importance of antihypertensive strategies that target nighttime SBP, but such an approach has not yet been validated in randomized clinical trials.
Unrecognized autonomous aldosterone production
Primary aldosteronism is the most common secondary cause of hypertension, estimated to underlie 8–13% of hypertension [20]. When primary aldosteronism is suspected, screening with a plasma aldosterone to renin ratio (ARR) is recommended, with positive screening tests subsequently verified using one of four commonly employed confirmation tests (intravenous saline suppression, oral sodium loading, fludrocortisone suppression or captopril challenge tests) [2,20]. The workup is complex with adrenal venous sampling being required to determine whether there is a unilateral or bilateral source of increased aldosterone production. Unilateral laparoscopic adrenalectomy, recommended for patients with unilateral hypersecretion, results in normalization or improvement in BP control in most patients [21]. For those who are unable or unwilling to undergo adrenalectomy or have demonstrated bilateral aldosterone production, specific treatment with a mineralocorticoid receptor antagonist (spironolactone or eplerenone), if administered in effective mineralocorticoid receptor blocking doses, leads to normalization of or marked improvement in BP [20].
Brown et al. [22**] reported a cross-sectional study characterizing the prevalence of non-suppressible, renin-independent aldosterone production, as well as overt primary aldosteronism, in relationship to BP. Every BP category from normal BP to resistant hypertension was characterized by a continuum of renin-independent aldosterone production, in which greater severity of production was associated with higher BP, kaliuresis, and lower serum potassium levels, indicative of both the clinical and biochemical manifestations of autonomous aldosterone production. In sodium loaded participants, mean adjusted levels of urinary aldosterone were 6.5 μg/24 h in normotensive individuals, 7.3 μg/24 h in stage 1 hypertension, 9.5 μg/24 h in stage 2 hypertension, and 14.6 μg/24 h in resistant hypertension and the corresponding prevalence estimates for biochemically overt primary aldosteronism were 11.3%, 15.7%, 21.6%, and 22.0%, respectively. The ARR had poor sensitivity and negative predictive value for detecting biochemically overt primary aldosteronism, at least in part due to demonstrated high measurement variability [22**, 23]. This landmark study [22**] demonstrates that the prevalence of primary aldosteronism is high and largely unrecognized. Beyond the categorical definition of overt primary aldosteronism, there exists a continuum of renin-independent aldosterone production that parallels severity of hypertension (Figure 2) [22**,24,25]. These findings, if confirmed, would redefine the primary aldosteronism syndrome and implicate autonomous aldosterone production, at least in part, in the pathogenesis of primary hypertension.
Figure 2:
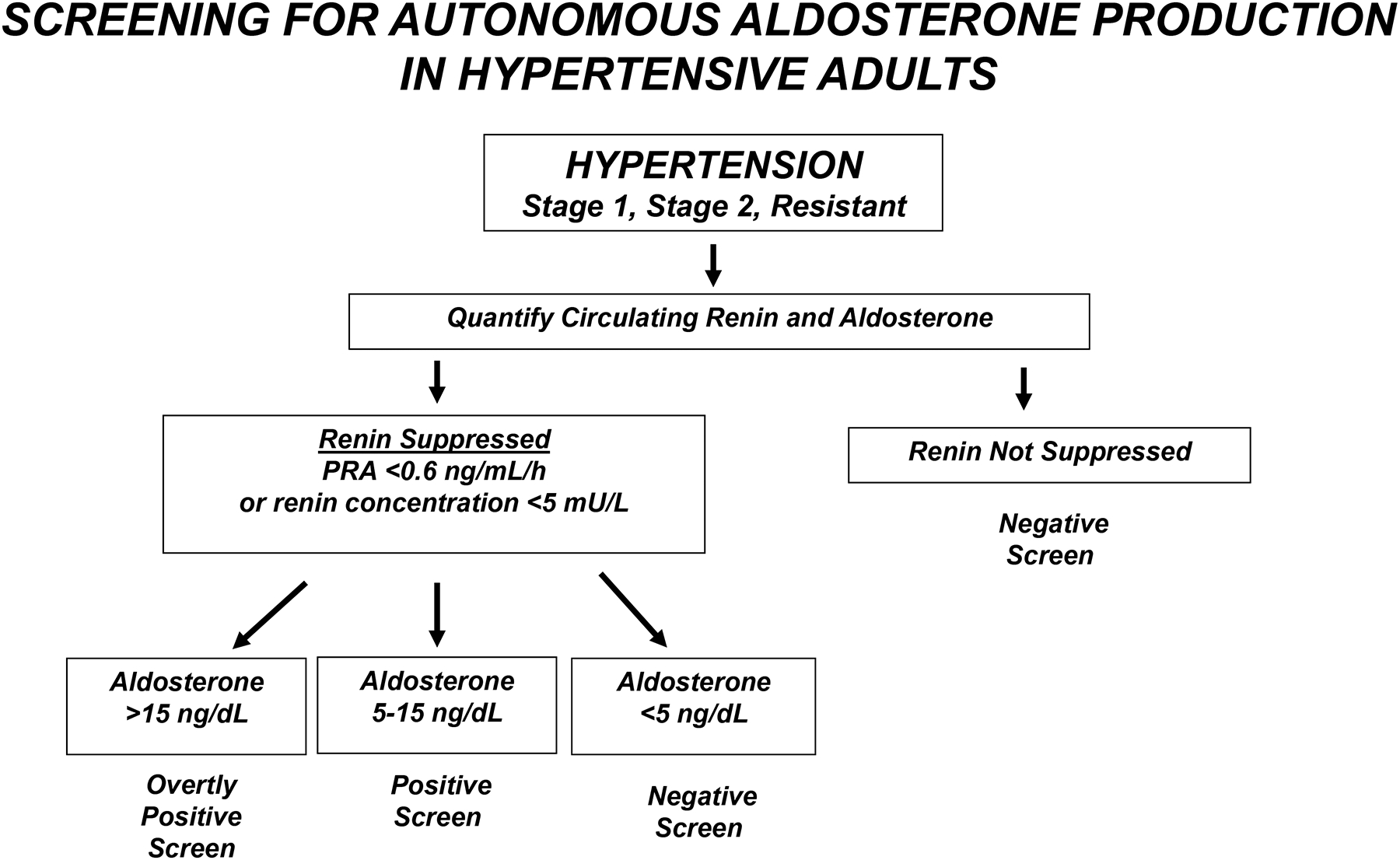
Proposed algorithm for screening adults with hypertension for renin-independent aldosterone production, as adapted on the basis of new information in References 22** and 25, with permission. Those with a positive screen may be considered for early mineralocorticoid receptor antagonist (spironolactone or eplerenone) treatment. Those with an overtly positive screen should be referred to a specialist for a formal workup of primary aldosteronism. PRA, plasma renin activity.
Effects of intensive BP reduction on cognitive function
Hypertension is a major modifiable risk factor for dementia. While observational studies have suggested prevention of cognitive decline and dementia with BP lowering, especially in middle-age adults, documentation of the effectiveness of BP reduction on cognitive function in randomized clinical trials has been inconclusive [26–30]. Examination of the effects of intensive vs standard BP lowering on cognitive function in older adults is critically important because this population has an increasing incidence of cognitive impairment and dementia, which commonly leads to progressive disability and high mortality [31–34].
The Systolic Blood Pressure Intervention Trial (SPRINT) trial included 2,636 community-dwelling adults age ≥ 75 years [35]. In this older subgroup, the SPRINT investigators demonstrated a 34% lower risk of developing the primary composite CVD outcome and a 33% lower risk of all-cause mortality with intensive (SBP goal <120 mm Hg) versus standard (SBP goal <140 mm Hg) treatment [numbers needed to treat (NNT) 27 and 41, respectively)]. The results were similar for those who were most frail or had impaired gait speed. Importantly, no difference in serious adverse events occurred, including falls or self-assessed quality of life, regardless of frailty status [35].
In a SPRINT Memory and Cognition in Decreased Hypertension (SPRINT MIND) analysis, the incidence of mild cognitive impairment (MCI), a precursor of dementia, was significantly reduced in the intensive compared to standard treatment group (HR 0.83; 95% CI, 0.70–0.99) [36**]. During extended trial and post-trial follow-up (median of 5.1 years), the composite of MCI and dementia was significantly less common in the intensive compared to standard treatment [HR 0.85 (95% CI, 0.74–0.97)] and there was a nonsignificant trend for benefit in dementia per se [HR 0.83 (95% CI, 0.67–1.04)] (Figure 3). The Kaplan-Meier curve separation by treatment arm occurred much later for probable dementia than for CVD events or MCI, suggesting that a longer follow up period with more events would be required to document a significant reduction in dementia, per se [36**].
Figure 3:
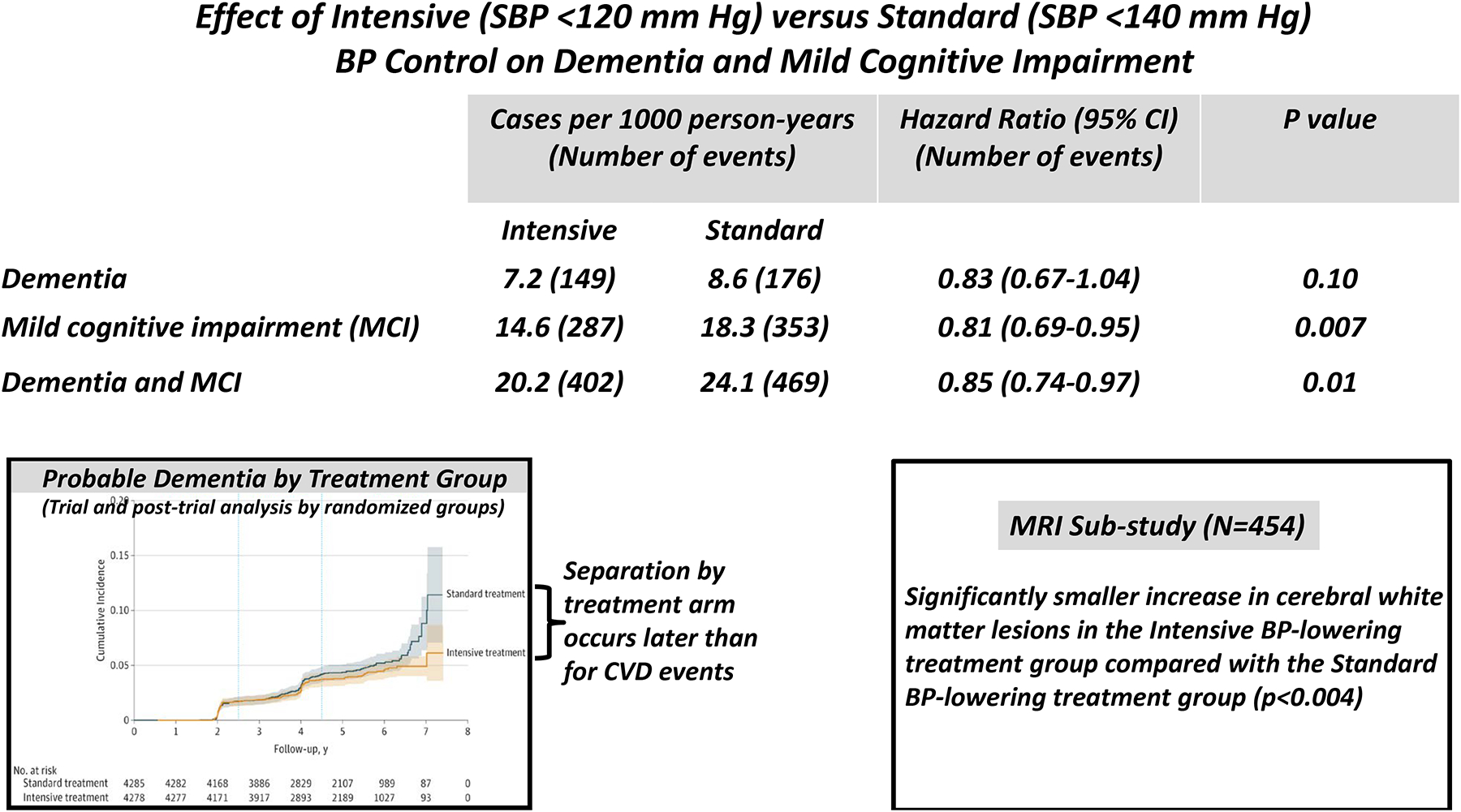
Summary of data from References 36** and 37* (the SPRINT-MIND trial) with permission.
During a median follow-up of 4 years, an MRI sub-study conducted in 670 SPRINT participants reported significantly less progression of cerebral small vessel ischemic disease as reflected by white matter lesions which are characteristically associated with Alzheimer’s disease, in the intensive compared to standard group (Figure 3) [37*]. A similar benefit was noted during intensive BP lowering in the INFINITY trial and during extended follow-up in the ACCORD trial [38,39]. Recently, the beneficial effect on major CVD events, mild cognitive impairment and death was reaffirmed in SPRINT participants age ≥ 80 years [40]. A preplanned SPRINT sub-study of the specific cognitive functions (ie. memory and processing speed) failed to demonstrate a significant intergroup difference in either function, likely due to failure to identify a specific subset of cognitive testing instruments that were effective in predicting dementia [41]. A systematic review and meta-analysis of 12 randomized trials including 92,135 patients, however, demonstrated that BP lowering was associated with a significant reduction in the odds ratio for dementia or cognitive impairment (odds ratio 0.93, 95% CI 0.88–0.98) [42]. Ongoing intensive vs standard BP lowering randomized trials will address the optimal BP target to prevent cognitive impairment and dementia [43]. In ambulatory non-institutionalized older adults these findings, taken together, support the 2017 ACC/AHA BP guideline recommendation that, in adults with hypertension, BP lowering is reasonable to prevent cognitive decline and dementia [2].
Conclusions
The association of ASCVD with SBP within the normal range defines optimal BP. New insights have been gained on the association of BP measurement parameters and techniques with CVD events and mortality. Adults with hypertension have a heretofore unrecognized high prevalence of autonomous aldosterone production and even normotensive persons may have underlying autonomous aldosterone production. Lowering BP may be one of the only ways in which cognitive impairment can be improved or prevented during the process of aging.
KEY POINTS.
Optimal blood pressure is defined by the association of atherosclerotic cardiovascular disease with systolic blood pressure levels within the normal range (<120 mm Hg).
Home blood pressure monitoring is encouraged based on its close association with left ventricular mass index.
Isolated diastolic hypertension should be monitored for progression, but should not be treated with antihypertensive medication because it is a benign condition.
Autonomous aldosterone production underlies a larger than expected proportion of hypertension, including stages 1 and 2 and resistant hypertension.
Blood pressure lowering below the 130/80 mm Hg target may prevent cognitive decline and dementia.
Acknowledgements
Sources of Funding
Dr. Carey is Principal Investigator and Project Director of an NIH Research Grant (R01-HL-128189) and Program Project Grant (P01-HL-074940), respectively. Dr. Whelton was supported by a National Institute of General Medical Sciences, Centers of Biomedical Research Excellence award NIGMS P30-GM-109036.
Footnotes
Disclosures
Dr. Carey was Vice-Chair of the 2017
ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults Writing Committee and Chair of the AHA resistant hypertension Scientific Statement Writing Committee. Dr. Whelton was Chair of the SPRINT Steering Committee and Chair of the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.
References
*Of special interest
** Of outstanding interest
- 1.Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1278–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison-Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner PK, Ovbiabele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. A guideline for the prevention, detection, evaluation and management of high blood pressure. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;171:e127–e248. doi: 10.1016/jack.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 3.**.Whelton SP, McEvoy JW, Shaw L, Psaty B, Lima JA, Budoff M, nasir K, Szklo M, Blumenthal RS, Blaha MJ. Association of normal systolic blood pressure with cardiovascular disease in absence of risk factors. JAMA Cardiol. 2020;5:1011–1018. doi: 10.1001/jamacardio20201731. [DOI] [PMC free article] [PubMed] [Google Scholar]; This seminal study in the MESA cohort demonstrates for the first time the association of increasing SBP in the normal range and the prevalence of ASCVD.
- 4.Jones DW. What is normal blood pressure? JAMA Cardiol. 2020;5:1018–1019. [DOI] [PubMed] [Google Scholar]
- 5.Gillman MW. Primordial prevention of cardiovascular disease. Circulation. 2015;131:599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muntner P, Shimbo D, Carey RM, Charleston J, Gaillard T, Meyers MG, Misra S, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB, Wright JR. Measurement of blood pressure in humans. A Scientific Statement of the American Heart Association. Hypertension. 2019;73: 73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banegas JR, Ruilope LM, de la Sierra P, Vinyoles E, Gurostidi M, de la Cruz J, Ruiz-Hurtado G, Segura J, Rodriguez-Artalejo F, Williams B. Relationship between clinic and ambulatory blood pressure measurements and mortality. N Engl J Med. 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 8.Retraction: Banegas JR, Ruilope LM, de la Sierra P, Vinyoles E, Gurostidi M, de la Cruz J, Ruiz-Hurtado G, Segura J, Rodriguez-Artalejo F, Williams B. Relationship between clinic and ambulatory blood pressure measurements and mortality. N Engl J Med. 2020;382:786. [DOI] [PubMed] [Google Scholar]
- 9.Shimbo D, Abdella M, Falson L, Townsend RR, Muntner P. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kario K, Shimbo D, Hoshide S, Wang J-G, Asayama K, Ohkubo T, Imai Y, McManus RJ, Kollias A, Niiranen TJ, Parati G, Williams B, Weber MA, Vongpatanasin W, Muntner P, Sterfiou GS. Emergence of home blood pressure-guided management of hypertension based on global evidence. Hypertension. 2019;74:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell NRC, Schutte AE, Varghese CV, Ordunez P, Zhang XH, Khan T, Sharman JE, Whelton PK, Parati G, Weber MA, Orías M, Jaffe MG, Moran AE, Plavnik FL, Ram VS, Brainin M, Owolabi MO, Ramirez AJ, Barbosa E, Bortolotto LA, Lackland DT. São Paulo call to action for the prevention and control of high blood pressure: 2020. J Clin Hypertens (Greenwich). 2019;21:1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.*.Schwartz JE, Muntner P, Kronish IM, Burg MM, Pickering TG, Bigger JT, Shimbo D. Reliability of office, home and ambulatory blood pressure measurements and their correlation with left ventricular mass. J Am Coll Cardiol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]; This small but well performed cohort study shows the superior reliability and predictability of left ventricular mass index of HBPM over ABPM and office BP measurements.
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Hypertension. 2003; 42:1206–52. [DOI] [PubMed] [Google Scholar]
- 14.Li Ym Wei FF, Thijs L, et al. International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) investigators. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. 2014;130:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi YJ, Kim SH, Kang SH, et al. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: a nationwide population-based study from Korea. Eur Heart J. 2019;40:724–731. [DOI] [PubMed] [Google Scholar]
- 16.Quinn Sm McEvoy JW. Systolic and diastolic blood pressure and cardiovascular outcomes. N Engl J Med. 2019;381:1690–1691. [DOI] [PubMed] [Google Scholar]
- 17.**.McEvoy JW, Daya N, Rahman F, Hoogeveen RC, Blumenthal RS, Shah AM, Banantyne CM, Coresh J, Selvin E. Association of isolated diastolic hypertension as defined by the 2017 ACC/AHA blood pressure guideline with incident cardiovascular outcomes. JAMA. 2020;323:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates the lack of association of IDH with cardiovascular events for the first time.
- 18.*.Yang WY, Melgarejo JD, Thijs L, Zheng Z-Y, Boggia J, Wei F-F, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cohort study demonstrates superior prediction of CVD events using ABPM over office BP measurement.
- 19.*.Kario K, Hoshide H, Kabutoya T, Nishizawa M, Yoshida T, Abe H, Katsuya T, Fujita Y, Okazaki O, Yano Y, Tomitani N, Kanegae H. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020:142:1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]; This population-based Japanese study shows the importance of nocturnal BP measurement in predicting CVD outcomes.
- 20.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis and treatment. J Clin Endocrinol Metab. 2016;101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 21.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5:688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.**.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The unrecognized prevalence of primary aldosteronism. Annals of Internal Medicine. 2020;173:10–20. doi: 10.7326/M20-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]; This landmark study demonstrates the unexpected high prevalence of non-suppressible (autonomous) aldosterone production in normotensive and hypertensive adults.
- 23.Yozamp N, Hundener GL, Moussa M, Underhill J, Fudim T, Sacks B, Vaidya A. Intraindividual variability of aldosterone concentrations in primary aldosteronism. Hypertension. 2020;120: doi: 10.1161/HYPERTENSIONAHA.120.16429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funder JW. Primary aldosteronism: at the tipping point. Ann Int Med. 2020;173:65–66. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya A, Carey RM. The evolution of primary aldosteronism: simplifying the clinical approach. J Clin Endocrinol Metab. 2020;105:doi: 10.1210/clinem/dgaa606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasan RS, Larson MG, Leip EP. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 27.Lewington S, Clarke R, Qizilbash N, Peto R, Co;;ins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 propective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 28.Gottesmann RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 30.Whitmer RA, Sidney S, Selby J, Sohnston SC, Yaffe K. Midlife cardiovascular factors and risk of dementia in late life. Neurology. 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 31.Freitag M, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, et al. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. 2006;37:33–37. [DOI] [PubMed] [Google Scholar]
- 32.Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson J, Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group. Intensive versus standard blood pressure control and cardiovascular disease outcomes in adults ≥ years: a randomized clinical trial. JAMA. 2016;86:1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.**.SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh MK, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT Jr, Wright CB. Effect of intensive versus standard blood pressure control on possible dementia: a randomized clinical trial. JAMA. 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]; The SPRINT MIND randomized clinical trial shows improvement in mild cognitive function of intensive vs standard BP lowering.
- 37.*.SPRINT MIND Investigators, Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group. Association of intensive versus standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]; This SPRINT MIND companion study shows reduction in cerebral white matter accumulation with intensive vs standard BP lowering.
- 38.White WB, Wakefield DB, Coscufo N, Guttmann CRG, Kaplan RF, Bohannon RW, Fellows D, Hall CB, Wolfson L. Effect of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation. 2019;140:1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray AM, Hsu F-C, Williamson JD, Bryan RN, Gerstein HC, Sullivan MD, Miller ME, Leng I, Lovato LL, Launer LJ, Action to Control Cardiovascular Risk in Diabetes Follow-On Memory in Diabetes (ACCORDION MIND) Investigators. ACCORDION MIND: Results of the observational extension of the ACCORD MIND randomized trial. Diabetologia. 2017;60:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pajewski NM, Berlowitz DR, Bress AP, Callahan KE, Cheung AK, Fine LJ, Gaussoin SA, Johnson KC, King J, Kuzman DW, Kostis JB, Lerner AJ, Lewis CE, Oparil S, Rahman M, Reboussin DM, Rocco MV, Snyder JK, Still C, Supiano MA, Wadley VG, Whelton PK, Wright JT Jr, Williamson D, Williamson JD. Intensive vs standard blood pressure control in adults 80 years or older: a secondary analysis of the Systolic Blood Pressure Intervention Trial. J Am Geriatr Soc. 2020;68:496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapp SR, Gaussoin SA, Sachs BC, Chelune G, Supiano MA, Lerner AJ, Wadley VG, Wilson VM, Fine LJ, Whittle JC, Auchus AP, Beddhu S, Berlowitz DR, Bress AP, Johnson KC, Krousel-Wood M, Martindale-Adams J, Miller EC, Rifkin DE, Snyder JK, Tamariz L, Wolfgram DF, Cleveland ML, Yang M, Nichols LO, Bryan RN, Reboussin DM, Williamson JD, Pajewski NM; SPRINT Research Group. Effects of intensive vs standard BP control on domain-specific cognitive function: a substudy of the SPRINT randomized controlled trial. Lancet Neurol. 2020;19:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, Bosch J, O’Donnell MJ, Canavan M. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020:323:1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorelick PB, Whelton PK, Sorond F, Carey RM. Blood pressure management in stroke. Hypertension. 2020;76;1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]


